Abstract
Along with expanding urbanization and industrialization, environmental pollution which negatively affects the surroundings, has been rising quickly. As a result, induces heavy metal contamination which poses a serious threat to living organisms of aquatic and soil ecosystems. Therefore, they are a need to ameliorate the effects cost by cost pollution on the environment. In this review, we explore methods employed to mitigate the effects caused by heavy metals on the environment. Many techniques employed to manage environmental pollution are tedious and very costly, necessitating the use of alternative management strategies to resolve this challenge. In this concept, bioremediation is viewed as a future technique, due to its environmental friendliness and cost-effective measures aligned with sustainable or climate-smart agriculture to manage contaminants in the environment. The technique involves the use of living entities such as bacteria, fungi, and plants to deteriorate toxic substances from the rhizosphere. Currently, bioremediation is thought to be the most practical, dependable, environmentally benign, and long-lasting solution. Although bioremediation involves different techniques, they are still a need to find the most efficient method for removing toxic substances from the environment. This review focuses on the origins of heavy metal pollution, delves into cost-effective and green technological approaches for eliminating heavy metal pollutants from the environment, and discusses the impact of these pollutants on human health.
Keywords: Anthropogenic activities, bioremediation, green technology, heavy metal, environmental pollution, living entities, phytoremediation
Introduction
Globally, the Industrial Revolution played a significant impact on the development of the economies of many different countries because it changed an economy that was predominately based on agriculture and handicrafts into one that was dominated by industry and machine manufacturing.1,2
In India, the Industrial Revolution had a pivotal role in the economic rise of developing countries. In the case of India, the Industrial Revolution commenced post-1850 and notably bolstered the rural economy. 3 However, this period of scientific and technological development had concomitantly brought pros and cons in the long run. As a result, it led to unprecedented outcomes due to human activities which were first ignored until the publication of Silent Spring by Rachel Carson on September 27, 1962. 4 The Silent Spring unveiled the mystery behind the use of synthetic chemical inputs and its negative impact on the environment. 5 Their effects were environmental pollution that was formally categorized as anthropogenic activities,6 -9 resulting from the dumping of industrial, home trash, and synthetic agricultural inputs. Therefore, to sustain the environment, alternative methods need to be employed to mitigate the effects induced by synthetic chemicals on the environment.
The anthropogenic activities, primarily emanating from the agricultural, industrial, and urbanization side, are currently releasing contamination to the rhizosphere or atmosphere which includes accumulation of heavy metals and other toxic fumigant chemicals that pose an environmental threat. 10 Heavy metal contamination is considered as one of the most critical environmental issues that reduce crop productivity and directly or indirectly jeopardizes the survival of almost all types of living entities on the planet. 11 The toxic metals absorbed by plants result in chemical residues on marketable produce causing mutagenic reactions which result in cancer in human beings. 12 Because wildlife depends on plants, they are also affected by heavy metal pollution, which disturbs the balance of mother nature and reduces biodiversity. 13 On the other side, pesticides used in plant protection also kill or affect the reproductive potential of untargeted organisms like beneficial nematodes, insects, birds and earthworms. 14 The prevention of heavy metal infiltration into terrestrial, atmospheric, and aquatic habitats as well as the remediation of damaged land are therefore imperative.
Heavy metals are a distinct group of metals that possess comparatively high densities, atomic numbers, and atomic weights within the periodic table.15,16 Typically, heavy metals are non-biodegradable and persist in the environment for several decades. 17 Heavy metals such as mercury (Hg), cadmium (Cd), lead (Pb), chromium (Cr), and arsenic (As) are considered to pose a significant threat to untargeted living entities due to their toxicity character, even at low concentrations. 18 As a result, bioremediation is viewed as a future technique to ameliorate the effects caused by pollution on the environment due to anthropogenic activities. This technique is suitable for remediating contaminants and it is eco-friendly. 19 Bioremediation involves the use of living entities such as bacteria: Acinetobacter sp., 20 Alcaligenes odorans, 21 Bacillus subtilis, 22 Corynebacterium propinquum, 23 Microbacterium sp., 24 Pseudomonas sp., P. putida, P. aeruginosa, 25 and Ralstonia sp. 26 to deteriorate toxic substances from the rhizosphere as well as the atmosphere. 27 It also employs the use of plants, technically known as green biotechnology where Brassica juncea, 28 Helianthus annuus, 29 Pteris vittate, 30 Salix viminalis, 31 and Solanum lycopersicum 32 plants were employed and shown the ability to extract or reduce heavy metals from the soil. This review summarizes a variety of bioremediation techniques, with a focus on their efficacy in thoroughly eradicating heavy metal pollution from the environment. It does so by doing a thorough analysis of the current literature.
The Principal Sources of Pollution
Heavy metals are released into the environment from various sources including mining, urbanization, chemical industry, sewage plants, pesticide plants, biomedical and unsafe agricultural practices (Figure 1) The United Nations Environment Program (UNEP/GPA) and the Global Plan of Action (GPA) recognize electronic waste (e-waste) which includes devices like mobile phones, tablets, computers, and smartwatches as a major threat to the environment and human well-being. This is primarily due to the presence of heavy metals like Hg, Cd, and Pb in electronic devices, which can pose serious risks to both the environment and human health if not properly disposed of UNEP/GPA 33 and Tchounwou et al. 34 The pollution levels of these heavy metals are influenced by industrial activities, geographic locations, regulatory oversight, and diverse sources. 35 For instance, Hg primarily emanates from coal combustion, electric/light bulb, wood preservatives, leather tanning, ointments, thermometers, adhesives and paints. 36 Cd often originates from industries like battery manufacturing, paint pigments, pesticides, galvanized pipes, plastics, polyvinyl and copper refineries. 37 Pb, an extremely toxic metal, commonly originates from substances like Pb-based paints, gasoline and mobile batteries. 38 Cr is emitted from a variety of industrial activities, including petroleum refining, electroplating, leather tanning, textile manufacturing, and pulp processing. 39 As, a naturally occurring element in the Earth’s crust, is released into the environment through a variety of human activities, including mining, agricultural practices, automobile exhaust and industrial dust, wood preservatives, and dyes. 40
Figure 1.
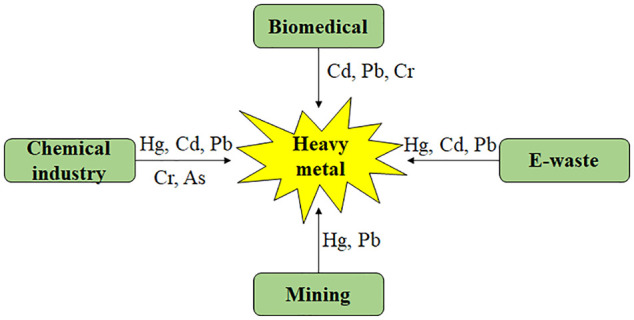
A schematic diagram illustrating the origins of heavy metal pollution.
Soil plays a vital role in supporting terrestrial ecosystems and their biodiversity. Heavy metals are prevalent pollutants within the soil environment, and their presence can adversely affect microorganisms, plants and animals. The European Environment Agency (EEA) has set limit values for soil pollutant levels of various heavy metals, including Hg (0.20 ppm), Cd (0.44 ppm), Pb (0.48 ppm), Cr (0.20 ppm), and As (0.11 ppm).41,42 According to World Health Organization (WHO) guidelines, the acceptable levels of heavy metal pollutants in drinking water are as stated: Hg—0.001 ppm, Cd—0.005 ppm, Pb—0.05 ppm, Cr—0.05 ppm, and As—0.05 ppm. 43 The Food and Agriculture Organization (FAO) of the United Nations (UN) and the WHO set maximum limits for the consumption of heavy metals, as higher levels can cause health problems. The permissible limits for heavy metals consumption through vegetables are as follows: Hg—0.05 mg/kg for all vegetables; Cd—0.2 mg/kg for leafy vegetables, 0.3 mg/kg for root vegetables, and 0.1 mg/kg for other vegetables; Pb—0.15 mg/kg for all vegetables; Cr—0.1 mg/kg for all vegetables, and As—0.1 mg/kg for all vegetables.44 -46
Managing Pollution
Several techniques are employed to decontaminate the environment from these pollutants and avert the entry of toxic metals into the environment. Nevertheless, these methods tend to be costly and exhibit suboptimal efficacy.47,48 The increasing concerns surrounding environmental contamination have initiated the development of suitable technologies to assess the presence and mobility of metals in soil, water, and wastewater (Figure 2). Private and government institutions face a technical challenge to removing contaminants from the environment. Phytoremediation has emerged as a popular and economical plant-based technology for effectively addressing environmental issues. The process entails utilizing plants to extract and remove elemental pollutants or lower their bioavailability in soil or water. 49 In modern science, this technology is widely accepted due to its eco-friendliness, affordability, and high effectiveness. 50 Phytoremediation takes advantage of the unique and selective uptake capabilities of plant root systems, coupled with the translocation, bioaccumulation, and contaminant degradation abilities of the entire plant body. 51 Both aquatic and terrestrial plant species have been harnessed to eliminate pollutants from the environment. 52
Figure 2.
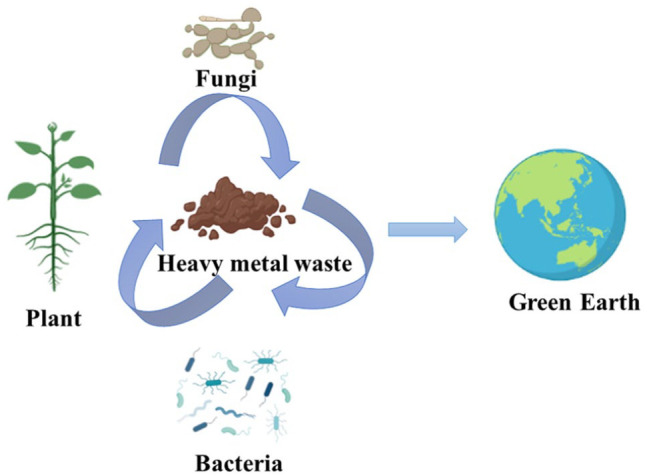
An illustrative diagram elucidating bioremediation, highlighting the crucial roles of plants, bacteria, and fungi (Created with BioRender.com).
More than 400 species have been identified as metal accumulators of Hg, Cd, Pb, Cr, As, and various radionuclides from contaminated soils (Tables 1–4). Arabidopsis sp. is well known for its metal tolerance and hyperaccumulation of Zn. 53 Aquatic plant species such as Azolla pinnata, Ceratophyllum demersum, Eichhornia crassipes, Lemna minor, Myriophyllum spicatum, Nasturtium officinale, Pistia stratiotes, Potamogeton pectinatus, Phragmites, Salvinia herzogii, Salvinia minima, Spirodela intermedia, Scirpus spp., and Typha latifolia, are of particular importance due to their high contaminant removal capacity.54 -58
Table 1.
Heavy metal accumulation in plants.
| Plant name | Contaminant | References |
|---|---|---|
| Arabidopsis halleri | Cd | Grignet et al 59 |
| Brassica juncea | Pb | Rathika et al 60 |
| Mentha aquatic | Cd, Pb | Zhang et al 61 |
| Nicotiana tabacum | Cd | Yang et al 62 |
| Pteris vittata | As | Zhu et al 63 |
| Salix spp. | Cd | Yang et al 64 |
| Sesbania drummondii | Pb | Valenti et al 65 |
| Solanum nigrum | Cd, Pb | He et al 66 and Li et al 67 |
| Tagetes patula | Cd, Pb | Zhang et al 61 |
| Vigna unguiculata | Pb | Narayanan et al 68 |
| Zeamays | Pb | Huang et al 69 |
Table 2.
Heavy metal accumulation in aquatic plants.
Table 3.
Heavy metal accumulation in genetically modified plants.
Table 4.
Heavy metal accumulation in ornamental plants.
Mechanism of phytoremediation
Phytoremediation encompasses several processes, including phytoextraction, phytoaccumulation, phytovolatilization, phytostabilization, and phytotransformation (Figure 3). 49 Phytoextraction is a technique that involves the absorption of organic and inorganic pollutants through the roots and stems. Besides, some particular plant species, like Brassica juncea, Cassia alata, Celosia argentea, Kummerowia striata, Helianthus annuus, Momordica charantia, Nicotiana tabacum, Salix mucronata, Salix viminalis, Solanum lycopersicum, Solanum melongena, Swietenia macrophylla, Pteris vittata, and Vigna unguiculata, have the potential to be used as suitable plant selections to enhance the phytoextraction process.83 -86 In phytostabilization, in this process, plants accumulate and immobilize heavy metals by binding with biomolecules. 87 Miscanthus giganteus, Avena sativa, and Sinapis alba can also help to stabilize heavy metal compounds in the soil. 88 There are several processes by which plants can reduce contaminants.
Figure 3.
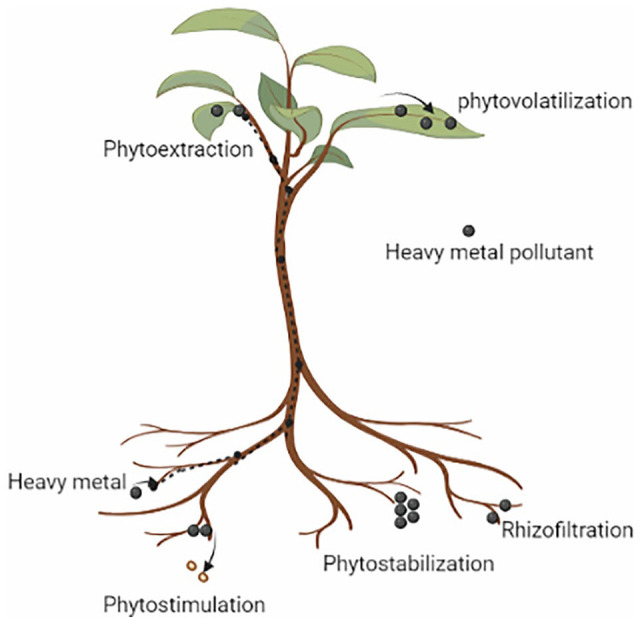
A schematic diagram depicting the underlying mechanisms of phytoremediation processes (Created with BioRender.com).
Phytoextraction
Phytoextraction, also called phytoaccumulation, involves the accumulation of heavy metals from earthland. In this method, the uptake and translocation of contaminants by plants root into the aerial portions of plants and deposited into vacuoles. The mechanism during the accumulation process is used to absorb and precipitate the toxic metals by metal-phytochelatin complex before translocating into the shoot, leaf and stem parts of the plant. The hyperaccumulator species accumulate a higher concentration of heavy metals.89,90
Rhizofiltration
Rhizofiltration involves the elimination of heavy metals using plant roots. Though it is comparable to phytoextraction, in this process, plants remove contaminants from wastewater or groundwater rather than soil. In this process, plant roots assimilate or adsorb pollutants from wastewater, groundwater, or surface water. Generally, aquatic plant species are employed to eliminate pollutants through rhizofiltration. Rhizofiltration is effective for removing Cd, Pb, and Cr, which are primarily accumulated in the roots. Sunflower, tobacco, and spinach exhibit promising potential in removing Pb from water. 91
Phytostabilization
Plant roots can limit the movement of heavy metals by phytostabilization, a process that reduces toxic effects. This process involves the capture of contaminants on the root surface using transport proteins or secondary metabolites. Furthermore, the process involves the breakdown of complex organic molecules into simpler ones by coupling them with protein, amino acid, and sugar derivatives. Black nightshade, sunflower, and cowpea are among the plant species that employ phytostabilization mechanisms. 92
Phytovolatilization
This process entails the uptake of contaminants by plants from the soil and their conversion into less toxic volatile compounds that are released into the atmosphere. The volatile compounds are primarily released from aerial plant parts such as stems and leaves. This mechanism is effective when the contaminants are less toxic. 93
Phytodegradation
Phytotransformation, also known as this process, refers to the absorption of contaminants by plants, which are then metabolized or broken down into less toxic compounds and translocated to various plant organs. The organic compounds are then degraded into non-toxic forms inside the plant tissue. 94
Microbial-assisted remediation of heavy metal
Microbial remediation is the process of using living microorganisms such as bacteria, fungi, and archaea to break down and detoxify various chemical and metallic hazardous wastes from the environment. 95 Bioremediation involves the direct application of microorganisms to the polluted site in order to facilitate the degradation of contaminants. Microorganisms are used in a variety of remediation techniques, including bioaugmentation and biostimulation. In bioaugmentation, specific microorganisms are added to a contaminated site to enhance the breakdown of contaminants. In biostimulation, the environmental conditions at the site are modified to promote the growth and activity of naturally occurring microorganisms that can degrade contaminants. Physical and chemical treatments are conventional remediation methods that have drawbacks such as high cost, heavy machinery, logistical glitches, and potential environmental toxicity. 96 In contrast, bioremediation technologies have seen significant growth and development, making it a promising method for treating soil and water contamination (Table 5). Among these methods, bioremediation of oil spills is the most lucrative and environment-friendly technique. 97
Table 5.
Bioremediation of heavy metal by microorganisms.
| Microorganism | Contaminant | References |
|---|---|---|
| Acinetobacter junii | As | Marwa et al 98 |
| Aeromonas sp. | Cr | Geng et al 99 |
| Aspergillus versicolor SPF-1 | Cd, Cr | Shukla et al 100 |
| Aspergillus fumigatus | Cd | Bhattacharya et al 101 |
| Bacillus flexus | As | Marwa et al 98 |
| Bacillus safensis | Cd | Li et al 102 |
| Cladosporium sp. | Cd | Văcar et al 103 |
| Cunninghamella elegans | Hg, Cd, Pb | Malik et al 104 |
| Microsporum sp. | Cd | Saini et al 105 |
| Lysinibacillus sphaericus CBAM5 | Pb, Cr | Páez-Vélez et al 106 |
| Paecilomyces sp. K32 | Cd | Pramanik et al 107 |
| Pseudomonas aeruginosa | Cr | Mat Arisah et al 108 |
| Pseudomonasfluorescens | Cd | Okpara-Elom et al 109 |
| Rhodopseudomonas palustris | Cr, Cd | Xiao et al 110 |
| Saccharomyces cerevisiae | Hg, Pb | Selamat et al 81 |
Fungi are used for the remediation of pollutants in mycoremediation, a type of bioremediation. Fungi play a vital role in cleaning up contaminated sites in both soil and aquatic ecosystems. 111 These microorganisms, which are widely present in nature, can thrive in a diverse range of environmental conditions. These microorganisms survive in extreme conditions and produce some extracellular ligninolytic enzymes like peroxidase and laccases. These enzymes help fungi to transform pollutants into non-toxic forms. Pollutants can be adsorbed by extracellular enzymes. 112 Diverse fungal species such as Aspergillus sp., Bjerkandera adusta, Coriolus versicolor, Cryptococcus sp. Hirschioporus laricinus, Inonotus hispidus, Mucor sp., Penicillium sp., Phanerochaete chrysosporium, Phlebia tremellosa, Phanerochaete chrysosporium, Pleurotus sp., and Trametes versicolor, have been reported for bioremediation.113,114
Role of genetical engineering microbes in bioremediation
The potential of microbes for bioremediation is vast but unexploited. Genetically engineered organisms are the best way to enhance bioremediation activity.115,116 Further research is required to formulate advanced bioremediation techniques in engineering that can effectively eliminate the complex mixtures of pollutants found at various sites. Several microbes use the contaminants as an energy source through their metabolic processes. Bacteria and fungi in the environment help to degrade or detoxify harmful substances. Modern science relies on biotechnology to facilitate the development of genetically modified organisms (GMOs), which can be instrumental in comparing them with their wild-type variant. GMOs possess the necessary protein machinery, which they utilize to uptake and regulate heavy metals through the implementation of gene regulatory elements such as promoters, binders, and terminators. These organisms produce a heavy metal binding protein that protects from toxicity by strongly binding to heavy metals (Figure 4). Mesorhizobium huakuii strain B3, produces phytochelatin protein which accumulates Cd as reported by Sriprang et al 117 . Bae et al 118 reported that P. putida 06909 produced metal-binding peptide (MBP) EC-20 that has a high affinity for Cd. Al Hasin et al 119 found that Methylococcus capsulatus can remediate Cr (VI). Wagner-Döbler 120 demonstrated that recombinant bacteria allow detoxifying Hg2+ to the non-toxic form of Hg0 through mercury reductase and subsequent release of Hg. The mechanism for detoxification of heavy metals is controlled by the mer operon gene that regulates transcription levels at both positive and negative. P. fluorescens HK44 was applied for large-scale field-based remediation of pollutants. 121 Patel et al 122 reported that the recombinant Caulobacter crescentus strain JS4022/p723-6H was able to eliminate Cd.
Figure 4.
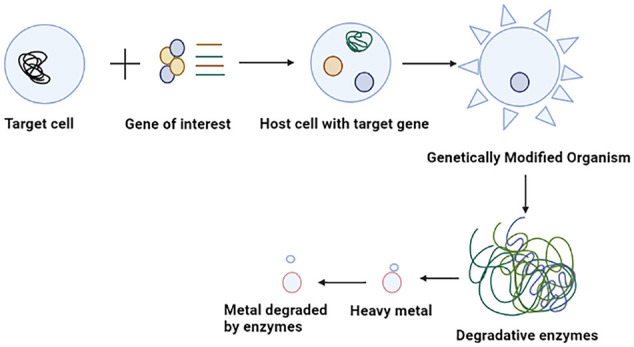
Illustrating the process of heavy metal degradation using genetically modified organisms (GMOs) (Created with BioRender.com).
According to several researchers, certain microbes can remove heavy metals from their environment by either accumulating them or developing a tolerance toward them. There are several microorganisms, including Acinetobacter sp., Alcaligenes odorans, Aspergillus niger (fungus), Aspergillus versicolor, Bacillus subtilis, Corynebacterium propinquum, Fomitopsis pinicola, Microbacterium sp, Pseudomonas sp., P. putida, P. aeruginosa, Ralstonia sp., and Streptomyces, that play a role in removing heavy metals.123 -126
Effect of Heavy Metal on Human Health
Certain edible crops can accumulate heavy metals, even in very small amounts. When these heavy metals enter our food chain, they disrupt the food pyramid and pose a threat to human health by causing cancer and liver diseases. Vegetables such as brinjal, gourd, spinach, coriander, tomato and pumpkin are particularly susceptible to heavy metal uptake by their roots, which can then be transported to the edible portions of the plant.127,128 As a result, consuming these vegetables that contain heavy metals can be extremely hazardous to human health. Alexander et al 129 carried out research involving vegetables cultivated in soil contaminated with heavy metals. Significant variations were observed among the vegetables in terms of the levels of metal accumulation. For Cd, lettuce exhibited a higher accumulation (8.6 mg/kg dry matter) compared to spinach (5.8 mg/kg dry matter), onion (3.6 mg/kg dry matter), carrot (2.0 mg/kg dry matter), pea (0.29 mg/kg dry matter), and French bean (0.07 mg/kg dry matter). Remarkably, lettuce recorded the highest concentration of Pb, nearly double that of onions, which held the second-highest average value. The sequence was as follows: lettuce (14.6 mg/kg dry matter) > onion (7.5 mg/kg dry matter) > carrot (5.8 mg/kg dry matter) > spinach (1.8 mg/kg dry matter) > pea (0.78 mg/kg dry matter) > French bean (0.34 mg/kg dry matter). A study conducted by Zhu et al, 63 revealed that the concentration of heavy metals in the edible parts of vegetables varied, with leafy vegetables having the highest amounts, followed by stalk vegetables, root vegetables, and solanaceous vegetables, and then legume vegetables and melon vegetables. Previous reports have also suggested that edible crops grown in industrial areas such as coal mines and petrochemical plants tend to contain higher levels of heavy metals. 130 Human exposure to heavy metals primarily occurs through the consumption of edible crops, which accounts for 90% of the exposure. The remaining 10% is attributed to the inhalation of polluted air particles as reported by Khan et al. 131
Excessive levels of heavy metals have the potential to pose harm to the body. They have the capacity to inflict damage on various organs such as the brain, muscles, nerves, liver, kidneys, and heart (Figure 5). Previous studies have specified that heavy metals can impair different organs within the human body, as illustrated in Table 6. The European Protection Agency (EPA) has reported that prolonged exposure to heavy metals can result in severe cancer. Research conducted by the WHO has shown that higher exposure to heavy metals puts 10% of women at risk of infertility.132,133
Figure 5.
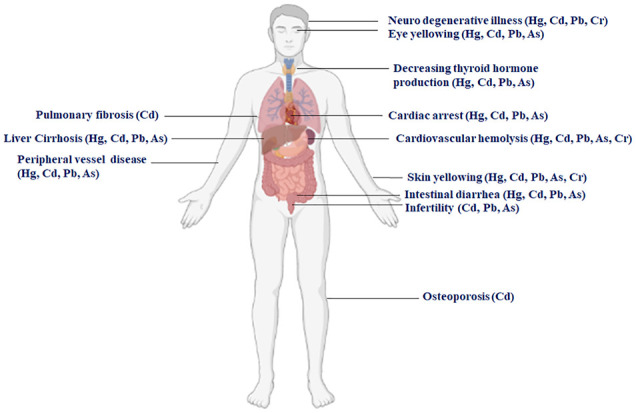
Illustrating the health implications of exposure to heavy metals on human well-being (Created with BioRender.com).
Table 6.
Effects of heavy metal contamination on human well-being.
| Heavy metals | Toxic effect | Reference |
|---|---|---|
| Hg, Cd, Pb, Cr | Neuro degenerative illness | Lee et al 134 and Wise et al 135 |
| Hg, Cd, Pb, As | Eye yellowing | Park 136 |
| Hg, Cd, Pb, As | Decreasing thyroid hormone production | Chen et al 137 |
| Hg, Cd, Pb, As | Cardiac arrest | Yang et al 138 |
| Hg, Cd, Pb, As, Cr | Cardiovascular hemolysis | Capitão et al 139 and Wilbur et al 140 |
| Cd | Pulmonary fibrosis | Hu et al 141 |
| Hg, Cd, Pb, As | Liver Cirrhosis | Kim et al 142 |
| Hg, Cd, Pb, As | Peripheral vessel disease | Patwa and Flora 143 |
| Hg, Cd, Pb, As, Cr | Skin yellowing | Balali-Mood et al 144 , Bissett et al 145 , Shimo et al 146 |
| Hg, Cd, Pb, As | Intestinal diarrhea | Balali-Mood et al 144 and Chen et al 147 |
| Cd, Pb, As | Infertility | Lei et al 148 and Lin et al 149 |
| Cd | Osteoporosis | Järup et al 150 |
Hg, a highly toxic metal found in air, water, and soil, is considered to be highly carcinogenic by the EPA. Hg exposure can result in various health problems, including Alzheimer’s disease, lung damage, and skin issues such as the common ailment. 151 Acrodynia is a common skin ailment in which skin color becomes pink. 152 Similarly, Cd is a highly toxic metal that causes bone damage and acute exposure can lead to renal dysfunction, while prolonged exposure to high levels of Cd can result in lung damage. Heavy metals such as these can also induce DNA damage, cause chromosome aberrations, and alter DNA replication and transcription.153 -155 Exposure to Cr over a long period can result in the formation of ulcers. Human activities have resulted in the contamination of the environment with heavy metals, which can have adverse effects on human health. Excessive uptake of heavy metals poses a significant threat to human health. The entering of heavy metals into the human body can initiate cancer by the production of reactive oxygen species (ROS) which mainly disrupts DNA molecules. Heavy metals can cause damage to specific organs within the human body. In an animal model of acute toxicity, Wister rats exposed to 1 mg/kg of Hg caused alterations in their kidneys. A study reported that oral exposure to Hg in rats resulted in diarrhea. Additionally, scientists found that guinea pigs exposed to 0.1 to 0.4 M of Pb increased serum endothelial and serum total protein levels, along with lung infection. 156 Male adult rats exposed continuously to Pb (0.4%) exhibited a significant reduction in white blood cell count, as reported by Mugahi et al. 157 Furthermore, the administration of Pb (10 mg/kg) was observed to increase the levels of lactate dehydrogenase and acid phosphatase in rats. 158 In rats, Patlolla et al 159 demonstrated that the administration of 10 mg/kg of Cr increased the levels of ROS and malondialdehyde in the liver and kidney. High doses of Cr(VI) caused the immune system to reduce, resulting in the development of allergic contact dermatitis.160,161 Fay et al 162 investigated Cd toxicity (0.6 mg/kg for 12 weeks) in the renal cortex of rats and found that Cd exposure significantly increased the volume of urine while decreasing the excretion of protein in urine. Cd toxicity can cause osteoporosis and bone fracture by increasing the dynamin-related protein, as demonstrated by Ma et al. 163 A close relationship between osteoporosis and high intake of Cd was also proven by Pouillot et al. 164
Conclusion
Recent technological advances have made bioremediation a more effective tool. This method is distinct and effective because it does not rely on chemicals or complex machinery. In the current study, bioremediation was shown to be a potential technique for resolving or reducing the negative effects of environmental contamination. Since it uses living entities to manage pollution, it cannot worsen the problem of heavy metal buildup or ozone depletion and is thought to be both environmentally friendly and economically effective, making it applicable to both emerging and developed nations globally. The results of the toxicity evaluation indicated that heavy metals constitute a substantial threat to living entities that are not specifically targeted. Therefore, funding ongoing research and innovation in bioremediation technologies is crucial for solving the 21st century’s expanding environmental issues.
Acknowledgments
Department of Biotechnology, The University of Burdwan, Burdwan, India, and the University of Limpopo’s Department of Plant Production, Soil Science and Agricultural Engineering, Green Biotechnologies Research Centre of Excellence, Private Bag X1106, Sovenga, 0727, South Africa, are gratefully acknowledged by the authors. Authors are sincerely acknowledged Tshepo S. Mashela, the University of Limpopo’s Department of Plant Production, Soil Science and Agricultural Engineering, Green Biotechnologies Research Centre of Excellence, Private Bag X1106, Sovenga, 0727, South Africa, for his invaluable assistance in manuscript preparation.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
CRediT Authorship Contribution Statement: SD: Conceptualization, Methodology, Validation, Visualization, Writing- Original draft, KWS, MM: Visualization, ARN: Writing- review and editing, IC: Supervision.
Data Availability: The manuscript contains all the necessary data to support the findings of this study.
Ethical Approval: This article does not contain any studies with human participants or animals performed by any of the authors.
References
- 1. Lucas RE. The industrial revolution: Past and future. Lectures on economic growth. Harvard University Press; 2002;109:188. [Google Scholar]
- 2. Lucas RE. Lectures on Economic Growth. Harvard University Press; 2002. [Google Scholar]
- 3. Oonk G. Industrialisation in India, 1850-1947: three variations in the emergence of indigenous industrialists. 2004. [Google Scholar]
- 4. Carson R. Silent Spring. Getty Publications Los; 2009:1962. [Google Scholar]
- 5. Paull J. The Rachel Carson letters and the making of Silent Spring. Sage Open. 2013;3:2158244013494861. [Google Scholar]
- 6. Al-Sulaiti MM, Soubra L, Al-Ghouti MA. The causes and effects of mercury and methylmercury contamination in the marine environment: a review. Curr Pollut Rep. 2022;8:249-272. [Google Scholar]
- 7. Allen RC. The industrial revolution in miniature: the spinning jenny in Britain, France, and India. J Econ Hist. 2009;69:901-927. [Google Scholar]
- 8. Lele U, Goswami S. The fourth industrial revolution, agricultural and rural innovation, and implications for public policy and investments: a case of India. Agric Econ. 2017;48:87-100. [Google Scholar]
- 9. Ward JR. The industrial revolution and British imperialism, 1750-1850. Econ Hist Rev. 1994;47:44-65. [Google Scholar]
- 10. Fei X, Lou Z, Xiao R, Ren Z, Lv X. Source analysis and source-oriented risk assessment of heavy metal pollution in agricultural soils of different cultivated land qualities. J Clean Prod. 2022;341:130942. [Google Scholar]
- 11. Hama Aziz KH, Mustafa FS, Omer KM, Hama S, Hamarawf RF, Rahman KO. Heavy metal pollution in the aquatic environment: efficient and low-cost removal approaches to eliminate their toxicity: a review. RSC Adv.2023;13:17595-17610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kamaruzaman NA, Jaafar MH, Mohideen M, Fatinathan S. Effects of treated and untreated sludge applications on human health, the environment and other ecological factors. In Makhtar MMZ, Shukor H, Yaser AZ. (Eds.). Microbial Fuel Cell (MFC) Applications for Sludge Valorization. Springer; 2023;23-42. [Google Scholar]
- 13. Jaishankar M, Tseten T, Anbalagan N, Mathew BB, Beeregowda KN. Toxicity, mechanism and health effects of some heavy metals. Interdiscip Toxicol. 2014;7:60-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ankit S, Kishor V, Bauddh K. Impacts of synthetic pesticides on soil health and non-targeted flora and fauna. In: Bauddh K, Kumar S, Singh RP & Korstad J (Eds.). Ecological and Practical Applications for Sustainable Agriculture. Springer Singapore, 2020;65-88. [Google Scholar]
- 15. Pichtel J. Oil and gas production wastewater: soil contamination and pollution prevention. Appl Environ Soil Sci. 2016;2016:1-24. [Google Scholar]
- 16. Pourret O, Bollinger JC, Hursthouse A. Heavy metal: a misused term? Acta Geochim. 2021;40:466-471. [Google Scholar]
- 17. Suman J, Uhlik O, Viktorova J, Macek T. Phytoextraction of heavy metals: a promising tool for clean-up of polluted environment? Front Plant Sci. 2018;9:1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di D, Tooki T, Zhou H, et al. Metal mixture and osteoporosis risk: insights from plasma metabolite profiling. Ecotoxicol Environ Saf. 2023;263:115256. [DOI] [PubMed] [Google Scholar]
- 19. Bala S, Garg D, Thirumalesh BV, et al. Recent strategies for bioremediation of emerging pollutants: a review for a green and sustainable environment. Toxics. 2022;10:484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu X, He L, Zhang X, et al. Bioremediation of petroleum-contaminated saline soil by Acinetobacter baumannii and Talaromyces sp. and functional potential analysis using metagenomic sequencing. Environ Pollut. 2022;311:119970. [DOI] [PubMed] [Google Scholar]
- 21. Cervantes PAM, Ziarati P, de Frutos Madrazo P. Bioremediation Encyclopedia of Sustainable Management. Springer; 2023:1-8. [Google Scholar]
- 22. Ganesh Kumar A, Manisha D, Nivedha Rajan N, et al. Biodegradation of phenanthrene by piezotolerant Bacillus subtilis EB1 and genomic insights for bioremediation. Mar Pollut Bull. 2023;194:115151. [DOI] [PubMed] [Google Scholar]
- 23. Toribio JA, Marrodán T, Fernández-Natal I. Orbital implant infection by Corynebacterium amycolatum. Orbit. 2017;36:344-346. [DOI] [PubMed] [Google Scholar]
- 24. Onder Erguven G, Tatar Serdar O, Yildirim NC. Evaluation of the efficiency of chlorpyrifos-ethyl remediation by Methylobacterium radiotolerans and Microbacterium arthrosphaerae using response of some biochemical biomarkers. Environ Sci Pollut Res. 2021;28:2871-2879. [DOI] [PubMed] [Google Scholar]
- 25. Maity JP, Chandra Samal A, Rajnish K, et al. Furfural removal from water by bioremediation process by indigenous Pseudomonas putida (OSBH3) and Pseudomonas aeruginosa (OSBH4) using novel suphala media: an optimization for field application. Groundwater Sustain Dev. 2023;20:100895. [Google Scholar]
- 26. Zhao Z, Oury BM, Xia L, et al. The ecological response and distribution characteristics of microorganisms and polycyclic aromatic hydrocarbons in a retired coal gas plant post-thermal remediation site. Sci Total Environ. 2023;857:159314. [DOI] [PubMed] [Google Scholar]
- 27. Sonawane JM, Rai AK, Sharma M, Tripathi M, Prasad RRam. Microbial biofilms: Recent advances and progress in environmental bioremediation. Sci Total Environ. 2022;824:153843. [DOI] [PubMed] [Google Scholar]
- 28. Mir AR, Alam P, Hayat S. Perspective of melatonin-mediated stress resilience and Cu remediation efficiency of Brassica juncea in Cu-contaminated soils. Front Plant Sci. 2022;13:910714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Panwar R, Mathur J. Comparative analysis of remediation efficiency and ultrastructural translocalization of polycyclic aromatic hydrocarbons in Medicago sativa, Helianthus annuus, and Tagetes erecta. Int J Phytoremediation. 2023;18:1-19. [DOI] [PubMed] [Google Scholar]
- 30. Yan Y, Yang J, Wan X, et al. Temporal and spatial differentiation characteristics of soil arsenic during the remediation process of Pteris vittata L. and Citrus reticulata Blanco intercropping. Sci Total Environ. 2022;812:152475. [DOI] [PubMed] [Google Scholar]
- 31. Nandillon R, Lebrun M, Miard F, et al. Co-culture of Salix viminalis and Trifolium repens for the phytostabilisation of Pb and as in mine tailings amended with hardwood biochar. Environ Geochem Health. 2022;44:1229-1244. [DOI] [PubMed] [Google Scholar]
- 32. Teng Y, Guan W, Yu A, et al. Exogenous melatonin improves cadmium tolerance in Solanum nigrum L. without affecting its remediation potential. Int J Phytoremediation. 2022;24:1284-1291. [DOI] [PubMed] [Google Scholar]
- 33. UNEP/GPA. The State of the Marine Environment: Trends and Processes. UNEP/GPA; 2006. [Google Scholar]
- 34. Tchounwou PB, Yedjou CG, Patlolla AK, Sutton DJ. Heavy metal toxicity and the environment. In: Luch A (Ed.). Molecular, Clinical and Environmental Toxicology. Experientia Supplementum. Springer, Basel. 2012;101:133-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bradl H. Sources and Origins of Heavy Metals Interface Science and Technology. Elsevier; 2005;6:1-27. [Google Scholar]
- 36. Mukherjee AB, Zevenhoven R, Bhattacharya P, Sajwan KS, Kikuchi R. Mercury flow via coal and coal utilization by-products: a global perspective. Resour Conserv Recycl. 2008;52:571-591. [Google Scholar]
- 37. Velusamy S, Roy A, Sundaram S, Kumar Mallick T. A review on heavy metal ions and containing dyes removal through graphene oxide-based adsorption strategies for textile wastewater treatment. Chem Rec. 2021;21:1570-1610. [DOI] [PubMed] [Google Scholar]
- 38. Rees N, Fuller R. The Toxic Truth: Children’s Exposure to Lead Pollution Undermines a Generation of Future Potential. UNICEF; 2020. [Google Scholar]
- 39. Choppala G, Bolan N, Park JH. Chromium contamination and its risk management in complex environmental settings. Adv Agron. 2013;120:129-172. [Google Scholar]
- 40. Jiang W, Meng L, Liu F, et al. Distribution, source investigation, and risk assessment of topsoil heavy metals in areas with intensive anthropogenic activities using the positive matrix factorization (PMF) model coupled with self-organizing map (SOM). Environ Geochem Health. 2023;45:6353-6370. [DOI] [PubMed] [Google Scholar]
- 41. Baritz R, Amelung W, et al. Soil Monitoring in Europe – Indicators and Thresholds for Soil Health Assessments. EEA Reports. Publications Office of the European Union; 8:2023. [Google Scholar]
- 42. European Union. Heavy Metals in Wastes, European commission on environment. European Commission DG ENV. E3. 2002. [Google Scholar]
- 43. WHO. Guidelines for Drinking-Water Quality. World Health Organization; 1:2004. [Google Scholar]
- 44. Kesson MT, Point CC, di Caracalla VDT. Joint FAO/WHO Food Standards Programme Codex Committee on Contaminants in Foods. World Health Organization; 2015. [Google Scholar]
- 45. Sharma A, Katnoria JK, Nagpal AK. Heavy metals in vegetables: screening health risks involved in cultivation along wastewater drain and irrigating with wastewater. Springerplus. 2016;5:488-516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu Y. General standard for contaminants and toxins in food and feed. Codex stan. 2014;193-1995. [Google Scholar]
- 47. Li C, Zhou K, Qin W, et al. A review on heavy metals contamination in soil: effects, sources, and remediation techniques. Soil Sediment Contam. 2019;28:380-394. [Google Scholar]
- 48. Qasem NAA, Mohammed RH, Lawal DU. Removal of heavy metal ions from wastewater: a comprehensive and critical review. NPJ Clean. 2021;4:1-15. [Google Scholar]
- 49. Liu Z, Tran K-Q. A review on disposal and utilization of phytoremediation plants containing heavy metals. Ecotoxicol Environ Saf. 2021;226:112821. [DOI] [PubMed] [Google Scholar]
- 50. Sharma P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: an update. Bioresour Technol. 2021;328:124835. [DOI] [PubMed] [Google Scholar]
- 51. Nedjimi B. Phytoremediation: a sustainable environmental technology for heavy metals decontamination. Appl Sci. 2021;3:1-19. [Google Scholar]
- 52. Huang D, Xiao R, Du L, et al. Phytoremediation of poly- and perfluoroalkyl substances: a review on aquatic plants, influencing factors, and phytotoxicity. J Hazard Mater. 2021;418:126314. [DOI] [PubMed] [Google Scholar]
- 53. Fasani E, DalCorso G, Zorzi G, Vitulo N, Furini A. Comparative analysis identifies micro-RNA associated with nutrient homeostasis, development and stress response in Arabidopsis thaliana upon high Zn and metal hyperaccumulator Arabidopsis halleri. Physiol Plant. 2021;173:920-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Das M, Bramhanand PS, Laxminarayana K. Performance and efficiency services for the removal of hexavalent chromium from water by common macrophytes. Int J Phytoremediation. 2021;23:1095-1103. [DOI] [PubMed] [Google Scholar]
- 55. Kumar S, Thakur N, Singh AK, Gudade BA, Ghimire D, Das S. Aquatic macrophytes for environmental pollution control phytoremediation technology for the Removal of Heavy Metals and Other Contaminants From Soil and Water. Elsevier; 2022:291-308. [Google Scholar]
- 56. Li J, Zheng B, Chen X, et al. The use of constructed wetland for mitigating nitrogen and phosphorus from agricultural runoff: a review. Water. 2021;13:476. [Google Scholar]
- 57. Prasad MNV. Prospects for manipulation of molecular mechanisms and transgenic approaches in aquatic macrophytes for remediation of toxic metals and metalloids in wastewaters. Transgenic Plant Technology for Remediation of Toxic Metals and Metalloids. Elsevier; 2019:395-428. [Google Scholar]
- 58. Tripathy PK, Mohapatra M, Pattnaik R, Tarafdar L, Panda S, Rastogi G. Macrophyte Diversity and Distribution in Brackish Coastal Lagoons: A Field Survey From Chilika, Odisha Coastal Ecosystems. Springer; 2022:325-358. [Google Scholar]
- 59. Grignet A, Sahraoui AL-H, Teillaud S, Fontaine J, Papin A, Bert V. Phytoextraction of Zn and Cd with Arabidopsis halleri: A focus on fertilization and biological amendment as a means of increasing biomass and Cd and Zn concentrations. Environ Sci Pollut Res. 2022;29:22675-22686. [DOI] [PubMed] [Google Scholar]
- 60. Rathika R, Srinivasan P, Alkahtani J, et al. Influence of biochar and EDTA on enhanced phytoremediation of lead contaminated soil by Brassica juncea. Chemosphere. 2021;271:129513. [DOI] [PubMed] [Google Scholar]
- 61. Zhang Y, Liu G, Gao S, Zhang Z, Huang L. Effect of humic acid on phytoremediation of heavy metal contaminated sediment. J Hazard Mater Adv. 2023;9:100235. [Google Scholar]
- 62. Yang Y, Ge Y, Tu P, et al. Phytoextraction of Cd from a contaminated soil by tobacco and safe use of its metal-enriched biomass. J Hazard Mater. 2019;363:385-393. [DOI] [PubMed] [Google Scholar]
- 63. Zhu G, Liu W, Wen Y, Liao X, Sun L. Potential of arsenate-reducing bacterial inoculants to enhance field-scale remediation of arsenic contaminated soils by Pteris vittata L. Ecol Eng. 2021;169:106312. [Google Scholar]
- 64. Yang W, Yang Y, Ding Z, Yang X, Zhao F, Zhu Z. Uptake and accumulation of cadmium in flooded versus non-flooded Salix genotypes: implications for phytoremediation. Ecol Eng. 2019;136:79-88. [Google Scholar]
- 65. Valenti M, Gonzalez M, Ruscitti M. Germination and in vitro regeneration of Sesbania punicea and Sesbania virgata: environmentally important plants. Physiol Rep. 2021;26:754-761. [Google Scholar]
- 66. He X, Zhang J, Ren Y, et al. Polyaspartate and liquid amino acid fertilizer are appropriate alternatives for promoting the phytoextraction of cadmium and lead in Solanum nigrum L. Chemosphere. 2019;237:124483. [DOI] [PubMed] [Google Scholar]
- 67. Li X, Zhang X, Wang X, Cui Z. Phytoremediation of multi-metal contaminated mine tailings with Solanum nigrum L. and biochar/attapulgite amendments. Ecotoxicol Environ Saf. 2019;180:517-525. [DOI] [PubMed] [Google Scholar]
- 68. Narayanan M, Ranganathan M, Kandasamy G, Kumarasamy S. Evaluation of interaction among indigenous rhizobacteria and Vigna unguiculata on remediation of metal-containing abandoned magnesite mine tailing. Arch Microbiol. 2021;203:1399-1410. [DOI] [PubMed] [Google Scholar]
- 69. Huang X, Luo D, Chen X, et al. Insights into heavy metals leakage in chelator-induced phytoextraction of Pb- and Tl-contaminated soil. Int J Env Res Pub. 2019;16:1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sebastian A, Deepa P, Prasad MNV. Azolla farming for sustainable environmental remediation. In: Prasad MNV (Ed.) Handbook of Assisted and Amendment: Enhanced Sustainable Remediation Technology. John Wiley & Sons Ltd., 2021;517-533. [Google Scholar]
- 71. Kaur S, Midha T, Verma H, et al. Bioremediation: A Favorable Perspective to Eliminate Heavy Metals From Polluted Soil Metagenomics to Bioremediation. Elsevier; 2023:209-230. [Google Scholar]
- 72. Shukla A, Srivastava S, D’Souza SF. An integrative approach toward biosensing and bioremediation of metals and metalloids. Int J Environ Sci. 2018;15:2701-2712. [Google Scholar]
- 73. Rai PK. Heavy metals/metalloids remediation from wastewater using free floating macrophytes of a natural wetland. Environ Technol Innov. 2019;15:100393. [Google Scholar]
- 74. Stefanidis K, Sarika M, Papastegiadou E. Exploring environmental predictors of aquatic macrophytes in water-dependent Natura 2000 sites of high conservation value: results from a long-term study of macrophytes in Greek lakes. Aquat Conserv Mar Freshw Ecosyst. 2019;29:1133-1148. [Google Scholar]
- 75. Nabuyanda MM, van Bruggen J, Kelderman P, Irvine K. Investigating Co, Cu, and Pb retention and remobilization after drying and rewetting treatments in greenhouse laboratory-scale constructed treatments with and without Typha angustifolia, and connected phytoremediation potential. J Environ Manag. 2019;236:510-518. [DOI] [PubMed] [Google Scholar]
- 76. Naqqash T, Aziz A, Babar M, et al. Lead-resistant Morganella morganii Rhizobacteria reduced lead toxicity in Arabidopsis thaliana by improving growth, physiology, and antioxidant activities. Agriculture. 2022;12:1155. [Google Scholar]
- 77. Raj D, Kumar A, Maiti SK. Mercury remediation potential of Brassica juncea (L.) czern. For clean-up of flyash contaminated sites. Chemosphere. 2020;248:125857. [DOI] [PubMed] [Google Scholar]
- 78. Samreen S, Khan AA, Khan MR, Ansari SA, Khan A. Assessment of phytoremediation potential of seven weed plants growing in chromium- and nickel-contaminated soil. Water Air Soil Pollut. 2021;232:1-18. [Google Scholar]
- 79. Mahmood-Ul-Hassan M, Yousra M, Saman L, Ahmad R. Floriculture: alternate non-edible plants for phyto-remediation of heavy metal contaminated soils. Int J Phytoremediation. 2020;22:725-732. [DOI] [PubMed] [Google Scholar]
- 80. Sangsuwan P, Prapagdee B. Cadmium phytoremediation performance of two species of chlorophytum and enhancing their potentials by cadmium-resistant bacteria. Environ Technol Innov. 2021;21:101311. [Google Scholar]
- 81. Selamat SN, Halmi MIEB, Abdullah SRS, Idris M, Hasan HA, Anuar N. Optimization of lead (Pb) bioaccumulation in Melastoma malabathricum L. by response surface methodology (RSM). Rend Lincei Sci Fis Nat. 2018;29:43-51. [Google Scholar]
- 82. Wei S, Xu L, Dai H, Hu Y. Ornamental hyperaccumulator Mirabilis jalapa L. Phytoremediating combine contaminated soil enhanced by some chelators and surfactants. Environ Sci Pollut Res. 2018;25:29699-29704. [DOI] [PubMed] [Google Scholar]
- 83. Hazarika A, Saikia S, Devi B, Yadav M, Yadav HS. Oxidoreductase Metalloenzymes as Green Catalyst for Phytoremediation of Environmental Pollutants Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants From Soil and Water. Elsevier; 2022:141-172. [Google Scholar]
- 84. Subašić M, Šamec D, Selović A, Karalija E. Phytoremediation of cadmium polluted soils: current status and approaches for enhancing. Soil Syst. 2022;6:3. [Google Scholar]
- 85. Vega A, Delgado N, Handford M. Increasing heavy metal tolerance by the exogenous application of organic acids. Int J Mol Sci. 2022;23:5438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kumar V, Shah, Shahi SK. (Eds.) Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants From Soil and Water. Elsevier; 2022:141. [Google Scholar]
- 87. Muthusamy L, Rajendran M, Ramamoorthy K, Narayanan M, Kandasamy S. Phytostabilization of Metal Mine Tailings—A Green Remediation Technology Phytoremediation Technology for the Removal of Heavy Metals and Other Contaminants From Soil and Water. Elsevier; 2022:243-253. [Google Scholar]
- 88. Hegedus C, Pașcalău S-N, Andronie L, Rotaru A-S, Cucu A-A, Dezmirean DS. The journey of 1000 leagues towards the decontamination of the soil from heavy metals and the impact on the soil–plant–animal–human chain begins with the first step: phytostabilization/phytoextraction. Agriculture. 2023;13:735. [Google Scholar]
- 89. Asgari Lajayer B, Khadem Moghadam N, Maghsoodi MR, Ghorbanpour M, Kariman K. Phytoextraction of heavy metals from contaminated soil, water and atmosphere using ornamental plants: mechanisms and efficiency improvement strategies. Environ Sci Pollut Res. 2019;26:8468-8484. [DOI] [PubMed] [Google Scholar]
- 90. Gul I, Manzoor M, Hashim N, et al. Challenges in microbially and chelate-assisted phytoextraction of cadmium and lead – a review. Environ Pollut. 2021;287:117667. [DOI] [PubMed] [Google Scholar]
- 91. Mohan I, Goria K, Dhar S, Kothari R, Bhau B, Pathania D. Phytoremediation of heavy metals from the biosphere perspective and solutions. Pollutants and Water Management: Resources, Strategies and Scarcity. 2021;16:95-127. [Google Scholar]
- 92. Li X, Zhu W, Meng G, Guo R, Wang Y. Phytoremediation of alkaline soils co-contaminated with cadmium and tetracycline antibiotics using the ornamental hyperaccumulators Mirabilis jalapa L. and Tagetes patula L. Environ Sci Pollut Res. 2020;27:14175-14183. [DOI] [PubMed] [Google Scholar]
- 93. Pouresmaieli M, Ataei M, Forouzandeh P, Azizollahi P, Mahmoudifard M. Recent progress on sustainable phytoremediation of heavy metals from soil. J Environ Chem Eng. 2022;10:108482. [Google Scholar]
- 94. Nebeská D, Trögl J, Ševců A, et al. Miscanthus x giganteus role in phytodegradation and changes in bacterial community of soil contaminated by petroleum industry. Ecotoxicol Environ Saf. 2021;224:112630. [DOI] [PubMed] [Google Scholar]
- 95. Yaashikaa PR, Kumar PS, Jeevanantham S, Saravanan R. A review on bioremediation approach for heavy metal detoxification and accumulation in plants. Environ Pollut. 2022;301:119035. [DOI] [PubMed] [Google Scholar]
- 96. Iwamoto T, Nasu M. Current bioremediation practice and perspective. J Biosci Bioeng. 2001;92:1-8. [DOI] [PubMed] [Google Scholar]
- 97. Pete AJ, Bharti B, Benton MG. Nano-enhanced bioremediation for oil spills: a review. ACS ES & T Eng. 2021;1:928-946. [Google Scholar]
- 98. Marwa N, Singh N, Srivastava S, Saxena G, Pandey V, Singh N. Characterizing the hypertolerance potential of two indigenous bacterial strains (Bacillus flexus and Acinetobacter junii) and their efficacy in arsenic bioremediation. J Appl Microbiol. 2019;126:1117-1127. [DOI] [PubMed] [Google Scholar]
- 99. Geng N, Xia Y, Lu D, et al. The bacterial community structure in epiphytic biofilm on submerged macrophyte Potamogetom crispus L. and its contribution to heavy metal accumulation in an urban industrial area in Hangzhou. J Hazard Mater. 2022;430:128455. [DOI] [PubMed] [Google Scholar]
- 100. Shukla SK, Paraneeiswaran A, Subba Rao T. Biosorption of Co-EDTA complex by Aspergillus versicolor SPF-1 strain isolated from solar salt pan. J Environ Chem Eng. 2020;8:103549. [Google Scholar]
- 101. Bhattacharya A, Gola D, Dey P, Malik A. Synergistic and antagonistic effects on metal bioremediation with increasing metal complexity in a hexa-metal environment by Aspergillus fumigatus. Int J Environ Health Res. 2020;14:761-770. [Google Scholar]
- 102. Li Q, Xing Y, Fu X, et al. Biochemical mechanisms of rhizospheric Bacillus subtilis-facilitated phytoextraction by alfalfa under cadmium stress - microbial diversity and metabolomics analyses. Ecotoxicol Environ Saf. 2021;212:112016. [DOI] [PubMed] [Google Scholar]
- 103. Văcar CL, Covaci E, Chakraborty S, et al. Heavy metal-resistant filamentous fungi as potential mercury bioremediators. J Fungi. 2021;7:386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Malik S, Kishore S, Shah MP, Kumar SA. A comprehensive review on nanobiotechnology for bioremediation of heavy metals from wastewater. J Basic Microbiol. 2022;62:361-375. [DOI] [PubMed] [Google Scholar]
- 105. Saini S, Gill JK, Kaur J, et al. Biosorption as environmentally friendly technique for heavy metal removal from wastewater. In: Qadri H, Bhat RA, Mehmood MA, Dar GH (Eds.) Fresh Water Pollution Dynamics and Remediation. Springer; 2020;167-181. [Google Scholar]
- 106. Páez-Vélez C, Rivas RE, Dussán J. Enhanced gold biosorption of Lysinibacillus sphaericus CBAM5 by encapsulation of bacteria in an alginate matrix. Metals. 2019;9:818. [Google Scholar]
- 107. Pramanik K, Mandal S, Banerjee S, Ghosh A, Maiti TK, Mandal NC. Unraveling the heavy metal resistance and biocontrol potential of Pseudomonas sp. K32 strain facilitating rice seedling growth under Cd stress. Chemosphere. 2021;274:129819. [DOI] [PubMed] [Google Scholar]
- 108. Mat Arisah F, Amir AF, Ramli N, et al. Bacterial resistance against heavy metals in Pseudomonas aeruginosa RW9 involving hexavalent chromium removal. Sustainability. 2021;13:9797. [Google Scholar]
- 109. Okpara-Elom IA, Onochie CC, Elom MO, Ezaka E, Elom O. Bioremediation of heavy metal polluted soil using plant growth promoting bacteria: an assessment of response. Bioremediat J. 2022;2:1-20. [Google Scholar]
- 110. Xiao X, Zhu Y, Gao Y, Fu J, Zhao Y, Zhao L. Inoculation of paddy soils with Rhodopseudomonas palustris enhanced heavy metal immobilisation. Plant Soil Environ. 2021;67:55-60. [Google Scholar]
- 111. Kumar V, Dwivedi SK. Mycoremediation of heavy metals: processes, mechanisms, and affecting factors. Environ Sci Pollut Res. 2021;28:10375-10412. [DOI] [PubMed] [Google Scholar]
- 112. Shourie A, Vijayalakshmi U. Fungal diversity and its role in mycoremediation. Geomicrobiol J. 2022;39:426-444. [Google Scholar]
- 113. Jebapriya GR, Gnanadoss JJ. Bioremediation of textile dye using white rot fungi: a review. Int J Curr Res Rev. 2013;5:1. [Google Scholar]
- 114. Singh P, Raghukumar C, Parvatkar RR, Mascarenhas-Pereira MB. Heavy metal tolerance in the psychrotolerant Cryptococcus sp. isolated from deep-sea sediments of the Central Indian Basin. Yeast. 2013;30:93-101. [DOI] [PubMed] [Google Scholar]
- 115. Irshad S, Xie Z, Mehmood S, Nawaz A, Ditta A, Mahmood Q. Insights into conventional and recent technologies for arsenic bioremediation: a systematic review. Environ Sci Pollut Res. 2021;28:18870-18892. [DOI] [PubMed] [Google Scholar]
- 116. Tonelli FMP, Lemos MS, Tonelli FCP. Genetically Modified Organisms as tools for water bioremediation freshwater pollution and aquatic ecosystems. Apple Academic Press; 2021:301-320. [Google Scholar]
- 117. Sriprang R, Hayashi M, Ono H, Takagi M, Hirata K, Murooka Y. Enhanced accumulation of Cd2+ by a Mesorhizobium sp. transformed with a gene from Arabidopsis thaliana coding for phytochelatin synthase. Appl Environ Microbiol. 2003;69:1791-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Bae W, Wu CH, Kostal J, Mulchandani A, Chen W. Enhanced mercury biosorption by bacterial cells with surface-displayed MerR. Appl Environ Microbiol. 2003;69:3176-3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Al Hasin A, Gurman SJ, Murphy LM, Perry A, Smith TJ, Gardiner PHE. Remediation of chromium(VI) by a methane-oxidizing bacterium. Environ Sci Technol. 2010;44:400-405. [DOI] [PubMed] [Google Scholar]
- 120. Wagner-Döbler I. Pilot plant for bioremediation of mercury-containing industrial wastewater. Appl Microbiol Biot. 2003;62:124-133. [DOI] [PubMed] [Google Scholar]
- 121. Ripp S, Nivens DE, Ahn Y, et al. Controlled field release of a bioluminescent genetically engineered microorganism for bioremediation process monitoring and control. Environ Sci Technol. 2000;34:846-853. [Google Scholar]
- 122. Patel J, Zhang Q, McKay RML, Vincent R, Xu Z. Genetic engineering of Caulobacter crescentus for removal of cadmium from water. Appl Biochem Biotechnol. 2010;160:232-243. [DOI] [PubMed] [Google Scholar]
- 123. Abdulsalam S, Adefila S, Bugaje I, Ibrahim S. Bioremediation of soil contaminated with used motor oil in a closed system. J Bioremediat Biodegrad. 2012;03:1-7. [Google Scholar]
- 124. Bahadure S, Kalia R, Chavan R. Comparative study of bioremediation of hydrocarbon fuels. Int J Biotechnol Bioeng Res. 2013;4:677-686. [Google Scholar]
- 125. Irankhah S, Abdi Ali A, Mallavarapu M, et al. Ecological role of Acinetobacter calcoaceticus GSN3 in natural biofilm formation and its advantages in bioremediation. Biofouling. 2019;35:377-391. [DOI] [PubMed] [Google Scholar]
- 126. Wasi S, Tabrez S, Ahmad M. Use of Pseudomonas spp. for the bioremediation of environmental pollutants: a review. Environ Monit Assess. 2013;185:8147-8155. [DOI] [PubMed] [Google Scholar]
- 127. Gupta N, Yadav KK, Kumar V, et al. Investigation of heavy metal accumulation in vegetables and health risk to humans from their consumption. Front Environ Sci. 2022;10:40. [Google Scholar]
- 128. Minhas PS, Saha JK, Dotaniya ML, Sarkar A, Saha M. Wastewater irrigation in India: current status, impacts and response options. Sci Total Environ. 2022;808:152001. [DOI] [PubMed] [Google Scholar]
- 129. Alexander PD, Alloway BJ, Dourado AM. Genotypic variations in the accumulation of Cd, Cu, Pb and Zn exhibited by six commonly grown vegetables. Environ Pollut. 2006;144:736-745. [DOI] [PubMed] [Google Scholar]
- 130. Haque MM, Niloy NM, Khirul MA, Alam MF, Tareq SM. Appraisal of probabilistic human health risks of heavy metals in vegetables from industrial, non-industrial and arsenic contaminated areas of Bangladesh. Heliyon. 2021;7:e06309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Khan A, Khan S, Khan MA, Qamar Z, Waqas M. The uptake and bioaccumulation of heavy metals by food plants, their effects on plants nutrients, and associated health risk: a review. Environ Sci Pollut Res. 2015;22:13772-13799. [DOI] [PubMed] [Google Scholar]
- 132. Abudawood M, Tabassum H, Alanazi AH, et al. Antioxidant status in relation to heavy metals induced oxidative stress in patients with polycystic ovarian syndrome (PCOS). Sci Rep. 2021;11:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Bhardwaj JK, Paliwal A, Saraf P. Effects of heavy metals on reproduction owing to infertility. J Biochem Mol Toxicol. 2021;35:e22823. [DOI] [PubMed] [Google Scholar]
- 134. Lee HJ, Park MK, Seo YR. Pathogenic mechanisms of heavy metal induced-Alzheimer’s disease. Toxicol Environ Health Sci. 2018;10:1-10. [Google Scholar]
- 135. Wise JP, Jr, Young JL, Cai J, Cai L. Current understanding of hexavalent chromium [Cr (VI)] neurotoxicity and new perspectives. Environ Int. 2022;158:106877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Park J-D. Heavy metal poisoning. Hanyang Med Rev. 2010;30:319-325. [Google Scholar]
- 137. Chen A, Kim SS, Chung E, Dietrich KN. Thyroid hormones in relation to lead, mercury, and cadmium exposure in the National Health and Nutrition Examination Survey, 2007–2008. Environ Health Perspect. 2013;121:181-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Yang A-M, Lo K, Zheng T-Z, et al. Environmental heavy metals and cardiovascular diseases: status and future direction. Chronic Dis Transl Med. 2020;6:251-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Capitão C, Martins R, Santos O, et al. Exposure to heavy metals and red blood cell parameters in children: a systematic review of observational studies. Front Pediatr. 2022;10:921239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Wilbur S, Abadin H, Fay M, Yu D, Tencza B, Ingerman L, Klotzbach J, James S. Toxicological profile for chromium. Atlanta (GA): Agency for Toxic Substances and Disease Registry (US); 2012. [PubMed] [Google Scholar]
- 141. Hu X, Fernandes J, Jones DP, Go YM. Cadmium stimulates myofibroblast differentiation and mouse lung fibrosis. Toxicology. 2017;383:50-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Kim D-W, Ock J, Moon K-W, Park C-H. Association between Pb, Cd, and Hg exposure and liver injury among Korean adults. Int J Env Res Pub. 2021;18:6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Patwa J, Flora SJS. Heavy metal-induced cerebral small vessel disease: insights into molecular mechanisms and possible reversal strategies. Int J Mol Sci. 2020;21:3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Balali-Mood M, Naseri K, Tahergorabi Z, Khazdair MR, Sadeghi M. Toxic mechanisms of five heavy metals: mercury, lead, chromium, cadmium, and arsenic. Front Pharmacol. 2021;12:643972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Bissett DL, Miyamoto K, Sun P, Li J, Berge CA. Topical niacinamide reduces yellowing, wrinkling, red blotchiness, and hyperpigmented spots in aging facial skin1. Int J Cosmet Sci. 2004;26:231-238. [DOI] [PubMed] [Google Scholar]
- 146. Shimo NA, Salam MA, Parvin M, Sultan MZ. Assessment of selected metals (chromium, lead and cadmium) in the hair of tannery workers at Hemayetpur, Bangladesh. J Elem Miner. 2023;4:100056. [Google Scholar]
- 147. Chen X, Wang S, Mao X, et al. Adverse health effects of emerging contaminants on inflammatory bowel disease. Front Public Health. 2023;11:1140786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Lei HL, Wei HJ, Ho HY, Liao KW, Chien LC. Relationship between risk factors for infertility in women and lead, cadmium, and arsenic blood levels: a cross-sectional study from Taiwan. BMC Public Health. 2015;15:1220-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Lin J, Lin X, Qiu J, You X, Xu J. Association between heavy metals exposure and infertility among American women aged 20–44 years: a cross-sectional analysis from 2013 to 2018 NHANES data. Front Public Health. 2023;11:1122183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Järup L, Alfvén T, Persson B, Toss G, Elinder CG. Cadmium may be a risk factor for osteoporosis. Occup Environ Med. 1998;55:435-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Harischandra DS, Ghaisas S, Zenitsky G, et al. Manganese-induced neurotoxicity: new insights into the triad of protein misfolding, mitochondrial impairment, and neuroinflammation. Front Neurosci. 2019;13:654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Shekhawat R, Meshram V. Acrodynia. StatPearls Publishing; 2021. [PubMed] [Google Scholar]
- 153. Coetzee JJ, Bansal N, Chirwa EMN. Chromium in environment, its toxic effect from chromite-mining and ferrochrome industries, and its possible bioremediation. Expo Health. 2020;12:51-62. [Google Scholar]
- 154. Kim HS, Kim YJ, Seo YR. An overview of carcinogenic heavy metal: molecular toxicity mechanism and prevention. J Cancer Prev. 2015;20:232-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Kothapalli CR. Differential impact of heavy metals on neurotoxicity during development and in aging central nervous system. Curr Opin Toxicol. 2021;26:33-38. [Google Scholar]
- 156. Farkhondeh T, Boskabady MH, Kohi MK, Sadeghi-Hashjin G, Moin M. Lead exposure affects inflammatory mediators, total and differential white blood cells in sensitized guinea pigs during and after sensitization. Drug Chem Toxicol. 2014;37:329-335. [DOI] [PubMed] [Google Scholar]
- 157. Mugahi MN, Heidari Z, Sagheb HM, Barbarestani M. Effects of chronic lead acetate intoxication on blood indices of male adult rat. DARU J Pharm Sci. 2003;11:147-151. [Google Scholar]
- 158. Abirami N, Raju VS, Rajathi K. Effect of Semecarpus anacardium against lead induced toxicity in rats. Anc Sci Life. 2007;27:24-27. [PMC free article] [PubMed] [Google Scholar]
- 159. Patlolla AK, Barnes C, Yedjou C, Velma VR, Tchounwou PB. Oxidative stress, DNA damage, and antioxidant enzyme activity induced by hexavalent chromium in Sprague-Dawley rats. Environ Toxicol. 2009;24:66-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Ahrensbøll-Friis U, Simonsen AB, Dahlin J, Isaksson M, Zachariae C, Johansen JD. Allergic contact dermatitis from dyes used in the temple of spectacles. Contact Derm. 2022;86:25-28. [DOI] [PubMed] [Google Scholar]
- 161. Bruynzeel DP, Hennipman G, van Ketel WG. Irritant contact dermatitis and chrome-passivated metal. Contact Derm. 1988;19:175-179. [DOI] [PubMed] [Google Scholar]
- 162. Fay M, Alt L, Ryba D, et al. Cadmium nephrotoxicity is associated with altered microRNA expression in the rat renal cortex. Toxics. 2018;6:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Ma Y, Ran D, Shi X, Zhao H, Liu Z. Cadmium toxicity: a role in bone cell function and teeth development. Sci Total Environ. 2021;769:144646. [DOI] [PubMed] [Google Scholar]
- 164. Pouillot R, Santillana Farakos S, Van Doren JM. Modeling the risk of low bone mass and osteoporosis as a function of urinary cadmium in U.S adults aged 50-79 years. Environ Res. 2022;212:113315. [DOI] [PubMed] [Google Scholar]


