Abstract
Background:
Epileptic seizures can occur throughout the course of multiple sclerosis (MS) and are associated with increasing disability progression over time. However, there are no data on whether epileptic seizures at the onset of MS also lead to increasing disability.
Objective:
To examine disease progression over time for MS patients with epileptic seizures at onset.
Methods:
We analyzed the data of 30,713 patients on the German Multiple Sclerosis Register in a case–control study for more than 15 years. MS patients with seizures at onset were further divided into subgroups with polysymptomatic and monosymptomatic onset to assess the impact of additional symptoms on disease progression.
Results:
A total of 46 patients had seizures as onset symptoms. Expanded Disability Status Scale (EDSS) within the first year was lower in the group with seizures at onset compared to controls (0.75 versus 1.6, p < 0.05), which changed until the last reported visit (3.11 versus 3.0). Both subgroups revealed increased EDSS progression over time compared to controls.
Conclusion:
Epileptic seizures at MS onset are associated with a higher amount of disability progression over time. Additional longitudinal data are needed to further clarify the impact of seizures on the pathophysiology of MS disease progression.
Keywords: epidemiology, epilepsy, multiple sclerosis
Introduction
Epileptic seizures occur in approximately 2–3% of patients with multiple sclerosis (MS), which exceeds the risk threefold compared to the general population.1–3 Comorbid epilepsy in MS is associated with disability, disease duration, and a higher extent of disease progression, all leading to increased mortality.3,4 The underlying cause of epilepsy is still a matter of debate, but imaging studies have suggested that cortical lesions, especially in the temporal lobe, are associated with a higher risk of epileptic seizures in MS.5,6 In addition to this disease-specific structural alteration, epileptic seizures can occur due to alternative etiologies like a traumatic brain injury or cerebral ischemia, which altogether account for approximately half of the cases in which both MS and epilepsy are present. 7
In the majority of patients, epileptic seizures have occurred after the diagnosis of MS. However, in a small number of cases, seizures emerged before an MS diagnosis, or they are the first manifestation of the disease. In a Norwegian study with a cohort of 19 patients with MS and epilepsy, one patient had epilepsy before the onset of MS and one at the onset 8 ; in a French cohort of 102 patients, 26 had epileptic seizures before MS and 7 at the onset 9 ; and in a German cohort of 59 patients, 22 patients had epilepsy before the onset of MS. 7
Several cohort studies outlined the positive association between epilepsy prevalence in MS and the Expanded Disability Status Scale (EDSS).2,3,10 The association between epilepsy and disability progression over time was particularly confirmed by a large register-based study. 3 The effect of epileptic seizures at the onset of MS on disease progression has rarely been investigated so far. In a very recent study by Selton et al., 11 MS patients with epileptic seizures at the onset of MS did not have a different disease progression compared to the MS control group.
Apart from the temporal relationship between MS and epilepsy, epileptic seizures theoretically can occur in patients with preexisting neurological symptoms such as motor or sensory impairment, or the absence of objective focal neurological disturbances due to clinical inapparent lesions. While it must be assumed in the former that there is already structural cortical damage, at least in motor or sensory areas, the number of cortical alterations that lead to epileptic seizures cannot be clarified in the latter. In the only study with data on EDSS in a group of patients with epileptic seizures as the first event of MS, the mean EDSS was 1.0, suggesting at least a minimal structural alteration. 11 Whether the presence of clinically measurable disability has any implication on the long-term disability progression remains unknown.
Here, we used the German Multiple Sclerosis Register (GMSR) with longitudinal data from more than 30,000 patients with MS to investigate patients with epileptic seizures as a symptom of onset. Because we were interested in differences between patients with or without preexisting disability, we further compared the subgroups of MS patients with seizures at MS onset as their only symptom and patients with further onset symptoms to examine whether differences at onset might influence disease progression over time.
Materials and methods
Study cohort
This analysis was based on the GMSR. The GMSR is a national database that was established in 2001 by the German MS Society (Deutsche Multiple Sklerose Gesellschaft, Bundesverband e.V., Hannover, Germany).12–14 All patients provided their written informed consent, and all data were collected in accordance with the Declaration of Helsinki. The registry has received ethical approval from independent local ethics committees.
Based on a data export from the GMSR (1 December 2021, 36,292 patients), a cohort of patients was defined with records of epilepsy and/or seizures as a symptom of clinical MS onset. Symptomatology of epilepsy is recorded via a sub-form of the ‘other symptoms’ option within the routinely collected MS onset symptoms in the GMSR. Predefined MS onset symptomatology includes the following domains: visual, brainstem, pyramidal, cerebellar, sensory, bladder, bowel, sexual dysfunction, and depression. Patients with unknown or incomplete onset symptomatology were excluded from the study (Figure 1).
Figure 1.
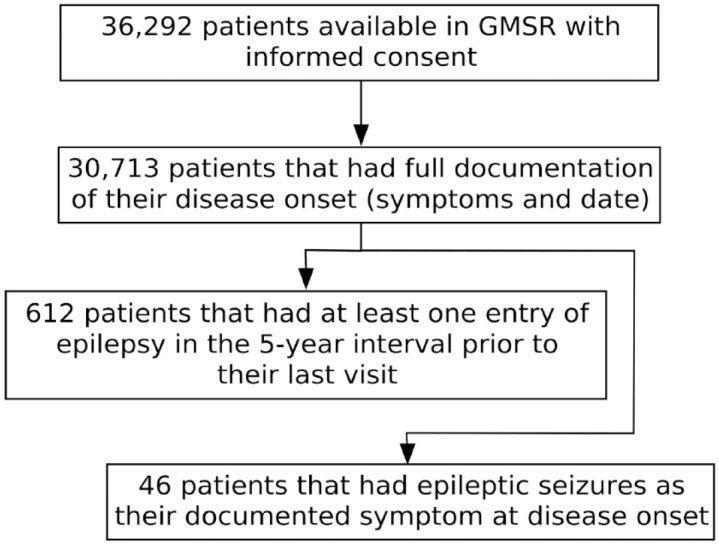
Flowchart showing the applied inclusion criteria.
GMSR, German MS register; N, number of patients.
Statistical analyses
Group characteristics and comparisons between people with MS with seizures as an onset symptom (MSSO) and people with MS without seizures at onset as controls (MSS-) were analyzed at the last follow-up (last entry into the database).
Subgroups of MSSO were formed based on the availability of further onset symptoms (e.g. a subgroup with epilepsy along other domains of onset symptoms) and were considered as patients with polysymptomatic onset (MSSOpoly). Conversely, patients with epilepsy as the only symptom at onset were considered monosymptomatic (MSSOmono).
Variables of interest were the age at onset, age at last follow-up, sex, time to diagnosis, EDSS scores, EDSS milestones, disease course, symptoms at onset, symptoms at last visit (including walking impairment), and working status.
Group comparisons were performed on cohorts matched by sex, age at onset, and current age to adjust for baseline inequalities, based on a 100:1 matching 15 since the number of controls was much higher than for the rare MSSO condition.
For comparison of disease progression, confirmed EDSS scores were collected from each patient. For long-term progression, we restricted analyses to patients who had documented scores over at least 15 years of disease duration. Time to disability level by EDSS milestones 4.0 and 6.0 was assessed as intervals between visits when the attainment of these levels occurred. Methods for interval-censored data were used to estimate disease progression as time-to-event analyses. Sensitivity analyses included generalized additive regression models for attainment of milestones as binomial endpoint as well as raw EDSS values.
Descriptive analyses were performed using R 4.0.5 (R Foundation for Statistical Computing, Vienna, Austria, including the packages: optmatch_0.9–13, compareGroups_4.4.1, mgcv_1.8-31, ggplot2_3.3.0, beeswarm_0.2.3, survival_3.2-10, icenReg_2.0.15) and statistical inference (i.e. confidence intervals) was carried out at a (descriptive) 5% type I error level.
Results
Demographics and prevalence
Datasets from 30,713 patients were analyzed with sufficient documentation of onset and clinical course. Out of these, 612 patients had at least one recorded entry of epilepsy in the 5-year interval prior to their last visit, resulting in a 5-year prevalence of 2% (Figure 1).
Out of the 30,713 patients, 46 MS patients had epileptic seizures as their documented symptom at disease onset. Prior to matching, MSSO patients had a numeric earlier disease onset compared to the controls without epilepsy [mean age 31.6 (±10.3) versus 33.1 (±10.7), respectively, p = 0.3].
Group comparison between MS patients with epilepsy at onset, monosymptomatic and polysymptomatic, and matched controls without epilepsy
MSSO patients typically differed without meeting statistical significance in onset symptoms other than epilepsy when compared to the matched MSS- control group (see Table 1). At their last visit, MSSO patients had more walking impairments (59%) on average compared to the MSS- patient controls (49%), albeit not statistically significant (p = 0.2). The EDSS within the first year was significantly lower in MSSO (mean 0.75) compared to the controls (mean 1.6, p = 0.019), which was no longer significant after 3 years (1.75 versus 2.07, p = 0.6), after 15 years (2.60 versus 2.90, p = 0.8), and at the last reported visit (3.11 versus 3.0, p = 0.8). 60% of MSSO patients were working at the last follow-up compared to 69% in MSS- (p = 0.3).
Table 1.
Patient characteristics including onset symptomatology stratified by groups.
| Variable | MSSO n = 46 |
Matched group n = 4600 |
p | MSSOpoly n = 31 |
Matched group n = 3100 |
p | MSSOmono n = 15 |
Matched group n = 1500 |
p | |
|---|---|---|---|---|---|---|---|---|---|---|
| Matching variables | Sex (female) | 67% | 67% | 1 | 81% | 81% | 1 | 40% | 40% | 1 |
| Age onset | 31.6 (10.3) | 31.7 (9.98) | 0.9 | 31.6 (9.56) | 31.6 (9.21) | 1 | 31.7 (12.1) | 31.9 (11.4) | 1 | |
| Age (last) | 45.2 (10.9) | 45.3 (10.7) | 0.9 | 45.8 (10.6) | 45.8 (10.3) | 1 | 44.1 (11.4) | 43.9 (11.7) | 0.9 | |
| Symptoms at onset (%) | Visual | 22 | 40 | 0.012 | 36 | 41 | 0.6 | – | 38 | – |
| Brainstem | 15 | 21 | 0.3 | 23 | 21 | 0.8 | – | 21 | – | |
| Motor | 36 | 38 | 0.8 | 53 | 38 | 0.11 | – | 37 | – | |
| Cerebellar | 26 | 22 | 0.6 | 39 | 22 | 0.074 | – | 22 | – | |
| Sensible | 22 | 58 | <0.001 | 35 | 59 | 0.018 | – | 57 | – | |
| Bladder dysfunction | 7 | 8 | 0.9 | 12 | 9 | 0.6 | – | 7 | – | |
| Depression | 17 | 14 | 0.7 | 26 | 15 | 0.2 | – | 13 | – | |
| Polysymptomatic | 67 | 42 | <0.001 | 100 | 41 | – | – | 43 | – |
MS, multiple sclerosis; MSSO, MS patients with seizures as an onset symptom; MSSOmono, patients with seizures as the only symptom at onset; MSSOpoly, patients with polysymptomatic onset.
In the subset MSSOpoly, differences, most of which were not statistically significant, could be obtained in onset symptoms and symptoms at the last visit, with a strong trend of a higher rate of walking impairments in MSSOpoly (68%) compared to MSS- (50%, p = 0.051). 58% of MSSOpoly were working at the last reported visit compared to 67% in MSS- (p = 0.4).
In the subset MSSOmono, patients, per definition, had no additional onset symptoms. The mean time to diagnosis was 1.46 in MSSOpoly and 1.02 in MSSOmono, while it was 1.71 (/1.65) in the matched groups. At the last visit, MSSOmono had significantly lower rates of spasticity (7%), pain (7%), and cognitive dysfunction (7%) compared to MSS- (29%, 24%, and 25%, respectively). A total of 64% of MSSOmono were working at the last reported visit compared to 72% in MSS- (p = 0.6). See Supplemental Table 1 for further details.
Disease progression between patients with epilepsy at onset and without epilepsy
A detailed analysis of EDSS progression over a 15-year period post-onset of MSSOpoly and MSSOmono compared to the matched MSS- groups is shown in Figures 2 and 3.
Figure 2.
Long-term comparison between MSSOpoly (a) and matched controls (b). The red/gray points in the figures show the raw EDSS values as beeplot (left y-axis). Red points indicate the first observation per patient. The solid lines show estimated proportions of patients who have not yet reached EDSS milestones of 4 (yellow) and 6 (orange; right y-axis).
EDSS, Expanded Disability Status Scale; MSSOpoly, multiple sclerosis patients with polysymptomatic onset.
Figure 3.
Long-term comparison between monosymptomatic MSSOmono (a) and matched controls (b). See Figure 2 for details.
MSSOmono, multiple sclerosis patients with seizures as the only symptom at onset.
The EDSS within the first year was significantly lower in MSSOpoly (mean 1.12) compared to the controls (mean 1.57, p = 0.028), which was not significant after 3 years (2.25 versus 2.16, p = 0.9), after 15 years (3.67 versus 2.95, p = 0.8), and at the last reported visit (3.60 versus 3.07, p = 0.22). For MSSOpoly, 0% of patients had an EDSS ⩾ 6.0 at disease onset, compared to 1% in controls. After 15 years of disease, 33% of MSSOpoly and 14% of controls had an EDSS ⩾ 6.0.
The EDSS within the first year was lower in MSSOmono (mean 0.0) compared to controls (mean 1.63), which was not significant after 3 years (1.25 versus 1.96, p = 0.5), after 15 years (1.00 versus 2.83, p = 1.0), and at last reported visit (2.13 versus 2.88, p = 0.2). For MSSOmono, 0% of patients had an EDSS ⩾ 6.0 at disease onset, compared to 1% in controls. After 15 years of disease, 0% of MSSOmono and 11% of controls had an EDSS ⩾ 6.0.
Discussion
For the first time, we have investigated MS patients with epileptic seizures at the onset of MS systematically both with respect to disability at disease onset and longitudinally. We revealed that patients with epileptic seizures at disease onset had a lower time to the diagnosis of MS, especially if seizures were not accompanied by other symptoms. With lower mean EDSS at MS onset and higher mean EDSS at the last entry, we demonstrated that epileptic seizures as onset symptoms are associated with an increase in disability over time compared to MS patients without epileptic seizures.
Out of the subgroup of 612 patients with MS and epilepsy, 46 had seizures at disease onset. Both the prevalence of epileptic seizures in MS patients and the prevalence of seizures as onset symptoms were comparable to the data of previous studies,2,3,8,9 with our data as the largest MS dataset in this field so far.
In this cohort, the age of patients with an epileptic seizure at onset was somewhat lower at MS onset compared with the age of patients without epileptic seizures at onset and compared with the general MS population in various MS registries. 14 Diagnostic approaches were made by neurologists with expertise in MS, but we cannot explicitly exclude that MS patients in our cohort might have had additional disorders leading to epileptic seizures. However, most other causes would have been detected on follow-up imaging or imaging at diagnosis. Furthermore, alternative causes of epilepsy, like cerebral ischemia, would occur at a later age, whereas others, like idiopathic epilepsies, would occur earlier.
The mean time to the diagnosis of MS was lower in patients with seizures at onset compared to the matched control group, which is especially interesting because the onset symptoms in almost all domains were either comparable or at a lower rate compared to patients in the control group. The neurological diagnostics after an epileptic seizure therefore seem to lead to a diagnostic setting that is sensitive to further clinical or paraclinical alterations leading to the diagnosis of MS, especially because the diagnostic in these patients usually contains an MRI. Interestingly, the highest difference in time to a diagnosis was seen in the patients with isolated epileptic seizures, with the diagnosis of MS after 1 year. Whether these differences are also due to the higher rate of a specific neurological diagnosis, or even neurological consultation, compared to other symptoms such as visual or sensory disturbances would be speculative, but quite conceivable.
The overall group of MS patients with seizures at onset did not show a different disability in the long-term comparison, which is in line with very recent data. 11 Furthermore, dividing the group of MS patients with seizures at onset into the polysymptomatic and monosymptomatic subgroups revealed different results in the long-term progression. In the polysymptomatic group, including patients with additional neurologic symptoms besides seizures at MS onset, most symptoms at onset and all symptoms at the last examination were more frequent compared to the control group, leading to a higher EDSS at the last visit. This is comparable to the data of MS patients with epileptic seizures in the later phase of MS, 3 suggesting that the higher amount of cortical pathology might drive disease activity. Furthermore, the polysymptomatic onset patients had a lower EDSS after 1 year of disease, with a higher difference in EDSS at the last visit with 2.5 EDSS points compared to 1.5 points in the matched control group, which was comparable to the EDSS differences in the monosymptomatic group with 2.1 EDSS points compared to 1.2 points. The EDSS at the last visit was lower in MSSOmono than in the control group, but the same EDSS difference was demonstrated between the first and last registry entries in the polysymptomatic group. This can be interpreted that MS patients in whom seizures are the first symptoms are in a very early stage of MS, with already existing disease activity leading to a loss of function, but which cannot be quantified by the usual measures such as the EDSS. Whether these patients have neurologic or neuropsychologic impairments that are not quantifiable with the EDSS is speculative, but there are some methodological concerns with the EDSS16–18 as well as data suggesting that patients with a radiologically isolated syndrome have measurable cognitive deficits similar to patients with a clinically isolated syndrome, 19 domains to which the EDSS is not sensitive. However, both the polysymptomatic and monosymptomatic MS patients had a lower rate of employment, additionally suggesting that these patients have a more progressive disease course. These results are somewhat different compared to very recent data showing no different disease progression in MS patients with an initial epileptic seizure compared to controls. 11 Unlike to our cohort, the cohort investigated by Selton et al. only included patients with preexisting (although very moderate) disability with an EDSS of 1. We here demonstrated that patients with an abnormal neurological status as measured by the EDSS, compared to patients without any deficit, that is, monosymptomatic, has an influence on the long-term prognosis, which might at least in part explain the different results.
This study has several limitations that should be considered. The main limitation is the small sample size. As there is no systematic assessment of epileptic seizures in MS, large registers like the GMSR are the best way to collect and assess commonalities and differences with regard to rare constellations like epileptic seizures at MS disease onset, but even with this approach, the statistical power is limited. Furthermore, register-based data collection is always performed in multiple centers with data heterogeneity, and epileptic seizures are recorded on the register without additional information about their frequency, alternative causes, or any other biomarkers. Furthermore, we did not consider different inclusion times with resulting approved drugs and their influence on disease progression. However, this cohort represents the largest real-world data set on this topic so far, provided by neurologists specializing in MS and assessed by our research group, specializing in register-based research for several decades.13,14 Based on the study design, we were not able to explain the pathophysiological causes of epileptic seizures and their role in disease progression in MS. Furthermore, we cannot exclude the possibility that other causes may have led to epileptic seizures. Epilepsy as a cortical network disorder 20 and imaging studies suggesting the role of cortical lesions in epilepsy in MS5,6 indicate that cortical pathology might be the leading cause, but more research, especially a combination of more detailed clinical, biomarker, and imaging research, is needed for a better understanding of these pathophysiological processes.
Supplemental Material
Supplemental material, sj-docx-1-tan-10.1177_17562864231192826 for Epileptic seizures at multiple sclerosis onset and their role in disease progression by Matthias Grothe, David Ellenberger, Paulus S. Rommer, Alexander Stahmann and Uwe K. Zettl in Therapeutic Advances in Neurological Disorders
Acknowledgments
We would like to thank all patients who have given their informed consent. Furthermore, this study would not have been possible without the efforts of the centers participating in the registry.
Footnotes
ORCID iDs: Matthias Grothe  https://orcid.org/0000-0002-7998-5310
https://orcid.org/0000-0002-7998-5310
Paulus S. Rommer  https://orcid.org/0000-0001-5209-6647
https://orcid.org/0000-0001-5209-6647
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Matthias Grothe, Department of Neurology, University Medicine Greifswald, Ferdinand-Sauerbruchstraße, Greifswald 17475, Germany.
David Ellenberger, German MS Register by the German MS Society, MS Research and Project Development gGmbH [MSFP], Hanover, Germany.
Paulus S. Rommer, Department of Neurology, Medical University of Vienna, Vienna, Austria Department of Neurology, Neuroimmunological Section, University of Rostock, Rostock, Germany.
Alexander Stahmann, German MS Register by the German MS Society, MS Research and Project Development gGmbH [MSFP], Hanover, Germany.
Uwe K. Zettl, Department of Neurology, Neuroimmunological Section, University of Rostock, Rostock, Germany
Declarations
Ethics approval and consent to participate: No dedicated ethics approval is required for this study, as it is covered by the ethics approval of the GMSR as a registry (IRB Julius-Maximilians-University of Würzburg; number of vote 142/12). The same applies to the informed consent of patients.
Consent for publication: Not applicable.
Author contributions: Matthias Grothe: Conceptualization; Writing – original draft; Writing – review & editing.
David Ellenberger: Formal analysis; Methodology; Visualization; Writing – review & editing.
Paulus S. Rommer: Writing – review & editing.
Alexander Stahmann: Project administration; Writing – review & editing.
Uwe K. Zettl: Supervision; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: MG received honoraria and travel reimbursements for attending meetings from Biogen, Celgene, Merck Serono, Novartis, Roche, Sanofi Genzyme, and TEVA. His research is funded by the German Ministry for Education and Research (BMBF), Deutsche Forschungsgemeinschaft (DFG), Merck Serono, and Novartis. None resulted in a conflict of interest. DE has nothing to disclose. AS has no personal pecuniary interests to disclose, other than being the lead of the German MS Registry, which receives (project) funding from a range of public and corporate sponsors, recently including The German Innovation Fund (G-BA), The German Retirement Insurance, The German MS Trust, The German MS Society, Biogen, BMS, Merck, Novartis, Roche, and Sanofi. None resulted in a conflict of interest. UKZ received honoraria and travel reimbursements for attending meetings from Alexion, Almirall, Bayer Health Care, Celgene, Sanofi Genzyme, Merck Serono, Novartis, Roche, and TEVA. His research is funded by the German Ministry for Education and Research (BMBF), the German Ministry for Economy (BMWi), Deutsche Forschungsgemeinschaft (DFG), and the European Union (EU). None resulted in a conflict of interest.
Availability of data and materials: Data of this study are accessible upon reasonable request by any qualified investigator under terms and conditions of the registry’s use-of-access policies and subject to informed consent of patients.
References
- 1. Marrie RA, Reider N, Cohen J, et al. A systematic review of the incidence and prevalence of sleep disorders and seizure disorders in multiple sclerosis. Mult Scler 2015; 21: 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burman J, Zelano J. Epilepsy in multiple sclerosis: a nationwide population-based register study. Neurology 2017; 89: 2462–2468. [DOI] [PubMed] [Google Scholar]
- 3. Grothe M, Ellenberger D, von Podewils F, et al. Epilepsy as a predictor of disease progression in multiple sclerosis. Mult Scler 2022; 28: 942–949. [DOI] [PubMed] [Google Scholar]
- 4. Mahamud Z, Burman J, Zelano J. Prognostic impact of epilepsy in multiple sclerosis. Mult Scler Relat Disord 2020; 38: 101497. [DOI] [PubMed] [Google Scholar]
- 5. Calabrese M, Grossi P, Favaretto A, et al. Cortical pathology in multiple sclerosis patients with epilepsy: a 3 year longitudinal study. J Neurol Neurosurg Psychiatry 2012; 83: 49–54. [DOI] [PubMed] [Google Scholar]
- 6. Calabrese M, Castellaro M, Bertoldo A, et al. Epilepsy in multiple sclerosis: the role of temporal lobe damage. Mult Scler 2017; 23: 473–482. [DOI] [PubMed] [Google Scholar]
- 7. Neuss F, von Podewils F, Wang ZI, et al. Epileptic seizures in multiple sclerosis: prevalence, competing causes and diagnostic accuracy. J Neurol 2021; 268: 1721–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Benjaminsen E, Myhr KM, Alstadhaug KB. The prevalence and characteristics of epilepsy in patients with multiple sclerosis in Nordland county, Norway. Seizure 2017; 52: 131–135. [DOI] [PubMed] [Google Scholar]
- 9. Catenoix H, Marignier R, Ritleng C, et al. Multiple sclerosis and epileptic seizures. Mult Scler 2011; 17: 96–102. [DOI] [PubMed] [Google Scholar]
- 10. Gasparini S, Ferlazzo E, Ascoli M, et al. Risk factors for unprovoked epileptic seizures in multiple sclerosis: a systematic review and meta-analysis. Neurol Sci 2017; 38: 399–406. [DOI] [PubMed] [Google Scholar]
- 11. Selton M, Mathey G, Soudant M, et al. Prognostic impact of epileptic seizures in multiple sclerosis varies according to time of occurrence and etiology. Eur J Neurol 2022; 29: 3537–3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rommer PS, Eichstadt K, Ellenberger D, et al. Symptomatology and symptomatic treatment in multiple sclerosis: results from a nationwide MS registry. Mult Scler 2019; 25: 1641–1652. [DOI] [PubMed] [Google Scholar]
- 13. Flachenecker P, Zettl UK, Gotze U, et al. [MS registry in Germany–design and first results of the pilot phase]. Nervenarzt 2005; 76: 967–975. [DOI] [PubMed] [Google Scholar]
- 14. Ohle LM, Ellenberger D, Flachenecker P, et al. Chances and challenges of a long-term data repository in multiple sclerosis: 20th birthday of the German MS registry. Sci Rep 2021; 11: 13340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hansen B, Fredrickson M, Pinelis Y. Matching in R using the optmatch and RItools packages, https://dept.stat.lsa.umich.edu/~bbh/cscarworksheet.pdf
- 16. Meyer-Moock S, Feng YS, Maeurer M, et al. Systematic literature review and validity evaluation of the Expanded Disability Status Scale (EDSS) and the Multiple Sclerosis Functional Composite (MSFC) in patients with multiple sclerosis. BMC Neurol 2014; 14: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ellenberger D, Flachenecker P, Haas J, et al. Is benign MS really benign? What a meaningful classification beyond the EDSS must take into consideration. Mult Scler Relat Disord 2020; 46: 102485. [DOI] [PubMed] [Google Scholar]
- 18. Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: the application of psychometric methods to clinical intuition. Brain 2000; 123: 1027–1040. [DOI] [PubMed] [Google Scholar]
- 19. Labiano-Fontcuberta A, Martinez-Gines ML, Aladro Y, et al. A comparison study of cognitive deficits in radiologically and clinically isolated syndromes. Mult Scler 2016; 22: 250–253. [DOI] [PubMed] [Google Scholar]
- 20. Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist 2012; 18: 360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tan-10.1177_17562864231192826 for Epileptic seizures at multiple sclerosis onset and their role in disease progression by Matthias Grothe, David Ellenberger, Paulus S. Rommer, Alexander Stahmann and Uwe K. Zettl in Therapeutic Advances in Neurological Disorders




