Abstract
In tissue engineering, the fate of a particular organ/tissue regeneration and repair mainly depends on three pillars - 3D architecture, cells used, and stimulus provided. 3D cell supportive structure development is one of the crucial pillars necessary for defining organ/tissue geometry and shape. In recent years, the advancements in 3D bio-printing (additive manufacturing) made it possible to develop very precise 3D architectures with the help of industrial software like Computer-Aided Design (CAD). The main requirement for the 3D printing process is the bio-ink, which can act as a source for cell support, proliferation, drug (growth factors, stimulators) delivery, and organ/tissue shape. The selection of the bio-ink depends upon the type of 3D tissue of interest. Printing tissues like bone and cartilage is always challenging because it is difficult to find printable biomaterial that can act as bio-ink and mimic the strength of the natural bone and cartilage tissues. This review describes different biomaterials used to develop bio-inks with different processing variables and cell-seeding densities for bone and cartilage 3D printing applications. The review also discusses the advantages, limitations, and cell bio-ink compatibility in each biomaterial section. The emphasis is given to bio-inks reported for 3D printing cartilage and bone and their applications in orthopedics and orthodontists. The critical/important performance and the architectural morphology requirements of desired bone and cartilage bio-inks were compiled in summary.
Keywords: Biomaterials, Bio-ink, 3D printing, Tissue engineering, Regenerative medicine, Drug delivery for bone tissue
1. Introduction
Regenerative medicine is the advanced branch of tissue engineering that mainly deals with the process of engineering, regenerating, and replacing diseased tissues or organs to establish/restore normal functions of the body. It is based on three main pillars; scaffold, cells, and stimulators (growth factors and other markers) [1]. In the past decade, regenerative medicine has advanced a lot, resulting in branching. The traditional approach for regenerative medicine-based therapies is top-down, where a porous 3D scaffold is first developed and then seeded with specific cells and stimulators (Fig. 1a) and allowed to proliferate in functional tissue slowly. In contrast, the new modular approach involves the use of cell aggregates/cell sheets/cells suspended in bio-inks assembled in a bio-compatible biomaterial-based 3D shape, which will allow the development of functional tissue (Fig. 1b). Both these approaches have their advantages and disadvantages, and one needs to choose the best approach depending on the type of tissue one plan to regrow. The top-down approach has the advantages like ease of 3D architecture development, pore size control, variety of processes available for scaffold development, and ease of sterilization. The drawbacks include lack of precision of 3D architecture development; scaffold-based sustain release of the stimuli is possible only with a specific scaffold development process (lyophilization). It was also reported that mass transfer and cell distribution are poor in the 3D matrix, which slows down the biodegradation rate and cell proliferation rate in the scaffold and, finally the limitation of multiple cell type seeding [2]. Many of the disadvantages of the top-down approaches were overcome with the relatively new bottom-up or modular approach. The limitations like precision architecture development, uniform cell distribution, use of multiple cell types, and sustained release of the stimuli were successfully addressed by the modular approach. Whereas, problems like efficient sterilization and 3D scaffold strength still pose a limitation to this approach [3]. The most successful and extensively evaluated technique among the different modular approaches is additive manufacturing or 3D bio-printing technique [4].
Fig. 1.
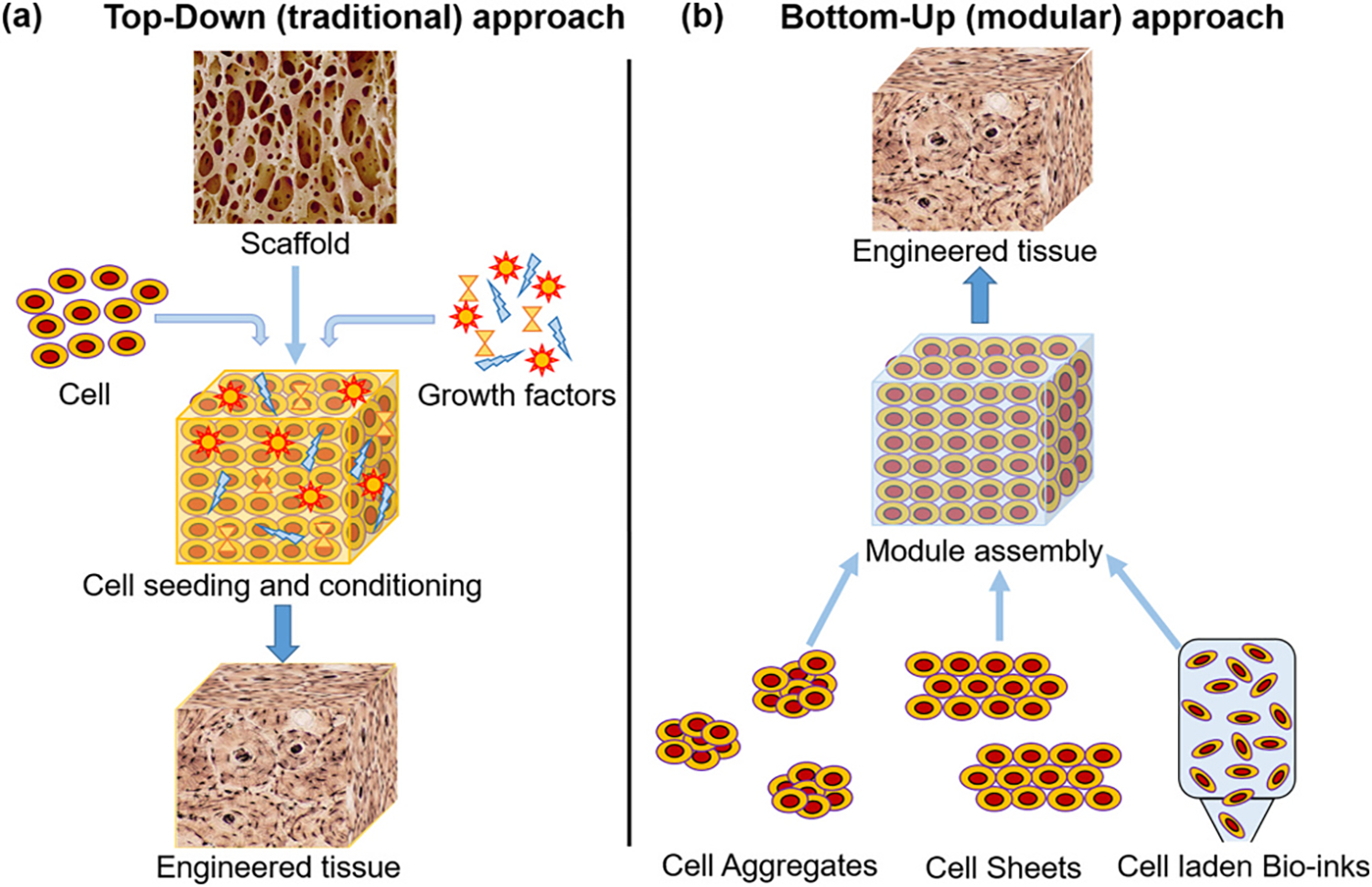
Tissue engineering (a) top-down (traditional) approach and (b) bottom-up (modular) approach [2].
1.1. Advantages and disadvantages of most common 3D bio-printing techniques
The advancements in additive manufacturing techniques provided many benefits to tissue engineering and regenerative medicine, mainly by providing Computer Aided Design (CAD) based precision 3D printing of a tissue architecture along with functional cells embedded (cell-laden) cell supportive hydrogel called bio-ink [5,6]. There is a plethora of 3D printing techniques available, but very few are compatible with bioprintings [7]. The most commonly used cell-laden bio-ink-based bioprinting techniques are Inkjet-based, Extrusion-based, Acoustic-based, Laser-assisted (LAD) techniques and Stereolithography (SLA) (Fig. 2). Inject printer-based and extrusion-based 3D bio-printing are well-established techniques. The inkjet printer has advantages like low printing cost, high (>85 %) cell viability, widely available printing heads and bio-inks, and high 3D printing speed, but it also possesses disadvantages like lack of precise printing due to low droplet directionality and low-density 3D structure due to less viscus (low biomaterial concentration) bio-ink. Whereas, the extrusion-based 3D bio-printer can print viscus (high density) 3D structures but lacks the resolution due to printing head aperture (generally >100 μm) and very low (40 %) viable cell density. These limitations restrict the use of both printers for specific soft tissue printing applications, which require precise printing with very fine nozzle tips but can be effectively used for certain hard tissues printed in >10 mm dimensions (Table 1) [8].
Fig. 2.
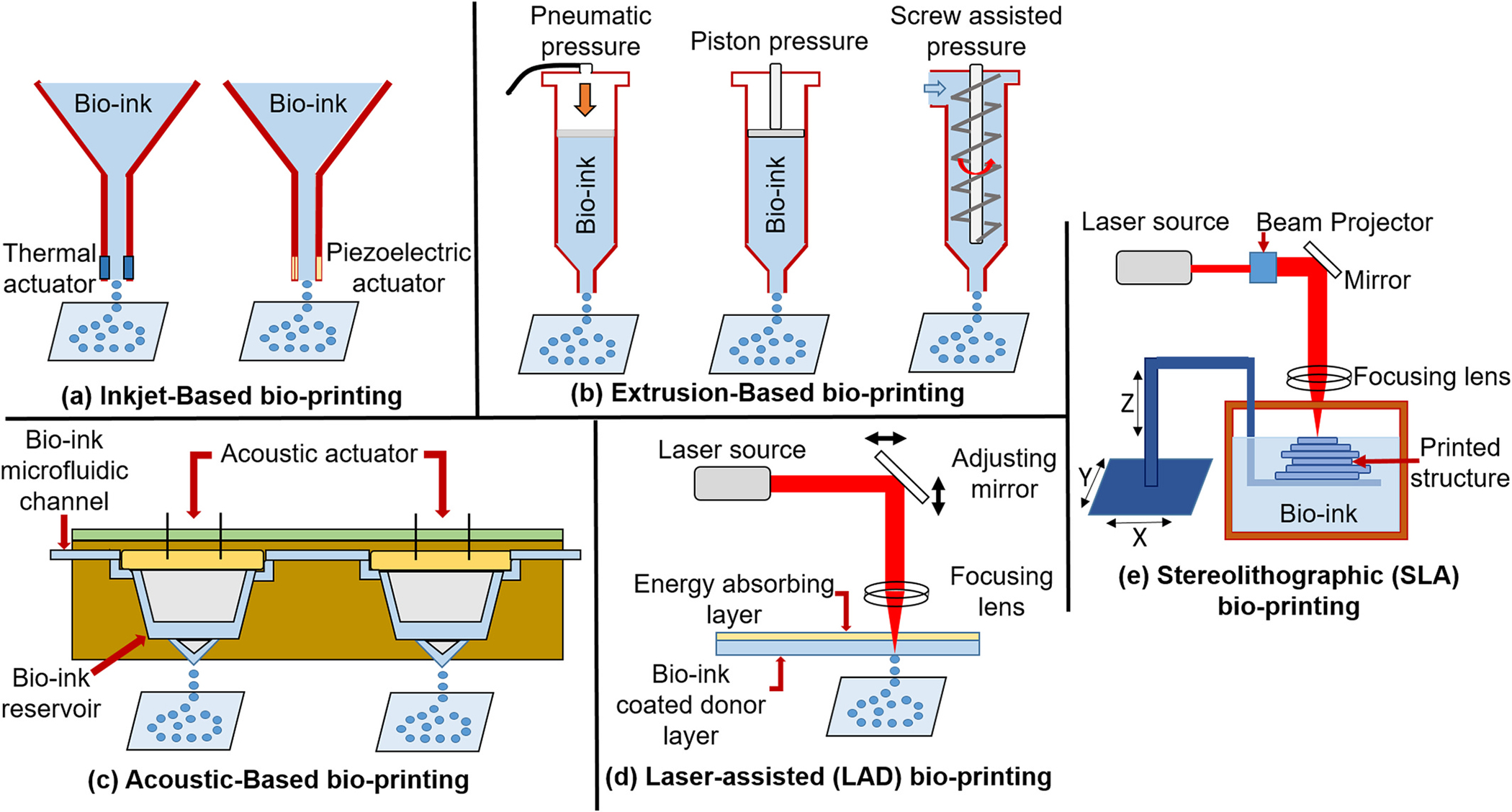
Different types of bio-printing techniques to print cell-laden bio-ink [4].
Table 1.
Bone and cartilage bio-inks developed using different polymer and combinations.
| Polymers of bio-ink | Loading cells density | Concentration of bioink (% wt/vol) | Method of gelation | Temperature range | Cell types used | Days required | Advantages (Adv) and disadvantages (Dadv) | Applications | Reference |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Agarose | 2.5 × 105/ml | 2 % | Ionic or thermal | 30–40 °C | Rabbit articular chondrocytes | 7 | Adv: cost-effective, good mechanical strength | Bone-tissue engineering | [23,24] |
| Agarose and its blend | 2.5 × 105/ml | 2 % | Thermal/ionic | 30–40 °C | Bone marrow stromal cells (BMSCs) | 7 | Dadv: Inferior cell adhesion. | ||
| Agarose-based gel | 3 × 105/ml | 2 % | Thermal/ionic X-linking. | 4–70 °C | SaOS-2 cells | 10 | Cartilage tissue engineering | [26] | |
| Collagen/alginate | 1 × 107/ml | 2 % collagen 8 % alginate | Ionic cross-linking | 37 °C | Primary chondrocytes | 14 | Adv: better cell adhesion Dadv: low mechanical strength, clogging, high cost, slow gelation. | Cartilage tissue engineering | [39] |
| Collagen/βTCP | 1 × 107/ml | 5 % collagen 20 % βTCP | Crosslinking | 37 °C | Preosteoblasts (MC3T3-E1) and human adipose stem cells(hASCs) | 21 | Adv: mechanically stable, satisfactorily viable, significant osteogenic activities higher levels of osteogenic gene expression Dadv: flow-ability decreased |
Bone tissue regeneration | [42] |
| Collagen | 2 × 107/ml | 4 % | pH-mediated | 37 °C | Chondrocytes | 40 | Adv: better cell adhesion Dadv: gelling and mechanical strength | Cartilage tissue engineering | [46] |
| Gelatin methacrylate (GelMA) | 1.5 × 107/ml | 15 % | Photo-polymerization | 37 °C | MC3T3-E1 preosteoblastic cells | 14 | Adv: mechanically strong, easy degeneration, possibility of blending, high printability Dadv: low viscosity resulting in poor resolution of printed structures | Bone tissue regeneration | [57] |
| Gelatine/hyaluronic acid/ glycerol | 1 × 106 ml | Gelatine – 10 %/hyaluronic acid – 3 %/glycerol – 10 % | Thermal | 37 °C | NIH3T3 fibroblast | 8 | Adv: cell attachment enhance, cost effective Dadv: mechanical strength |
Cartilage Tissue engineering | [60] |
| NICE (nanoengineered ionic covalent entanglement) (GelMA+ kCA+ nSi) | 2.5 × 103/ml | 7.5 % GelMA, 1 % kCA, and 2 % nSi | Crosslinking | 37 °C | Human mesenchymal stem cells (hMSCs), osteoblasts | 60 | Adv: precise control over printability, mechanically resilient Dadv: requirement of physiological temperature, weak mechanical properties |
Cartilage tissue engineering and regenerative medicine. | [65] |
| Gelatin–alginate + cellulose nanofibrils (CNF) and bioactive glass (BaG) | 5 × 105/ml | Ge-5 % Alg-4 % CNF-0.25 % BaG-1 % | Crosslinking | 37 °C | Human bone marrow-derived mesenchymal stem cells(hBMSCs), human osteoblast-like cells (Saos2) | 14 | Adv: improved rheological properties Dadv: increased viscosity and death of cells immediately after printing. |
Bone tissue engineering | [66] |
| GelMA-BMP | 5 × 106/ml | FITC-BMP-SH were mixed with 5 % GelMA hydrogel 0.2 wt% photoinitiator (Irgacure 2959) in DI water | UV light crosslinking | 37 °C | Dental pulp stem cells (DPSCs) | 21 | Adv: high cell viability, high calcification, printability and dental specific microenvironment Dadv: short half-life of BMP in body |
Regenerative medicine and dentistry | [67] |
| Alginate/NFC | 2 × 106/ml | Alginate – 4 %/NFC – 6 % | Ionic cross linking | 37 °C | induced pluripotent stem cells (iPSCs) | 40 | Adv: develop cartilage lesions, maintain pluripotency Dadv: | Cartilage tissue engineering | [85] |
| Alginate and methycellulose (plasma-alg-mc) | MSC – 5 × 106/ ml HOB -2.65 × 106/ml hDPSC- 4 × 106/ml | 3 % alginate 9 % methylcellulose |
Lyophilization | 37 °C | Mesenchymal stromal cells (MSC), Human preosteoblasts (hOB) and human dental pulp stem cells (hDPSC) and | 35 | Adv: excellent plotting properties,high shape fidelity, resembles the native mineral of bone, stimulated osteogenic differentiation Dadv: Stability and cross contamination |
Tissue engineered bone grafts | [86] |
| Alginate/graphene oxide | 3 × 106/ml | 3 % alginate 0.05 % GO | Ionic cross linking | 37 °C | Mesenchymal stem cells (MSCs), osteogenic tissues | 12 | Adv: highly stable, improved structural stability, high cell viability, stimulated osteogenic differentiation Dadv: clogging of needle | Cartilage tissue engineering | [89] |
| Alginate-polyvinyl alcohol (PVA)-hydroxyapatite (HA) | 1 × 106/ml | Alginate – 1.5 %, PVA – 1 %, HA – 5 % | Ionic cross linking | 37 °C | NIH3T3 fibroblast | 14 | Adv: fast gelation, cost effective, shape fidelity Dadv: clogging of needle, inferior cell adhesion |
Cartilage tissue engineering | [76] |
| Hydroxyapatite alginate aerogel | 6.5 × 103/ml | Hydroxyapatite – 16 %, Alginate – 6 % | Supercritical drying | 37 °C | Mesenchymal stem cells (MSCs) | 13 | Adv: fast gelation, cost effective, shape fidelity Dadv: clogging of needle |
Bone tissue engineering | [92] |
| Hyaluronic acid with collagen | 6 × 106/ml | 0.5 %HA 4 % collagen | Photoinitiated gelation | 37 °C | Mesenchymal stromal cells (MSCs) | In-vivo animal model | Adv: cell growth enhancement, angiogenesis Dadv: shape fidelity |
Osteochondral tissue regeneration | [122] |
| Hyaluronic acid TGF-1 tethering | 20 × 106/ml | HA-0.5 % | Crosslinking | 37 °C | Mesenchymal stromal cells (MSCs) | 21 | Adv: cell growth enhancement, chondrogenic differentiation Dadv: shape fidelity | Cartilage tissue engineering | [124] |
| Hyaluronic acid with methylcellulose | 3 × 105/ml | 2 %HA 5–9%MC | Thermal | 37 °C | Marrow stromal cells | 15 | Adv: highly tuneable mechanincal properties, higher cell viability and stability Dadv: low mechanical properties, fast degradation | Cartilage and bone tissue engineering | [126] |
| Silk fibroin (SF), gelatin (GEL), hyaluronic acid (HA), and tricalcium phosphate (TCP), human platelet-rich plasma (PRP) | 5 × 104 ml | SF-6.8 %,GEL-4 %, HA-2 %,TCP-10 % | Ggenipin crosslinking | 37 °C | Human adipose derived mesenchymal stem cells (HADMSC) | 14 | Adv: good cell adhesion Dadv: long process. |
Bone tissue engineering | [133] |
| Silk/PEG | 2.5 × 106/ml | 5–10 % | Thermal, chemical | 37 °C | Fibroblast and human bone marrow | 84 | Adv: good cell adhesion, low cost. Dadv: modification required, long process. |
Bone tissue engineering. | [135] |
| Aldehyde hyaluronic acid (AHA) and N-carboxymethyl chitosan (CMC), two were composited with gelatin (GEL)–alginate (ALG) | 1.5 × 106/ml | GEL– 5 %/ALG – 1 %/CMC - 0.375 %/AHA – 1 % | Ionic crosslinking | 37 °C | NIH/3T3 fibroblasts | 29 | Adv: improved structural stability, high cell viability, osteogenic differentiation and ossification Dadv: mechanical strength |
Cartilage tissue engineered grafts | [151] |
| Carboxymethyl Chitosan-EDTA | 1 × 105/ml | 2 % | Ionic crosslinking | 37 °C | Rabbit chondrocytes | 12 | Adv: improved structural stability, mechanical strength Dadv: low high cell viability |
Cartilage tissue engineering | [154] |
| ECM/AMP (extracellular matrix + Amorphous magnesium phosphates) | 1 × 106/ml | (ECM-α) 2 % octapeptide FEFEFKFK 98 % water 1 % AMP | Ionic crosslinking | 37 °C | Dental pulp stem cells (DPSCs) | 42 | Adv: improved osteogenic differentiation, increase in the bone density Dadv: clogging of needle | Regeneration of patient-specific bone tissue for regenerative dentistry. | [169] |
| Sheet constructs (fibronectin and laminin) in undecane and silicone oil blend | 3 × 107/ml | 5 μl sheet constructs in (35:65 v:v) oil blend | Multi-cellular aggregates | 37 °C | HEK-293 T and ovine mesenchymal stem cells (oMSCs) | HEK-293 T - 7d oMSCs – 35 d | Adv: good compatibility. Dadv: low mechanical properties and cell adhesion. |
Bone and cartilage tissue engineering. | [187] |
To overcome the limitations of inkjet-based and extrusion-based 3D printers, recently, more advanced acoustic-based, laser-based printers, and stereolithographic (SLA) techniques were developed. The laser-based printer is one of the favorable choices for 3D printing due to very high ‘viable cell’ densities (>95 %) in the developed 3D matrix, it can process highly viscous bio-inks and provides a very high degree of precision and resolution. Although this technique has many advantages, it also bears two significant disadvantages: processing time and high cost. The SLA technique cures the bio-ink layer-by-layer using specific wavelength Ultraviolet (UV) light. This technique is very accurate, fast, and has very high ‘viable cell’ densities (>90 %), but it also has disadvantages like the use of high-intensity UV light, complicated post-curing procedures, and a limited number of photo-curable biomaterial [9]. The comparatively new acoustic-based printing has advantages like high-resolution noninvasive cellular aggregate distribution printing, simple, highly biocompatible, and low power-consuming techniques. However, patterns of acoustic waves in printing head channels need to be pre-determined according to the transducers and geometry of the shape to be printed and can’t be changed suddenly to generate complex patterns [10]. Because of the advantages and disadvantages discussed for different frequently used techniques, the researcher needs to select the best bio-printing technique suitable to the cell-laden bio-ink to get optimized 3D printing balancing good viable cell density, well-resolved 3D architecture for better mass transfer and time and cost of printing.
1.2. Properties of bio-ink
According to Williams et al., Bio-inks can be classified into four types; namely support, fugitive (sacrificial), structural, and functional bio-inks. Support bio-inks are generally the biomaterials that support cells during printing and act as an extracellular similar matrix (eg. agarose, chitosan, etc.). Fugitive or sacrificial bio-inks are the biomaterials used in bio-printing along with support or functional bio-ink and can be removed easily from the 3D architecture to form internal channels (eg. Poly Vinyl Alcohol, Pluronic, etc.). Structural bio-inks are the biomaterials that provide structural integrity to 3D printed architecture and can also act as sustained sacrificial bio-ink (eg. Polycaprolactone, poly(lactic acid), Hydroxyapatite, etc.). Functional bio-inks provide biochemical, electrical, or mechanical stimuli to modify the printed cell behavior (eg. Nano functionalized, growth factor-loaded biomaterials) [11].
According to Groll et al., a simplified classification of bio-inks and biomaterial inks can be provided based on the bio-fabrication definition. Thus, bio-inks can be defined as the medium formulated using suitable biomaterials hydrogel for cell forms (cells, spheroids of cell or micro-tissues) based 3D bio-printing (eg. Cell laden GelMA, Collagen, protein-polymer composites, biopolymer composites, etc.). Whereas biomaterial inks are hydrogel formulations of biomaterials that are used to print biodegradable 3D architecture in which cells are seeded (eg. Alginate, Hydroxyapatite, chitosan, PLA, PCA, and composites thereof printed without cells) [12].
As explained above, bio-inks mainly comprise biocompatible biomaterials-based hydrogels and cells/cell aggregates in combinations. The ideal bio-ink should possess some basic properties for projected 3D printing of the tissue architecture (Fig. 3a). Bio-ink should (i) provide sufficient strength to the 3D structure and simultaneously should have porosity to support cell proliferation; (ii) should have sufficient visco-elasticity to draw precise shapes with perfect fidelity; (iii) should be bio-compatible and bio-degradable; (iv) for some types of printers, it should have shear thinning property; (v) in-situ gelation is the most desired property of the bio-ink though it can be enhanced using external stimuli like electromagnetic radiations (UV, visible, lasers) and cross-linking solutions (Ca, aldehydes, etc.) and (vi) it should be permeable to gases and nutrient to support the proliferating cells through the 3D matrix [13].
Fig. 3.
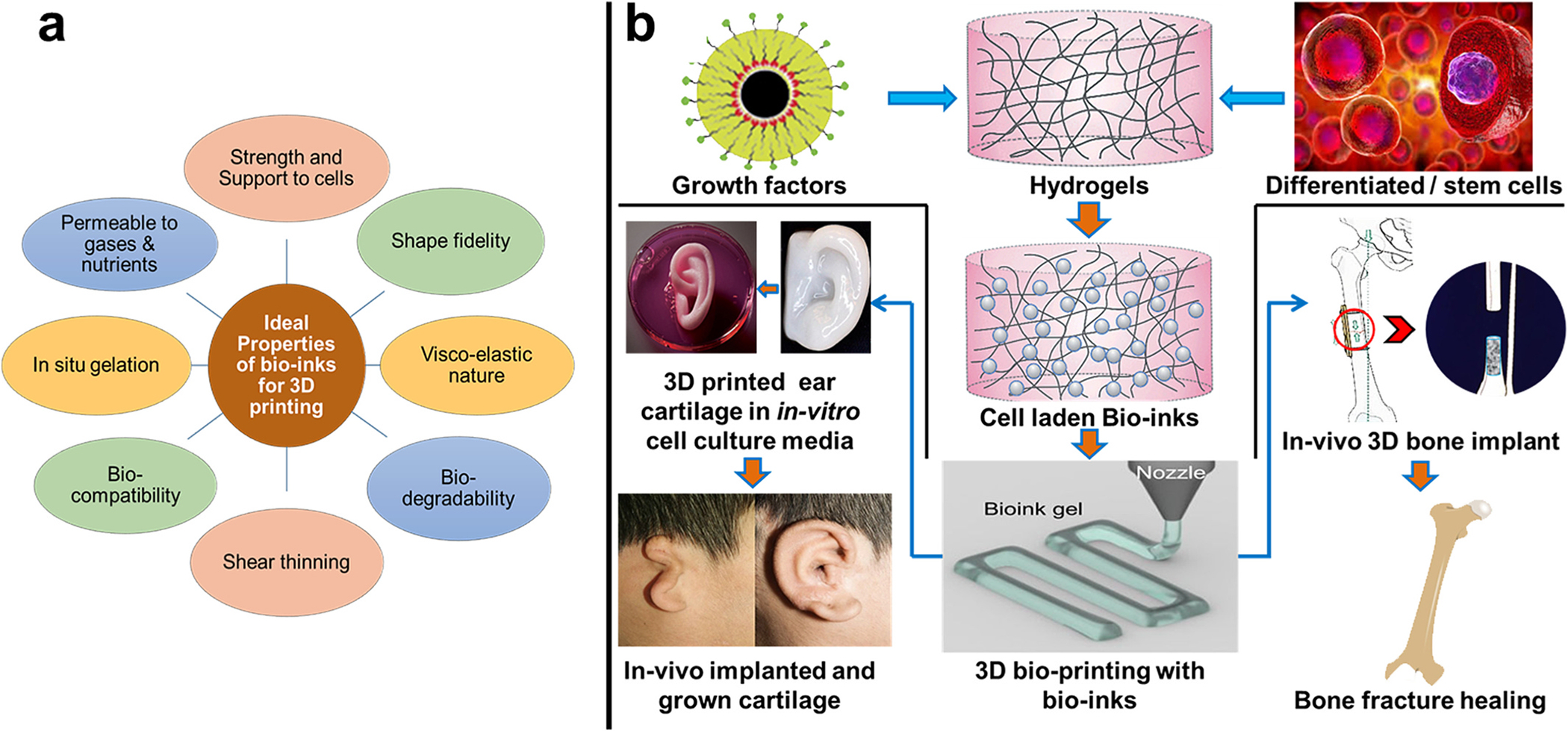
(a) Properties of ideal bio-inks that facilitate printing of 3D architecture for tissue engineering and regenerative medicine applications; (b) the cell-laden and/or functionalized bio-ink development and 3D bio-printing of bone and cartilage regeneration.
Developing and optimizing the cell-laden bio-ink formulation is the crucial step in bio-printing. Considering all the required properties for bio-ink, cartilage, and bone structure, bio-ink development for considerable load and shear stress-bearing 3D structure is a further challenging domain. Various natural, synthetic, semisynthetic, and composite biomaterials have been developed and tested for cartilage and bone bio-ink and 3D printing applications. However, a smaller number of biomaterials were able to meet the maximum ideal bio-ink properties for successful bio-printing of these tissues. This review attempts to provide a detailed overview of the most successful reports and recent updates on the cartilage and bone bio-ink developments (Fig. 3b). Further, different natural, semisynthetic, and composite biomaterials used for the development of the cartilage and bone bio-ink are described in detail. Table 1 describes the cell density supported, printers used for bio-printing, and types of tissues printed and provided comparative information on the capacity of different biomaterials to act as cartilage and bone bio-ink.
1.3. Cell response toward the bio-inks
The cells respond to different biomaterials differently, the formulation of bio-ink for bone and cartilage using a single biomaterial is always challenging as no natural biomaterial can satisfy all desired characteristics (Fig. 3a) of bio-inks. In general, compared to single biomaterial-based bio-ink, multicomponent bio-inks are preferred as they can combine the ideal properties of different biomaterials in one desired composite that can form bio-ink. Every biomaterial has different toxicity to different cells thus, the composite or block biomaterials are developed to keep the cell viability to the maximum. The concentration of the biomaterials/composites used to form hydrogel bio-inks is very important. To achieve optimal viscosity, flowability and shape fidelity of 3D architecture the composition and concentration of the biomaterials are very important. At the same time, the optimum concentration should generate a bio-ink that should have good oxygen permeation/gas diffusibility to avoid hypoxic conditions that can kill the cell population and reduce the cell density in the printed bio-ink [14]. These types of conditions are frequently observed with bone and cartilage bio-inks as they require high biopolymer density for load-bearing and shape-fidelity properties. The printing conditions of bone bio-inks with high-density hydrogel and high viscosity are comparatively harsh (higher piston/pneumatic pressure). This might lead to excessive pressure on bio-ink dispersed cells leading to cell viability and cell density loss post-printing. This might be the reason that many of the bone and cartilage 3D printing research papers report bio-inks that have very good cell viability and post-printing cell densities but lacked structural accuracy and mechanical strength. Researchers are working to overcome this limitation and some researchers have recently suggested the use of nanohydroxyapatite, the use of crosslinkers, or the use of higher initial cell densities as a reasonable solution to it [15]. In addition to this printing, speed is also very important and the stress time on the cells loaded in bio-ink will be more with slower printing speed and pressure will be higher with faster printing speed ultimately affecting the cell viability in printed architecture. The presence of the biologically active molecule (growth factors, vitamins, etc.) in bio-ink favors and directs the embedded cells to proliferate into a tissue. Nanocomposites like nanohydroxyapatite can act as a viscosity enhancer, biological stimulator, and strength enhancer in bone bio-inks [16]. The cells present in the 3D bone or cartilage architecture proliferate in the 3D matrix. While proliferating if the cells are exposed to multiple stimuli (pressure/stress, pH, electrical, etc.), their morphology, functionality, and ECM matrix composition get augmented to do the tissue desired functions [17,18].
2. Natural biomaterials for cartilage and bone bio-inks
The majority of bio-inks are developed from natural biomaterial or composite biomaterial-based hydrogels. These materials provide specific cell binding sites essential for cell proliferation; they are biocompatible and biodegradable. They can be selectively modified to get desired 3D bio-printing properties with maximum viable cell density, superior shape printability, and optimized 3D architecture strength. The natural biomaterials like agarose, gelatin alginate, collagen, hydroxyapatite (HAp), hyaluronic acid (HA), extracellular matrix (ECM), and their composites with other biomaterials (natural/synthetic) will be discussed in the following sub-sections [19]. In advanced materials, the use of fibrin, silk, cellulose, and cell aggregates/cell sheets will be discussed.
2.1. Agarose
One of the most widely used support biomaterials for 3D bioprinting is a naturally derived linear polysaccharide called agarose. It contains disaccharides 3,6,-anhydro-L-galactopyranose, and d-galactose as the repeating units of the chain. Agarose is considered one of the most used candidates for bioprinting because, apart from the high viscosity and shape fidelity, it is also known for the ease of manipulating mechanical properties, rapid gelation kinetics, and also for the potential for further chemical functionalization [20]. There has been evidence of using agarose to engineer cartilage constructs by promoting cell proliferation and matrix production [21,22]. Benya and Shaffer had shown how helpful agarose was in supporting the cell functionality and dedifferentiation of rabbit chondrocytes. They reported the survival of 80 % of cells and the transition of morphology from flattened to spherical anchorage-independent cells in culture along with type II collagen and cartilage-specific proteoglycan synthesis (Table 1) [23]. Another advantage agarose has over other gels like alginate is its rapid gelation without adding any polymerization agents (Table 1) [24]. Researchers have also seen that by using a combination of functional biomaterials, for example, collagen, chitosan, or even alginate, along with the gel, they can form stable 3D constructs and support fibroblast and endothelial cell growth. Lopez-Marcial and coworkers had seen that the blend of agarose and alginate at a 3:2 ratio (with calcium as a crosslinker) demonstrated 70 % cell viability and matrix production over the same period for cartilage tissue engineering. On comparing its mechanical and rheological properties, it was found to be statistically similar to those of Pluronic (a hydrogel with established printing capabilities) for all tests [25]. Alginate-agarose hydrogels, stabilized with gelatin, have also been proven capable of creating a biologically inert matrix that can be used for encapsulating and 3D bioprinting of bone- cells. The addition of calcium salt of polyphosphate [polyP·Ca2+-complex] to alginate/gelatin/agarose/SaOS-2 cell scaffold not only marked an increase in cell proliferation but also opened new doors for the application of 3D bioprinting in bone tissue engineering (Table 1) [26].
Agarose and agarose blended bio-inks printability was further established as researchers tried to embed human mesenchymal stromal cells in printable type I collagen- and chitosan–agarose blends, which were then induced to differentiate toward osteoblasts and adipocytes. By comparing both, it was found that the combination of type I collagen and agarose with varying ratios proved to be the most suitable bio-ink for a broad range of 3D-printed mesenchymal tissues [24]. Recently Campos and coworkers proposed a novel concept using a synchronized dual bioprinting approach by combining two distinct printing strategies for mechanically and biologically improved substitutes for cartilage tissue engineering. Mechanical stability is achieved using a bio-ink prepared by combining equal parts of agarose and alginic acid solutions for micro-extrusion printing. For the cell-loaded functional bio-ink, the type I collagen solution was blended with agarose stock solutions and was bio-printed using cell-compatible drop-on-demand (DoD) bioprinting [27]. The use of collagen along with agarose is not only limited to bones and cartilage but has also been extrapolated to dental pulp regeneration. This specific approach enabled the bioprinting of dental pulp cells by using a blend of agarose and collagen-based bio-inks with suitable rheological, structural, and biological properties, which allowed for vasculogenesis in the root canal. These results were conclusive in maintaining the biological function of the tooth [28].
Not only other bio-inks like alginate and collagen but there has been evidence of using other substances as bio-fabricants too. In one of the recent reports by Nadernezhad and coworkers, the engineered agarose-nano silicate bio-ink served as a new generation of hydrogel bio-ink for extrusion 3D bioprinting with tunable flow properties and bioactivity was reported. The influence of the addition of nano silicates on agarose’s bioactivity was proved successful as the nanocomposite bio-ink showed significant improvement in the metabolic activity of encapsulated cells [29]. Recently, Zhimin and co-workers summarized different stem cell-laden hydrogel bio-ink for bone and cartilage tissue engineering reported to date. They have compiled different natural polymer composites that were developed and tested for bone and cartilage 3D printing applications as bio-inks [30]. The use of agarose and its composite for bone and cartilage 3D printing and tissue engineering was recently summarized by Pedroza-González and his team [31].
In summary, agarose has high biocompatibility and extremely stable mechanical properties required for shape fidelity after gelation, and it doesn’t require polymerizing agents or cross linkers to help in gelation, unlike other hydrogels. However, there are some limitations to use agarose as a bio-ink. Agarose biopolymer lack cell binding sites in their backbones, resulting in bioactivity loss and demanding the attention of biochemists to incorporate some binding sites in the backbone [32]. The second limitation is that the thermal crosslinking mechanism of agarose is also dependent on polymer concentration. High-concentration agarose solutions rapidly form gels and result in a higher shear rate during extrusion and reduced cell viability in bio-ink this limitation demanded engineering intervention to develop new printing heads and printing protocols [33].
2.2. Collagen
Collagen, typically isolated from natural sources, is the major protein component of animal bone, cartilage, skin, and connective tissue, accounting for about 20–30 % of total body proteins. It is a fibrous, structural protein made of three parallel polypeptide strands in a left-handed, polyproline II-type (PPII) helical conformation. They coil around each other with a one-residue stagger to form a right-handed triple helix [34]. Collagen is biodegradable, biocompatible, and a major component of the extracellular matrix (ECM), due to which its application in the biomedical field has been rapidly growing and widely explored [35]. Due to its favorable mechanical properties, collagen, and its derivatives are actively used as bio-inks for 3D printing for hard tissue engineering, namely for bone, cartilage, and osteochondral repair and regeneration. However, at lower temperatures, it is liquid in nature and forms fibers at higher temperatures and neutral pH which pose some limitations [36]. Therefore, in the last few years, several methodologies have been developed to improve the printability of collagen-based bio-ink by adding fabricants like synthetic polymers, thus enhancing the bio-ink rheological properties [37].
Kim and coworkers reported using a crosslinking reagent, genipin, and collagen for bioprinting 3D osteoblast-like cells (MG63) and human adipose stem cells (hASCs). They observed that on the addition of 1 mM of genipin solution, the embedded cells had 95 % cell viability, along with an increase in cell proliferation after printing. The cells also exhibited increased osteogenic activities compared to the conventional controls. These results substantiated the great potential of applications for collagen-based bio-inks in tissue regeneration [38].
The individual hydrogels viz, agarose, sodium alginate, and collagen have their uses and applications. Yang and coworkers compared all the dual combinations of these hydrogels for 3D bioprinting and cartilage repairs. Their reports suggested that sodium alginate and collagen composite (SA/COL) facilitated cell adhesion, accelerated cell proliferation, and enhanced the expression of cartilage-specific genes such as Acan, Col2al, and Sox9, thus paving the way for a promising future of collagen composite in cartilage engineering (Table 1) [39].
The importance of collagen as a bio-ink was further established by using 3D-bio-printed agarose-collagen hydrogel with a high-collagen ratio. This study suggested that if the concentration of collagen in the hydrogel composite is increased, it induces and supports the positive cell morphology change and renders the scaffold suitable for Mesenchymal stem cells (MSCs)-based osteogenic differentiation compared to low-collagen blends [40].
To overcome the structural shortcomings of collagen bio-ink, polycaprolactone (PCL) was used as a fortification additive to form a novel hydrogel bio-composite. This hydrogel was further supplemented with a bio-ceramic (hydroxyapatite (HA)/β-tricalcium-phosphate (TCP)) and growth factors (like recombinant human bone morphogenetic protein-2 [rhBMP-2] and platelet-rich plasma [PRP]). This blend enhanced the in-vitro cellular activities of the osteoblast-like cells (MG63) and collagen synthesis [41]. Many studies have reported the use of β-tricalcium-phosphate (TCP) as a growth factor, indicating its role in triggering osteogenic differentiation. One recent study has suggested that a composite of fibrillated collagen, cells, and 20 % by weight of the bio-ceramic (β-TCP). This is capable of building 3D porous cell-laden composite structure with high cellular responses, viz. cell viability, proliferation, and differentiation using pre-osteoblasts (MC3T3-E1) and human adipose stem cells (hASCs), which were confirmed by using DAPI/phalloidin staining (Table 1) [42]. Another crosslinking agent popularly used with collagen is tannic acid (TA). It was reported that on the addition of 0–3 wt% TA in the cell-laden collagen scaffolds of pre-osteoblasts (MC3T3-E1) cells, the mechanical strength, and biocompatibility of the scaffold was significantly enhanced, and cell viability observed was 95 % [43]. Recently, a study reported treating deep dental carious lesions by removing the infected tissue and filling the root canal with cell-loaded collagen-based bio-inks using a hand-held in-situ bio-printing device [44]. A similar approach was used for dentoalveolar tissue engineering to reconstruct the alveolar bone using bio-inks and bio-printing devices [45]. In one of the recent studies, chondrocytes laden with 4 % collagen bio-ink were developed and tested for de novo cartilage formation. Extrusion-based bio-printing was used for the 3D scaffold development. Initially, the cells (97.7 % cell survival) in the scaffold were allowed to proliferate in cell culture media then the scaffolds were grafted subcutaneously. The growth of the cartilage tissue was observed for 40 days, and it was concluded that the scaffold with cells, post subcutaneous grafting, was converted to cartilage-like tissue with distinctive structure, high content of glycosaminoglycans and high type II collagen (Fig. 4) (Table 1) [46]. Advanced pure collagen and collagen-composite-based bio-inks were also reported for improvised cartilage and bone-like tissue development [47,48].
Fig. 4.
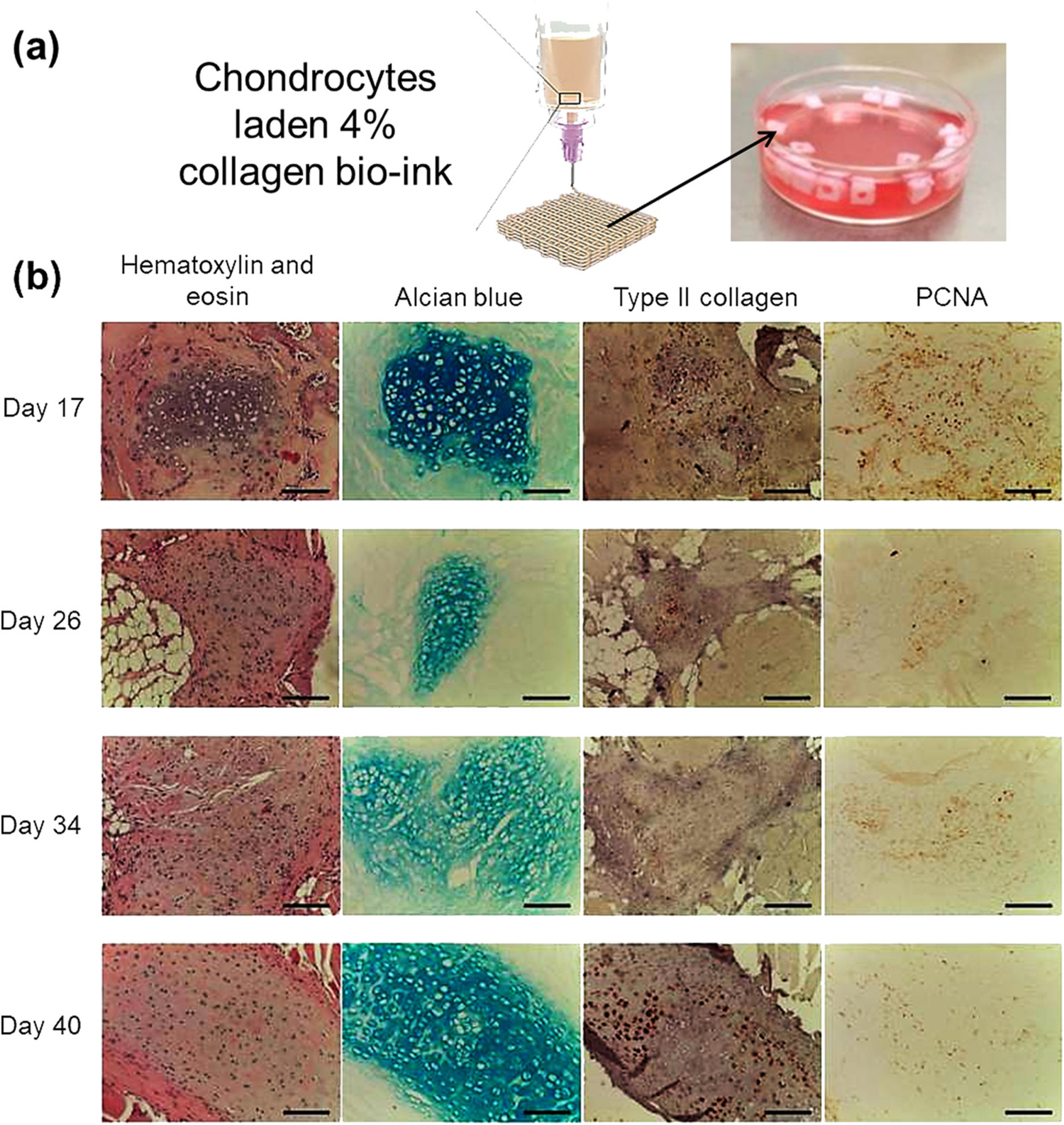
(a) Development of 3D architecture using chondrocytes laden 4 % collagen bio-ink followed by initial culturing in the cell culture media; (b) monitoring of cartilage tissue formation post subcutaneous scaffold grafting [46].
Overall, collagen is the component of human ECM and hence is a natural hydrogel choice for bio-ink. It is biodegradable and has very weak antigenicity with superior biocompatibility. It readily forms fibers with extra strength and stability through its self-aggregation and cross-linking. These advantages made collagen and collagen-based products the first choice of clinicians and biologists [49]. But it has poor mechanical strength and is ineffective in the management of infected sites. Among other limitations, one of the major problems with collagen is that it remains in the liquid state at low temperatures and forms fibrous structures with increasing temperature and neutral pH. It also requires GA and EDC as crosslinking agents to enhance mechanical properties, which are a bit cytotoxic. This major limitation requires biochemist and polymer engineer intervention to improve desired properties while keeping toxicity low [50]. Other limitations include weak mechanical properties, shape fidelity, and batch-to-batch quality variation. These limitations can be subdued with the modifications implemented by engineers and biochemists [51].
2.3. Gelatin
One of the most important constituents of a hydrogel frequently used in bio-printing is Gelatin; it is extremely biodegradable and has been used for several pharmaceutical and medical purposes like soft and hard gelatin capsules, dietary food supplements, and wound sealing glues [52]. It is derived by partial hydrolysis of a fibrous protein - collagen- a primary constituent of ECM (extracellular matrix). The amino acid composition of gelatin is repeating units of Gly-X-Y triplets, where X is mostly proline and Y is mostly hydroxyl-proline [53]. Over the years, the global demand for gelatin has increased significantly, especially due to its applications in hydrogels and bone grafts [54].
The most used form of gelatin is Gel-MA, which contains methacrylamide and methacrylate groups attached to gelatin to form a composite. It is developed by the UV polymerization process in presence of photoinitiators to form covalently cross-linked hydrogels [55]. Gel-MA, as compared to alginate or agarose gels, has demonstrated superior printability, generating structures with greater fidelity and also supported the development of a more fibrocartilage-like tissue, which could be proved by the development of a tissue containing both types I and type II collagen [56]. In one of the most recent articles by Irmak and his co-workers, it was reported that Gel-MA hydrogels contributed significantly to enhance various factors viz., the cell viability, adhesion, proliferation, alkaline phosphatase (ALP) activity, and mineral deposition of MC3T3-E1 preosteoblastic cells (Table 1) [57].
Gel-MA hydrogels support the viability of stem cells as well as the differentiation of chondrocytes and also provide a wide range of mechanical properties depending on several cross-linking parameters making it one of the most eligible biomaterials for cartilage tissue engineering. When it is combined with viscosity-enhancing additives such as HA and/or a reinforcing support structure, such as PCL, it can be fabricated to aid in the engineering of human cartilage [58]. In one such report, Noh and his co-workers developed a bio-ink having desired properties for rheology, morphology, swelling, cytocompatibility, and having the capacity to deliver small molecular drugs using gelatin, hyaluronic acid (HA) and hydroxyethyl acrylate (HEA) [59]. Shin and co-workers, on similar lines, have developed and reported a gelatin-based bio-ink and optimized this bio-ink for 3D bio-printing by combining it with hyaluronic acid and glycerol. The cytocompatibility test showed that the developed bio-ink provided an appropriate physical and biological environment for printing living NIH3T3 fibroblast cells (Table 1) [60]. A study by Kundu and his team has also demonstrated how MSC-laden constructs could be manipulated for specific applications by bio-fabricating them with PCL, to control 3D construct mechanical properties [56,61].
In addition to PCL and HA, the use of other fabricants has also been extensively used in combination with Gel-MA as bio-inks for the 3D bio-printing of osteoblasts. One example is the inclusion of synthetic nano clay, Laponite (LPN), and a gelatin methacryloyl (Gel-MA) bio-ink. This mixture enhanced shape fidelity retention and presented ideal characteristics for skeletal bio-fabrication applications. One of the most interesting observations was that LPN addition did not significantly affect the hydrogel stiffness and, in fact, increased cell proliferation and osteogenic differentiation, which was proven by matrix formation over 3 weeks in the absence of any osteogenic factors [62].
Some studies showed Strontium carbonate nanoparticles as one of the most suitable bio-fabricants. One study reported a nano-composite bio-ink (Sr-Gel-MA) comprising of strontium‑carbonate (Sr) nanoparticles and low concentration (5 w/v%) gelatin-methacryloyl (Gel-MA) hydrogel for 3D bioprinting of low-stiffness cell-laden scaffolds with high shape fidelity and osteogenic factors. The structures produced from this bio-ink retained their physical and mechanical properties, had enhanced printability and exhibited excellent shape fidelity and high cell viability (>95 %) post-fabrication. Furthermore, tests like alkaline phosphatase, osteocalcin (OCN), and collagen type-I (Col I) expression showed that ‘Sr’ addition resulted in enhanced osteogenic differentiation of hMSCs [63]. The effect of UV crosslinking time and the concentration of Gel-MA on cell survival and proliferation was studied, and it was reported that mechanical properties were good with 45 s UV exposure and 10 % Gel-MA concentration. Whereas the viability of HTR-8/SVneo cells was very good up to 60s UV exposure of cell-laden Gel-MA bio-ink. It was reported that 45 s UV exposure of 5 % Gel-MA bio-ink showed optimized cell survival and proliferation with optimized mechanical properties (Fig. 5) [64].
Fig. 5.
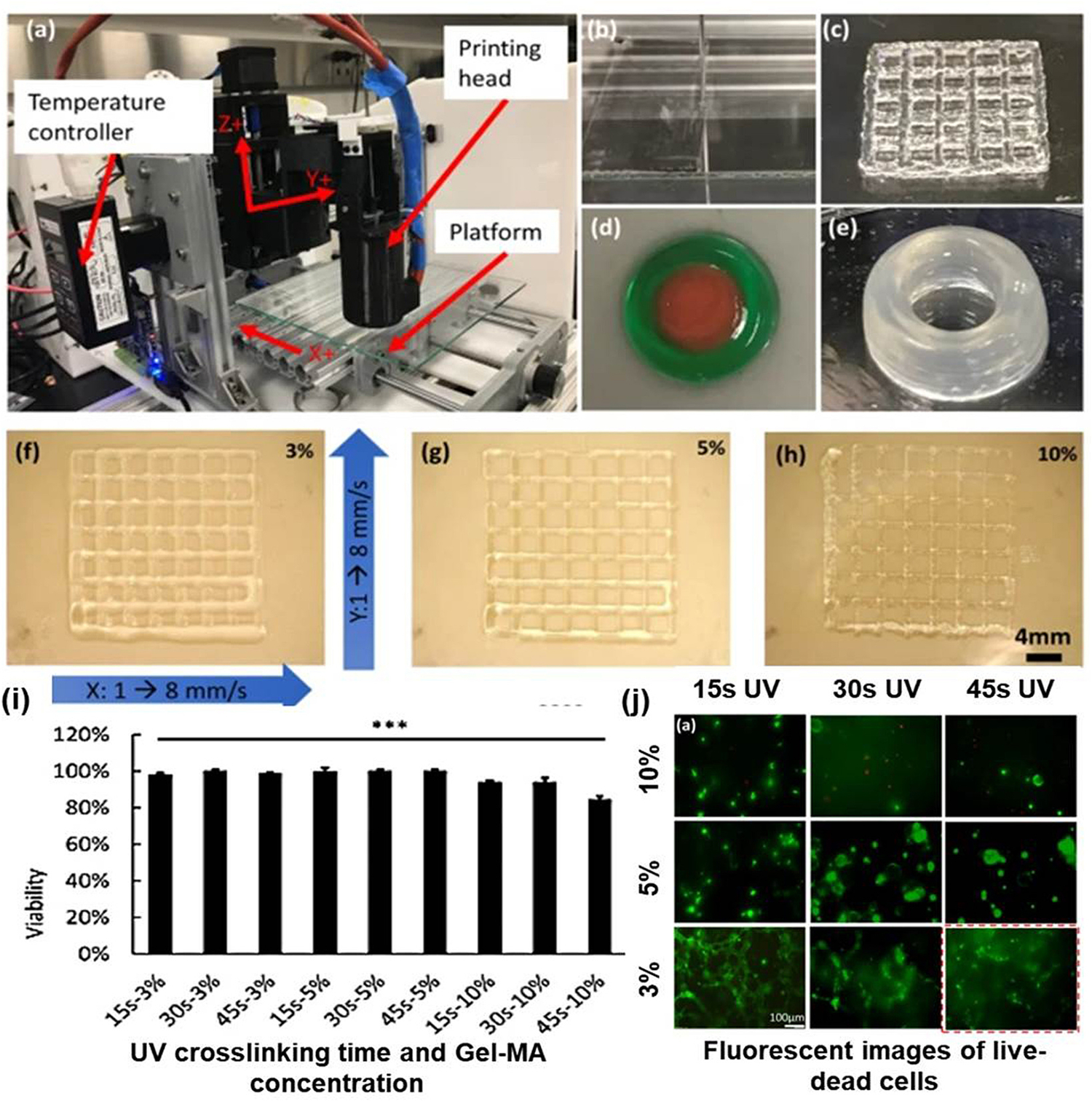
(a) Bio-printing system configuration; (b–e) various 3D architectures (sheet, lattice double ring, and tube) developed using Gel-MA; (f–h) grid architecture developed using different concentrations (3, 5, 10 %) of Gel-Ma at 1–8 mm/s stage speed in x and y plane; (i) cell survival study in 3D architecture at different concentrations (3, 5, 10 %) of Gel-; (j) fluorescent images of live-dead cells in Gel-MA samples printed and crosslinked under conditions of differing Gel-MA concentration and UV crosslinking time [64].
Most advanced bio-inks developed to improve mechanical properties and printability without compromising biocompatibility is a nanoengineered ionic covalent entanglement (NICE) bio-ink formulation. Human mesenchymal stem cells (hMSCs) laden NICE bio-ink enabled researchers to develop patient-specific implantable 3D scaffold for craniomaxillofacial bone defects that did not require external growth factors (Table 1) [65].
Many studies suggest using a composite hydrogel made of alginate and gelatin to strengthen cell biomaterial interaction and cell detection. Hence it was suggested that a combination might provide adequate physicochemical properties for maintaining the viability of human stem cells, bone cells, and mouse fibroblasts during and after the printing process. This, along with the influence of wood-based cellulose nanofibrils (CNF) and bioactive glass (BaG) proved highly appropriate to bone tissue engineering applications despite being non-biodegradable (Table 1) [66].
These studies are limited to bone and cartilage and extrapolate to bioengineering dental constructs. A novel bone morphogenetic protein (BMP) peptide-tethering bio-ink formulation was developed which was successfully conjugated into the gelatin methacrylate (Gel-MA)-based bio-inks and showed robust expression of dentin sialophosphoprotein and osteocalcin in the dental constructs. The cell viabilities were >90 % and also had good printability (Table 1) [67]. Recently, methacrylate cartilage ECM-based hydrogel/bio-ink (cECM-MA) was proposed to overcome the disadvantage of the non-gelling nature of type II collagen at body temperature. Similarly, gelatin–alginate was used to form a blended with de-cellularized elastic cartilage to improve the mechanical properties of new bio-ink [68–70].
The above studies provide an extensive idea of the importance of gelatin-based bio-inks and their different bio-fabrications, which have uncountable applications in the 3D bioprinting of bone, dental, and cartilage tissue engineering. In addition to the exceptionally biodegradable, biocompatible, and safe-to-use properties of gelatin, it is commercially available with different bloom strengths. The aqueous gelatin solution forms thermo-reversible hydrogels below their upper critical solution temperatures. These advantages made gelatin (specifically Gel-MA) a commercially available product for a clinical application like bio-glue/bio-adhesive used in various surgical procedures [71,72]. Gel-MA has limitations due to its low viscosity and narrow bio-fabrication window. It results in poor extrusion and shapes fidelity at even high concentrations. This limitation invited intervention by polymer scientists and engineers to modify the biopolymer properties as well as printers [73,74].
2.4. Alginate
A natural biomaterial obtained from brown algae also referred to as alginic acid or algin, has found numerous applications in biomedical engineering owing to its biocompatibility properties and ease of gelation with the help of divalent Ca2+ cations [75]. These are naturally occurring, negatively charged polymers extracted from the brown algae predominately by adding alkaline solutions [76,77]. It is a linear (unbranched) chain made up of non-repeating copolymers, viz. β-D-mannuronic acid and its C5-epimer α-L-guluronic acid linked via β−1,4-glycosidic bonds and fractional precipitation with manganese and calcium salts have shown that the ratio of guluronic to manuronic acid varies between different alginate sources [78]. The biosynthesis of the alginate pathway can be divided into four different stages: (i) synthesis of precursor substrate, (ii) polymerization and cytoplasmic membrane transfer, (iii) periplasmic transfer and modification, and (iv) export through the outer membrane [79]. Depending on their wide range of molecular weights (32,000 and 400,000 g/mol), different concentrations of alginates can be prepared. The viscosity of an aqueous alginate solution depends on the concentration of the polymer and the MW distribution [80]. The overall gel stiffness also depends on alginate concentrations and the chelating cation concentration [81]. Due to their extreme versatility, they have been used as cell delivery vehicles and a support matrix for tissue engineering, as depots for the local presentation of growth factors or other drugs, and as model ECMs for in-vitro cell experiments [82].
Owing to its vast properties, alginate has several advantages to be used as bio-inks for 3D bio-printing assisted with a bit of bio fabrication with constituents like nanocellulose, methylcellulose, and graphene oxide. One such study was performed on human chondrocytes to support cartilage bio-printing. In this study, the researchers identified a mitogenic hydrogel system based on alginate sulfate, which supports the chondrocyte phenotype. To convert this hydrogel alginate sulfate to a printable bio-ink, it was combined with nanocellulose to promote cell spreading, proliferation, and collagen II synthesis by the encapsulated cells [83]. Markstedt and co-workers published another article that supported this claim in 2015, which reported a bio-ink formed by combining the alginate’s cross-linking ability and nano-fibrillated celluloses (NFC) shear thinning properties provided positive results with living soft tissue with cells. Human chondrocytes, which were bio-printed using the combination of the non-cytotoxic, nanocellulose along with alginate, served as a successful bio-ink exhibiting 86 % cell viability after 7 days of 3D culture [84]. Nguyen and co-workers 3D printed iPSCs loaded Alginate-NFC bio-ink co-cultured with irradiated chondrocytes that supported cartilage regeneration. It could be used successfully in a 60/40 (NFC/A) ratio for cartilage lesions that might otherwise progress to secondary osteoarthritis. It not only maintained the pluripotency but also marked an increase in cell number and cell densities which advocate positivity in the case of 3D bio-inks (Fig. 6) (Table 1) [85].
Fig. 6.
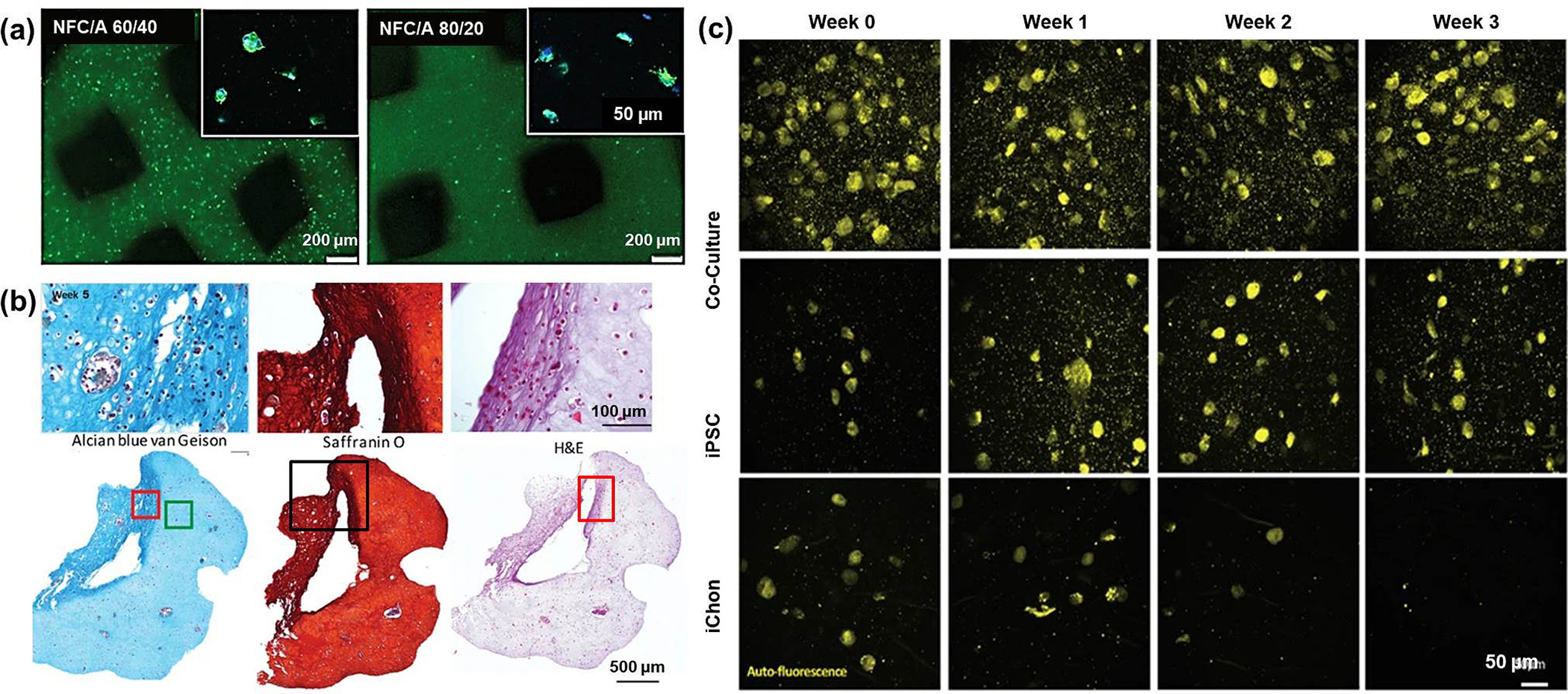
(a) Day 5 post printing – better cell viability (green fluorescence) observed with 60:40 NFC: A (scale 200 μm); cell proliferation (blue – DAPI and green – actin) shown in the upper right corner (scale 50 μm); (b) Alvin blue, Safranin O, hematoxylin and eosin (H&E) stained zoomed and complete 3D architecture images (100 μm and 500 μm) of iPSCs with iChons at 5th week; (c) two-photon excitation fluorescence microscopy images of co-culture, oPSC, and iChons after 0, 1, 2 and 3 weeks (scale 50 μm) [85].
Another common constituent used with alginate to stabilize the bio-ink, as mentioned earlier, is methylcellulose. Ahlfed and his colleagues used the blending of the plasma with 3 w/v% alginates and 9 w/v% methylcelluloses to create this pasty bio-ink (plasma-alg-mc) which then he further bio-plotted with mesenchymal stromal cells (MSC) and primary osteoprogenitor cells to spread within the bio-ink. This novel bio-ink was seen not only to promote excellent cell viability and cell attachment resulting in the formation of intercellular interactions, but also the addition of calcium phosphate cement (CPC) in the second step, formed a synergistic system supporting adhesion, proliferation, and osteogenic differentiation of bone cells (Table 1) [86]. In another study, the same group mentioned using a synthetic nanosilicate clay, called Laponite, along with alginate and methylcellulose, to build up scaffolds utilizing the extrusion-based 3D plotting method. This blending allowed easy extrusion, achieving scaffolds with high printing fidelity [87].
Kosik-Koziol and his co-workers gave an idea about 3D printed cartilage structures of polylactic acid fibers incorporated with alginate, and it is observed that because of PLA fibers, the mechanical properties of the 3D structures improved three times more than the alginate 3D construct (young’s modulus) alone. From the above study, it was observed that the fibers reinforced alginate structures maintained the spherical morphology of the incorporated human chondrocyte cells during in-vitro studies for up to 14 days [88]. In another study, it was revealed that the addition of GO (Graphene oxide) (0.05–1.0 mg/ml) to 3 % alginate significantly enhanced the printing performances of the alginate bio-ink by increasing the structural stability, cell viability, and osteogenic induction of the printed MSCs (Table 1) [89].
Some recent studies mentioned a specific combination of alginate with polyvinyl alcohol (PVA) and hydroxyapatite (HA). This formulation was proven novel due to its osteoconductive and biodegradable nature and its optimal rheological properties for 3D bio-printing of mouse calvaria 3T3-E1 (MC3T3) cells [90]. Hydroxyapatite has also been used as a bio-fabricant for the regeneration of ‘complex bone defects’ which is still a significant clinical challenge. The process involves a gene-activated bio-ink developed by using RGD-γ-irradiated alginate and nano-hydroxyapatite (nHA) complexed with plasmid DNA (pDNA). This mixture used in appropriate combination with bone marrow-derived mesenchymal stem cells (MSCs) and co-printed with a polycaprolactone supporting mesh provided mechanical stability to the construct and robust osteogenesis of encapsulated MSCs in-vitro, with enhanced levels of matrix deposition and mineralization [91].
Supplementing alginate hydrogels with methacrylate (Ma)-decellularized extracellular matrix (dECM) derived from bone tissues has also been shown to give good printability and cell viability results of 3D cell-laden structures. Moreover, the biologically improved microenvironment of alginate-based cell-laden structures formed using this method demonstrated a substantial improvement in the osteogenic differentiation of the human adipose-derived stem cells that were laden in the bio-ink [92]. Thus, the recent studies above have shown a great potential for alginate-based bio-ink in tissue-specific tissue engineering applications [93]. Recently there are reports on the M/G [D-mannuronic acid (M) and L-guluronic acid (G)] ratio determining the crosslinking conditions and mechanical properties of the alginate-based bio-ink helping the researchers to tune the ratio for desired characteristics for specific tissue engineering [94]. Along with this, the composite gel of alginate can act as a drug delivery matrix delivering medicaments and growth factors to the immobilized cells and the surrounding tissue if implanted/injected in-vivo [95,96].
One of the most significant limitations is that ionically crosslinked alginate hydrogels lose >60 % of mechanical strength within 15 h of exposure to phosphate buffers and about 40 % in 9 days of in-vitro cell culture. This limitation is very important from a biologist’s perspective if alginate bio-ink is tested for cartilage or bone. The time mentioned is not sufficient for bone or cartilage cells to proliferate to their highest capacity and start showing desired tissue characteristics. Biochemists and polymer engineers work on this problem by increasing the concentration of high molecular-weight alginate. This increases the viscosity of the pre-gelled solution before gel formation and might affect cell viability. Similarly, increasing the concentration of low MW alginate might facilitate shear thinning and ease of flow but it increases the stiffness of hydrogel [94]. It also has different responses to human and animal cells, thus the preclinical and clinical scientists need to work on some correlation mechanism for the results with animal cells to Human cells. A lack of bioactive binding sites and resistance to protein adsorption requires the intervention of polymer chemists to modify alginate to be more biologically responsive [97,98].
2.5. Hydroxyapatite
Hydroxyapatite (HAp) is a biomaterial that has a mineral composition matching the bone. It was proven that composite biomaterials developed as bone implants have better in-vivo integration with natural bone. [99–101]. It was also proven that the use of nanocrystalline hydroxyapatite (nHAp) in bioprinting to develop 3D architecture as subchondral bone layers significantly improved mechanical properties and enhanced cell adhesion, growth, and differentiation [102,103].
Hydroxyapatite (5 % w/w) was also used in combination with other prominent hydrogels viz methacrylated gelatin and hyaluronic acid to exhibit a significant positive effect on bone matrix development. Encapsulation of primary human adipose-derived stem cells in the hydrogel showed excellent printability and integrity [104].
A novel bio-ink was prepared by combining Nano hydroxyapatite (nHAp) and deproteinized bovine bone (DBB), which were dispersed into collagen (Col) to prepare the bio-ink for 3D printing. This was done to make a bone substitute closest to natural bone structure and composition and prepare a porous architecture of the gel. This blend proved successful as it was not only biocompatible but also had a positive effect on the osteogenic differentiation of the human bone marrow-derived mesenchymal stem cells (hBMSCs) [105]. Nano hydroxyapatite particles and collagen mixture have also been used for extrusion printing applications by making a homogeneous suspension. This study also provides a path for the future potential of this Collagen/nano-HAp bio-ink to reproduce the architecture of natural bone by exploiting the combination of 3D printing technologies [106]. As mentioned earlier, Hydroxyapatite is a critical osteogenic component which is why it was used in many studies as a fabricant to all gelatin-based bio-inks viz. only gelatin, GEL-MA, and acetylated GEL-MA. It was also reported that the addition of HAp in the bio-ink significantly improved the printing properties of the hydrogel and in 30-day culture, enhanced collagen type I, alkaline phosphatase, and fibronectin (proteins associated with bone matrix) deposition were observed [107].
Recently, 3D-printed alginate-hydroxyapatite aerogel scaffolds were developed and tested for bone tissue engineering applications. This 3D aerogel exhibited textural and biological properties, and the printed scaffold showed high shape fidelity for bone tissue engineering (Fig. 7) [108]. Similarly, hydroxyapatite/collagen/chitosan composite hydrogel printed to the 3D scaffold was reported to show restoration of defected maxillofacial mandible bone [109]. In recent advances, HAp was formulated in nano form to enhance its biological properties. The recent studies on the nanometric hydroxyapatite particles as an active ingredient for bio-inks were summarized by Ojeda and coworkers [110–113].
Fig. 7.
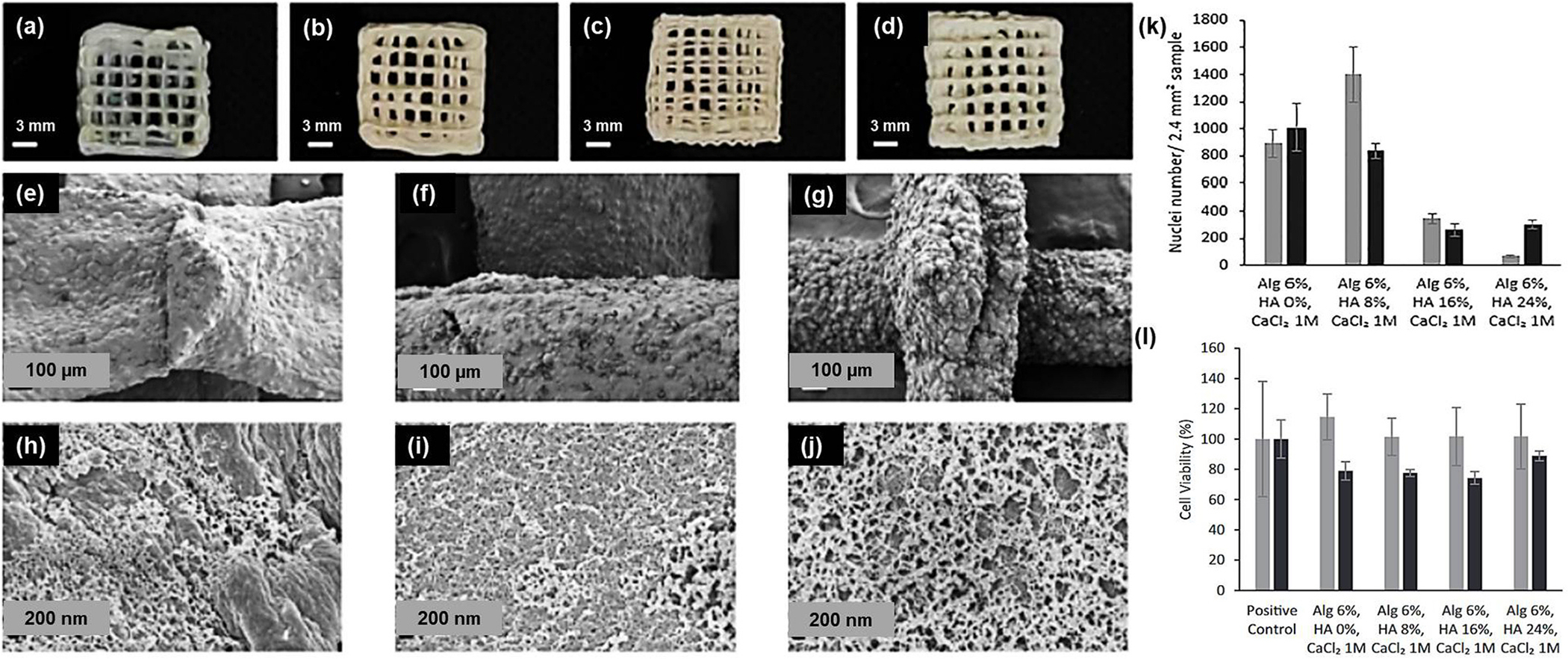
3D-printed alginate-HAp aerogels - images (scale 3 mm) and SEM pictures (scale 100 μm and 200 μm) – alginate: HAp (a) 6:0; (b, e, h) 6:8; (c, f, i) 6:16 and (d, g, j) 6: 24. Gelation using 1 M CaCl2 solution; (k) % volume shrinkage of different bio-ink ratios; (l) % cell viability of BALB/c3T3 mouse embryo cells. [92].
The chemical composition of Hydroxyapatite is similar to the mineral composition of bone; thus, it is more biocompatible and osteoconductive. Even though many successful bone implants have had HAp as a constituent of scaffolds, HAp causes aggregation and clumping due to entropy leading to inconsistent shape fidelity of the 3D architecture generated by bio-ink and also reduces the surface area of the particles [114]. These limitations can be reduced by doping the bio-ink with another mineral like magnesium that helped the bio-ink to print high-strength 3D architecture [115].
2.6. Hyaluronic acid
Necas and his colleagues reported different physiological and pathological functions, basic pharmacological properties, and the clinical use of Hyaluronic acid (HA). HA is a naturally occurring mucopolysaccharide present in all living organisms. It is abundant in connective tissues and cartilages and is a component of the natural extracellular matrix (ECM) [116]. HA can be modified in many ways, and the properties of the resulting materials, including hydrophobicity and biological activity, can be adjusted according to the tissue-specific conditions and were rigorously studied for tissue engineering applications. Due to such robust properties, its applications in 3D bio-printing have also been reported many times [117,118]. HA has been extrapolated for the treatment of Osteoarthritis and, sometimes, rheumatoid arthritis, thus suggesting its potential role in the scope of bone and tissue regeneration [119]. This claim is also supported by a study that has shown that in-vitro and in-vivo cultures of mesenchymal stem cells (MSC)-laden HA hydrogels permitted chondrogenesis, which are early markers of tissue regeneration [120].
One particular study mentioned a functional hybrid bio-ink with fast gelation time and biological function using HA. For fast gelation, tyramine was conjugated to the amine group of hyaluronic acids with an enzymatic crosslinking reaction. Also, for application to complex tissue such as bone, angiogenic peptides such as substance P (SP) and Ac-SDKP (SDKP) and osteogenic peptides such as BMP-7-derived peptides (BMP–7D) and osteopontin (OPN) were immobilized on acrylate HA. These fabrications resulted in optimal mechanical properties, high cell viability, and an increase in the differentiation rate of hMSC cells [121].
Shim and co-workers have shown a 3D printing process that uses a hydrogel comprised of atelocollagen and supramolecular hyaluronic acid (HA) containing human mesenchymal stromal cells. This specific construct was implanted in the rabbit knee joint and showed outstanding osteochondral tissue regenerative ability. The novelty of this blend lies in the principle that allowed two different types of ECM hydrogels to be easily printed and stacked into one multilayered construct without potentially harmful chemical cross-linking (Table 1) [122]. In one recent study, a composite bio-ink of polylactic acid and HA was developed and cartilage regeneration was reported based on significant cell proliferation, matrix deposition, and chondrogenic gene expression [123]. The use of transforming growth factor-beta 1 (TGF-1) with HA in the form of bio-ink (where TGF-1 acted as covalent tethering) was reported to show chondrogenic differentiation from Bone marrow-derived mesenchymal stromal cells (MSCs) in the 3D matrix, and TGF-1 was acted as growth factor (Fig. 8) (Table 1) [124]. Xing and co-workers have recently published a review on various modifications and characteristics of HA that rendered HA a potential biopolymer for bone bio-ink [125]. Law and coworkers also recently suggested that hyaluronic acid and methylcellulose bio-ink has great potential as biomimetic bio-ink for cartilage and bone tissue engineering (Table 1) [126].
Fig. 8.
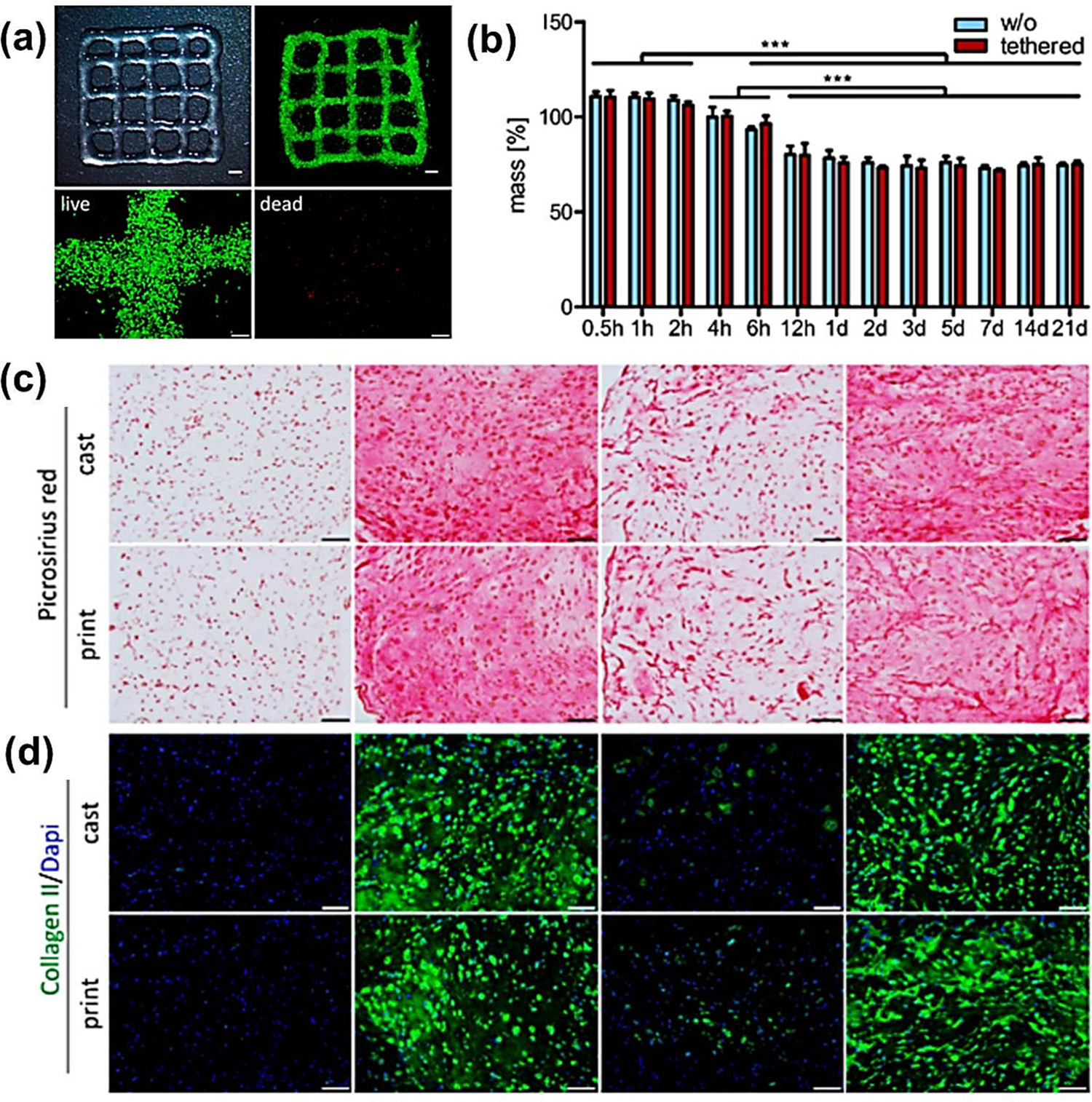
3D printing and bio-ink characterization (a) a 3D printed HA bio-ink grid (scale 2 mm), live (calcein-AM - green) dead (EthD-III – red) staining of the zoomed grid (scale 200 μm); (b) three week (Histological and immunohistochemical (IHC) staining based) swelling analysis (with and without) tethered TGF-β1. [Mean ± standard deviation (n = 3), significancy *** (p < 0.001)]. (c) Picrosirius red staining and (d) IHC staining for collagen type II. Nuclei - DAPI (Scale 100 μm) [124].
Since HA is an important component of the ECM, it is an endogenous component involved in cellular signaling, wound repair, morphogenesis, and matrix organization. Gels made of HA do not cause skin irritation and hypersensitive reactions. They have excellent biocompatibility, non-toxic nature, and tunability of properties and degradation, which have led to HA hydrogels as an ideal candidate for molecule delivery applications. The molecular weight (MW) of HA plays a vital role in body functions, as we can see that low MW HA promotes the production of inflammatory mediators, whereas high MW HA inhibits the production of pro-inflammatory mediators. HA is usually used along with other biomaterials as pure and unmodified HA at working concentrations is unsuitable for producing printable bio-inks. HA in water solutions gives viscous shear-thinning preparations having no yield stress and no shape retention upon printing. If this limitation is subdued high molecular weight HA can be a more suitable candidate for cartilage bio-ink [127].
2.7. Silk-based bio-inks
Silk is a robust material with inherent spinnability and adequate cytocompatibility properties. Silk’s favorable or most exploited properties are that it can completely remove the high temperature or toxic organic solvent requirement into the biomaterial as it can induce sol-to-gel transition by changing the secondary conformations. These applications make silk an appropriate biomaterial for bio-inks [128,129].
Silk has been used in combination with gelatin as a novel bio-ink for optimized rheological properties and improved printability. The use of nasal tissue-derived mesenchymal progenitor cells when cultured with this bio-ink-based 3D architecture, significant cell viability, and multilineage differentiation was observed. [130]. The cell proliferation and influence of the redifferentiation of the cells can be governed by different ratios of gelatin and silk. Another advantage of this modular and novel bio-ink was that it could flow through the nozzle without clogging, facilitating scaffold manufacturing. Therefore, this novel bio-ink has much potential in tissue engineering and regenerative medicine [131]. A recent article by Chameettachal and co-workers suggested that tyrosinase cross-linked silk-gelatin bio-ink offered a suitable material composition for 3D bio-printing of cartilage constructs. The study showed that hMSC-laden constructs in this novel bio-ink showed up-regulated hypoxia which positively regulated the expression of chondrogenic markers over chondrocyte-laden constructs [132]. The combination of gelatin and silk-based bio-inks has also been used in combination with Hyaluronic acid (HA) and human platelet-rich plasma (PRP) to upregulate the gene expression levels of late osteogenic markers successfully, thus suggesting its applications on the HADMSC osteogenic differentiation and the regenerative efficacy after implantation in the bone defect animal models (Fig. 9) (Table 1) [133]. Studies have shown that 3D bio-printed silk-gelatin constructs could provide adequate cellular attachment, proliferation, and even articular cartilage differentiation. This property is attributed to the regulatory role of silk-gelatin bio-ink during chondrogenic differentiation of bone marrow stromal cells (BMSCs) in controlling Hedgehog (Hh) and Wnt signaling pathways [134].
Fig. 9.
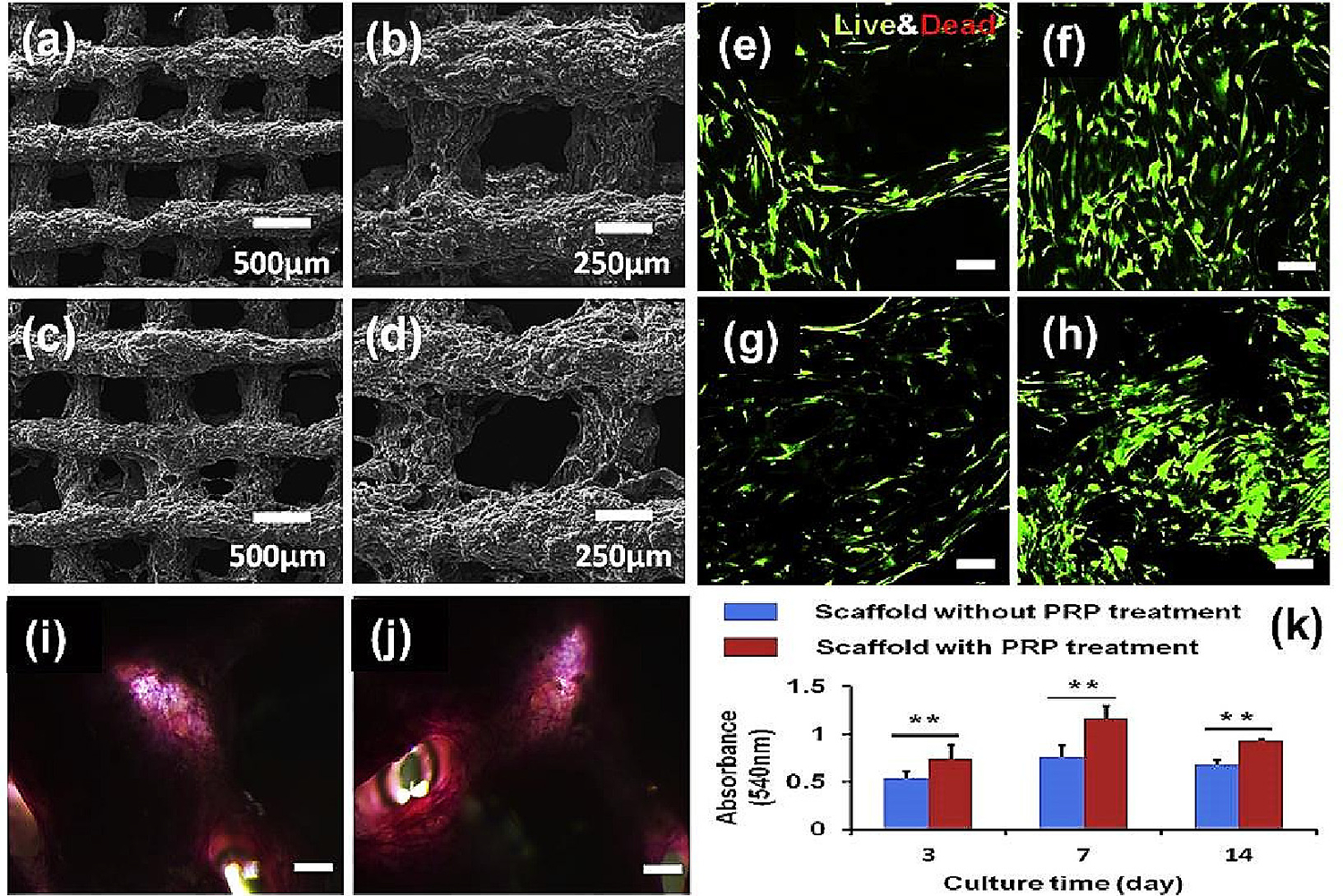
(a, b and c, d) SEM images of SF-based composite scaffolds (without and with PRP treatment respectively, scale 500 and 250 μm); (e, g and f, h) 7 and 14 days Live/Dead images of scaffolds without and with PRP treatment respectively (scale 100 μm); (I nd j) SF/GEL/HA/TCP hybrid scaffold ALP staining images without and with PRP treatment respectively (scale 500 μm); (k) cell proliferation using MTT assay without and with PRP treatment (n = 6; **p < 0.01) [133].
Silk/polyethylene glycol (PEG) hydrogels have also been used as bio-inks for 3D printing in tissue engineering as they have the property to induce silk β-sheet structure formation, thus promoting gelation and water insolubility. When human bone marrow mesenchymal stem cells are printed using this bio-ink, the cells grow faster, especially as the concentrations of the silk were increased (Table 1) [135]. Some studies have also shown the use of strontium-doped nanoapatite as ceramic additives along with silk hydrogel networks. This bio-ink combination improved osteoinduction and osteocyte maturation, enabling better diffusion and promoting cellular crosstalk within the constructs. This hybrid silk-based cartilage and bone bio-ink increased the differentiation of chondrocytes and osteoblasts, preferentially within the bio-printed osteochondral constructs [136].
In summary, silk is a robust material with good spinnability, and it also possesses good cytocompatibility. The advantage of using silk is that it is possible to change the secondary conformations of the silk fibroin polymers by controlling the shear. This property saves the user from requiring high temperatures or toxic organic solvents into the material. Though regenerated silk fibroin (SF) protein-based scaffolds support chondrogenic differentiation of human mesenchymal progenitor cells, it causes frequent choking of nozzles due to shear-induced β-sheet crystallization. These limitations require engineering as well as polymer chemist intervention to assure smooth bio-ink flow through the nozzle. This can be achieved by blending SF with Sodium alginate which dampens the sheer pressure on the β-sheets and avoids crystallization in the nozzle [137]. Silk obtained from Bombyx mori is devoid of cell adhesion motifs, ultimately required for cell attachment and proliferation. Another limitation of SF-based bio-inks is the weakening of the bioactivity of silk when it is re-dissolved in organic solvents to obtain higher concentrations [138]. This can be efficiently managed by adding silk sericin (SS) and other protein bases biomaterials as a copolymer with SF [139].
2.8. Chitosan
Chitosan is the second most abundantly available natural polymer after cellulose and has been reported widely for many pharmaceutical, biological, biotechnological, medical, and regenerative medicine applications. Chitosan alone or in combination with other natural, semisynthetic, synthetic forms and modified forms was used to develop bio-inks [140]. Recently, Tamo and co-workers reported the development of Cell-Laden nano cellulose/chitosan-based bio-inks that enhanced osteogenic cell differentiation [141]. Butler and his team demonstrated that starch and chitosan could be composited for a bio-ink that showed good neurogenic potential [142]. Mora-Boza and team studied different bio-printing strategies affecting the 3D scaffold construct like mechanical strength, biocompatibility, and shape fidelity [143]. The ‘Chitoink’ is the first ever commercialized chitosan-based bio-ink for 3D bio-printing manufactured by CELLINK. Recently, many reports have been on printable hydrogels for cartilage and bone tissue engineering [144,145]. Guoke and his co-workers recently reported the development and use of hydrogels for bone and dental tissue regeneration [146]. Chitosan and composite hydrogels were reported for a variety of tissue engineering and therapeutic applications in-vivo and in-vitro and can be easily modified as bio-inks for the respective tissue engineering applications [147–150].
In one recent report, aldehyde hyaluronic acid (AHA) and N-carboxymethyl chitosan (CMC), two were composited with gelatin (GEL)–alginate (ALG) ink to form GEL–ALG/CMC/AHA bio-ink. This bio-ink was printed at room temperature and was reinforced with a calcium chloride solution. The bio-ink so formed showed 91.38 ± 1.55 % cell viability on the 29th day (Table 1) [151]. Similarly, there is a report of the development of collagen/chitosan-functionalized graphene oxide hydrogel that can provide a 3D matrix for neural stem/precursor cells (NS/PCs) and can also serve as cell-laden bio-ink for 3D bio-printing. It was observed that NS/PCs showed excellent survival, proliferation, and migration capacity in this developed hydrogel [152]. The potential use of chitosan and its derivatives (carboxymethylated, acylated, quaternary ammonium, thiolated, and grafted chitosan) alone or in a combination of other natural and synthetic polymers for the development of bio-inks was highlighted in a recent review [153]. Recent work on carboxymethyl chitosan bio-ink suggested that carboxymethyl chitosan alone can be a very good bio-ink supporting the chondrocytes cells as well as helping them to proliferate and synthesize collagen II and mimic cartilage-like 3D structure (Fig. 10) (Table 1) [154]. Chitosan (CS)/3D-printed poly(ε-caprolactone) (PCL) hybrid hydrogel was developed, and the scaffolds were cured with tetrahedral framework nucleic acid (TFNA). This hydrogel and synovial MSCs (SMSCs) showed the proliferation and chondrogenic differentiation of SMSCs in the hydrogel. The same observations were reported for in-vivo (injected into the articular cavity) chondrocyte regeneration. Thus, the hydrogel is a potential candidate for cartilage bio-ink [155].
Fig. 10.
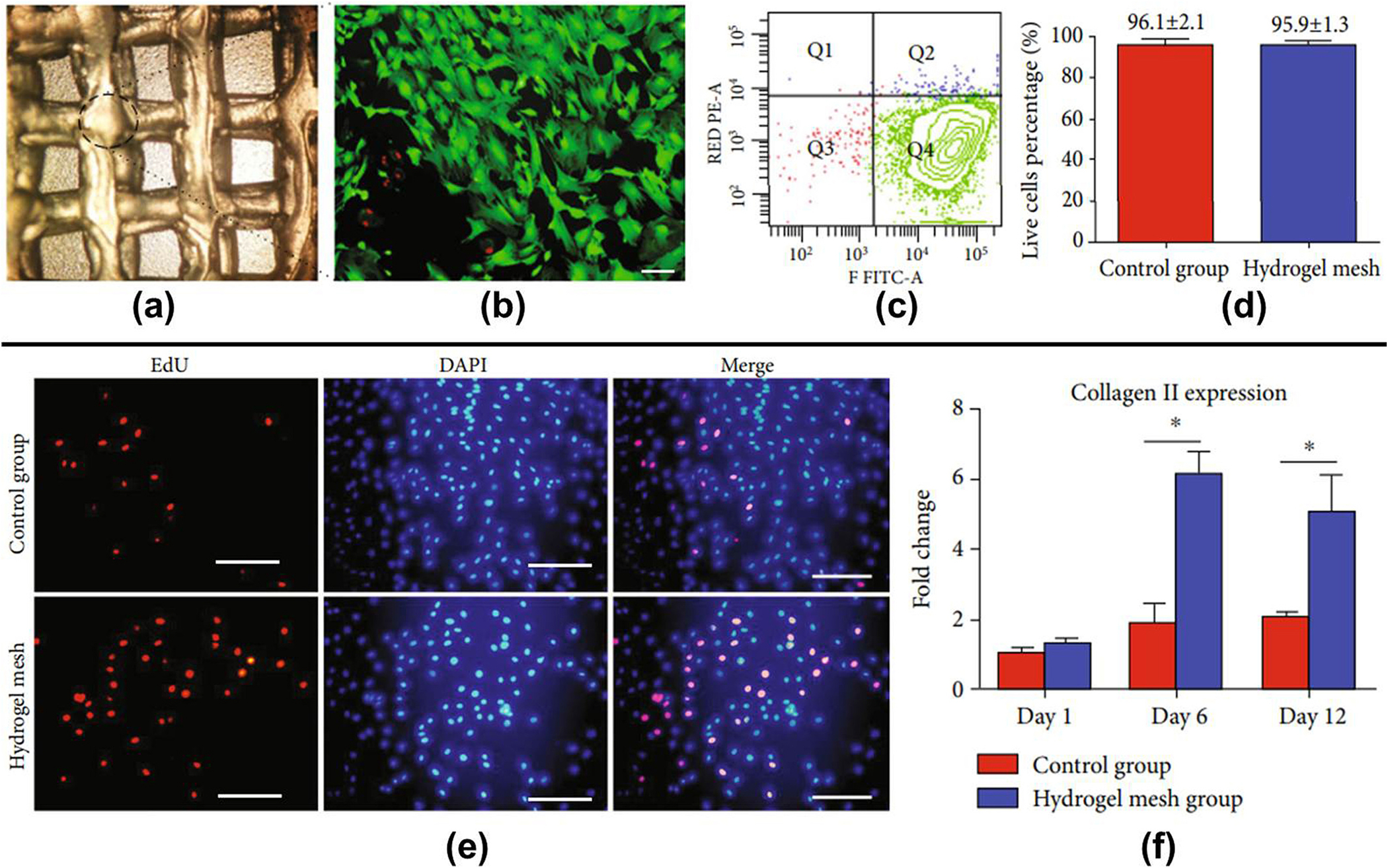
(a) Carboxymethyl chitosan bio-ink based 3D printed architecture; (b) live/dead staining of chondrocytes in 3D architecture (scale 100 μm); (c) bio-ink cell viability based on flow cytometry result; (d) quantification of cell viability in both groups; (e) EdU/DAPI staining of chondrocytes in both groups (scale 100 μm); (f) collagen II marker expression [154].
One of the recent studies reported the use of visible light curable glycol chitosan [water-soluble methacrylated glycol chitosan (MeGC)] cell-laden bio-ink for bone tissue engineering. The developed bio-ink showed good shape fidelity due to photo-curing and has shown cell viability above 92 %, a proliferation above 96 %, and a hemolysis level below 2 %, proving it a good candidate for bone tissue engineering [156]. A cell-laden bio-ink was prepared using thermogelling chitosan, glycerophosphate, hydroxyethyl cellulose, and cellulose nanocrystals (CNCs). It contained pre-osteoblast cells (MC3T3-E1), and it has been reported that the addition of CNCs and cells (5 million cells/ml) significantly improved the gelation time [(<7 s) at 37 °C], the viscosity of bio-ink and the mechanical properties of 3D printed chitosan scaffolds. It was also observed that scaffolds supported the upregulation of alkaline phosphatase activity, higher calcium mineralization, and extracellular matrix formation [157]. The applications of chitosan and modified chitosan composites were recently reported for 3D-printed bone tissue engineering applications with an emphasis on mechanical, antimicrobial, and metal-chelating properties. This study also focused on the use of carboxymethyl chitosan which is a water-soluble chitosan derivative for a variety of applications including bone and cartilage. In a separate report, it was stated that the combination of bioactive molecules with chitosan-based bio-composites can support stem cell differentiation and proliferation, accelerate tissue regeneration, angiogenesis, and vascularization, and also provide desired near in-vivo mechanical properties [158,159].
Overall, chitosan is a positive-charged, low-cost natural polymer with good biodegradability, biocompatibility, fluid absorption/retention, non-toxicity, mucoadhesive, hemostatic, and antimicrobial properties. The structural modifications and composite of chitosan render it with adjustable properties necessary for different tissue engineering applications. The cross-linking and concentrations of chitosan can control the drug and growth factor release properties. The 3D hydrogel matrix provides the porous structure for cell attachment, survival, and proliferation. However, the shape fidelity and mechanical properties of only chitosan bio-ink are low. It needs to be combined (composite) with other biomaterials to get the desired mechanical property for bone tissue engineering. The shape fidelity can also be achieved by chemical modification and composite formation of chitosan [160]. In addition, the biodegradation time of high molecular weight chitosan and its composite is also high. The original chitosan, being deacetylated product of chitin, is not water-soluble and requires mild acidic conditions for its solubilization. This restricts its use in many tissue engineering applications. To overcome this problem chemically modified chitosan (carboxymethyl chitosan) is being tried by polymer chemists and engineers as it is water soluble and retains maximum chitosan properties [161].
3. Potential new candidates for bio-inks
3.1. Extracellular matrix (ECM)
The components of the ECM include collagen, fibrin, gelatin, and HA, which have been used as bio-inks widely because of the mechanical, biophysical, and biochemical properties they provide to the cells. But the Organ-derived de-cellularized ECM (dECM) ensures the integrity of the bio-printed structure, and after mixing it with either natural or synthetic materials, it has been shown to have potential large-scale applications as bio-ink [162]. In one of the recent articles, the authors presented a novel bio-ink from de-cellularized extracellular matrices (dECMs) for bio-printing cell-laden constructs. This dECM bio-ink is conducive to the growth of different 3D structured tissue, including adipose, cartilage, and heart tissues [163]. The significant difference between the other materials and ECM is that the former is not capable of recreating the complexity and microenvironment necessary for cell-cell and cell-matrix interaction for 3D cellular organization necessary for developing a tissue. But in the case of the latter, it can very consistently recapitulate all the features of the natural extracellular matrix (ECM) [164]. The potential of dECM as a bio-ink is getting more popular because it has been established as a biomaterial that preserves tissue’s native environment, promotes cell proliferation, and provides cues for cell differentiation. It has been used extensively in the utilization of bone and cartilage through applications as scaffolds, particles, and supplementary factors in bone and cartilage tissue engineering [165].
As seen in the case of other bio-inks, these biomaterials are combined with other biomaterials to produce the most appropriate bio-ink for 3D printing of the cells. Similarly, even ECM can be bio-fabricated with other suitable materials. One such study is presented in an article by Lee and co-workers where biocompatible and nontoxic natural hydrogels like collagen/extracellular matrix (ECM) and alginate were copolymerized to develop a bio-ink to bio-print 3D porous structure laden with pre-osteoblasts and human adipose stem cells (hASCs). Assessment of cell viability via DAPI/phalloidin staining and cell migration, showed positive outcomes in the case of both pre-osteoblast cells and hASCs [166].
In another article, Rathan and team have shown the benefits of functionalizing alginate bio-inks with cartilage extracellular matrix (cECM) to enhance the chondrogenesis of encapsulated MSCs. This combination significantly improved its chondro-inductive potential and promoted robust chondrogenesis of the MSCs. In the same article, authors also observed that when this cECM-functionalized bio-ink was deposited into a 3D-printed PCL network, biomimetic constructs with mechanical properties comparable to native cartilage could be developed [167]. It was reported that ECM cannot be printed alone as it forms mechanically inferior 3D architecture. It was suggested that ECM can be reinforced using polycaprolactone (PCL) and co-printed with an ECM material as a framework to enhance the structural stability of the printed scaffold [168].
Dubey and his co-workers have established a novel bio-ink combination using ECM-based hydrogel containing 2 % octapeptide FEFEFKFK and 98 % water with AMP (Amorphous magnesium phosphates) particles to realize high cell function with desirable bio-printability. The Cell-laden AMP-modified bio-printed constructs showed an improved cell morphology and osteogenic differentiation of dental pulp stem cells (DPSCs) encapsulated in the bio-ink, as well as in-vivo bone regeneration (Table 1) [169]. Further, a novel bio-ink by combining de-cellularized extracellular matrix (dECM) with one of the most used natural hydrogels, agarose, with 3 T3 fibroblast cells also showed an appropriate environment for cell proliferation [170].
One of the major limitations of ECM is that it is difficult to print and even a printed ECM often has poor mechanical properties. Also, to use ECM as bio-inks and in regenerative therapy, the excised tissue must first undergo de-cellularization to remove cellular components, leaving behind only the noncellular ECM that can be used for therapeutic applications. The decellularization process is a limiting process where the damage to ECM needs to minimize while removing maximum cellular material.
3.2. Fibrin-based bio-inks
Fibrin, a natural component of blood, is obtained from fibrinogen, a fibrous glycoprotein that is essential for hemostasis, wound healing, inflammation, angiogenesis, and other biological functions. It forms a blood clot on being converted to fibrin, which is important for wound healing [171]. Fibrin-based bio-inks have been used in 3D bioprinting of human induced pluripotent stem cells (hiPSC), and their differentiation into mature neural phenotypes, as well as providing a suitable environment for the cell to survive and differentiate [172]. It was reported that fibrin and its co-biopolymers have been used as 3D support materials for stem cells as well as differentiated cells to regenerate bone, cartilage, cardiac tissue, tendons, and ligaments apart from neural tissues [173]. Not a lot is known concerning fibrin-based bio-ink for bone till now, but its regenerative properties show good potential. Though fibrinogen alone has a limited capacity being qualified as a bio-ink; due to its poor rheological properties combining fibrinogen solution with other printable biomaterials like gelatin and alginate was suggested. Bio-inks with tunable bio-printing properties could be achieved that could facilitate extrusion and maintain post-printing 3D shape integrity with this approach [174].
However, fibrin-based bio-inks also have some limitations. The most significant limitation of fibrin-based bio-inks is that they possess poor mechanical properties and undergo fast disintegration. They require the presence of a proteinase inhibitor for structural stabilization for up to 4 weeks in-vitro. Other limitations, like the shrinkage of the gel that happens during the formation of flat sheets, low mechanical stiffness, and its rapid degradation before the proper formation of tissue-engineered structures, were also reported.
3.3. Cellulose-based bio-inks
Cellulose, one of the most important natural polymers and an almost inexhaustible raw material, is a linear polysaccharide composed of β(1 → 4) linked D-glucose units obtained from the biosynthesis of plants or bacteria [175]. When cellulosic extracts are prepared in dimensions of the nanometer range, it is termed nanocellulose, which is again subdivided into nano-fibrillated cellulose, cellulose nanocrystals, and bacterial cellulose [176]. Nano cellulosic materials display many interesting properties for application in tissue engineering, including cost-effectiveness, sustainability, environmental friendliness, biocompatibility, biodegradability, and non-toxicity. Therefore, it can be used as bio-inks for 3D bio-printing in tissue engineering, drug delivery, and wound healing [177].
A novel bio-ink prepared with cellulose nanofibril hydrogel with native or synthetic calcium-containing particles was reported to help repair bone defects. Such 3D bio-printed constructs exhibit high porosity due to the shear thinning properties of cellulose nanofibrils which serves as an excellent property for improving printing fidelity [178]. Cellulose nanofibrils are known for their unique properties, which include sustainability, biodegradability, biocompatibility, high surface area, good strength properties, and abundant availability. The viscosity of pure alginate hydrogel was only about 16 % of the initial value, as compared to 66 % on fabricating it with cellulose nanofibrils. Apart from rheological properties, the combination also helped in increasing mechanical strength as well. The overall applications of the novel bio-ink T-CNF/SA (TEMPO-oxidized cellulose nanofibrils/Sodium alginate) showed a promising future for bone tissue engineering [179]. A novel bio-ink composition consisting of Nanocellulose-alginate-(hyaluronic acid) was reported to withstand short autoclave cycles and be used for bone and cartilage tissue engineering applications [180]. The advantage of cellulose-based bio-ink is that the viscosity of NFC changes exponentially with concentration, which is ideal for creating 3D-printed free-standing, biocompatible constructs. They can control their mechanical behavior to adapt to the cell microenvironment and cell behavior. The limitations of nanocellulose-based bio-inks are attributed due to the lesser understanding of the properties of NFC and the lack of universally accepted protocols for sample preparations and characterizations.
3.4. Cell sheet and cell aggregates as bio-inks
Cell aggregates-based bio-ink consists of numerous cells used for the development of 3D printed structures. The spheroids were dispensed into the biocompatible scaffolds, allowing them to fuse by the self-assembly process [181,182]. It is a “scaffold-free” approach that has recently gained attention due to its ability to recapitulate tissue biology using self-assembly, mimicking the embryonic development process. This procedure has been used for creating cartilage strands as building units to bio-print articular cartilage tissue [183]. As compared to the previous technologies, in which cell proliferation, differentiation, and ECM production can lead to deformation, shrinking, and/or thickening of the printed tissue constructs as they mature, the scaffold-free 3D bioprinting not only makes it possible to print isolated cells without a biomaterial carrier but also makes a significant contribution to advancement in regenerative medicine [184].
Just like cell aggregates, cell sheet-based bio-inks are also scaffold-free. Bakirci and co-workers have developed this novel bio-ink where researchers used poly (N-isopropylacrylamide) (PNIPAAm) coated detached cell sheets to prepare cell sheet-based bio-ink and bio-printed to form various shapes. The results showed an increase in structural integrity compared to cell aggregates [185].
Some studies have shown that combining bioprinting and Mesenchymal stromal cell (MSC)-laden polylactic acid microcarrier technology allowed the extensive expansion of cells by forming multi-cellular aggregates. The combination improved the compressive modulus of the hydrogel constructs, facilitated cell adhesion, and supported osteogenic differentiation and bone matrix deposition by MSCs. These studies pointed out the potential of this novel micro carrier-based bio-fabrication approach for bone and osteochondral constructs [186]. Similarly, 3D bio-printing with aqueous droplets containing mammalian cells was studied to produce patterned cell sheet constructs in oil. In this study, ovine mesenchymal stem cells (oMSCs) were printed at high densities (107 cells/ml) with very high droplet resolution (1nL) in around 200 μm 3D geometries. These oMSCs-laden 3D architectures showed differentiation into collagen type II-rich cartilage-like structures (Fig. 11) (Table 1) [187].
Fig. 11.
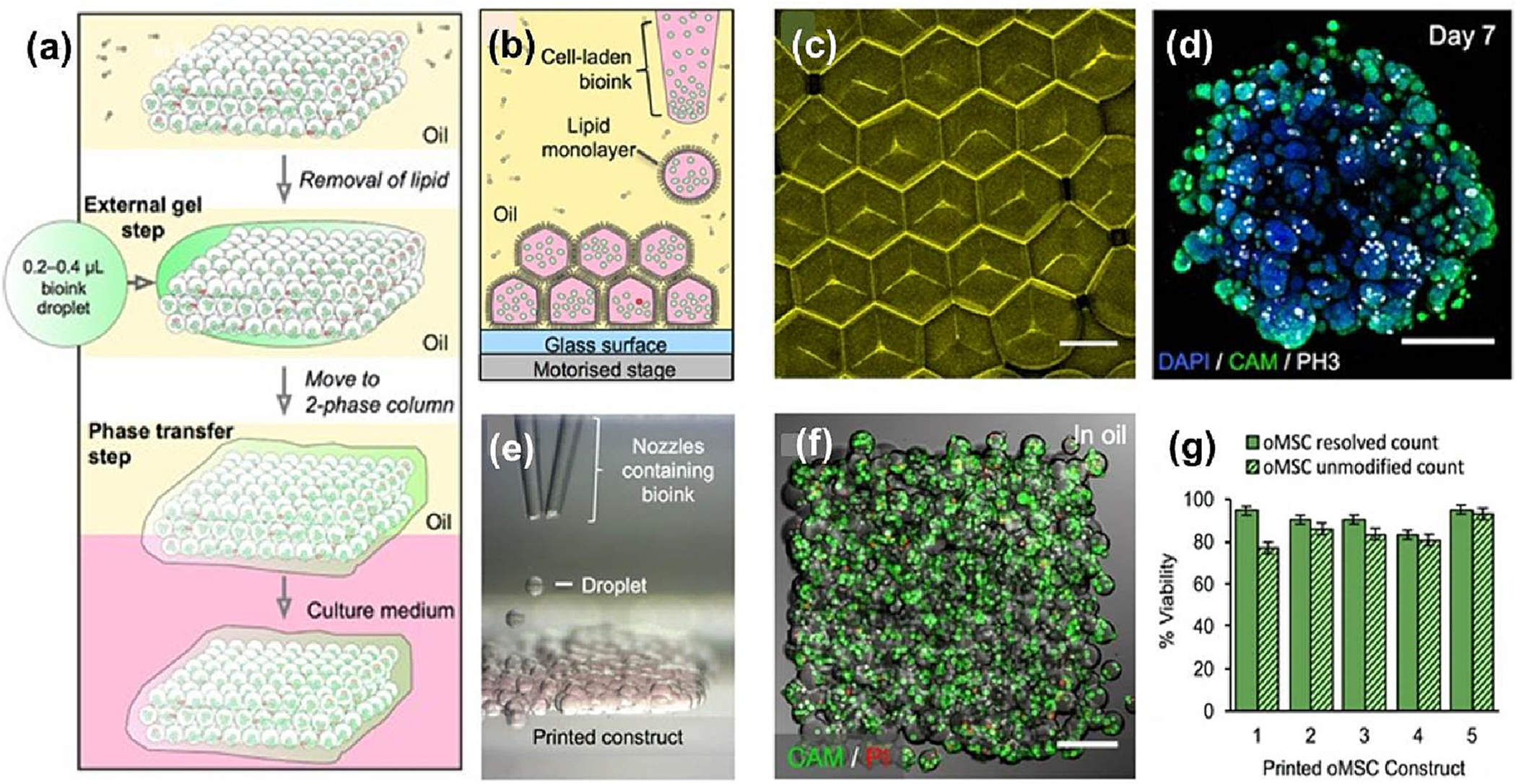
(a) Schematic for the develop cell sheet-based 3D architecture using a two-phase system; (b) layers of 3D printed cellular constructs; (c) bilayered droplet interface (11 × 14 × 7 droplets) [interface – yellow due to sulforhodamine-101 (scale 100 μm) as seen in fluorescent confocal micrograph]; (d) seventh-day z-stack 3D image (nuclei – DAPI, blue and cytoplasm - CAM, green; scale 200 μm) (e) bright-field micrograph of cell layers patterned and containing two cell types forming junction (glass nozzle d ≈ 150 μm and droplet size d ≈ 130 μm); (f) live/dead cell staining of a printed HEK-293 T cellular construct (11 × 14 × 2 droplets) under oil ≈700 cells (4 × 107 cells ml−1 scale 150 μm); (g) graph of oMSC viabilities [187].
This scaffold-free technology generates tissue strands that are not only bio-printable, but also facilitate rapid fusion and maturation through self-assembly, enable bioprinting in solid form, do not need a liquid delivery medium during extrusion, and do not require a support molding structure during bio-printing for cell aggregation and fusion. It reduces the use of toxic or harmful chemicals, which might decrease the viability of cells or cause other side effects. One of this approach’s limitations is that printing large, clinically relevant grafts requires the encapsulation of high amounts of cells, which are difficult to obtain using these bio-inks. Also, these hydrogels are too soft and lack mechanical strength.
4. Summary
The literature compiled here suggests that despite rigorous efforts in developing ideal bone and cartilage bio-inks the development of ideal bone or cartilage 3D tissue construct is still a challenge. The ideal expectations from the literature for bone and cartilage bio-inks requirements are summarized in Fig. 12. It can be understood that depending on the biomaterials both bone and cartilage bio-inks have some common performance requirements like shear-thinning property, extrudability, self-supporting ability, laminar flow in printing, biodegradability, biocompatibility and external stimulus responsiveness needed to be optimized independently. At the same time, both types of bio-inks need to be optimized for architectural morphologies like interconnecting pores and controlled swelling necessary for cells’ survival and proliferation.
Fig. 12.
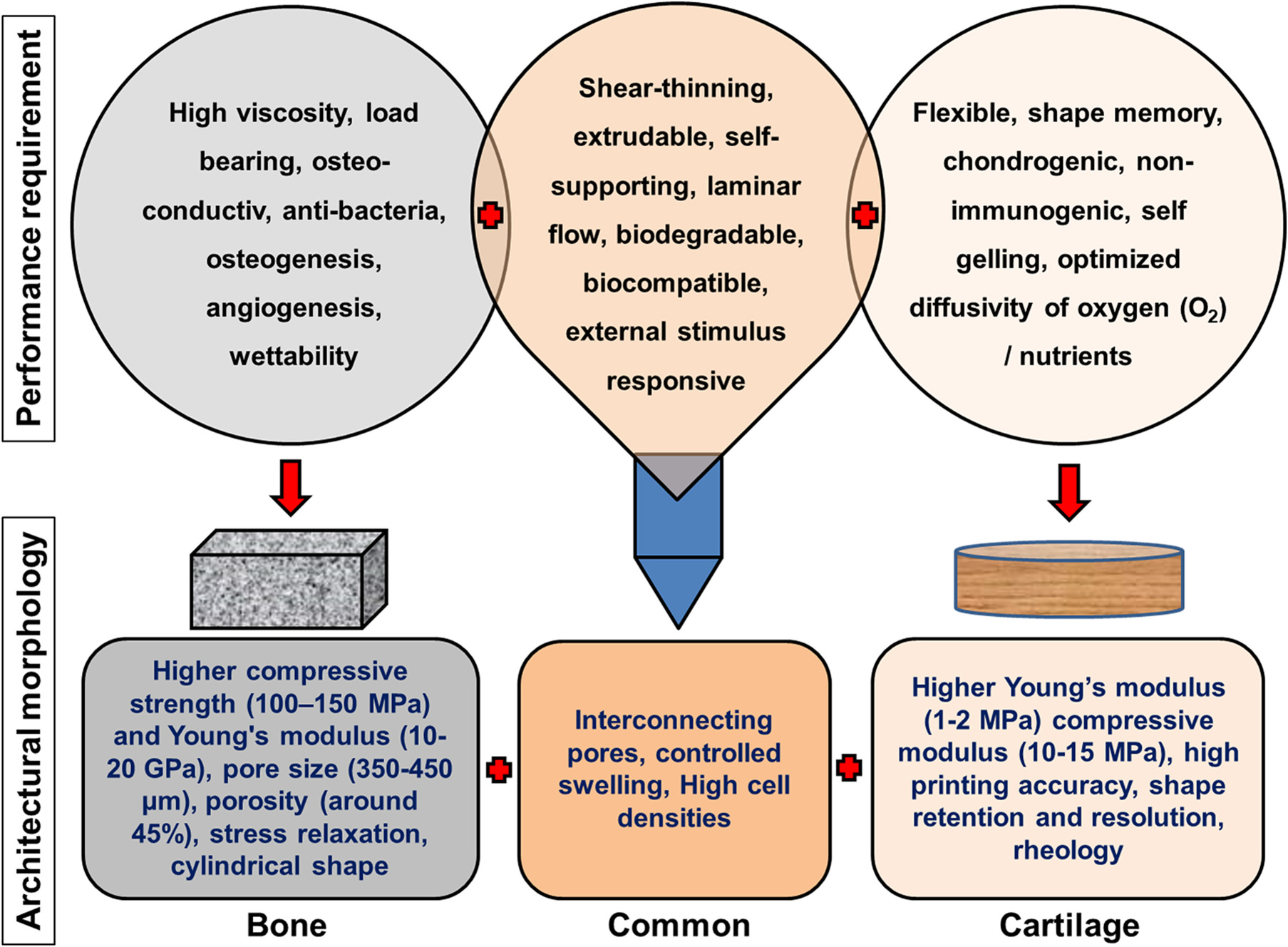
The performance and architectural morphology requirements for the success of bone and cartilage bio-inks [188–191].
In the case of bone bio-inks the ideal performance needs to be optimized for high viscosity, post-printing load-bearing ability/capacity, osteoconductivity, anti-bacteria property, osteogenic property, angiogenic property, and wettability. At the same time higher compressive strength (100–150 MPa - natural bone) and Young’s modulus (10–20 GPa – natural bone), pore size in the range of 350–450 μm, porosity (around 45 %), stress relaxation property, precise cylindrical shape are the important architectural morphologies that need to be optimized with different biomaterials based bio-inks [188,189].
For cartilage bio-ink flexibility, shape memory, chondrogenicity, non-immunogenicity, self-gelling, and optimized diffusivity of oxygen (O2)/nutrients are important performance requirements that needed to be optimized. At the same time, the higher Young’s modulus (1.03 ± 0.48 MPa – natural cartilage) compressive modulus (10.60 ± 3.62 MPa - natural cartilage), high printing accuracy, high shape retention and resolution, and rheology for shape fidelity are the important architectural morphologies that are needed to be optimized with different biomaterials based cartilage bio-inks [190,191].
5. Future directions
The need of the hour for replacing defective/damaged organs/tissue and the scarcity of organ donors intensified the efforts to design and develop new techniques to cater to these needs. One such advanced technology is 3D bio-printing/additive manufacturing, which is reaching new horizons in tissue engineering and regenerative medicine every day. The 3D bio-printing technique with advanced 3D/4D bioreactors is advancing the experimental regenerative medicine science to practicality. 3D bio-printing also showed vast promising applications in diagnostics and drug delivery. The important component of 3D bio-printing is bio-ink, which can be developed with various natural, synthetic, and composite biomaterial-based hydrogels. This review presented the progress of natural bio-ink development related to cartilage, bone, and dental tissue regeneration. These studies are critical in addressing orthopedic issues like bone fractures, major bone defects, and bone correction/reconstruction surgery.
In recent years 3D printing technology is explored for meniscus and intervertebral disc cartilage regeneration. Meniscal tears are frequently observed in knee injuries and osteoarthritis. The position of the meniscus is between the joints and that demanded a high compressive modulus of the bio-ink. At the same time, depending on patient to patient the size of the 3D architecture need to be precisely printed and faster cell proliferation is desired for early regeneration of the cartilage is expected from the developed bio-inks. Due to increasing numbers of meniscus cartilage damage patients, the 3D printing of the meniscus cartilage has become a prime research area. Thus the majority of the cartilage printing performance and architectural morphology requirements were anticipated for meniscus cartilage regeneration [192,193]. Similarly, the intervertebral disc is also subjected to excessive compressive stress, and due to postural and accidental conditions these intervertebral discs damage and give rise to the nucleus pulposus by damaging the annulus fibrosus pressuring the spinal cord leading to many painful complications. In recent years many studies were reported developing 3D printed constructs for a replacement for the damaged intervertebral disc. Thus, both meniscus cartilage and intervertebral disc regeneration are the desired research areas in the 3D cartilage printing domain [194–196].
The bio-inks can also be explored with drug (growth factors and medicaments) loaded nanoparticles for localized drug delivery. The role of low-intensity electric currents (LIC), also known as the current of injury, in enhancing cell proliferation in 3D constructs was previously established [197]. Novel electricity conducting bio-inks can be explored to enhance cartilage and bone regeneration in in-vitro and in-vivo experiments [198]. Further, as mentioned previously, the diagnostic applications of the cartilage and osteoblast cell-laden bio-inks as organoids and 3D porous constructs can provide valuable information about the toxicities and effects of different experimental variables (materials, physical strains, secondary metabolites, and drug formulations) can be effectively established.
This review mainly covers natural polymer-based hydrogels, but along with natural polymers, synthetic polymers and the composite of natural and synthetic polymers were also reported for different cartilage, joint, and bone-related therapeutic and tissue engineering applications. The most commonly used synthetic polymers used in bone, cartilage, and teeth bio-ink applications are aminocaproic acid, polycaprolactone, aminosalicylic acid, polycaprolactone (PCL), polylactic acid-glycolic acid copolymer (PLGA), polyglycolic acid (PGA), polyvinylpyrrolidone (PVP), polylactic acid (PLA), etc. [199–201].
Thus, bio-inks can be efficiently developed and effectively implemented for various therapeutic, diagnostic, and regenerative purposes and should be continuously explored in the orthopedic field.
Acknowledgment
The authors acknowledge financial support from the Blazer Foundation for Regenerative Medicine and Disability Research Lab and Nanomedicine Labs at the Department of Biomedical Sciences, UIC College of Medicine at Rockford. The authors also acknowledge NIH R01 - AR070181, NIH R03 - R03NS111554, and Department of Health Science Education (UIC-Rockford) funding for financial support.
Footnotes
Declaration of competing interest
The authors declare that they have no competing interests.
Data availability
Data will be made available on request.
References
- [1].Mao AS, Mooney DJ, Regenerative medicine: current therapies and future directions, Proc. Natl. Acad. Sci. U. S. A. 112 (2015) 14452–14459, 10.1073/pnas.1508520112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Tiruvannamalai-Annamalai R, Armant DR, Matthew HW, A glycosaminoglycan-based, modular tissue scaffold system for rapid assembly of perfusable, high cell density, engineered tissues, PloS one 9 (2014), e84287, 10.1371/journal.pone.0084287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Burke M, Carter BM, Perriman AW, Bioprinting: uncovering the utility layer-by-layer, J. 3D Print. Med. 1 (2017) 165–179, 10.2217/3dp-2017-0006. [DOI] [Google Scholar]
- [4].Matai I, Kaur G, Seyedsalehi A, McClinton A, Laurencin CT, Progress in 3D bioprinting technology for tissue/organ regenerative engineering, Biomaterials 226 (2020), 119536, 10.1016/j.biomaterials.2019.119536. [DOI] [PubMed] [Google Scholar]
- [5].Papaioannou TG, Manolesou D, Dimakakos E, Tsoucalas G, Vavuranakis M, Tousoulis D, 3D bioprinting methods and techniques: applications on artificial blood vessel fabrication, Acta Cardiol. Sin. 35 (2019) 284–289, 10.6515/ACS.201905_35(3).20181115A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ji S, Almeida E, Guvendiren M, 3D bioprinting of complex channels within cell-laden hydrogels, Acta Biomater. 95 (2019) 214–224, 10.1016/j.actbio.2019.02.038. [DOI] [PubMed] [Google Scholar]
- [7].Sundaramurthi D, Rauf S, Hauser CAE, 3D bioprinting technology for regenerative medicine applications, Int. J. Bioprinting 2 (2016) 9–26, 10.18063/IJB.2016.02.010. [DOI] [Google Scholar]
- [8].Kačarević Z, Alkildani P.Rider S., Retnasingh S, Smeets R, Jung O, Ivanisšević Z, Barbeck M, An introduction to 3D bioprinting: possibilities, challenges and future aspects, Materials (Basel, Switzerland) 11 (2018) 2199, 10.3390/ma11112199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Bishop ES, Mostafa S, Pakvasa M, Luu HH, Lee MJ, Wolf JM, Ameer GA, He TC, Reid RR, 3-D bioprinting technologies in tissue engineering and regenerative medicine: current and future trends, Genes Dis. 4 (2017) 185–195, 10.1016/j.gendis.2017.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Sriphutkiat Y, Kasetsirikul S, Ketpun D, Zhou Y, Cell alignment and accumulation using the acoustic nozzle for bioprinting, Sci. Rep. 9 (2019) 17774, 10.1038/s41598-019-54330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Williams D, Thayer P, Martinez H, Gatenholm E, Khademhosseini A, A perspective on the physical, mechanical, and biological specifications of bioinks and the development of functional tissues in 3D bioprinting, Bioprinting 9 (2018) 19–36, 10.1016/j.bprint.2018.02.003. [DOI] [Google Scholar]
- [12].Groll J, Burdick JA, Cho D-W, Derby B, Gelinsky M, Heilshorn SC, Jüngst T, Malda J, Mironov VA, Nakayama K, Ovsianikov A, Sun W, Takeuchi S, Yoo JJ, Woodfield TBF, A definition of bioinks and their distinction from biomaterial inks, Biofabrication 11 (2019), 013001, 10.1088/1758-5090/aaec52. [DOI] [PubMed] [Google Scholar]
- [13].Gungor-Ozkerim PS, Inci I, Zhang YS, Khademhosseini A, Dokmeci MR, Bioinks for 3D bioprinting: an overview, Biomater Sci. 6 (2018) 915–946, 10.1039/C7BM00765E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hölzl K, Lin S, Tytgat L, Vlierberghe SV, Gu L, Ovsianikov A, Bioink properties before, during and after 3D bioprinting, Biofabrication 8 (2016), 032002, 10.1088/1758-5090/8/3/032002. [DOI] [PubMed] [Google Scholar]
- [15].Schwab A, Levato R, D’Este M, Piluso S, Eglin D, Malda J, Printability and shape Fidelity of bioinks in 3D bioprinting, Chem. Rev. 120 (2020) 11028–11055, 10.1021/acs.chemrev.0c00084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mo X, Zhang D, Liu K, Zhao X, Li X, Wang W, Nano-hydroxyapatite composite scaffolds loaded with bioactive factors and drugs for bone tissue engineering, Int. J. Mol. Sci. 24 (2023) 1291, 10.3390/ijms24021291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Vázquez-Aristizabal P, Perumal G, García-Astrain C, Liz-Marzán LM, Izeta A, Trends in tissue bioprinting, cell-laden bioink formulation, and cell tracking, ACS Omega 7 (2022) 16236–16243, 10.1021/acsomega.2c01398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Pan RL, Martyniak K, Karimzadeh M, Gelikman DG, DeVries J, Sutter K, Coathup M, Razavi M, Sawh-Martinez R, Kean TJ, Systematic review on the application of 3D-bioprinting technology in orthoregeneration: current achievements and open challenges, J. Exp. Ortop. 9 (2022) 95, 10.1186/s40634-022-00518-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Yang Z, Yi P, Liu Z, Zhang W, Mei L, Feng C, Tu C, Li Z, Stem cell-laden hydrogel-based 3D bioprinting for bone and cartilage tissue engineering, Front. Bioeng. Biotechnol. 10 (2022), 865770, 10.3389/fbioe.2022.865770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Nadernezhad A, Khani N, Skvortsov GA, Toprakhisar B, Bakirci E, Menceloglu Y, Unal S, Koc B, Multifunctional 3D printing of heterogeneous hydrogel structures, Sci. Rep. 6 (2016) 33178, 10.1038/srep33178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Mauck RL, Soltz MA, Wang CCB, Wong DD, Chao PH, Valhmu WB, Hung CT, Ateshian GA, Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels, J. Biomech. Eng. 122 (2000) 252–260, 10.1115/1.429656. [DOI] [PubMed] [Google Scholar]
- [22].DeKosky BJ, Dormer NH, Ingavle GC, Roatch CH, Lomakin J, Detamore MS, Gehrke SH, Hierarchically designed agarose and Poly(Ethylene Glycol) interpenetrating network hydrogels for cartilage tissue engineering, Tissue Eng. Part C Methods 16 (2010) 1533–1542, 10.1089/ten.tec.2009.0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Benya PD, Shaffer JD, Dedifferentiated chondrocytes reexpress the differentiated collagen phenotype when cultured in agarose gels, Cell 30 (1982) 215–224, 10.1016/0092-8674(82)90027-7. [DOI] [PubMed] [Google Scholar]
- [24].Duarte Campos DF, Blaeser A, Korsten A, Neuss S, Jäkel J, Vogt M, Fischer H, The stiffness and structure of three-dimensional printed hydrogels direct the differentiation of mesenchymal stromal cells toward adipogenic and osteogenic lineages, Tissue Eng. A 21 (2015) 740–756, 10.1089/ten.tea.2014.0231. [DOI] [PubMed] [Google Scholar]
- [25].López-Marcial GR, Zeng AY, Osuna C, Dennis J, García JM, O’Connell GD, Agarose-based hydrogels as suitable bioprinting materials for tissue engineering, ACS Biomater Sci. Eng. 4 (2018) 3610–3616, 10.1021/acsbiomaterials.8b00903. [DOI] [PubMed] [Google Scholar]
- [26].Neufurth M, Wang X, Schröder HC, Feng Q, Diehl-Seifert B, Ziebart T, Steffen R, Wang S, Müller WEG, Engineering a morphogenetically active hydrogel for bioprinting of bioartificial tissue derived from human osteoblast-like SaOS-2 cells, Biomaterials 35 (2014) 8810–8819, 10.1016/j.biomaterials.2014.07.002. [DOI] [PubMed] [Google Scholar]
- [27].Duarte Campos DF, Philip MA, Gürzing S, Melcher C, Lin YY, Schöneberg J, Blaeser A, Theek B, Fischer H, Betsch M, Synchronized dual bioprinting of bioinks and biomaterial inks as a translational strategy for cartilage tissue engineering, 3D Print. Addit. Manuf. 6 (2019) 63–71, 10.1089/3dp.2018.0123. [DOI] [Google Scholar]
- [28].Duarte Campos DF, Zhang S, Kreimendahl F, Köpf M, Fischer H, Vogt M, Blaeser A, Apel C, Esteves-Oliveira M, Hand-held bioprinting for de novo vascular formation applicable to dental pulp regeneration, Connect. Tissue Res. 61 (2020) 205–215, 10.1080/03008207.2019.1640217. [DOI] [PubMed] [Google Scholar]
- [29].Nadernezhad A, Caliskan OS, Topuz F, Afghah F, Erman B, Koc B, Nanocomposite bioinks based on agarose and 2D nanosilicates with tunable flow properties and bioactivity for 3D bioprinting, ACS Appl. Bio Mater. 2 (2019) 796–806, 10.1021/acsabm.8b00665. [DOI] [PubMed] [Google Scholar]
- [30].Zhimin Y, Ping Y, Zhongyue L, Wenchao Z, Lin M, Chengyao F, Chao T, Zhihong L, Stem cell-laden hydrogel-based 3D bioprinting for bone and cartilage tissue engineering, Front. Bioeng. Biotechnol. 10 (2022), 865770, 10.3389/fbioe.2022.865770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Pedroza-González SC, Rodriguez-Salvador M, Pérez Benítez BE, Alvarez MM, Santiago GT, Bio-inks for 3D bioprinting: a scientometric analysis of two decades of progress, Int. J. Bioprinting 7 (2021) 333, 10.18063/ijb.v7i2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Wenger L, Radtke CP, Gerisch E, Kollmann M, Niemeyer CM, Rabe KS, Hubbuch J, Systematic evaluation of agarose- and agar-based bioinks for extrusion-based bioprinting of enzymatically active hydrogels, Front. Bioeng. Biotechnol. 10 (2022), 928878, 10.3389/fbioe.2022.928878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Chrenek J, Kirsch R, Scheck K, Willerth SM, Protocol for printing 3D neural tissues using the BIO X equipped with a pneumatic printhead, STAR Protocols 3 (2022), 101348, 10.1016/j.xpro.2022.101348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Shoulders MD, Raines RT, Collagen structure and stability, Annu. Rev. Biochem. 78 (2009) 929–958, 10.1146/annurev.biochem.77.032207.120833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lee CH, Singla A, Lee Y, Biomedical applications of collagen, Int. J. Pharm. 221 (2001) 1–22, 10.1016/S0378-5173(01)00691-3. [DOI] [PubMed] [Google Scholar]
- [36].Hospodiuk M, Dey M, Sosnoski D, Ozbolat IT, The bio-ink: a comprehensive review on bioprintable materials, Biotechnol. Adv. 35 (2017) 217–239, 10.1016/j.biotechadv.2016.12.006. [DOI] [PubMed] [Google Scholar]
- [37].Marques CF, Diogo GS, Pina S, Oliveira JM, Silva TH, Reis RL, Collagen-based bioinks for hard tissue engineering applications: a comprehensive review, J. Mater. Sci. Mater. Med. 30 (2019) 32, 10.1007/s10856-019-6234-x. [DOI] [PubMed] [Google Scholar]
- [38].Kim YB, Lee H, Kim GH, Strategy to achieve highly porous/biocompatible macroscale cell blocks, using a Collagen/Genipin-bioink and an optimal 3D printing process, ACS Appl. Mater. Interfaces 8 (2016) 32230–32240, 10.1021/acsami.6b11669. [DOI] [PubMed] [Google Scholar]
- [39].Yang X, Lu Z, Wu H, Li W, Zheng L, Zhao J, Collagen-alginate as bio-ink for three-dimensional (3D) cell printing based cartilage tissue engineering, Mater. Sci. Eng. C 83 (2018) 195–201, 10.1016/j.msec.2017.09.002. [DOI] [PubMed] [Google Scholar]
- [40].Duarte Campos DF, Blaeser A, Buellesbach K, Sen KS, Xun W, Tillmann W, Fischer H, Bioprinting organotypic hydrogels with improved mesenchymal stem cell remodeling and mineralization properties for bone tissue engineering, Adv. Healthc. Mater. 5 (2016) 1336–1345, 10.1002/adhm.201501033. [DOI] [PubMed] [Google Scholar]
- [41].Kim W, Jang CH, Kim G, Optimally designed collagen/polycaprolactone biocomposites supplemented with controlled release of HA/TCP/rhBMP-2 and HA/TCP/PRP for hard tissue regeneration, Mater. Sci. Eng. C. 78 (2017) 763–772, 10.1016/j.msec.2017.04.144. [DOI] [PubMed] [Google Scholar]
- [42].Kim W, Kim G, Collagen/bioceramic-based composite bioink to fabricate a porous 3D hASCs-laden structure for bone tissue regeneration, Biofabrication 12 (2019), 015007, 10.1088/1758-5090/ab436d. [DOI] [PubMed] [Google Scholar]
- [43].Lee J, Yeo M, Kim W, Koo Y, Kim GH, Development of a tannic acid cross-linking process for obtaining 3D porous cell-laden collagen structure, Int. J. Biol. Macromol. 110 (2018) 497–503, 10.1016/j.ijbiomac.2017.10.105. [DOI] [PubMed] [Google Scholar]
- [44].Amoli MS, EzEldeen M, Jacobs R, Bloemen V, Materials for dentoalveolar bioprinting: current state of the art, Biomedicines 10 (2022) 71, 10.3390/biomedicines10010071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mitchell J, Lo KWH, Small molecule-mediated regenerative engineering for craniofacial and dentoalveolar bone, Front. Bioeng. Biotechnol. 10 (2022) 1003936, 10.3389/fbioe.2022.10039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Beketov EE, Isaeva EV, Yakovleva ND, Demyashkin GA, Arguchinskaya NV, Kisel AA, Lagoda TS, Malakhov EP, Kharlov VI, Osidak EO, Domogatsky SP, Ivanov SA, Shegay PV, Kaprin AD, Bioprinting of cartilage with bioink based on high-concentration collagen and chondrocytes, Int. J. Mol. Sci. 22 (2021) 11351, 10.3390/ijms222111351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Isaeva EV, Beketov EE, Yuzhakov VV, Arguchinskaya NV, Kisel AA, Malakhov EP, Lagoda TS, Yakovleva ND, Shegai PV, Ivanov SA, Kaprin AD, The use of collagen with high concentration in cartilage tissue engineering by means of 3D-bioprinting, Cell Tiss. Biol. 15 (2021) 493–502, 10.1134/S1990519X21050059. [DOI] [Google Scholar]
- [48].Rico-Llanos GA, Borrego-González S, Moncayo-Donoso M, Becerra J, Visser R, Collagen type I, biomaterials as scaffolds for bone tissue engineering, Polymers 13 (2021) 599, 10.3390/polym13040599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li Z, Ruan C, Niu X, Collagen-based bioinks for regenerative medicine: fabrication, application and prospective, Med. Novel Technol. Devices 17 (2023), 100211, 10.1016/j.medntd.2023.100211. [DOI] [Google Scholar]
- [50].Ebrahimi Z, Irani S, Ardeshirylajimi A, Seyedjafari E, Enhanced osteogenic differentiation of stem cells by 3D printed PCL scaffolds coated with collagen and hydroxyapatite, Sci. Rep. 12 (2022) 12359, 10.1038/s41598-022-15602-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Dong C, Lv Y, Application of collagen scaffold in tissue engineering: recent advances and new perspectives, Polymers (Basel) 8 (2016) 42, 10.3390/polym8020042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Yamamoto M, Ikada Y, Tabata Y, Controlled release of growth factors based on biodegradation of gelatin hydrogel, J. Biomater. Sci. Polym. Ed. 12 (2001) 77–88, 10.1163/156856201744461. [DOI] [PubMed] [Google Scholar]
- [53].Schrieber R, Gareis H, Gelatine Handbook: Theory and Industrial Practice, 1st ed., Wiley, Weinheim, Germany, June 2007. 10.1002/9783527610969. [DOI] [Google Scholar]
- [54].Karim AA, Bhat R, Fish gelatin: properties, challenges, and prospects as an alternative to mammalian gelatins, Food Hydrocoll. 23 (2009) 563–576, 10.1016/j.foodhyd.2008.07.002. [DOI] [Google Scholar]
- [55].Yue K, Trujillo-de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A, Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels, Biomaterials 73 (2015) 254–271, 10.1016/j.biomaterials.2015.08.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Daly AC, Critchley SE, Rencsok EM, Kelly DJ, A comparison of different bioinks for 3D bioprinting of fibrocartilage and hyaline cartilage, Biofabrication 8 (2016), 045002, 10.1088/1758-5090/8/4/045002. [DOI] [PubMed] [Google Scholar]
- [57].Irmak G, Demirtas TT ¸, M. Gümüşderelioǧlu, Highly methacrylated gelatin bio-ink for bone tissue engineering, ACS Biomater Sci. Eng. 5 (2019) 831–845, 10.1021/acsbiomaterials.8b00778. [DOI] [PubMed] [Google Scholar]
- [58].Schuurman W, Levett PA, Pot MW, van Weeren PR, Dhert WJ, Hutmacher DW, Melchels FP, Klein TJ, Malda J, Gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs: gelatin-methacrylamide hydrogels as potential biomaterials for fabrication of tissue-engineered cartilage constructs, Macromol. Biosci. 13 (2013) 551–561, 10.1002/mabi.201200471. [DOI] [PubMed] [Google Scholar]
- [59].Noh I, Kim N, Tran HN, Lee J, Lee C, 3D printable hyaluronic acid-based hydrogel for its potential application as a bioink in tissue engineering, Biomater. Res. 23 (2019) 3, 10.1186/s40824-018-0152-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Shin JH, Kang HW, The development of gelatin-based bio-ink for use in 3D hybrid bioprinting, Int. J. Precis. Eng. Manuf. 19 (2018) 767–771, 10.1007/s12541-018-0092-1. [DOI] [Google Scholar]
- [61].Kundu J, Shim JH, Jang J, Kim SW, Cho DW, An additive manufacturing-based PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering: PCL-alginate-chondrocyte bioprinted scaffold for cartilage tissue engineering, J. Tissue Eng. Regen. Med. 9 (2015) 1286–1297, 10.1002/term.1682. [DOI] [PubMed] [Google Scholar]
- [62].Cidonio G, Alcala-Orozco CR, Lim KS, Glinka M, Mutreja I, Kim YH, Dawson JI, Woodfield TBF, Oreffo ROC, Osteogenic and angiogenic tissue formation in high fidelity nanocomposite laponite-gelatin bioinks, Biofabrication 11 (2019), 035027, 10.1088/1758-5090/ab19fd. [DOI] [PubMed] [Google Scholar]
- [63].Alcala-Orozco CR, Mutreja I, Cui X, Kumar D, Hooper GJ, Lim KS, Woodfield TBF, Design and characterisation of multi-functional strontium-gelatin nanocomposite bioinks with improved print fidelity and osteogenic capacity, Bioprinting 18 (2020), e00073, 10.1016/j.bprint.2019.e00073. [DOI] [Google Scholar]
- [64].Ding H, Illsley NP, Chang RC, 3D bioprinted GelMA based models for the study of trophoblast cell invasion, Sci. Rep. 9 (2019) 18854, 10.1038/s41598-019-55052-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Chimene D, Miller L, Cross LM, Jaiswal MK, Singh I, Gaharwar AK, Nanoengineered osteoinductive bioink for 3D bioprinting bone tissue, ACS Appl. Mater. Interfaces 12 (2020) 15976–15988, 10.1021/acsami.9b19037. [DOI] [PubMed] [Google Scholar]
- [66].Ojansivu M, Rashad A, Ahlinder A, Massera J, Mishra A, Syverud K, Finne-Wistrand A, Miettinen S, Mustafa K, Wood-based nanocellulose and bioactive glass modified gelatin–alginate bioinks for 3D bioprinting of bone cells, Biofabrication 11 (2019), 035010, 10.1088/1758-5090/ab0692. [DOI] [PubMed] [Google Scholar]
- [67].Park JH, Gillispie GJ, Copus JS, Zhang W, Atala A, Yoo JJ, Yelick PC, Lee SJ, The effect of BMP-mimetic peptide tethering bioinks on the differentiation of dental pulp stem cells (DPSCs) in 3D bioprinted dental constructs, Biofabrication 12 (2020), 035029, 10.1088/1758-5090/ab9492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Benwood C, Chrenek J, Kirsch RL, Masri NZ, Richards H, Teetzen K, Willerth SM, Natural biomaterials and their use as bioinks for printing tissues, Bioengineering 8 (2021) 27, 10.3390/2Fbioengineering8020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Behan K, Dufour A, Garcia O, Kelly D, Methacrylated cartilage ECM-based hydrogels as injectables and bioinks for cartilage tissue engineering, Biomolecules 12 (2022) 216, 10.3390/2Fbiom12020216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Huang CC, Tuning gelatin–alginate bioink properties by introducing new decellularized elastic cartilage scaffolds for bioinspired composite membranes in orthopedics, Polym. Bull. 80 (2023) 3279–3291, 10.1007/s00289-022-04211-4. [DOI] [Google Scholar]
- [71].Rajabi N, Kharaziha M, Emadi R, Zarrabi A, Mokhtari H, Salehi S, An adhesive and injectable nanocomposite hydrogel of thiolated gelatin/gelatin methacrylate/Laponite® as a potential surgical sealant, J. Colloid Interface Sci. 564 (2020) 155–169, 10.1016/j.jcis.2019.12.048. [DOI] [PubMed] [Google Scholar]
- [72].Barroso IA, Man K, Robinson TE, Cox SC, Ghag AK, Photocurable GelMA adhesives for corneal perforations, Bioengineering 9 (2022) 53, 10.3390/bioengineering9020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shie MY, Lee JJ, Ho CC, Yen SY, Ng HY, Chen YW, Effects of gelatin methacrylate bio-ink concentration on mechano-physical properties and human dermal fibroblast behavior, Polymers (Basel) 12 (2020) 1930, 10.3390/polym12091930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Gan X, Li C, Sun J, Zhang X, Zhou M, Deng Y, Xiao A, GelMA/κ-carrageenan double-network hydrogels with superior mechanics and biocompatibility, RSC Adv. 13 (2023) 1558–1566, 10.1039/D2RA06101E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Clark DE, Green HC, Alginic Acid and Process of Making Same, 1935. US patent no. US2036922A. [Google Scholar]
- [76].Smidsrod O, Skjakbrk G, Alginate as immobilization matrix for cells, Trends Biotechnol. 8 (1990) 71–78, 10.1016/0167-7799(90)90139-O. [DOI] [PubMed] [Google Scholar]
- [77].Lee KY, Mooney DJ, Alginate: properties and biomedical applications, Prog. Polym. Sci. 37 (2012) 106–126, 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Szekalska M, Puciłowska A, Szymańska E, Ciosek P, Winnicka K, Alginate: current use and future perspectives in pharmaceutical and biomedical applications, Int. J. Polym. Sci. 8 (2016) 1–17, 10.1155/2016/7697031. [DOI] [Google Scholar]
- [79].Remminghorst U, Rehm BHA, Bacterial alginates: from biosynthesis to applications, Biotechnol. Lett. 28 (2006) 1701–1712, 10.1007/s10529-006-9156-x. [DOI] [PubMed] [Google Scholar]
- [80].Kong HJ, Lee KY, Mooney DJ, Decoupling the dependence of rheological/mechanical properties of hydrogels from solids concentration, Polymer 43 (2002) 6239–6246, 10.1016/S0032-3861(02)00559-1. [DOI] [Google Scholar]
- [81].Lee KY, Rowley JA, Eiselt P, Moy EM, Bouhadir KH, Mooney DJ, Controlling mechanical and swelling properties of alginate hydrogels independently by cross-linker type and cross-linking density, Macromolecules 33 (2000) 4291–4294, 10.1021/ma9921347. [DOI] [Google Scholar]
- [82].Augst AD, Kong HJ, Mooney DJ, Alginate hydrogels as biomaterials, Macromol. Biosci. 6 (2006) 623–633, 10.1002/mabi.200600069. [DOI] [PubMed] [Google Scholar]
- [83].Müller M, Öztürk E, Arlov Ø, Gatenholm P, Zenobi-Wong M, Alginate sulfate-nanocellulose bioinks for cartilage bioprinting applications, Ann. Biomed. Eng. 45 (2017) 210–223, 10.1007/s10439-016-1704-5. [DOI] [PubMed] [Google Scholar]
- [84].Markstedt K, Mantas A, Tournier I, Martínez H, Ávila D, Gatenholm Hägg P, 3D bioprinting human chondrocytes with nanocellulose-alginate bioink for cartilage tissue engineering applications, Biomacromolecules 16 (2015) 1489–1496, 10.1021/acs.biomac.5b00188. [DOI] [PubMed] [Google Scholar]
- [85].Nguyen D, Hägg DA, Forsman A, Ekholm J, Nimkingratana P, Brantsing C, Kalogeropoulos T, Zaunz S, Concaro S, Brittberg M, Lindahl A, Gatenholm P, Enejder A, Simonsson S, Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink, Sci. Rep. 7 (2017) 658, 10.1038/s41598-017-00690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Ahlfeld T, Cubo-Mateo N, Cometta S, Guduric V, Vater C, Bernhardt A, Akkineni Lode A, Gelinsky M, A novel plasma-based bioink stimulates cell proliferation and differentiation in bioprinted, mineralized constructs, ACS Appl. Mater. Interfaces 12 (2020) 12557–12572, 10.1021/acsami.0c00710. [DOI] [PubMed] [Google Scholar]
- [87].Ahlfeld T, Cidonio G, Kilian D, Duin S, Akkineni AR, Dawson JI, Yang S, Lode A, Oreffo ROC, Gelinsky M, Development of a clay based bioink for 3D cell printing for skeletal application, Biofabrication 9 (2017), 034103, 10.1088/1758-5090/aa7e96. [DOI] [PubMed] [Google Scholar]
- [88].Kosik-Kozioł A, Costantini M, Bolek T, Szöke K, Barbetta A, Brinchmann J, Święszkowski W, PLA short sub-micron fiber reinforcement of 3D bioprinted alginate constructs for cartilage regeneration, Biofabrication 9 (2017), 044105, 10.1088/1758-5090/aa90d7. [DOI] [PubMed] [Google Scholar]
- [89].Choe G, Oh S, Seok JM, Park SA, Lee JY, Graphene oxide/alginate composites as novel bioinks for three-dimensional mesenchymal stem cell printing and bone regeneration applications, Nanoscale 11 (2019) 23275–23285, 10.1039/C9NR07643C. [DOI] [PubMed] [Google Scholar]
- [90].Bendtsen ST, Quinnell SP, Wei M, Development of a novel alginate-polyvinyl alcohol-hydroxyapatite hydrogel for 3D bioprinting bone tissue engineered scaffolds: development of alginate-pva-ha hydrogel for 3d bioprinting, J. Biomed. Mater. Res. 105 (2017) 1457–1468, 10.1002/jbm.a.36036. [DOI] [PubMed] [Google Scholar]
- [91].Cunniffe GM Gonzalez-Fernandez T, Daly A, Sathy BN, Jeon O, Alsberg E, Kelly DJ, Three-dimensional bioprinting of polycaprolactone reinforced gene activated bioinks for bone tissue engineering, Tissue Eng. A 23 (2017) 891–900, 10.1089/ten.tea.2016.0498. [DOI] [PubMed] [Google Scholar]
- [92].Lee J, Hong J, Kim W, Kim GH, Bone-derived dECM/alginate bioink for fabricating a 3D cell-laden mesh structure for bone tissue engineering, Carbohydr. Polym. 250 (2020), 116914, 10.1016/j.carbpol.2020.116914. [DOI] [PubMed] [Google Scholar]
- [93].Jia J, Richards DJ, Pollard S, Tan Y, Rodriguez J, Visconti RP, Trusk TC, Yost MJ, Yao H, Markwald RR, Mei Y, Engineering alginate as bioink for bioprinting, Acta Biomater. 10 (2014) 4323–4331, 10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Gorroñogoitia I, Urtaza U, Zubiarrain-Laserna A, Alonso-Varona A, Zaldua AM, A study of the printability of alginate-based bioinks by 3D bioprinting for articular cartilage tissue engineering, Polymers 14 (2022) 354, 10.1016/j.actbio.2014.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Chejara DR, Mabrouk M, Kumar P, Choonara YE, Kondiah PPD, Badhe RV, du Toit LC, Bijukumar D, Pillay V, Synthesis and evaluation of a sodium Alginate-4-aminosalicylic acid based microporous hydrogel for potential viscosupplementation for joint injuries and arthritis-induced conditions, Mar. Drugs 15 (2017), E257, 10.3390/md15080257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Chejara DR, Mabrouk M, Badhe RV, Kumar P, Choonara YE, du Toit LC, Pillay V, A bio-injectable algin-aminocaproic acid thixogel with tri-stimuli responsiveness, Carbohydr. Polym. 135 (2016) 324–333, 10.1016/j.carbpol.2015.08.097. [DOI] [PubMed] [Google Scholar]
- [97].Kostenko A, Connon CJ, Swioklo S, Storable cell-laden alginate based bioinks for 3D biofabrication, Bioengineering 10 (2023) 23, 10.3390/bioengineering10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Wei Q, Zhou J, An Y, Li M, Zhang J, Yang S, Modification, 3D printing process and application of sodium alginate based hydrogels in soft tissue engineering: a review, Int. J. Biol. Macromol. 232 (2023), 123450, 10.1016/j.ijbiomac.2023.123450. [DOI] [PubMed] [Google Scholar]
- [99].Yazdanpanah Z, Johnston JD, Cooper DML, Chen X, 3D bioprinted scaffolds for bone tissue engineering: state-of-the-art and emerging technologies, Front. Bioeng. Biotechnol. 10 (2022), 824156, 10.3389/fbioe.2022.824156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Kurashina K, Kurita H, Wu Q, Ohtsuka A, Kobayashi H, Ectopic osteogenesis with biphasic ceramics of hydroxyapatite and tricalcium phosphate in rabbits, Biomaterials 23 (2002) 407–412, 10.1016/S0142-9612(01)00119-3. [DOI] [PubMed] [Google Scholar]
- [101].Woodard JR, Hilldore AJ, Lan SK, Park CJ, Morgan AW, Eurell JA, Clark SG, Wheeler MB, Jamison RD, Wagoner Johnson AJ, The mechanical properties and osteoconductivity of hydroxyapatite bone scaffolds with multi-scale porosity, Biomaterials 28 (2007) 45–54, 10.1016/j.biomaterials.2006.08.021. [DOI] [PubMed] [Google Scholar]
- [102].Nowicki M, Zhu W, Sarkar K, Rao R, Zhang LG, 3D printing multiphasic osteochondral tissue constructs with nano to micro features via PCL based bioink, Bioprinting 17 (2020), e00066, 10.1016/j.bprint.2019.e00066. [DOI] [Google Scholar]
- [103].Radulescu DE, Neacsu IA, Grumezescu AM, Andronescu E, Novel trends into the development of natural hydroxyapatite-based polymeric composites for bone tissue engineering, Polymers 14 (2022) 899, 10.3390/polym14050899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Wenz A, Borchers K, Tovar GEM, Kluger PJ, Bone matrix production in hydroxyapatite-modified hydrogels suitable for bone bioprinting, Biofabrication 9 (2017), 044103, 10.1088/1758-5090/aa91ec. [DOI] [PubMed] [Google Scholar]
- [105].Li Q, Lei X, Wang X, Cai Z, Lyu P, Zhang G, Hydroxyapatite/Collagen three-dimensional printed scaffolds and their osteogenic effects on human bone marrow-derived mesenchymal stem cells, Tissue Eng. A 25 (2019) 1261–1271, 10.1089/ten.tea.2018.0201. [DOI] [PubMed] [Google Scholar]
- [106].Montalbano G, Molino G, Fiorilli S, Vitale-Brovarone C, Synthesis and incorporation of rod-like nano-hydroxyapatite into type I collagen matrix: a hybrid formulation for 3D printing of bone scaffolds, J. Eur. Ceram. Soc. 40 (2020) 3689–3697, 10.1016/j.jeurceramsoc.2020.02.018. [DOI] [Google Scholar]
- [107].Leucht A, Volz A-C, Rogal J, Borchers K, Kluger PJ, Advanced gelatin-based vascularization bioinks for extrusion-based bioprinting of vascularized bone equivalents, Sci. Rep. 10 (2020) 5330, 10.1038/s41598-020-62166-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Iglesias-Mejuto A, García-González CA, 3D-printed alginate-hydroxyapatite aerogel scaffolds for bone tissue engineering, Mater. Sci. Eng. C 131 (2021), 112525, 10.1016/j.msec.2021.112525. [DOI] [PubMed] [Google Scholar]
- [109].Rahman MS, Rana MM, Spitzhorn LS, Akhtar N, Hasan MZ, Choudhury N, Fehm T, Czernuszka JT, Adjaye J, Asaduzzamanet SM, Fabrication of biocompatible porous scaffolds based on hydroxyapatite/collagen/chitosan composite for restoration of defected maxillofacial mandible bone, Prog. Biomater. 8 (2019) 137–154, 10.1007/s40204-019-0113-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Ojeda E, García-Barrientos Á, Martínez de Cestafe N, Alonso JM, Pérez-González R, Sáez-Martínez V, Nanometric hydroxyapatite particles as active ingredient for bioinks: a review, Macromol. 2 (2022) 20–29, 10.3390/macromol2010002. [DOI] [Google Scholar]
- [111].Wenz A, Janke K, Hoch E, Tovar G, Borchers K, Kluger P, Hydroxyapatite-modified gelatin bioinks for bone bioprinting, BioNanoMaterials 17 (2016) 179–184, 10.1515/bnm-2015-0018. [DOI] [Google Scholar]
- [112].Heid S, Boccaccini AR, Advancing bioinks for 3D bioprinting using reactive fillers: a review, Acta Biomater. 113 (2020) 1–22, 10.1016/j.actbio.2020.06.040. [DOI] [PubMed] [Google Scholar]
- [113].Lindsey J, Allen NB, Abar B, Danilkowicz R, Adams SB, 3D bioprinted GelMA-gelatin-hydroxyapatite osteoblast laden composite hydrogels for bone tissue engineering, J. Orthop. Experience Innov. (2022) (Conference Proceedings), https://journaloei.scholasticahq.com/article/36645-3d-bioprinted-gelma-gelatin-hydroxyapatite-osteoblast-laden-composite-hydrogels-for-bone-tissue-engineering. [Google Scholar]
- [114].Shi H, Zhou Z, Li W, Fan Y, Li Z, Wei J, Hydroxyapatite based materials for bone tissue engineering: a brief and comprehensive introduction, Crystals 11 (2021) 149, 10.3390/cryst11020149. [DOI] [Google Scholar]
- [115].Shao H, Jing Z, Xia P, Zhang T, Nian Z, Liu W, Zhu J, Gong Y, Zhou R, He Y, Yao Q, 3D-printed magnesium-doped wollastonite/nano-hydroxyapatite bioceramic scaffolds with high strength and anti-tumor property, Mater. Des. 225 (2023), 111464, 10.1016/j.matdes.2022.111464. [DOI] [Google Scholar]
- [116].Necas J, Bartosikova L, Brauner P, Kolar J, Hyaluronic acid (hyaluronan): a review, Vet. Med. 53 (2008) 397–411, 10.17221/1930-VETMED. [DOI] [Google Scholar]
- [117].Burdick JA, Prestwich GD, Hyaluronic acid hydrogels for biomedical applications, Adv. Mater. 23 (2011) H41–H56, 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Huang YT, Imura M, Nemoto Y, Cheng CH, Yamauchi Y, Block-copolymer-assisted synthesis of hydroxyapatite nanoparticles with high surface area and uniform size, Sci. Technol. Adv. Mater. 12 (2011), 045005, 10.1088/1468-6996/12/4/045005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Goa KL, Benfield P, Hyaluronic acid: a review of its pharmacology and use as a surgical aid in ophthalmology, and its therapeutic potential in joint disease and wound healing, Drugs 47 (1994) 536–566, 10.2165/00003495-199447030-00009. [DOI] [PubMed] [Google Scholar]
- [120].Chung C, Burdick JA, Influence of three-dimensional hyaluronic acid microenvironments on mesenchymal stem cell chondrogenesis, Tissue Eng. A 15 (2009) 243–254, 10.1089/ten.tea.2008.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Lee J, Kim BS, Cho Y-S, Lee S-H, Park Y, Development and evaluation of hyaluronic acid-based hybrid bio-ink for tissue regeneration, Tissue Eng. Regen. Med. 15 (2018) 761–769, 10.1007/s13770-018-0144-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Shim J-H, Jang K-M, Hahn SK, Park JY, Jung H, Oh K, Park KM, Yeom J, Park SH, Kim SW, Wang JH, Kim K, Cho DW, Three-dimensional bioprinting of multilayered constructs containing human mesenchymal stromal cells for osteochondral tissue regeneration in the rabbit knee joint, Biofabrication 8 (2016), 014102, 10.1088/1758-5090/8/1/014102. [DOI] [PubMed] [Google Scholar]
- [123].Antich C, de Vicente J, Jiménez G, Chocarro C, Carrillo E, Montañez E, Gálvez-Martín P, Marchal JA, Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs, Acta Biomater. 106 (2020) 114–123. [DOI] [PubMed] [Google Scholar]
- [124].Hauptstein J, Forster L, Nadernezhad A, Groll J, Teßmar J, Blunk T, Tethered TGF-1 in a hyaluronic acid-based bioink for bioprinting cartilaginous tissues, Int. J. Mol. Sci. 23 (2022) 924, 10.3390/ijms23020924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Xing F, Zhou C, Hui D, Du C, Wu L, Wang L, Wang W, Pu X, Gu L, Liu L, Xiang Z, Zhang X, Hyaluronic acid as a bioactive component for bone tissue regeneration: fabrication, modification, properties, and biological functions, Nanotechnol. Rev. 9 (2020) 1059–1079, 10.1515/ntrev-2020-0084. [DOI] [Google Scholar]
- [126].Law N, Doney B, Glover H, Qin Y, Aman ZM, Sercombe TB, Liew LJ, Dilley RJ, Doyle BJ, Characterisation of hyaluronic acid methylcellulose hydrogels for 3D bioprinting, J. Mech. Behav. Biomed. Mater. 77 (2018) 389–399, 10.1016/j.jmbbm.2017.09.031. [DOI] [PubMed] [Google Scholar]
- [127].Szymański T, Semba JA, Mieloch AA, Cywoniuk P, Kempa M, Rybka JD, Hyaluronic acid and multiwalled carbon nanotubes as bioink additives for cartilage tissue engineering, Sci. Rep. 13 (2023) 646, 10.1038/s41598-023-27901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ghosh S, Parker ST, Wang X, Kaplan DL, Lewis JA, Direct-write assembly of microperiodic silk fibroin scaffolds for tissue engineering applications, Adv. Funct. Mater. 18 (2008) 1883–1889, 10.1002/adfm.200800040. [DOI] [Google Scholar]
- [129].Chawla S, Midha S, Sharma A, Ghosh S, Silk-based bioinks for 3D bioprinting, Adv. Healthc. Mater. 7 (2018), 1701204, 10.1002/adhm.201701204. [DOI] [PubMed] [Google Scholar]
- [130].Das S, Pati F, Choi Y-J, Rijal G, Shim JH, Kim SW, Ray AR, Cho DW, Ghosh S, Bioprintable, cell-laden silk fibroin–gelatin hydrogel supporting multilineage differentiation of stem cells for fabrication of three-dimensional tissue constructs, Acta Biomater. 11 (2015) 233–246, 10.1016/j.actbio.2014.09.023. [DOI] [PubMed] [Google Scholar]
- [131].Das S, Pati F, Chameettachal S, Pahwa S, Ray AR, Dhara S, Ghosh V, Enhanced redifferentiation of chondrocytes on microperiodic silk/gelatin scaffolds: toward tailor-made tissue engineering, Biomacromolecules 14 (2013) 311–321, 10.1021/bm301193t. [DOI] [PubMed] [Google Scholar]
- [132].Chameettachal S, Midha S, Ghosh S, Regulation of chondrogenesis and hypertrophy in silk fibroin-gelatin-based 3D bioprinted constructs, ACS Biomater Sci. Eng. 2 (2016) 1450–1463, 10.1021/acsbiomaterials.6b00152. [DOI] [PubMed] [Google Scholar]
- [133].Wei L, Wu S, Kuss M, Jiang X, Sun R, Reid P, Qin X, Duan B, 3D printing of silk fibroin-based hybrid scaffold treated with platelet rich plasma for bone tissue engineering, Bioact. Mater. 4 (2019) 256–260, 10.1016/j.bioactmat.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Chawla S, Kumar A, Admane P, Bandyopadhyay A, Ghosh S, Elucidating role of silk-gelatin bioink to recapitulate articular cartilage differentiation in 3D bioprinted constructs, Bioprinting 7 (2017) 1–13, 10.1016/j.bprint.2017.05.001. [DOI] [Google Scholar]
- [135].Zheng Z, Wu J, Liu M, Wang H, Li C, Rodriguez MJ, Li G, Wang X, Kaplan DL, 3D bioprinting of self-standing silk-based bioink, Adv. Healthc. Mater. 7 (2018), 1701026, 10.1002/adhm.201701026. [DOI] [PubMed] [Google Scholar]
- [136].Moses JC, Saha T, Mandal BB, Chondroprotective and osteogenic effects of silk-based bioinks in developing 3D bioprinted osteochondral interface, Bioprinting 17 (2020), e00067, 10.1016/j.bprint.2019.e00067. [DOI] [Google Scholar]
- [137].Bari E, Di Gravina GM, Scocozza F, Perteghella S, Frongia B, Tengattini S, Segale L, Torre ML, Conti M, Silk fibroin bioink for 3D printing in tissue regeneration: controlled release of MSC extracellular vesicles, Pharmaceutics 15 (2023) 383, 10.3390/pharmaceutics15020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Wang Q, Han G, Yan S, Zhang Q, 3D printing of silk fibroin for biomedical applications, Materials 12 (2019) 504, 10.3390/ma12030504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Xie M, Lian L, Mu X, Luo Z, Garciamendez-Mijares CE, Zhang Z, López A, Manríquez J, Kuang X, Wu J, Sahoo JK, González FZ, Li G, Tang G, Maharjan S, Guo J, Kaplan DL, Zhang YS, Volumetric additive manufacturing of pristine silk-based (bio) inks, Nat. Commun. 14 (2023) 210, 10.1038/s41467-023-35807-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Taghizadeh M, Taghizadeh A, Yazdi MK, Zarrintaj P, Stadler FJ, Ramsey JD, Habibzadeh S, Hosseini Rad S, Naderi G, Saeb MR, Mozafari M, Schubert US, Chitosan-based inks for 3D printing and bioprinting, Green Chem. 24 (2022) 62–101, 10.1039/D1GC01799C. [DOI] [Google Scholar]
- [141].Tamo AK, Tran TA, Doench I, Jahangir S, Lall A, David L, Peniche-Covas C, Walther A, Osorio-Madrazo A, 3D printing of cellulase-laden cellulose nanofiber/chitosan hydrogel composites: towards tissue engineering functional biomaterials with enzyme-mediated biodegradation, Materials 15 (2022) 6039, 10.3390/ma15176039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Butler HM, Naseri E, MacDonald DS, Tasker RA, Ahmadi A, Optimization of starch- and chitosan-based bio-inks for 3D bioprinting of scaffolds for neural cell growth, Materialia 12 (2020), 100737, 10.1016/j.mtla.2020.100737. [DOI] [Google Scholar]
- [143].Mora-Boza A, Włodarczyk-Biegun MK, del Campo A, Vázquez-Lasa B, San Román J, Chitosan-based inks: 3D printing and bioprinting strategies to improve shape fidelity, mechanical properties, and biocompatibility of 3D scaffolds, Biomecánica 27 (2019) 7–16, 10.5821/sibb.27.1.9199. [DOI] [Google Scholar]
- [144].Han X, Chang S, Zhang M, Bian X, Li C, Li D, Advances of hydrogel-based bio-printing for cartilage tissue engineering, Front. Bioeng. Biotechnol. 9 (2021), 746564, 10.3389/2Ffbioe.2021.746564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Demirtas TT¸, Irmak G, Gumusderelioglu M, Bioprintable form of chitosan hydrogel for bone tissue engineering, Biofabrication 9 (2017), 035003, 10.1088/1758-5090/aa7b1d. [DOI] [PubMed] [Google Scholar]
- [146].Guoke T, Zhihong T, Wusi Z, Xing W, Changgui S, Yi L, Hailong H, Rui C, Xiaojian Y, Recent advances of chitosan-based injectable hydrogels for bone and dental tissue regeneration, Front. Bioeng. Biotechnol. 8 (2020), 587658, 10.3389/fbioe.2020.587658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [147].Badhe RV, Nanda RK, Chejara DR, Choonara YE, Kumar P, du Toit LC, Pillay V, Microwave-assisted facile synthesis of a new tri-block chitosan conjugate with improved mucoadhesion, Carbohydr. Polym. 130 (2015) 213–221, 10.1016/j.carbpol.2015.05.027. [DOI] [PubMed] [Google Scholar]
- [148].Badhe RV, Nanda RK, Chejara DR, Choonara YE, Kumar P, du Toit LC, Pillay V, A composite chitosan-gelatin bi-layered, biomimetic macroporous scaffold for blood vessel tissue engineering, Carbohydr. Polym. 3 (2017) 1215–1225, 10.1016/j.carbpol.2016.09.095. [DOI] [PubMed] [Google Scholar]
- [149].Gupta A, Kheur S, Badhe RV, Raj T, Bhonde R, Jaisinghani A, Vyas N, Patil VR, Alhazmi YA, Parveen S, Baeshen HA, Patil H, Assessing the potential use of chitosan scaffolds for the sustained localized delivery of vitamin D, Saudi J. Biol. Sci. 28 (2021) 2210–2215, 10.1016/j.sjbs.2021.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Levengood SL, Zhang M, Chitosan-based scaffolds for bone tissue engineering, J. Mater. Chem. B 2 (2014) 3161–3184, 10.1039/C4TB00027G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Chen H, Fei F, Li X, Nie Z, Zhou D, Liu L, Zhang J, Zhang J, Fei Z, Xu T, A facile, versatile hydrogel bioink for 3D bioprinting benefits long-term subaqueous fidelity, cell viability and proliferation, Regen. Biomater. 8 (2021), rbab026, 10.1093/rb/rbab026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Rezaei A, Aligholi H, Zeraatpisheh Z, Gholami A, Mirzaei E, Collagen/chitosan-functionalized graphene oxide hydrogel provide a 3D matrix for neural stem/precursor cells survival, adhesion, infiltration and migration, J. Bioact. Compat. Polym. 36 (2021) 296–313, 10.1177/08839115211022453. [DOI] [Google Scholar]
- [153].Kołodziejska M, Jankowska K, Klak M, Wszoła M, Chitosan as an underrated polymer in modern tissue engineering, Nanomaterials 11 (2021) 3019, 10.3390/nano11113019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].He Y, Derakhshanfar S, Zhong W, Li B, Lu F, Xing M, Li X, Characterization and application of carboxymethyl chitosan-based bioink in cartilage tissue engineering, J Nanomater. 2020 (2020), 2057097, 10.1155/2020/2057097. [DOI] [Google Scholar]
- [155].Li P, Fu L, Liao Z, Peng Y, Ning C, Gao C, Zhang D, Sui X, Lin Y, Liu S, Hao C, Guo Q, Chitosan hydrogel/3D-printed poly(ε-caprolactone) hybrid scaffold containing synovial mesenchymal stem cells for cartilage regeneration based on tetrahedral framework nucleic acid recruitment, Biomaterials 278 (2021), 121131, 10.1016/j.biomaterials.2021.121131. [DOI] [PubMed] [Google Scholar]
- [156].Chang HK, Yang DH, Ha MY, Kim HJ, Kim CH, Kim SH, Choi JW, Chun HJ, 3D printing of cell-laden visible light curable glycol chitosan bioink for bone tissue engineering, Carbohydr. Polym. 287 (2022), 119328, 10.1016/j.carbpol.2022.119328. [DOI] [PubMed] [Google Scholar]
- [157].Maturavongsadit P, Narayanan LK, Chansoria P, Shirwaiker R, Benhabbour SR, ACS Appl. Bio Mater Cell-laden nanocellulose/chitosan-based bioinks for 3D bioprinting and enhanced osteogenic cell differentiation,. 4 (2021) 2342–2353, 10.1021/acsabm.0c01108. [DOI] [PubMed] [Google Scholar]
- [158].Szulc M, Lewandowska K, Biomaterials based on chitosan and its derivatives and their potential in tissue engineering and other biomedical applications-a review, Molecules 28 (2023) 247, 10.3390/molecules28010247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Aguilar A, Zein N, Harmouch E, Hafdi B, Bornert F, Offner D, Clauss F, Fioretti F, Huck O, Benkirane-Jessel N, Hua G, Application of chitosan in bone and dental engineering, Molecules 24 (2019) 3009, 10.3390/molecules24163009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Gwak MA, Lee SJ, Lee D, Park SA, Park WH, Highly gallol-substituted, rapidly self-crosslinkable, and robust chitosan hydrogel for 3D bioprinting, Int. J. Biol. Macromol. 227 (2023) 493–504, 10.1016/j.ijbiomac.2022.12.124. [DOI] [PubMed] [Google Scholar]
- [161].Mohabatpour F, Duan X, Yazdanpanah Z, Tabil XL, Lobanova L, Zhu N, Papagerakis S, Chen X, Papagerakis P, Bioprinting of alginate-carboxymethyl chitosan scaffolds for enamel tissue engineering in vitro, Biofabrication 15 (2023), 015022, 10.1088/1758-5090/acab35. [DOI] [PubMed] [Google Scholar]
- [162].Choudhury D, Tun HW, Wang T, Naing MW, Organ-derived decellularized extracellular matrix: a game changer for bioink Manufacturing? Trends Biotechnol. 36 (2018) 787–805, 10.1016/j.tibtech.2018.03.003. [DOI] [PubMed] [Google Scholar]
- [163].Pati F, Cho D-W, Bioprinting of 3D tissue models using decellularized extracellular matrix bioink, in: Koledova Z (Ed.), 3D Cell Culture. Methods in Molecular Biology 1612, Springer, New York, 2017, pp. 381–390, 10.1007/978-1-4939-7021-6_27. [DOI] [PubMed] [Google Scholar]
- [164].Pati F, Jang J, Ha DH, Kim SW, Rhie JW, Shim JH, Kim DH, Cho DW, Printing three-dimensional tissue analogues with decellularized extracellular matrix bioink, Nat. Commun. 5 (2014) 3935, 10.1038/ncomms4935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Kim YS, Majid M, Melchiorri AJ, Mikos AG, Applications of decellularized extracellular matrix in bone and cartilage tissue engineering, Bioeng. Transl. Med. 4 (2019) 83–95, 10.1002/btm2.10110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Lee HJ, Kim YB, Ahn SH, Lee JS, Jang CH, Yoon H, Chun W, Kim GH, A new approach for fabricating Collagen/ECM-based bioinks using preosteoblasts and human adipose stem cells, Adv. Healthc. Mater. 4 (2015) 1359–1368, 10.1002/adhm.201500193. [DOI] [PubMed] [Google Scholar]
- [167].Rathan S, Dejob L, Schipani R, Haffner B, Möbius ME, Kelly DJ, Fiber reinforced cartilage ECM functionalized bioinks for functional cartilage tissue engineering, Adv. Healthc. Mater. 8 (2019), 1801501, 10.1002/adhm.201801501. [DOI] [PubMed] [Google Scholar]
- [168].Jung CS, Kim BK, Lee J, Min B-H, Park S-H, Development of printable natural cartilage matrix bioink for 3D printing of irregular tissue shape, Tissue Eng. Regen. Med. 15 (2018) 155–162, 10.1007/s13770-017-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Dubey N, Ferreira JA, Malda J, Bhaduri SB, Bottino MC, Extracellular Matrix/Amorphous magnesium phosphate bioink for 3D bioprinting of craniomaxillofacial bone tissue, ACS Appl. Mater. Interfaces 12 (2020) 23752–23763, 10.1021/acsami.0c05311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Özden P, Decellularized extracellular matrix- agarose hybrid bioink development for 3d bioprinting applications, Master’s thesis Submitted to the Graduate School of Engineering and Natural Sciences, Sabanci University, Tuzla, Turkey, 2018, https://research.sabanciuniv.edu/id/eprint/36987/1/10208752_IpeknazOzden.pdf. [Google Scholar]
- [171].Weisel JW, Litvinov RI, Fibrin formation, structure and properties, Subcell Biochem. 82 (2017) 405–456, 10.1007/978-3-319-49674-0_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Abelseth E, Abelseth L, De la Vega L, Beyer ST, Wadsworth SJ, Willerth SM, 3D printing of neural tissues derived from human induced pluripotent stem cells using a fibrin-based bioink, ACS Biomater Sci. Eng. 5 (2019) 234–243, 10.1021/acsbiomaterials.8b01235. [DOI] [PubMed] [Google Scholar]
- [173].Ahmed TAE, Dare EV, Hincke M, Fibrin: a versatile scaffold for tissue engineering applications, Tissue Eng. B Rev. 14 (2008) 199–215, 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- [174].de Melo BAG, Jodat YA, Cruz EM, Benincasa JC, Shin SR, Porcionatto MA, Strategies to use fibrinogen as bioink for 3D bioprinting fibrin-based soft and hard tissues, Acta Biomater. 117 (2020) 60–76, 10.1016/j.actbio.2020.09.024. [DOI] [PubMed] [Google Scholar]
- [175].Klemm D, Kramer F, Moritz S, Lindström T, Ankerfors M, Gray D, Dorris A, Nanocelluloses: a new family of nature-based materials, Angew. Chem. Int. Ed. 50 (2011) 5438–5466, 10.1002/anie.201001273. [DOI] [PubMed] [Google Scholar]
- [176].Naderi A, Nanofibrillated cellulose: properties reinvestigated, Cellulose 24 (2017) 1933–1945, 10.1007/s10570-017-1258-1. [DOI] [Google Scholar]
- [177].Piras CC, Fernańdez-Prieto S, De Borggraeve WM, Nanocellulosic materials as bioinks for 3D bioprinting, Biomater Sci. 5 (2017) 1988–1992, 10.1039/C7BM00510E. [DOI] [PubMed] [Google Scholar]
- [178].Badhe RV, Nipate SS, in: Pillay V, Kumar P, du Toit L (Eds.), Cellulosic Bioink for 3D printing Applications (Chapter 5) in Advanced 3D-Printed Systems and Nanosystems for Drug Delivery and Tissue Engineering, Elsevier, Amsterdam, Netherlands, 2020, pp. 109–137, 10.1016/B978-0-12-818471-4.00005-4 (ISBN: 978-0-12-818471-4). [DOI] [Google Scholar]
- [179].Abouzeid RE, Khiari R, Beneventi D, Dufresne A, Biomimetic mineralization of three-dimensional printed Alginate/TEMPO-oxidized cellulose nanofibril scaffolds for bone tissue engineering, Biomacromolecules 19 (2018) 4442–4452, 10.1021/acs.biomac.8b01325. [DOI] [PubMed] [Google Scholar]
- [180].Lafuente-Merchan M, Ruiz-Alonso S, Espona-Noguera A, Galvez-Martin P, Lopez-Ruiz E, Marchal JA, Lopez-Donaire ML, Zabala A, Ciriza J, Saenz-del-Burgo L, Pedraz JL, Development, characterization and sterilisation of nanocellulose-alginate-(hyaluronic acid)- bioinks and 3D bioprinted scaffolds for tissue engineering, Mater. Sci. Eng. C 126 (2021), 112160, 10.1016/j.msec.2021.112160. [DOI] [PubMed] [Google Scholar]
- [181].Jakab K, Damon B, Neagu A, Kachurin A, Forgacs G, Three-dimensional tissue constructs built by bioprinting, Biorheology 43 (2006) 509–513. [PubMed] [Google Scholar]
- [182].De Pieri A, Rochev Y, Zeugolis DI, Scaffold-free cell-based tissue engineering therapies: advances, shortfalls and forecast, npj Regen. Med. 6 (2021) 18, 10.1038/s41536-021-00133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Yu Y, Moncal KK, Li J, Peng W, Rivero I, Martin JA, Ozbolat IT, Three-dimensional bioprinting using self-assembling scalable scaffold-free “tissue strands” as a new bioink, Sci. Rep. 6 (2016) 28714, 10.1038/srep28714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Jeon O, Lee YB, Jeong H, Lee SJ, Wells D, Alsberg E, Individual cell-only bioink and photocurable supporting medium for 3D printing and generation of engineered tissues with complex geometries, Mater. Horiz. 6 (2019) 1625–1631, 10.1039/C9MH00375D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Bakirci E, Toprakhisar B, Zeybek MC, Ince GO, Koc B, Cell sheet based bioink for 3D bioprinting applications, Biofabrication 9 (2017), 024105, 10.1088/1758-5090/aa764f. [DOI] [PubMed] [Google Scholar]
- [186].Levato R, Visser J, Planell JA, Engel E, Malda J, Mateos-Timoneda MA, Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers, Biofabrication 6 (2014), 035020, 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- [187].Graham AD, Olof SN, Burke MJ, Armstrong JPK, Mikhailova EA, Nicholson JG, Box SJ, Szele FG, Perriman AW, Bayley H, High-resolution patterned cellular constructs by droplet-based 3D printing, Sci. Rep. 7 (2017) 7004, 10.1038/s41598-017-06358-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Wang C, Huang W, Zhou Y, He L, He Z, Chen Z, He X, Tian S, Liao J, Lu B, Wei Y, Wang M, 3D printing of bone tissue engineering scaffolds, Bioact. Mater. 5 (2020) 82–91, 10.1016/j.bioactmat.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Li Z, Wang Q, Liu G, A review of 3D printed bone implants, Micromachines 13 (2022) 528, 10.3390/mi13040528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Yang X, Li S, Ren Y, Qiang L, Liu Y, Wang J, Dai K, 3D printed hydrogel for articular cartilage regeneration, Compos. Part B 237 (2022), 109863, 10.1016/j.compositesb.2022.109863. [DOI] [Google Scholar]
- [191].Szychlinska MA, Bucchieri F, Fucarino A, Ronca A, D’Amora U, Three-dimensional bioprinting for cartilage tissue engineering: insights into naturally-derived bioinks from land and marine sources, J. Funct. Biomater. 13 (3) (2022) 118, 10.3390/jfb13030118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [192].Stocco E, Porzionato A, De Rose E, Barbon S, De Caro R, Macchi V, Meniscus regeneration by 3D printing technologies: current advances and future perspectives, J. Tissue Eng. 13 (2022), 10.1177/20417314211065860, 20417314211065860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Deng X, Chen X, Geng F, Tang X, Li Z, Zhang J, Wang Y, Wang F, Zheng N, Wang P, Yu X, Hou S, Zhang W, Precision 3D printed meniscus scaffolds to facilitate hMSCs proliferation and chondrogenic differentiation for tissue regeneration, J. Nanobiotechnol. 19 (2021) 400, 10.1186/s12951-021-01141-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Jiang Y, Shi K, Zhou L, He M, Zhu C, Wang J, Li J, Li Y, Liu L, Sun D, Feng G, Yi Y, Zhang L, 3D-printed auxetic-structured intervertebral disc implant for potential treatment of lumbar herniated disc, Bioact. Mater. 20 (2022) 528–538, 10.1016/j.bioactmat.2022.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [195].Yoshida M, Turner PR, Cabral JD, Intervertebral disc tissue engineering using additive manufacturing, Gels 9 (2023) 25, 10.3390/gels9010025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Sun B, Lian M, Han Y, Mo X, Jiang W, Qiao Z, Dai K, A 3D-bioprinted dual growth factor-releasing intervertebral disc scaffold induces nucleus pulposus and annulus fibrosus reconstruction, Bioact. Mater. 6 (2021) 179–190, 10.1016/j.bioactmat.2020.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Badhe RV, Nipate SS, Low-intensity current (LIC) stimulation of subcutaneous adipose derived stem cells (ADSCs) - a missing link in the course of LIC based wound healing, Med. Hypotheses 125 (2019) 79–83, 10.1016/j.mehy.2019.02.039. [DOI] [PubMed] [Google Scholar]
- [198].Badhe RV, Godse AK, Shinkar AS, Kharat A, Patil V, Gupta A, Kheur S, Development and characterization of conducting-polymer-based hydrogel dressing for wound healing, Turk. J. Pharm. Sci. 18 (2021) 483–491, 10.4274/tjps.galenos.2020.44452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Li N, Guo R, Zhang ZJ, Bioink formulations for bone tissue regeneration, Front. Bioeng. Biotechnol. 9 (2021), 630488, 10.3389/fbioe.2021.630488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [200].Khoeini R, Nosrati H, Akbarzadeh A, Eftekhari A, Kavetskyy T, Khalilov R, Ahmadian E, Nasibova A, Datta P, Roshangar L, Deluca DC, Davaran S, Cucchiarini M, Ozbolat IT, Natural and synthetic bioinks for 3D bioprinting, Adv. NanoBiomed Res. 1 (2021), 2000097, 10.1002/anbr.202000097. [DOI] [Google Scholar]
- [201].Mabrouk M, Bijukumar D, Mulla JAS, Chejara DR, Badhe RV, Kumar P, Choonara YE, du Toit LC, Pillay V, Enhancement of the biomineralization and cellular adhesivity of polycaprolactone-based hollow porous microspheres via dopamine bio-activation for tissue engineering applications, Mater. Lett. 161 (2015) 503–507, 10.1016/j.matlet.2015.08.146. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.


