Abstract
Purpose
To compare the survival outcomes of postoperative adjuvant aspirin with surgery alone in patients with hepatitis B virus (HBV)-related hepatocellular carcinoma (HCC) and portal vein tumor thrombus (PVTT).
Methods
From June 2013 to July 2015, an open-label, randomized controlled study was conducted in patients with resectable HBV-related HCC and PVTT. Patients were randomly assigned to undergo surgical resection and postoperative adjuvant aspirin (n = 40) or hepatectomy alone (n = 40). The primary end point was overall survival (OS). The secondary end points were time to recurrence of primary tumor (t-TTR) and time to recurrence of PVTT (p-TTR). The expression levels of COX1 and COX2 in surgical specimens of the aspirin group were correlated with patients’ survival.
Results
The median OS were 16.2 and 13.4 months for the adjuvant aspirin and surgery alone groups, respectively. The median t-TTR were 5.3 and 3.2 months for the adjuvant aspirin and surgery alone groups, respectively. There was no significant difference in the OS and t-TTR between the two groups of patients (P = 0.078 and 0.336, respectively). The median p-TTR were 12.0 months and 5.4 months for the adjuvant aspirin group and the surgery alone group, respectively. Patients in the adjuvant aspirin group had markedly longer p-TTR (P = 0.001). Increased expressions of COX1 or COX2 in tumor tissues denoted better prognosis for patients receiving adjuvant aspirin.
Conclusion
For patients with resectable HBV-related HCC and PVTT, postoperative adjuvant aspirin significantly prolonged time to recurrence of PVTT than surgery alone. Expression of COX1 or COX2 may predict survival in these patients.
Keywords: Hepatocellular carcinoma, Portal vein tumor thrombus, Hepatitis B virus, Surgery, Aspirin, Overall survival, Recurrence
List of abbreviations
- HCC
hepatocellular carcinoma
- PVTT
portal vein tumor thrombus
- OS
overall survival
- t-TTR
time to recurrence of primary tumor
- p-TTR
time to recurrence of portal vein tumor thrombus
- BCLC
Barcelona Clinic Liver Cancer
- EASL
European Association for the Study of the Liver
- HBV
hepatitis B virus
1. Introduction
Hepatocellular carcinoma (HCC) is the sixth most common cancer and the third most common cause of cancer-related mortality in the world [1]. HCC is inclined to invade into the portal vein system, with the subsequent formation of portal vein tumor thrombus (PVTT). The incidence of PVTT has been reported to range from 10% to 40% when the first diagnosis of HCC [2], and from 44% to 62% at autopsy in HCC patients [3]. PVTT represents a significant poor prognostic factor for HCC patients [4,5]. If left untreated, the median overall survival (OS) is only 2–4 months for these patients. According to the BCLC staging system and EASL guidelines, HCC patients with PVTT are classified as advanced stage, and systemic therapy, including sorafenib, lenvatinib and atezolizumab plus bevacizumab, is recommended as the first-line treatment strategy [1,[6], [7], [8]]. However, long-term survival probabilities of these patients treated with systemic drugs vary significantly based on the extent of PVTT [9,10]. Recent evidence has demonstrated that surgery may result in better survival outcomes than nonsurgical treatments in selected HCC patients with PVTT [[11], [12], [13]]. Nevertheless, the high incidence of HCC and PVTT recurrences after hepatectomy is still a bottleneck to achieve optimal survival outcomes. The application of postoperative adjuvant therapy may become a promising approach to decrease postoperative HCC and PVTT recurrence rates.
Aspirin, an anti-platelet drug commonly prescribed in clinical practice, has gained much attention recently. It has been reported to be associated with reduced risk of multiple cancers, including colorectal, esophageal, breast, lung, and prostate cancers [[14], [15], [16]]. Recently, a prospective study with a large sample size also suggests that long-term aspirin use is associated with a dose-dependent reduction in HCC risk [17]. Moreover, an increasing number of studies have shown that postoperative adjuvant aspirin is closely related to a lower recurrence risk, improved liver function, and better prognosis for HCC patients compared with liver resection alone [[18], [19], [20]], which provides a basis for the application of aspirin as a promising adjuvant therapeutic modality. However, the impact of adjuvant use of aspirin on the postoperative long-term survival of HBV-related HCC patients with PVTT is still unclear.
This open-label, randomized controlled trial was conducted to assess the role of postoperative adjuvant aspirin in patients with HBV-related HCC and PVTT.
2. Methods
2.1. Study design
This randomized, open-label, single-center controlled clinical trial (RCT) was designed to evaluate the efficacy of postoperative adjuvant aspirin treatment in patients with HBV-related HCC associated with PVTT. This study was approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital (approval number: EHBHKY2013-001-1) and conformed to the principles of 1975 Declaration of Helsinki. Detailed information of the trial protocol was explained to every participant, and written informed consent was obtained from all the patients before randomization. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) statement. This study has been registered in the Chinese Clinical Trials Registry (ChiCTR) and the registration number is ChiCTR-TRC-13003357. The research data were used only for publication or shared with other researchers. The identity and medical information of participants were kept strictly confidential.
2.2. Participants and recruitment
Consecutive patients with newly diagnosed HCC associated with PVTT who had underwent R0 hepatectomy at the Eastern Hepatobiliary Surgery Hospital were candidates for enrollment. The diagnosis of HCC was based on preoperative biopsy or the noninvasive approaches of the European Association for the Study of Liver guidelines [1]. The diagnosis of PVTT was based on characteristic imaging features [21,22]. Recruitment lasted from June 1, 2013 to July 1, 2015.
2.3. Classification of PVTT
PVTT was divided into 4 types according to the extent of PVTT in the portal vein system: type I, tumor thrombus involving the segmental branches of the portal vein or above; type II, tumor thrombus involving the right- or left-side of the main portal vein; type III, tumor thrombus involving the main portal vein; and type IV, tumor thrombus extending to the superior mesenteric vein [11,23].
2.4. Eligibility criteria
The key eligibility criteria included age between 20 and 70 years; HBV-related HCC and PVTT diagnosed by the diagnostic criteria as mentioned above; technically resectable primary HCC (single tumor <10 cm in maximum diameter or multiple lesions confined to one hemiliver); an Eastern Cooperative Oncology Group performance status score of 0–1; Child-Pugh class A liver function; absence of macroscopic hepatic vein or bile duct tumor thrombus; no evidence of extrahepatic spread or distant metastases; white blood cell count >4*109/L, platelet counts >50*109/L, and a prothrombin time prolongation of less than 5 s; and adequate function of vital organs.
The key exclusion criteria were unresectable HCC or patients not feasible for surgery (ECOG-PS score ≥2, maximum tumor diameter ≥10 cm, multiple disseminated nodules involved left and right hemilivers), a history of other malignancies within the past 5 years, a history of spontaneous tumor rupture, any previous anti-cancer treatment within 1 year, severe or uncontrolled concomitant diseases, acute or active infectious diseases, an active gastric or duodenal ulcer, hepatitis C virus (HCV) or HIV infection, and pregnancy or breastfeeding.
2.5. Sample size estimation
The sample size was estimated on the basis of a 3-year OS of 17% in the surgery alone group based on our previous retrospective studies and an expected 10% increase in the 3-year OS with aspirin standard therapy added to curative surgery. Hence, the minimum sample size was 36 for each group (two-sided, α = 0.05; β = 0.10; power, 90%), and 10% of patients were added to compensate for any loss to follow-up. Thereby, 40 patients were needed in each of the group at least.
2.6. Randomization and masking
All eligible patients were randomly assigned to receive either adjuvant aspirin or not after surgery in a 1:1 ratio. A computer-based randomization code was generated by a staff member from the data center outside the study team before the beginning of randomization. The randomization code used permuted blocks of treatment group allocations without stratification. Allocation concealment was performed using sequentially numbered, opaque, sequestered envelops. After written informed consent was obtained from an eligible patient, the staff member of the data center opened a sequentially placed envelop and informed the investigators of the grouping of the patient. Randomization was conducted within 1 week after eligibility confirmation, and surgery began within 1 week thereafter.
2.7. Interventions
The surgical procedures for HCC with PVTT have been described in previous reports [24]. Generally, partial hepatectomy was performed under general anesthesia using a right-side subcostal incision with a midline extension. Intraoperative ultrasonography (IOUS) was routinely performed to evaluate tumor number and size, the relationship of the tumor with adjacent major vascular structures, and the extent of PVTT. The clamp crushing method was adopted to carry out liver parenchymal transection. The intermittent Pringle's maneuver was applied to occlude the blood inflow of the liver if necessary. Thrombectomy was performed according to the location and extent of PVTT. For patients with PVTT confined to the ipsilateral branches of the portal vein (Cheng's type I/II), PVTT was resected en bloc with the tumor. When the tumor thrombus extended beyond the resection plane (Cheng's type III/IV), thrombectomy was carried by extracting the PVTT from the opened stump of the portal vein. After flushing with normal saline and confirming that no residual thrombus remained, the stump was closed with a continuous suture. All the operations were carried out by a single experienced surgical team.
The patients in the adjuvant aspirin group received aspirin after two weeks of surgery when the general condition, liver and renal functions of patients recovered. Aspirin was continuously administered orally at a standard 75 mg dose daily for at least 90 days until unacceptable toxicities occurred. In our hospital, low-dose aspirin (75 mg) was recommended for primary cardiovascular prevention or secondary risk reduction. Considering that HCC patients with PVTT may complicated with abnormal coagulation status or portal vein hypertension, higher-dose aspirin was not prescribed for HCC patients with PVTT to reduce risk of bleeding.
Patient visits were scheduled every month for the first 3 month and then once every 3 months thereafter to monitor safety and drug accountability. Dose reductions and treatment interruptions were allowed according to drug-related severe toxicity as recommended, and only grade 3–4 gastrointestinal bleeding was considered to be aspirin-related severe toxicity.
2.7.1. Assessment of outcomes and follow-up
The primary endpoint was OS, which was defined as the time from random assignment to tumor-related death. One of the secondary outcomes was time to recurrence of primary tumor (t-TTR), which was defined as the time from random assignment to the time when a recurrent tumor confined to liver was first diagnosed. The other secondary outcome was time to recurrence of PVTT (p-TTR), which was defined as the time from random assignment to the time when an appearance of tumor thrombus located in the branches or main trunk of portal vein was first diagnosed. The routine follow-up investigations, including routine blood examination, liver and renal function tests, alpha fetoprotein (AFP), chest radiography and abdominal ultrasound (US), were performed every month for the first 3 month and once every 3 months thereafter. Once recurrence of primary tumor or PVTT is highly suspected based on AFP elevation and typical US images, computed tomography (CT) or magnetic resonance imaging (MRI) was subsequently performed to make a definite diagnosis of disease recurrences.
The diagnosis of HCC and PVTT recurrence was based on typical imaging features on CT and/or MRI with or without abnormal AFP levels. If the recurrence was confirmed, locoregional or systemic treatments, such as transarterial chemoembolization (TACE), radiofrequency ablation (RFA), radiotherapy, and molecular targeted drugs, were used depending on the characteristics of the recurrent diseases and the general condition of the patient. The data were analyzed on an intention-to-treat basis. This study was censored on October 1, 2019.
2.7.2. Immunohistochemistry assay for COX1 and COX2
HCC tissues were collected only from the 30 specimens of the 40 patients in the aspirin group after surgical and postoperative adjuvant aspirin treatment. Considering aspirin was non-selective inhibitor of cyclooxygenase (COX)1 and COX2, immunohistochemistry (IHC) staining for COX1 and COX2 was performed based on the method used in a previous report [25]. The extent and intensity of staining were automatically examined by Vectra 2 system (PerkinElmer, USA) and were estimated using H-score as previously described [26]. According to the median score of COX1/2, the patients were categorized as the COX1/2 low and high expression groups. The relationship between long-term survival outcomes and the expression level of COX1 or COX2 was analyzed.
2.8. Statistical analysis
All the baseline clinicopathological variables were transformed into categorical data and presented as frequencies and percentages. Variables were compared using the chi-square test or Fisher's exact test as appropriate. Survival analysis for time-to-event curves were generated by the Kaplan-Meier method and the differences were compared by the means of log-rank test. The hazard ratio (HR) for survival and its 95% confidence interval (CI) were calculated using a Cox proportional hazards regression model. A statistical significance was set at a P value of less than 0.05. Statistical analyses were performed using the SPSS software, version 25.0 (SPSS, Chicago, IL, USA).
3. Results
3.1. Study population
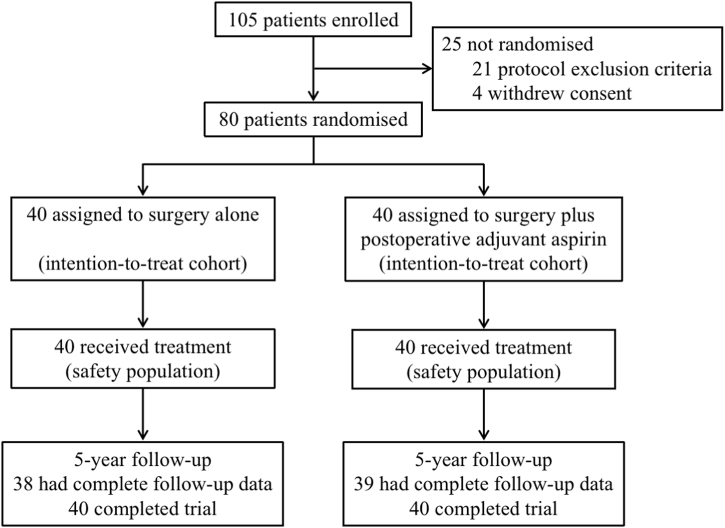
Between June 1, 2013 and July 1, 2015, 105 patients with HCC and PVTT assessed for eligibility were enrolled in our hospital, among whom 25 patients were excluded from the study (21 fulfilled the exclusion criteria of study protocol, 4 withdrew consent). The remaining 80 patients were randomly assigned to the postoperative adjuvant aspirin group (40 participants) and surgery alone group (40 participants), and they were included in the intention-to-treat analysis (Fig. 1). The baseline characteristics were well balanced between the adjuvant aspirin and surgery alone groups (Table 1).
Fig. 1.
Patients flow diagram.
Table 1.
Baseline characteristics of patients.
| Characteristics | Patients, No. (%) |
P-value | |
|---|---|---|---|
| Control (n = 40) | Aspirin (n = 40) | ||
| General status | |||
| Age, years | 0.356 | ||
| ≤50 | 27 (67.5) | 23 (57.5) | |
| >50 | 13 (32.5) | 17 (42.5) | |
| Sex | 1.000 | ||
| Male | 37 (92.5) | 37 (92.5) | |
| Female | 3 (7.5) | 3 (7.5) | |
| ECOG performance status | 0.340 | ||
| 0 | 29 (72.5) | 25 (62.5) | |
| 1 | 11 (27.5) | 15 (37.5) | |
| Laboratory findings | |||
| Viral serology | |||
| Positive for HBsAg | 35 (87.5) | 37 (92.5) | 0.456 |
| Positive for HBeAg | 11 (27.5) | 11 (27.5) | 1.000 |
| HBV DNA load, IU/mL | 0.654 | ||
| <2000 | 18 (45.0) | 20 (50.0) | |
| ≥2000 | 22 (55.0) | 20 (50.0) | |
| Anti-viral treatment, yes | 11 (27.5) | 8 (20.0) | 0.431 |
| AFP, ng/ml | 0.648 | ||
| <400 | 15 (37.5) | 17 (42.5) | |
| ≥400 | 25 (62.5) | 23 (57.5) | |
| Total bilirubin, μmol/L | 0.171 | ||
| ≤17.1 | 21 (52.5) | 27 (67.5) | |
| >17.1 | 19 (47.5) | 13 (32.5) | |
| Albumin, g/L | 0.166 | ||
| <35 | 4 (10.0) | 1 (2.5) | |
| ≥35 | 36 (90.0) | 39 (97.5) | |
| ALT, U/L | 0.228 | ||
| ≤44 | 25 (62.5) | 30 (75.0) | |
| >44 | 15 (37.5) | 10 (25.0) | |
| Prothrombin time, s | 0.152 | ||
| ≤13 | 30 (75.0) | 35 (87.5) | |
| >13 | 10 (25.0) | 5 (12.5) | |
| Platelet count, × 109/L | 0.329 | ||
| <100 | 10 (25.0) | 14 (35.0) | |
| ≥100 | 30 (75.0) | 26 (65.0) | |
| Surgical findings | |||
| Type of hepatectomy | 0.469 | ||
| Major | 29 (72.5) | 26 (65.0) | |
| Minor | 11 (27.5) | 14 (35.0) | |
| Anatomic resection, yes | 14 (35.0) | 16 (40.0) | 0.644 |
| Hilar clamping time, minutes | 0.531 | ||
| <15 | 5 (12.5) | 7 (17.5) | |
| ≥15 | 35 (87.5) | 33 (82.5) | |
| Intraoperative blood loss, mL | 0.813 | ||
| ≤800 | 26 (65.0) | 27 (67.5) | |
| >800 | 14 (35.0) | 13 (32.5) | |
| PVTT types | 0.469 | ||
| Ⅰ | 4 (10.0) | 9 (22.5) | |
| Ⅱ | 23 (57.5) | 18 (45.0) | |
| Ⅲ | 11 (27.5) | 11 (27.5) | |
| Ⅳ | 2 (5.0) | 2 (5.0) | |
| Pathologic findings | |||
| Tumor diameter, cm | 0.340 | ||
| >5 | 29 (72.5) | 25 (62.5) | |
| ≤5 | 11 (27.5) | 15 (37.5) | |
| Tumor number | 0.644 | ||
| Multiple | 2 (5.0%) | 3 (7.5%) | |
| Single | 38 (95.0%) | 37 (92.5%) | |
| Tumor encapsulation | 0.592 | ||
| No/Incomplete | 30 (75.0) | 32 (80.0) | |
| Complete | 10 (25.0) | 8 (20.0) | |
| Satellite nodules, yes | 24 (60.0) | 22 (55.0) | 0.651 |
| Liver cirrhosis, yes | 8 (20.0) | 7 (17.5) | 0.775 |
| Microvascular invasion, yes | 37 (92.5) | 36 (90.0) | 0.692 |
| Recurrence findings | |||
| Primary tumor recurrence | 0.793 | ||
| Yes | 30 (75.0) | 31 (77.5) | |
| No | 10 (25.0) | 9 (22.5) | |
| PVTT recurrence | 0.045 | ||
| Yes | 36 (90.0) | 29 (72.5) | |
| No | 4 (10.0) | 11 (27.5) | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; AFP, α-fetoprotein; ALT, alanine aminotransferase; PVTT, portal vein tumor thrombus.
Positive for HBsAg was the predominant cause of liver disease (90%). Increased serum AFP levels were found in 48 (60%) patients. There were 55 (68.8%) patients underwent major hepatectomy. Most patients had a single tumor (93.8%). Tumors larger than 5 cm in diameter were found in 54 (67.5%) patients. The number of patients with type I, II, III and IV PVTT were 13 (16.3%), 41 (51.3%), 22 (27.5%) and 4 (5%), respectively. Seventy-three (91.3%) patients had microvascular invasion. Primary tumor recurrence was found in 61 (76.3%) patients, and PVTT recurrence was encountered in 65 (81.3%) patients. Notably, the percentage of PVTT recurrence was lower in the adjuvant aspirin group than the control group (72.5% vs. 90.0%; P = 0.045). The median follow-up duration was 19 months (range, 8–73 months). At the time of censor of this study, there were still three survivors in the aspirin group and two survivors in the control group, respectively.
3.1.1. Overall survival (OS)
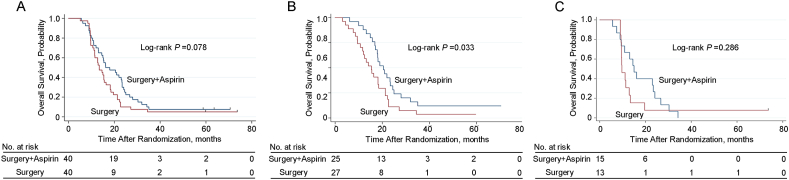
As shown in Table 2, the median OS were 16.2 months and 13.4 months for the adjuvant aspirin group and the surgery alone group, respectively (hazard ratio, 0.664; 95% CI, 0.419–1.052). There was no significant difference in the OS between the two groups of patients (P = 0.078, Fig. 2A). The corresponding 1-, 2- and 3-year OS rates were 70.0%, 40.0%, and 10.0%, and 70.0%, 37.5% and 10.0%, respectively (Fig. 2A). On subgroup analysis of the type of PVTT, patients with type I and II PVTT in the adjuvant aspirin group had a better OS compared with the surgery alone group (20.5 months vs. 15.4 months, P = 0.033) (Fig. 2B). Nevertheless, there was no significant difference in the OS between the two groups of patients with type III and IV PVTT (15.0 months vs. 9.7 months, P = 0.286) (Fig. 2C).
Table 2.
Survival outcomes of patients.
| Outcomes | Median Time (95% CI), months |
HR (95% CI)a | P-value | |
|---|---|---|---|---|
| Control (n = 40) | Aspirin (n = 40) | |||
| Overall survival | ||||
| Entire group | 13.4 (14.0–16.4) | 16.2 (8.3–24.1) | 0.664 (0.419–1.052) | 0.081 |
| Type I and II PVTT group | 15.4 (14.316.4) | 20.5 (12.7–28.3) | 0.638 (0.358–0.945) | 0.036 |
| Type III and IV PVTT group | 9.7 (7.8–11.7) | 15.0 (11.2–18.8) | 0.653 (0.297–1.439) | 0.291 |
| t-Time to recurrence | ||||
| Entire group | 3.2 (1.3–5.1) | 5.3 (4.5–6.1) | 0.782 (0.472–1.297) | 0.341 |
| Type I and II PVTT group | 5.4 (1.7–9.2) | 5.3 (3.3–7.4) | 0.876 (0.469–1.635) | 0.677 |
| Type III and IV PVTT group | 2.9 (2.2–3.6) | 5.3 (3.3–7.3) | 0.620 (0.262–1.468) | 0.277 |
| p-Time to recurrence | ||||
| Entire group | 5.4 (3.7–7.0) | 12.0 (4.8–19.3) | 0.425 (0.258–0.700) | 0.001 |
| Type I and II PVTT group | 7.2 (5.3–9.2) | 14.9 (5.4–24.3) | 0.410 (0.215–0.781) | 0.007 |
| Type III and IV PVTT group | 2.7 (0.0–6.8) | 8.9 (2.5–15.3) | 0.382 (0.169–0.862) | 0.020 |
Abbreviations: HR, hazard ratio; t-Time to recurrence, Time to recurrence of primary tumor; p-Time to recurrence, Time to recurrence of portal vein tumor thrombus. aCox proportional hazards regression model for the aspirin group with the placebo group as a reference.
Fig. 2.
Kaplan–Meier analysis for OS. (A) OS for HBV-related HCC patients with PVTT with or without adjuvant aspirin (40 patients vs. 40 patients) after surgery (P = 0.078); (B) OS for patients with HBV-related HCC and type I and II PVTT with or without adjuvant aspirin (25 patients vs. 27 patients) after surgery (P = 0.033); (C) OS for patients with HBV-related HCC and type III and IV PVTT with or without adjuvant aspirin (15 patients vs. 13 patients) after surgery (P = 0.286).
3.2. Time to recurrence of primary tumor (t-TTR)
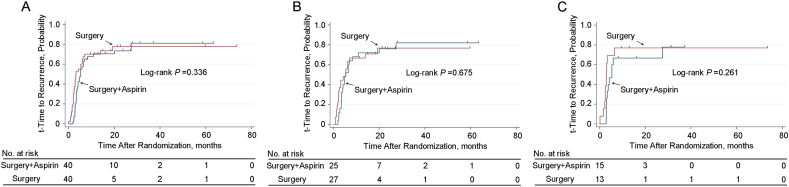
As shown in Table 2, the median t-TTR were 5.3 months and 3.2 months for the adjuvant aspirin group and the surgery alone group, respectively (hazard ratio, 0.782; 95% CI, 0.472–1.297). There was no significant difference in the t-TTR between the two groups of patients (P = 0.336, Fig. 3A). On subgroup analysis of the type of PVTT, the t-TTR outcomes were comparable between the adjuvant aspirin and surgery alone groups with type I and II PVTT (5.3 months vs. 5.4 months, P = 0.675) (Fig. 3B). Moreover, there was no significant difference in the t-TTR between the two groups of patients with type III and IV PVTT (5.3 months vs. 2.9 months, P = 0.261) (Fig. 3C).
Fig. 3.
Kaplan–Meier analysis for t-TTR. (A) t-TTR for HBV-related HCC patients with PVTT with or without adjuvant aspirin (40 patients vs. 40 patients) after surgery (P = 0.336); (B) t-TTR for patients with HBV-related HCC and type I and II PVTT with or without adjuvant aspirin (25 patients vs. 27 patients) surgery (P = 0.675); (C) t-TTR for patients with HBV-related HCC and type III and IV PVTT with or without adjuvant aspirin (15 patients vs. 13 patients) surgery (P = 0.281). t-TTR, time to recurrence of primary tumor.
3.3. Time to recurrence of PVTT (p-TTR)
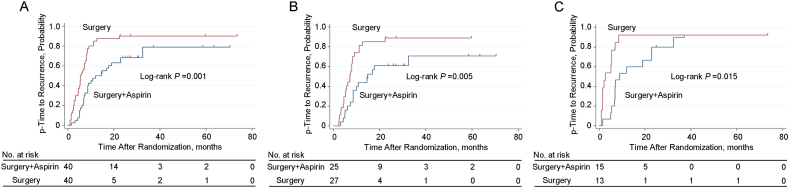
As shown in Table 2, the median p-TTR were 12.0 months and 5.4 months for the adjuvant aspirin group and the surgery alone group, respectively (hazard ratio, 0.425; 95% CI, 0.258–0.700). Patients in the adjuvant aspirin group had significantly longer p-TTR compared with the surgery alone group (P = 0.001, Fig. 4A). On subgroup analysis of the type of PVTT, patients with type I and II PVTT in the adjuvant aspirin group had markedly longer p-TTR compared with the surgery alone group (14.9 months vs. 7.2 months, P = 0.005) (Fig. 4B). Similarly, the p-TTR was markedly longer in the adjuvant aspirin group than the surgery alone group with type III and IV PVTT (8.9 months vs. 2.7 months, P = 0.015) (Fig. 4C).
Fig. 4.
Kaplan–Meier analysis for p-TTR. (A) p-TTR for HBV-related HCC patients with PVTT with or without adjuvant aspirin (40 patients vs. 40 patients) after surgery (P = 0.001); (B) p-TTR for patients with HBV-related HCC and type I and II PVTT with or without adjuvant aspirin (25 patients vs. 27 patients) after surgery (P = 0.005); (C) p-TTR for patients with HBV-related HCC and type III and IV PVTT with or without adjuvant aspirin (15 patients vs. 13 patients) after surgery (P = 0.015). p-TTR, time to recurrence of portal vein tumor thrombus.
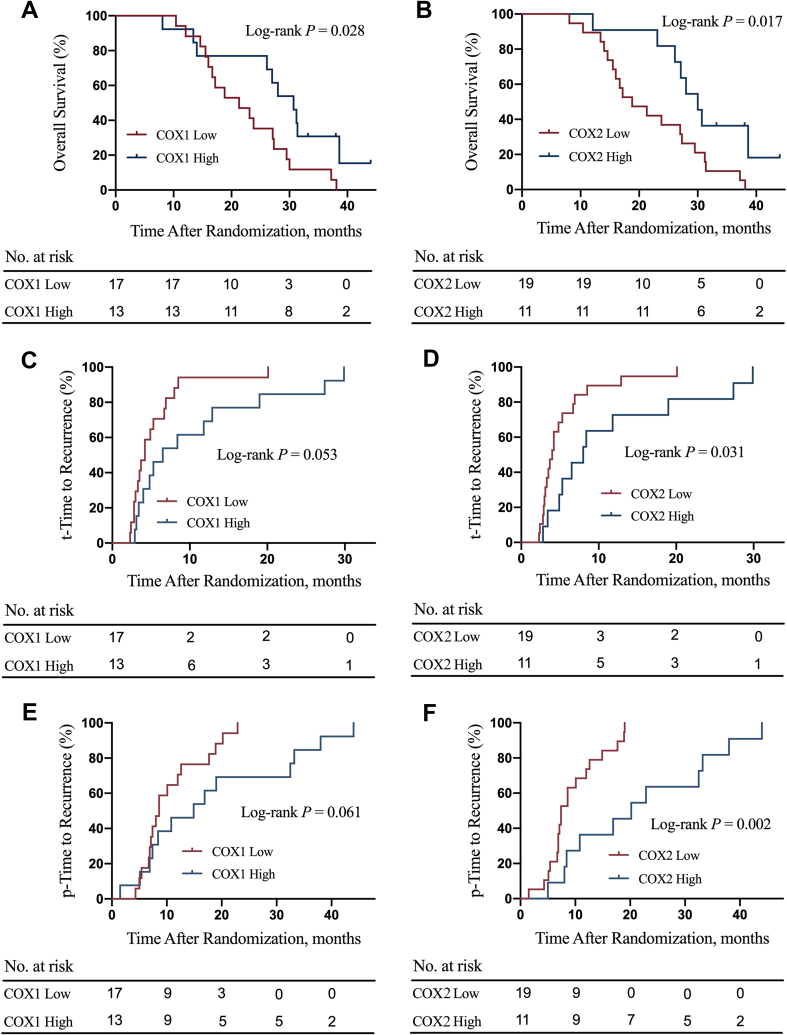
3.4. Association of COX-1 (2) expression with survival outcomes
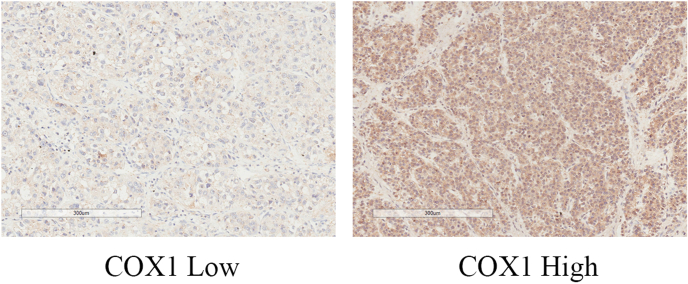
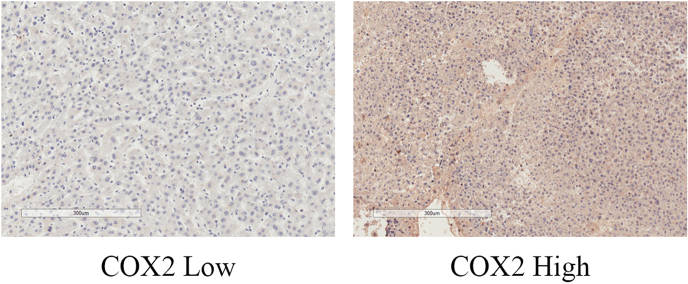
The associations of tissue expression levels of COX1/2 with patients’ survival outcomes were further investigated. The COX1/2 high expression group had significantly better OS compared with the COX1/2 low expression group (P = 0.028, Fig. S1A; P = 0.017, Fig. S1B). The COX1 high expression group had a trend of statistically significantly lower t-TTR compared with the COX1 low expression group (P = 0.053, Fig. S1C). The COX2 high expression group had markedly lower t-TTR compared with the COX2 low expression group (P = 0.031, Fig. S1D). The COX1 high expression group had a marginally significantly lower p-TTR compared with the COX1 low expression group (P = 0.061, Fig. S1E). The COX2 high expression group had a markedly lower p-TTR compared with the COX2 low expression group (P = 0.002, Fig. S1F). The representative immunohistochemistry image of COX1 low and high expression levels is shown in Fig. S2. The typical immunohistochemistry picture of COX2 low and high expression levels is shown in Fig. S3.
3.4.1. Adverse events
Table 3 summarizes Grade 1–2 and Grade 3 toxicities for all the patients who underwent adjuvant aspirin. Dyspepsia, bruising, nose bleed, anaemia, bleeding gums, lower gastrointestinal bleeding, and haematuria were the most common toxicities, but these were all Grade 1–2. No serious toxicities with Grade 3 were observed in these patients.
Table 3.
Toxicities reported of patients with HCC and PVTT who underwent adjuvant aspirin after surgery.
| Grade 1–2 | Grade 3 | |
|---|---|---|
| Dyspepsia | 2 | 0 |
| Bruising | 2 | 0 |
| Nose bleeding | 1 | 0 |
| Anaemia | 2 | 0 |
| Bleeding gums | 2 | 0 |
| Lower gastrointestinal bleeding | 1 | 0 |
| Haematuria | 1 | 0 |
| Allergic reaction to aspirin | 0 | 0 |
| Upper gastrointestinal bleeding | 0 | 0 |
4. Discussions
This present study is the first open-label, randomized comparative trial to determine the role of adjuvant aspirin after partial hepatectomy in patients with HBV-related HCC and PVTT. Postoperative adjuvant aspirin yielded comparable OS and t-TTR compared to surgery alone for HBV-related HCC patients with PVTT. Notably, postoperative adjuvant aspirin treatment prolonged 6.6 months of the median p-TTR (12.0 months and 5.4 months in the adjuvant aspirin and surgery alone groups, respectively). In subgroup analysis, the protective effect of postoperative adjuvant aspirin on p-TTR still existed whether for patients with type I and II PVTT or for patients with type III and IV PVTT. Interestingly, postoperative adjuvant aspirin yielded marginally significant benefit of OS for HBV-related HCC patients with PVTT, and conferred significantly better OS for patients with type I and II PVTT. No patients who received adjuvant aspirin developed serious acute adverse events.
Despite the existing controversy among experts from the West and the East on the treatment strategy for patients with HCC and PVTT [1,21,27], many tertiary liver referral centers in Eastern Asia consider surgery to be a potentially curative treatment which provides optimal survival outcomes for selected HCC patients with PVTT [12,23,28,29]. Partial hepatectomy for such patients is safe, with the surgical mortality rates ranging from 0% to 10.0% [30]. However, the long-term prognosis after curative surgery remains disappointing mainly due to the high HCC recurrence rate. Currently, emerging postoperative adjuvant therapies, including TACE and radiotherapy, have been proposed to reduce recurrence and prolong the survival in HCC patients with PVTT. Several prospective and retrospective studies have demonstrated that postoperative adjuvant TACE could reduce tumor recurrence and render survival benefits for HCC patients with vascular invasion [[31], [32], [33]]. Recently, a randomized clinical trial conducted by our research team found that postoperative intensity modulated radiation therapy (IMRT) significantly improved the overall survival outcomes of these patients [34].
Aspirin is a non-steroidal anti-inflammatory drug (NSAID) and is prescribed not only as an antipyretic analgesic but also as an anti-platelet drug. Lannacone et al. [35]. reported that the administration of aspirin reduced the development of HCC via blocking platelet activation in a mouse model. Experimental [36] and clinical [37] data collectively suggest that aspirin may prevent or delay the progression of liver disease and hepatocarcinogenesis through various mechanisms including prevention of platelet degranulation, modulation of bioactive lipids, and inhibition of the proinflammatory cyclooxygenase enzyme. Many prospective and retrospective cohort studies also demonstrated that aspirin may reduce the risk of HCC development [[37], [38], [39]]. Moreover, the protective role of adjuvant aspirin on the recurrence and survival outcomes of HCC patients after surgery has been proposed by several studies. One large sample size study which included 442 patients with antiplatelet therapy and 1768 patients without antiplatelet therapy showed that use of aspirin was associated with better RFS and OS among patients with HBV-related HCC after liver resection [20]. Young et al. [18]. also reported that aspirin can lower the recurrence risk in HBV-related HCC patients after curative resection.
In this present study, however, the protective effects of adjuvant aspirin in preventing the primary tumor recurrence and prolonging the overall survival outcomes were not observed in patients with HBV-related HCC and PVTT after surgery. However, the survival benefits of adjuvant aspirin appeared when patients with PVTT were in a relative early stage, namely, type I and II PVTT stage. One reason may be that once PVTT develops to a more advanced stage, the protective effect of postoperative adjuvant aspirin was counteracted by the deterioration of liver function or rapid progression of ascites caused by the obstruction of main portal vein. Nevertheless, postoperative adjuvant aspirin provided considerable benefits in decreasing the recurrence of PVTT, and the anti-platelet effect of aspirin may explain this novel finding. Platelet has been reported to promote the extravasation and metastasis of HCC cells from primary lesion and trigger cancer progression during the interaction between platelets and cancer cells in the bloodstream [40]. In addition, one previous retrospective research from our group echoes the findings of the present study, which demonstrated that HCC patients associated with PVTT with lower platelet counts exhibited a better prognosis [41]. Besides, aspirin mainly inhibits its target of cyclooxygenases, including COX1 and COX2, to play its biological functions. Our study demonstrated that COX1 or COX2 overexpression in tumor tissues was associated with better survival outcomes in HBV-related HCC patients with PVTT who underwent adjuvant aspirin. More fundamental and clinical studies are required to validate this exploratory funding.
This study has several limitations. First, this is a single-center study with relatively small samples. A multi-center research which includes more patients is needed to further validate the impact of adjuvant aspirin on the prognosis of these patients. Second, this study is conducted in patients with HBV-related HCC, which requires validation from HCC patients with other different etiologies. Third, salvage treatments, including local and systemic therapies, may affect the long-term outcomes. These subsequent treatments were used to treat HCC recurrence for ethical reasons.
In conclusion, for patients with resectable HBV-related HCC and PVTT, postoperative adjuvant aspirin significantly prolonged time to recurrence of PVTT than surgery alone, whereas the overall survival and time to recurrence of HCC were not remarkably improved. COX1 or COX2 may serve as a useful marker to predict survival in these patients using aspirin.
Statement of ethics
This single-center, open-label, randomized controlled study was conducted from June 1, 2013 to July 1, 2015 at the Eastern Hepatobiliary Surgery Hospital. This study was approved by the ethics committees of the Eastern Hepatobiliary Surgery Hospital (EHBHKY2013-001-1) and conducted in accordance with the Declaration of Helsinki (1975). Written informed consents were obtained from all the patients prior to randomization. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) statement and was registered in the Chinese Clinical Trial Registry (ChiCTR-TRC-13003357).
Funding
This trial was supported by the State Key Program of National Natural Science Foundation of China (No: 81730097); National Natural Science Foundation of China (No:82072618; 82103483); Shanghai sailing program (20YF1459700).
Author contribution statement
Conceived and designed the experiments: Shu-Qun Cheng, Wan Yee Lau, Chong-De Lu; Performed the experiments: Chong-De Lu, Lei Wang, Xu-Biao Wei, Wei-Xing Guo; Analyzed and interpreted the data: Chong-De Lu, Ya-Bo Jiang, Jin-Kai Feng, Lei Wang, Bin Zhou, Xiao-Lu Lin, Wei-Xing Guo; Contributed reagents, materials, analysis tools or data: Chong-De Lu, Lei Wang, Xu-Biao Wei; Wrote the paper: Chong-De Lu, Ya-Bo Jiang, Jin-Kai Feng.
Data availability statement
Data will be made available on request.
Ethics statement
This study was approved by the Institutional Ethics Committee of the Eastern Hepatobiliary Surgery Hospital (approval number: EHBHKY2013-001-1) and conformed to the principles of 1975 Declaration of Helsinki. Detailed information of the trial protocol was explained to every participant, and written informed consent was obtained from all the patients before randomization. This trial followed the Consolidated Standards of Reporting Trials (CONSORT) statement. This study has been registered in the Chinese Clinical Trials Registry (ChiCTR) and the registration number is ChiCTR-TRC-13003357. The research data were used only for publication or shared with other researchers. The identity and medical information of participants were kept strictly confidential.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.All of the authors who participate in this clinical trial declare no potential conflicts of interest.
Acknowledgements
None.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e20015.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
figs1.
figs2.
figs3.
References
- 1.EASL Clinical Practice Guidelines Management of hepatocellular carcinoma. J. Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 2.Lau W.Y., Sangro B., Chen P.J., Cheng S.Q., Chow P., Lee R.C., et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology. 2013;84(5):311–318. doi: 10.1159/000348325. [DOI] [PubMed] [Google Scholar]
- 3.Primary liver cancer in Japan. Clinicopathologic features and results of surgical treatment. Ann. Surg. 1990;211(3):277–287. [PMC free article] [PubMed] [Google Scholar]
- 4.Bruix J., Sherman M. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53(3):1020–1022. doi: 10.1002/hep.24199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schöniger-Hekele M., Müller C., Kutilek M., Oesterreicher C., Ferenci P., Gangl A. Hepatocellular carcinoma in Central Europe: prognostic features and survival. Gut. 2001;48(1):103–109. doi: 10.1136/gut.48.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Llovet J.M., Ricci S., Mazzaferro V., Hilgard P., Gane E., Blanc J.F., et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008;359(4):378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 7.Kudo M., Finn R.S., Qin S., Han K.H., Ikeda K., Piscaglia F., et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163–1173. doi: 10.1016/S0140-6736(18)30207-1. [DOI] [PubMed] [Google Scholar]
- 8.Finn R.S., Qin S., Ikeda M., Galle P.R., Ducreux M., Kim T.Y., et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N. Engl. J. Med. 2020;382(20):1894–1905. doi: 10.1056/NEJMoa1915745. [DOI] [PubMed] [Google Scholar]
- 9.Cheng A.L., Kang Y.K., Chen Z., Tsao C.J., Qin S., Kim J.S., et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi: 10.1016/S1470-2045(08)70285-7. [DOI] [PubMed] [Google Scholar]
- 10.Shuqun C., Mengchao W., Han C., Feng S., Jiahe Y., Guanghui D., et al. Tumor thrombus types influence the prognosis of hepatocellular carcinoma with the tumor thrombi in the portal vein. Hepato-Gastroenterology. 2007;54(74):499–502. [PubMed] [Google Scholar]
- 11.Shi J., Lai E.C., Li N., Guo W.X., Xue J., Lau W.Y., et al. A new classification for hepatocellular carcinoma with portal vein tumor thrombus. J Hepatobiliary Pancreat Sci. 2011;18(1):74–80. doi: 10.1007/s00534-010-0314-0. [DOI] [PubMed] [Google Scholar]
- 12.Kokudo T., Hasegawa K., Matsuyama Y., Takayama T., Izumi N., Kadoya M., et al. Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J. Hepatol. 2016;65(5):938–943. doi: 10.1016/j.jhep.2016.05.044. [DOI] [PubMed] [Google Scholar]
- 13.Zhang X.P., Gao Y.Z., Chen Z.H., Chen M.S., Li L.Q., Wen T.F., et al. An eastern hepatobiliary surgery hospital/portal vein tumor thrombus scoring system as an aid to decision making on hepatectomy for hepatocellular carcinoma patients with portal vein tumor thrombus: a multicenter study. Hepatology. 2019;69(5):2076–2090. doi: 10.1002/hep.30490. [DOI] [PubMed] [Google Scholar]
- 14.Ye X.F., Wang J., Shi W.T., He J. Relationship between aspirin use after diagnosis of colorectal cancer and patient survival: a meta-analysis of observational studies. Br. J. Cancer. 2014;111(11):2172–2179. doi: 10.1038/bjc.2014.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coyle C., Cafferty F.H., Rowley S., MacKenzie M., Berkman L., Gupta S., et al. ADD-ASPIRIN: a phase III, double-blind, placebo controlled, randomised trial assessing the effects of aspirin on disease recurrence and survival after primary therapy in common non-metastatic solid tumours. Contemp. Clin. Trials. 2016;51:56–64. doi: 10.1016/j.cct.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Joharatnam-Hogan N., Cafferty F., Hubner R., Swinson D., Sothi S., Gupta K., et al. Aspirin as an adjuvant treatment for cancer: feasibility results from the Add-Aspirin randomised trial. Lancet Gastroenterol Hepatol. 2019;4(11):854–862. doi: 10.1016/S2468-1253(19)30289-4. [DOI] [PubMed] [Google Scholar]
- 17.Simon T.G., Ma Y., Ludvigsson J.F., Chong D.Q., Giovannucci E.L., Fuchs C.S., et al. Association between aspirin use and risk of hepatocellular carcinoma. JAMA Oncol. 2018;4(12):1683–1690. doi: 10.1001/jamaoncol.2018.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Young S.H., Chau G.Y., Lee I.C., Yeh Y.C., Chao Y., Huo T.I., et al. Aspirin is associated with low recurrent risk in hepatitis B virus-related hepatocellular carcinoma patients after curative resection. J. Formos. Med. Assoc. 2020;119(1 Pt 2):218–229. doi: 10.1016/j.jfma.2019.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Boas F.E., Brown K.T., Ziv E., Yarmohammadi H., Sofocleous C.T., Erinjeri J.P., et al. Aspirin is associated with improved liver function after embolization of hepatocellular carcinoma. AJR Am. J. Roentgenol. 2019;213(3):1–7. doi: 10.2214/AJR.18.20846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee P.C., Yeh C.M., Hu Y.W., Chen C.C., Liu C.J., Su C.W., et al. Antiplatelet therapy is associated with a better prognosis for patients with hepatitis B virus-related hepatocellular carcinoma after liver resection. Ann. Surg Oncol. 2016;23(Suppl 5):874–883. doi: 10.1245/s10434-016-5520-9. [DOI] [PubMed] [Google Scholar]
- 21.Cheng S., Chen M., Cai J., Sun J., Guo R., Bi X., et al. Chinese expert consensus on multidisciplinary diagnosis and treatment of hepatocellular carcinoma with portal vein tumor thrombus (2018 edition) Liver Cancer. 2020;9(1):28–40. doi: 10.1159/000503685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wei X.B., Xu J., Li N., Yu Y., Shi J., Guo W.X., et al. The role of three-dimensional imaging in optimizing diagnosis, classification and surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. HPB (Oxford) 2016;18(3):287–295. doi: 10.1016/j.hpb.2015.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shi J., Lai E.C., Li N., Guo W.X., Xue J., Lau W.Y., et al. Surgical treatment of hepatocellular carcinoma with portal vein tumor thrombus. Ann. Surg Oncol. 2010;17(8):2073–2080. doi: 10.1245/s10434-010-0940-4. [DOI] [PubMed] [Google Scholar]
- 24.Peng Z.W., Guo R.P., Zhang Y.J., Lin X.J., Chen M.S., Lau W.Y. Hepatic resection versus transcatheter arterial chemoembolization for the treatment of hepatocellular carcinoma with portal vein tumor thrombus. Cancer. 2012;118(19):4725–4736. doi: 10.1002/cncr.26561. [DOI] [PubMed] [Google Scholar]
- 25.Tiwari S.K., Shaik A.S., Shaik A.P., Alyousef A.A., Bardia A., Habeeb M.A., et al. Gene expression patterns of COX-1, COX-2 and iNOS in H. Pylori infected histopathological conditions. Microb. Pathog. 2019;135 doi: 10.1016/j.micpath.2019.103634. [DOI] [PubMed] [Google Scholar]
- 26.Stack E.C., Wang C., Roman K.A., Hoyt C.C. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods. 2014;70(1):46–58. doi: 10.1016/j.ymeth.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 27.Lu J., Zhang X.P., Zhong B.Y., Lau W.Y., Madoff D.C., Davidson J.C., et al. Management of patients with hepatocellular carcinoma and portal vein tumour thrombosis: comparing east and west. Lancet Gastroenterol Hepatol. 2019;4(9):721–730. doi: 10.1016/S2468-1253(19)30178-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang K., Guo W.X., Chen M.S., Mao Y.L., Sun B.C., Shi J., et al. Multimodality treatment for hepatocellular carcinoma with portal vein tumor thrombus: a large-scale, multicenter, propensity mathching score analysis. Medicine (Baltim.) 2016;95(11) doi: 10.1097/MD.0000000000003015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu P.H., Lee Y.H., Hsia C.Y., Hsu C.Y., Huang Y.H., Chiou Y.Y., et al. Surgical resection versus transarterial chemoembolization for hepatocellular carcinoma with portal vein tumor thrombosis: a propensity score analysis. Ann. Surg Oncol. 2014;21(6):1825–1833. doi: 10.1245/s10434-014-3510-3. [DOI] [PubMed] [Google Scholar]
- 30.Sakamoto K., Nagano H. Surgical treatment for advanced hepatocellular carcinoma with portal vein tumor thrombus. Hepatol. Res. 2017;47(10):957–962. doi: 10.1111/hepr.12923. [DOI] [PubMed] [Google Scholar]
- 31.Sun J.J., Wang K., Zhang C.Z., Guo W.X., Shi J., Cong W.M., et al. Postoperative adjuvant transcatheter arterial chemoembolization after R0 hepatectomy improves outcomes of patients who have hepatocellular carcinoma with microvascular invasion. Ann. Surg Oncol. 2016;23(4):1344–1351. doi: 10.1245/s10434-015-5008-z. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X.P., Liu Y.C., Chen Z.H., Sun J.X., Wang K., Chai Z.T., et al. Postoperative adjuvant transarterial chemoembolization improves outcomes of hepatocellular carcinoma associated with hepatic vein invasion: a propensity score matching analysis. Ann. Surg Oncol. 2019;26(5):1465–1473. doi: 10.1245/s10434-019-07223-z. [DOI] [PubMed] [Google Scholar]
- 33.Chen Z.H., Zhang X.P., Zhou T.F., Wang K., Wang H., Chai Z.T., et al. Adjuvant transarterial chemoembolization improves survival outcomes in hepatocellular carcinoma with microvascular invasion: a systematic review and meta-analysis. Eur. J. Surg. Oncol. 2019;45(11):2188–2196. doi: 10.1016/j.ejso.2019.06.031. [DOI] [PubMed] [Google Scholar]
- 34.Sun J., Yang L., Shi J., Liu C., Zhang X., Chai Z., et al. Postoperative adjuvant IMRT for patients with HCC and portal vein tumor thrombus: an open-label randomized controlled trial. Radiother. Oncol. 2019;140:20–25. doi: 10.1016/j.radonc.2019.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Iannacone M., Sitia G., Narvaiza I., Ruggeri Z.M., Guidotti L.G. Antiplatelet drug therapy moderates immune-mediated liver disease and inhibits viral clearance in mice infected with a replication-deficient adenovirus. Clin. Vaccine Immunol. 2007;14(11):1532–1535. doi: 10.1128/CVI.00298-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kern M.A., Schubert D., Sahi D., Schöneweiss M.M., Moll I., Haugg A.M., et al. Proapoptotic and antiproliferative potential of selective cyclooxygenase-2 inhibitors in human liver tumor cells. Hepatology. 2002;36(4 Pt 1):885–894. doi: 10.1053/jhep.2002.36125. [DOI] [PubMed] [Google Scholar]
- 37.Lee T.Y., Hsu Y.C., Tseng H.C., Yu S.H., Lin J.T., Wu M.S., et al. Association of daily aspirin therapy with risk of hepatocellular carcinoma in patients with chronic hepatitis B. JAMA Intern. Med. 2019;179(5):633–640. doi: 10.1001/jamainternmed.2018.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin S., Lee S.H., Lee M., Kim J.H., Lee W., Lee H.W., et al. Aspirin and the risk of hepatocellular carcinoma development in patients with alcoholic cirrhosis. Medicine (Baltim.) 2020;99(9) doi: 10.1097/MD.0000000000019008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liao Y.H., Hsu R.J., Wang T.H., Wu C.T., Huang S.Y., Hsu C.Y., et al. Aspirin decreases hepatocellular carcinoma risk in hepatitis C virus carriers: a nationwide cohort study. BMC Gastroenterol. 2020;20(1):6. doi: 10.1186/s12876-020-1158-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Franco A.T., Corken A., Ware J. Platelets at the interface of thrombosis, inflammation, and cancer. Blood. 2015;126(5):582–588. doi: 10.1182/blood-2014-08-531582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y.Q., Wang K., Zhang X.P., Wei X.B., Jiang Y.B., Hu Y.R., et al. Thrombocytopenia: a prognostic factor for hepatocellular carcinoma patients with portal vein tumor thrombus after hepatectomy. J. Gastroenterol. Hepatol. 2019;34(7):1214–1221. doi: 10.1111/jgh.14537. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.