Abstract
Phthalate acid esters (PAE) are used as additives in the formulation of plastics, to increase their flexibility and transparency. They can migrate from plastic packaging to food, then cause endocrine disruption in consumers. This migration depends on the conditions of use defined for each plastic. Non-food plastics are likely to release more PAE than food-grade plastics. In Cameroon, non-food grade plastics such as old paint buckets are used by people to preserve liquid food. The present work aimed at studying the conditions and mechanism of migration of total PAE from paint buckets to pap. For this purpose, the effects of seven factors were determined through Plackett-Burman experimental design. The interactions of the most influential factors were determined through a full factorial design. The conditions of the migration of total PAE were obtained via face-centered composite design. Then experimental results of migration kinetics were modelled according to equations of pseudo-first order, pseudo-second order and intra-particle diffusion. The results revealed that the most influential factors were pH, temperature and contact time. The effects of these factors are non-linear, and their interactions have to be considered. When pap is preserved in paint buckets according to the conditions: temperature of pap >70 °C, pH of pap ≤4 or ≥10 and contact time > 2 h, as is the case in donut shops in Cameroon, the amount of total PAE released is greater than 50 μg/L. Migration of total PAE from paint buckets to pap is best described by the pseudo-second order model.
Keywords: PAE, Non-food plastics, Pap, Experimental design, Migration, Kinetic
1. Introduction
Plastics containers are either food grade or non-food grade. When they are food grade, they are intended to be in contact with food. Therefore, safety controls have to be carried out, the choice of plastic checked and the conditions of use defined. Same level of safety tests are not carried out with non-food grade containers [1]. However, in Africa, precisely in Cameroon, this distinction of plastic containers is not commonly applied. All plastics containers are re-used in food storage. For example, old paint buckets are cleaned to store water in households, or pap in donut-bean shops [2]. The population is therefore exposed to a potential health risk due to usage of unsafe materials for food services [3].
In fact, the formulation process of plastic materials uses various additives that are essential for the physicochemical and mechanical properties of the whole. Among these additives are phthalate acid esters (PAEs), chemical molecules derived (salts or esters) from phthalic acid, which all have a diester structure. The main role of PAEs is to increase the plasticity, flexibility and transparency of plastics. The most common PAEs are BBP (Benzylbutyl phthalate), DBP (Dibutyl phthalate), DEP (Diethyl phthalate), DEHP (di-2-ethylhexyl phthalate), DNOP (di-n-Octyl phthalate) and DINP (di-isononyl phthalate) [4]. Although they help to improve the plastic properties, they have harmful effect on human health as well. They are associated with fertility problems and adverse effects in the development of newborns. Moreover, PAE are endocrine disruptors and carcinogens [[5], [6], [7]]. Phthalates such as DBP have been associated with toxicity to the neural, reproductive and developmental systems [8]. Although several sources of exposure exist, dietary source through contaminated food and beverages is the main pathway of human exposure to phthalates [9]. In fact, phthalates can migrate from plastic materials to food in contact. This migration may occur during food processing, packaging, storage and transport [10].
In the context of strict control of pollutants and safeguarding human health, European and American states have established regulations on certain phthalates, including DEHP, DBP and BBP. Since 2007, the latter have been banned from use in the formulation of plastic materials in Europe (Directives 2004/93/EC; 2005/84 EC and 2007/19/EC) [6]. The European Food Safety Authority (EFSA) has defined the tolerable daily intake (TDI) at 0.01 mg/kg body weight per day for DBP, 0.5 mg/kg body weight per day for BBP, and 0.05 mg/kg body weight per day for DEHP, based on toxicological studies [11,12]. On the other hand, the US Food and Drug Administration established a specific migration limit (SML) of DBP and BBP at 0.3 mg/kg and 30.0 mg/kg of simulant that is in contact with food packaging respectively [8]. The legislation is not established for all phthalates, because the health effects of all phthalates have not been studied. In view of their structural similarity, it is better to quantify total phthalates during dietary exposure studies [13,14].
Studies showed that migration of phthalates from plastic materials to food depends on several factors such as the nature of food and to the containers or its treatment [6,15]. The factors that stand out are temperature, amount of electrolyte (NaCl), contact time, number of washes, polarity or acidity/basicity of the food.
[16,17,18,19]. There is an evidence that the migration of phthalates is high in fatty food than is acid or aqueous food [20,21]. In this line [10], showed that high fat content promotes migration of PAEs into milk packaged in plastic bottles [18]. observed that the migration of phthalates in beverages is accelerated in acid medium (pH < 5) by modifying the structure of the plastic polymer. Similar reports showed that migration of phthalates increases with temperature, contact time, dry matter content and hydrophobic nature of food [22,23,15,19]. On contrary, studies show that the successive washing of plastic containers contributes to reduce the content of additives that they release, due to the depolymerisation of the polymer [24,25]. Indeed, a high amount of additives is released during the first washes, then it decreases as the plastic container is washed [24,26].
Although several studies show that contact time increases the migration of phthalates into food, some authors reported no significant effect [27], while others observed a food composition-dependant effect [8]. Thus, the influence of contact time depends on the nature of food. Hence, rather than studying the effect of each factor separately, it could be interesting to consider interaction between factors. To study the effects of factors simultaneously, experimental design modeling was found to be an important tool for reducing the number of experiments and exhibiting interaction between influencing parameters [28].
Several studies have focused on modeling the migration of phthalates from plastic material into food using mathematical models and food simulant such as water, ethanol, acetic acid, hexane or isooctane [19,29,30]. [30] showed that using food simulant in evaluation of migration kinetics of additives leads to an overestimation of migration data by multiplying the real levels of migrated compound by 50 times. The same trend was observed when using Fick's equations in modeling of migration of phthalates [16]. Interestingly, Liu, Li, Zhao et al. (2020) showed that migration kinetic was best described by the pseudo-first order model. Similar studies focused on these kinetic models by using plastics made of thin materials such as PVC films or drinking bottles [22,19,31]. However, plastic food packaging is not only made of thin materials. This is the case of paint buckets used for food preservation in Cameroon. Hence, the selection of a kinetics model to study migration of phthalate from plastic containers into food system should be made carefully. To the best of our knowledge, this was the first study focus on the migration of PAE from non-food plastic containers used in food preservation in developing countries [2]. Moreover, it was a first approach that identified the interactions between the main factors that influence the migration of PAE from plastic to food.
The present work aimed to study the conditions and mechanism of migration of phthalates from paint buckets to pap, by determining the effects of the most influential factors and their interactions, as well as the best kinetic model.
2. Materials and methods
2.1. Reagents and standards
Six phthalates were chosen to produce a mixed solution: di-ethylhexylphthalate (DEHP) (≥98%), di-isononylphthalate (DINP) (≥98%), di-isobutylphthalate (DBP) (≥98%), di-isooctylphthalate (DNOP) (≥98%), benzylbutylphthalate (BBP) (≥98%) and di-isodecylphtalate (DIDP) (≥98%). The stock solution containing total phthalates at 1 mg/mL was prepared in methanol and stored in 2 mL amber vials at 4 °C. The working solutions were obtained by appropriate dilution of the stock solution. Di-ethylhexylphtalate-d4 (DEHP-d4) (99.1% D; 98% purity) was the internal standard. All phthalates were purchased from Supelco-Sigma Aldrich (Diegem, Belgium).
Triethylamine (TEA) (>99%) and (3-bromopropyl)trimethylammonium bromide (BPTAB) (97%) used for phthalate derivatization were purchased from Merck (Darmstadt, Germany). Sodium hydroxide pellets (99%), hydrochloric acid (37%) and formic acid (98%) were purchased from Merck (Darmstadt, Germany). All these reagents were of analytical grade. The organic solvents used were of HPLC or LC grade (Ethyl acetate, methanol, and acetonitrile) and were purchased from Merck (Darmstadt, Germany). The ultrapure water was produced by the Milli-Q purifier from Merck Millipore (Bedford, MA, USA). The 2 N NaOH solution was prepared by dissolving 8 g of pellet in 100 mL of ultrapure water. The 2 N HCl solution was obtained by diluting 37% HCl six times. Corn starch (CAS: 9005-25-8) and sucrose (≥99.5%; CAS: 57-50-1) were purchased from Sigma Aldrich (Milan, Italy). Sodium chloride (NaCl) (≥99.5%; CAS: 7647-14-5) was purchased from Merck (Darmstadt, Germany).
2.2. Equipment
The ME 235-P balance (Sartorius, Germany) was used to weigh test sample. The Seven easy pH-meter (Mettler Toledo, France) was used to pH determination. The IT vortex (Star LAB, France) was used to homogeneize samples or extracts contained in tubes or vials. The magnetic heating stirrer IKA RCT basic (IKA-Werke GmbH & Co.KG, Germany) equipped with a temperature probe was used for the preparation of pap.The Corio C water bath (Julabo, Germany) was used for constant temperature heating. The Universal 320 R Centrifuge (Hettich, Germany) allowed the centrifugation of extract in vials. An ACQUITY ultra high performance liquid chromatography system coupled to a Waters XevoTQ triple quadrupole tandem mass spectrometer (Waters Co., USA) was used for the analysis of derivatized compound.
2.3. Samples
Samples of this study are old plastic paint buckets most used in households and donut shops in Cameroon for preservation of drinking water and pap. The pap at 5 or 10 g of starch/L was prepared for phthalate migration studies. To obtain the pap, ultrapure water contained in a 1 L beaker was placed on a heating plate until it boiled. In parallel, the desired amount of starch was weighed into beaker then a little ultrapure water was added and stirred using a glass rod. Then the mixture was poured into boiling ultrapure water, stirring to cook the starch. Depending on the desired temperature, the pap was either used hot or cooled in an ice bath.
For the migration studies, the buckets were cut into 2.5 cm × 5.0 cm pieces to allow them to fit into the 150 mL glass bottles containing pap or water. In each bottle, 8 pieces (1 dm2 of internal surface) of buckets were introduced. Each piece has 2 mm of thickness and an average weight of 2.08 ± 0.15 g. In case of the study called “real situation”, the pap is directly introduced in the paint buckets, and given volumes are taken at inters of time to carry out the analysis. This study was carried out in particular for the kinetic study of migration.
2.4. Study of the phthalate migration from paint buckets to pap
The methodology for studying the migration of total PAEs from paint buckets to Pap is shown in Fig. 1. The main steps of the study were: Screening of factors, interaction study of the most influential factors, determination of optimum phthalate migration conditions, and kinetic study.
Fig. 1.
Methodology for studying the migration of total PAEs from paint bucket to Pap.
2.4.1. Screening of factors that influence the phthalate migration
The screening of the factors by Plackett-Burman experimental design helps to classify them according to their effects. Factors that influence the migration of phthalate from packaging to food as pap were selected according to literature. These factors are starch content, salt content, sucrose content, pH, temperature, time and number of washes. The levels of different pap composition factors (salt content and sucrose content) were determined according to personal observation and literature [32]. The pH was defined by considering that people prepare pap either with lemon (acidic) or with alkaline rock salt called “kanwa” (basic). High temperature (90 °C) was defined considering that hot pap is stored in old paint buckets in some donut shops of Cameroon. Low temperature reflects the situation where drinking water with ice are stored (4 °C) in old paint buckets in some Cameroun households. The starch content was revised downwards compared to real pap, in order to reduce the complex extraction step. It is therefore a light pap or ultrapure water, to look for the effect of the matter amount. The contact time was set at 1 h (low level) and 72 h (high level), in order to comply with the real storage conditions in households. The number of washes was chosen to highlight the use of these buckets over a long period.
In the experimental design, the real values of the factors are encoded. Code value 1 or +1 indicates the highest level. Value −1 indicates the lowest level, and value 0 is the center of the experimental domain. Central points improve the statistical analysis and ensure a homogeneous distribution of the experimental error over all the tests. Table 1 shows the Plackett-Burman design for screening.
Table 1.
Coded matrix and test matrix of the Plackett-Burman design.
| A |
B |
C |
D |
E |
F |
G |
||
|---|---|---|---|---|---|---|---|---|
| N° | pH | T°C | Starch (g/L) | NaCl (%) | Sucrose (%) | Time (h) | Washes | Response |
| Coded matrix | ||||||||
| 1 | 1 | 1 | 1 | −1 | 1 | −1 | −1 | |
| 2 | −1 | 1 | 1 | 1 | −1 | 1 | −1 | |
| 3 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | |
| 4 | 1 | −1 | −1 | 1 | 1 | 1 | −1 | |
| 5 | −1 | 1 | −1 | −1 | 1 | 1 | 1 | |
| 6 | 1 | −1 | 1 | −1 | −1 | 1 | 1 | |
| 7 | 1 | 1 | −1 | 1 | −1 | −1 | 1 | |
| 8 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | |
| 9 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 11 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 12 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Test matrix | ||||||||
| 1 | 10 | 90 | 10 | 0 | 10 | 1 | 1 | 46 |
| 2 | 4 | 90 | 10 | 2.5 | 0 | 72 | 1 | 9.6 |
| 3 | 4 | 4 | 10 | 2.5 | 10 | 1 | 50 | 6.9 |
| 4 | 10 | 4 | 0 | 2.5 | 10 | 72 | 1 | 8.4 |
| 5 | 4 | 90 | 0 | 0 | 10 | 72 | 50 | 10.4 |
| 6 | 10 | 4 | 10 | 0 | 0 | 72 | 50 | 9.5 |
| 7 | 10 | 90 | 0 | 2.5 | 0 | 1 | 50 | 24.5 |
| 8 | 4 | 4 | 0 | 0 | 0 | 1 | 1 | 3.4 |
| 9 | 7 | 47 | 5 | 1.25 | 5 | 36.5 | 25 | 12.3 |
| 10 | 7 | 47 | 5 | 1.25 | 5 | 36.5 | 25 | 12.2 |
| 11 | 7 | 47 | 5 | 1.25 | 5 | 36.5 | 25 | 12.6 |
| 12 | 7 | 47 | 5 | 1.25 | 5 | 36.5 | 25 | 12.4 |
2.4.2. Interaction study
After the screening, the four most influential factors were retained to look for possible interactions. The interaction study was carried out using full factorial design. The levels of each factor were the same as presented in the screening study. Four runs at the center of the domain were also performed for repeatability testing.
2.4.3. Determination of optimum phthalate migration conditions
The three most influential factors taken from the full factorial design are then used in the face-centered composite design, in order to obtain the optimum conditions for phthalates migration from paint buckets to food. A quadratic model was estimated and validated.
2.4.4. Migration kinetics of phthalates from paint buckets
The study of the migration kinetics of phthalates from paint buckets to pap was carried out according to two procedures, in order to understand the transfer mechanism. In the first procedure, a 150 mL glass bottles containing 100 mL of pap were placed in water bath at the set temperature (50, 70 and 90 °C). When the temperature of the pap reached that of the set, eight pieces of buckets (see Section 2.3) were introduced, the bottles were closed with the lids, then the stopwatch was started. In the second procedure called “in real situation” (RS), the pap was prepared in a large aluminum pot using 1 g of corn starch and 10 L of ultrapure water. The resulting pap at 95 ± 2 °C was poured into paint buckets. The latter were closed, and after the set time, pap was sampled and used for phthalates analysis.
The kinetic study was carried out for 180 min (3 h). The set times were 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 105, 120, 135, 150, 165 and 180 min. Time t = 0 min was set when the pap is still in aluminum pot. The temperature of pap was recorded at each set time in RS. The trials were performed in duplicate. The concentrations of total phthalates obtained after UPLC-MS/MS were used to calculate the parameters of the pseudo-first order, pseudo-second order and intraparticle diffusion models. The two first models were described in Eqs. (1), (2) respectively by Langengren (1898) cited by Ref. [33]. The intraparticle diffusion model was described in Equation (3) [34].
-
❖
Pseudo-first order model
| (1) |
-
❖
Pseudo-second order model
| (2) |
-
❖
Intraparticle diffusion model
| (3) |
where: qt and qe are the amounts of phthalate in pap at time t and at equilibrium respectively. k1 (min−1), k2 (L μg min−1) and k (L μg min−1) are the rate constants of the pseudo-first order, pseudo-second order and intraparticle diffusion models. C is a constant.
2.4.5. Validation of models
Validation of models is done through the calculation of absolute average deviation (AAD), adjusted coefficient of determination (R2aj), bias factor (Bf) and exactitude factor (Af1) according to Eqs. (4), (5), (6), (7) respectively [28].
| (4) |
| (5) |
| (6) |
| (7) |
where Yexp is the experimental value; Ycal is the calculated value; n is the number of experiments. These parameters have to fulfill the following conditions [28]:
-
❖
AAD has to be as close to zero as possible
-
❖
R2 aj has to be greater than 0.85
-
❖
Bf and Af1 have to be between 0.75 and 1.2.
2.5. Analysis of phthalates
Before analysis, the glassware was cleared of contamination. It was rinsed with methanol-water (50/50) and shook in an ultrasonic tank for 30 min. Then it was rinsed with ultrapure water. Finally, the glassware was heated for 2 h at 400 °C.
2.5.1. Hydrolysis and extraction
The method was based to alkaline hydrolysis of phthalates and liquid extraction with ethylacetate [13]. Briefly, 10 mL of liquid sample and about 2 μg of internal standard (250 μL of 20 μM DEHP-d4 in methanol) were put in 50 mL test tube. Then few drops to 1 mL of 2 N NaOH was added in the mixture to reach pH 12. The tubes were heated in a water bath at 90 °C for 30 min. Then, they were removed and left to stand for 15 min at room temperature, before adding 2 N HCl (a few drops to 2 mL) so that the pH became close to 2. This acidification was the end of the transformation of PAEs into phthalic acid. The resulting phthalic acid was extracted though liquid-liquid extraction with ethyl acetate. In the previous 50 mL test tube, 2 mL of ethyl acetate was added and the mixture was subjected to 10 s of vortex for homogenization. Then the polar phase was collected using 1000 μL micropipette, and transferred to 25 mL test tube. Extraction with ethyl acetate was repeated a second time and the polar phase was added to the same 25 mL tube. The collected extract was then ready for derivatization. The extraction recovery was determined for pap by spiking with the mix of PAEs at concentration of 1, 10 and 100 μg/L. The tests were repeated five times at each level of concentration. The amount of total PAEs in pap before and after spiking was recorded. The recovery corresponds to the ratio (%) of the difference between the two amounts and the amount of total PAEs in spiking standard solution.
2.5.2. Derivatization
The obtained phthalic acid was derivatized with (3-bromopropyl) trimethylammonium bromide (BPTAB) as described by Ref. [35]. After derivatization, 1.5 mL of the water/acetonitrile/formic acid mixture (89.9/10/0.1) was added in a vial tube before UPLC-MS/MS analysis. The mix phthalate solutions at different concentrations followed the same steps as samples to get the calibration curve. Each solution and each sample, contained an internal standard (DEHP-d4). The ratios between the area of derivatized phthates (PA-BPTAB) and that of internal standard (PA-BPTAB-d4) versus concentration of total phthalates allowed to draw the calibration curve. Pap was spiked with a mixed solution of phthalates at three concentration levels to determine the extraction recovery. Before analysis, the glassware was rinsed with methanol-water (50/50), then with ultrapure water. Finally, the glassware was heated at 400 °C for 2 h.
2.5.3. UPLC-MS/MS analysis
The UPLC-MS/MS method for quantification of total phthalates via PA-BPTAB took place in two steps, chromatographic separation and detection through tandem mass spectrometry (MS/MS). Chromatographic separation was carried out using a Waters Acquity™ UPLC™ separation module associated with a Waters isolator column (P/N: 186003975). The column was a Waters Acquity UPLC BEH C18 column (2.1 mm × 50 mm; particle size, 1.7 μm). The mobile phase was (A) water and methanol (B) [35]. The flow rate was 0.3 mL min−1 under isocratic conditions. The injection volume was 2 μL and the column oven was maintained at 25 °C. The column was equilibrated for 2 min before the next injection. MS/MS detection and quantification were carried out using a Waters Xevo™ TQD triple-quadrupole mass spectrometer with an Electrospray ionization interface operating in positive ion mode (ESI+). A multiple-reaction monitoring (MRM) was used. The cone gas and desolvation gas was Nitrogen (purity, 99.9%) at a flow rate of 50 L/h and 1200 L/h, respectively. The capillary voltage was 1.5 kV. The electrospray source block and desolvation temperatures were set at 150 °C and 600 °C, respectively. Ultra-high-purity argon was used as the collision gas. Ion energies 1 and 2 were set at −0.5 and 0.2 respectively. The cone voltage and collision energy were optimized for maximum signal intensity, and two transitions were selected from ionization of PA-BPTAB.
Reagent blank was systematically made to ensure that samples were not contaminated by the medium and materials. After injection of ten samples, a reagent blank and a standard were systematically injected in UPLC-MS/MS. The limits of detection (LOD) and quantification (LOQ) were obtained by determining the mean (Yo) and the standard deviation (SDo) of ten blank tests. The LOD was Yo+3*SDo, and the LOQ was Yo+10*SDo.
2.6. Data processing
The acquisition and processing of chromatographic analysis data were carried out with Masslynx software version 4.1, then exported to Excel 2016. The processing of kinetic data was performed with Excel 2016. The R 4.0.1 and Design Expert 13.0 software were used for statistical analysis (analysis of variance and whisker plot) and design modeling respectively.
3. Results and discussion
3.1. Extraction recovery of phthalates in pap
The recovery of total phthalates in pap ranges from 78 to 95%. These values are comparable to those commonly presented by other authors (75–110%) [36,37]. At low doping level (1 μg/L), the recovery is lower with a greater standard deviation, due to experimental error.
The limits of detection (LOD) and quantification (LOQ) of total phthalates analysis were 0.4 μg/L and 0.9 μg/L respectively. The LOD was comparable to that of some authors who studied phthalates migration from different plastics material to food [22,38,8] (Table 6). The method has higher LOD than that of [39] (0.4 > 0.002–0.03 μg/L) (Table 6). However, observation of the phthalate content commonly found in samples or released during migration studies, shows that the LOD of the method is sufficient for this analysis [15,40,41]. Methods with a limit of detection lower or higher than the PAE contents encountered in the samples could imply high dilution or concentration of these, which could increase the uncertainties of the results.
Table 6.
Status of work on the migration of phthalates from plastics to food.
| Type of material | Type of polymer | Matrice | Number of PAEs studied | Method of analysis | LOD (μg/L) | Factors | Range/Value | Study of factor interactions ? | Kinetic Modeling | Reference |
|---|---|---|---|---|---|---|---|---|---|---|
| Plastic paint bucket | PP | Pap | Total | UPLC-MS/MS | 0.4 | Temperature Contact time pH %Sucrose %NaCl Starch (g/L) Number of washes |
4–90 °C 1–72 h 4–10 0–10% 0–5% 0–10 1–50 |
Yes | Second order kinetic model | This work |
| Wrap film | PP | Food simulants | 16 | GC-MS | 0.1 | Temperature Contact time Microwave conditions Type of simulant |
−18–40 °C 1–10 days 100–720 w Distillated water 3% acetic acid 10% ethanol Isooctane n-hexane |
No | First order kinetic model | [22] |
| Lunch boxes | PP | Food simulants | 16 | GC-MS | 2 | Temperature Contact time Type of simulant |
4–60 °C 1–50 h 1–3 days Distillated water 3% acetic acid 10% ethanol Isooctane n-hexane |
No | First order kinetic model | [21] |
| Bottles | PET | 3% acetic acid | 01 | GC-MS | Not mentioned | Temperature Contact time |
25–40 °C 1–120 days |
No | Fick's second law | [23] |
| Films | PVC | Fatty food simulants | 05 | GC-FID | Not mentioned | Temperature Contact time Type of simulant |
20–60 °C 1–10 days n-nexane Isooctane 95% ethanol |
No | Fick's second law | [16] |
| Plastic containers | PET | Pickled vegetables | 06 | GC-MS | 120–190 | Temperature Storage time pH Sun light exposure |
/ 1–365 days 4–6 / |
No | Exponential fit | [42] |
| Barreled water and bottled water | PC and PET | Water | 06 | GC-MS | 0.2 | Temperature Storage time |
25–60 °C 1–40 days 1–7 days |
No | Exponential fit | (C [38]. |
| Plastic containers | PET | Acidic juice | 06 | GC-MS | 0.0011 to 0.0023 | Temperature Storage time pH Sun light exposure |
/ 1–365 days 2–4 / |
No | Exponential fit | [39] |
| Plastic packaging | PVDC | Food simulants | 01 | GC-MS | Not mentioned | Temperature Contact time Type of simulant |
25–80 °C 2–10 h Heptane Ethanol Distillated water |
No | Artificial neural network | [48] |
| Plastic containers | PP | Water | 02 | GC-MS | 0.08–0.31 | Time of use Microwave conditions |
New – Old 210–700 w |
No | / | [8] |
| Plastic packaging | Not mentioned | Convenience foods | 18 | GC-MS | Not mentioned | Temperature Contact time |
−4.3–25.1 1–150 days |
No | / | [17] |
| Bags, films, Bottles and containers | PP | Food simulants | 4 | GC-MS | Not mentioned | Temperature Contact time Type of simulant |
5–80 °C 0.5–4 h Distillated water Olive oil |
No | / | [20] |
PC: Polycarbonate; PP: Polypropylene; PET: Polyethyle terephthalate; PVDC: Poly(vinylidene chloride); PVC: Polyvinyl chloride. GC-MS: Gas chromatography- Mass spectrometry; GC-FID: Gas chromatography with flame ionization detector; UPLC-MS/MS: Ultra high performance liquid chromatography - tandem mass spectrometry.
3.2. Effects of factors
Table 1 shows the test matrices of the screening design. The validation criteria reported in Table 2 conform to the reference values. The estimated equation of the model is:
| PA-BPTAB = 14.0 + 7.26A + 7.79B + 3.16C-2.49D + 3.09E- 5.36F-2 G | (8) |
Where A is the pH, B the temperature, C the amount of starch, D he amount of salt, E the amount of sucrose, F the contact time, and G the number of washes. These are the coded factors.
Table 2.
Validation of models.
| Plackett-Burman design | Full factoriel design | Face-centered composite design | Validation criteria | |
|---|---|---|---|---|
| Ajusted R2 | 0.963 | 0.981 | 0.989 | >0.850 |
| AAD | 0.012 | 0.006 | 0.072 | ≈0.0 |
| Bias (Bf) | 0.973 | 0.993 | 0.982 | 0.75–1.2 |
| Exactitude (Af) | 1.119 | 1.028 | 1.058 | 0.75–1.2 |
Eq. (8) shows that temperature and pH are the most important factors (and equal values) and increase the migration of total phthalates. These factors are followed in importance by the contact time, but the latter has a negative contribution. The contribution of sucrose is half of the first two factors. The number of washes and the salt content seem to be the least important factors. Several authors show that temperature and contact time increase the migration of phthalates to food [39,15,31]. In fact, when the temperature increases, the free energy of the high-energy molecular segment in polypropylene is sufficient to enhance the mobility of PAEs which are low molecular weight substances [21]. Other studies showed that the storage time was not affect the concentration levels of PAEs in food [27].
The effect of pH is contradictory from one author to another [42]. showed that the extent of PAEs’ migration was greater under acidic conditions and occurred faster. For their part [43], noticed that the lowest levels of phthalates were found in the least acidic juice [44]. showed that some phthalates are usually degraded more than 20% by hydrolysis under acidic and high temperature conditions.
The starch and sucrose contents are part of the influence on the nature of the food [22]. observed that foods with low polarity are more likely to cause additional swelling, permeation and diffusion in the polyethylene film upon contact with each other and affected the migration of PAEs [16]. revealed that fatty food simulants had no impact on the migration rate at 60 °C. But at 20 and 40 °C, more the food simulant are non polar, more the migration rate is higher [17]. showed that under the same conditions, with increasing time, PAEs were prone to migrate into foods rich in fat. Thus, the effect of the nature of food depends on temperature or contact time.
Eq. (8) shows that high NaCl content decreases the amount of migrated PAEs. On the contrary [45], revealed that the migration of PAEs increased with NaCl content. Concerning the number of washes, Eq. (8) shows that it is one of the least influential factors, and when it increases the amount of total migrated PAEs decreases. However, other authors showed that the successive washing of plastic containers contributes to decrease the content of released additives [24,25]. Indeed, during the first washes, a high amount of additives is released, then it decreases as the plastic container is washed, due to the depolymerisation of the polymer [26].
The contributions of the factors to the direction of the evolution of migration as presented in Eq. (8) are not definitive. Indeed, the differences between the factors are not very important, and there may be interactions. The probable presence of the interactions may explain the contradictory conclusion found in the literature.
3.3. Interactions between the most influential factors
Table 3 presents the test matrices of full factorial design. The validation coefficients in Table 2 show that the data obtained are valid. Eq. (9) corresponds to the model with order 2 interactions obtained from the full factorial design.
| (9) |
where A is the pH, B the temperature, C the contact time and D the starch content. These are the coded factors.
Table 3.
Test matrices of the full factorial design and the face-centered composite design.
| Factor 1 |
Factor 2 |
Factor 3 |
Factor 4 |
Responses |
|
|---|---|---|---|---|---|
| Test matrix of the full factorial design | |||||
| N° | A:pH | B:Temperature (°C) | C: Contact time (h) | D:Starch (g/L) | PA-BPTAB (μg/L) |
| 1 | 10 | 4 | 72 | 0 | 22.0 |
| 2 | 10 | 4 | 1 | 0 | 17.2 |
| 3 | 10 | 90 | 72 | 0 | 28.7 |
| 4 | 4 | 4 | 72 | 0 | 46.3 |
| 5 | 10 | 90 | 72 | 10 | 21.4 |
| 6 | 4 | 4 | 72 | 10 | 45.9 |
| 7 | 4 | 90 | 1 | 10 | 49.2 |
| 8 | 4 | 4 | 1 | 10 | 18.9 |
| 9 | 10 | 90 | 1 | 10 | 31.8 |
| 10 | 10 | 4 | 1 | 10 | 21.9 |
| 11 | 4 | 90 | 72 | 10 | 62.1 |
| 12 | 10 | 90 | 1 | 0 | 25.2 |
| 13 | 4 | 4 | 1 | 0 | 3.3 |
| 14 | 10 | 4 | 72 | 10 | 9.9 |
| 15 | 4 | 90 | 72 | 0 | 63.9 |
| 16 | 4 | 90 | 1 | 0 | 20.1 |
| 17 | 7 | 47 | 36.5 | 5 | 28.5 |
| 18 | 7 | 47 | 36.5 | 5 | 28.9 |
| 19 | 7 | 47 | 36.5 | 5 | 29.0 |
| 20 |
7 |
47 |
36.5 |
5 |
28.2 |
| Test matrix of the face-centered composite design | |||||
| N° |
A: pH |
B:Temperature (°C) |
C: Contact time (h) |
PA-BPTAB (μg/L) |
|
| 1 | 7 | 47 | 36.5 | 9.3 | |
| 2 | 7 | 47 | 72 | 29.6 | |
| 3 | 7 | 90 | 36.5 | 25.2 | |
| 4 | 4 | 4 | 72 | 14.5 | |
| 5 | 4 | 90 | 1 | 31.7 | |
| 6 | 10 | 4 | 72 | 23.9 | |
| 7 | 4 | 90 | 72 | 60,8 | |
| 8 | 4 | 47 | 36.5 | 15.0 | |
| 9 | 7 | 4 | 36.5 | 1.3 | |
| 10 | 7 | 47 | 36.5 | 9.3 | |
| 11 | 10 | 47 | 36.5 | 15.5 | |
| 12 | 7 | 47 | 1 | 8.5 | |
| 13 | 10 | 90 | 72 | 60.5 | |
| 14 | 10 | 90 | 1 | 20.4 | |
| 15 | 4 | 4 | 1 | 10.4 | |
| 16 | 7 | 47 | 36.5 | 9.1 | |
| 17 | 10 | 4 | 1 | 8.4 | |
There are interactions between pH, temperature, contact time and nature of the food (Table 4). The effects of the factors taken two by two are globally significant (p < 0.05). Therefore, the migration of phthalates from paint buckets to food cannot be explained by studying only the main effects of each of these factors. This results is similar to those of [17] who remarked that temperature and time influenced the migration simultaneously and it was difficult to separate these two variables in the daily life situation.
Table 4.
Effects and interactions of the most influential factors.
| Factors | Effects of the factors | Estimated coefficients | Standard error | P-value |
|---|---|---|---|---|
| A-pH | −16.44 | −8.22 | 0.4186 | <0.0001 |
| B-Temperature | 14.62 | 7.32 | 0.4186 | <0.0001 |
| C-time | 14.08 | 7.04 | 0.4186 | <0.0001 |
| D-Starch | 4.30 | 2.15 | 0.4186 | 0.0068 |
| AB | −5.60 | −2.80 | 0.4186 | 0.0026 |
| AC | −17.60 | −8.80 | 0.4186 | <0,0001 |
| AD | −6.32 | −3.16 | 0.4186 | 0.0016 |
| BC | −1.62 | −0.81 | 0.4186 | 0.1242 |
| BD | 2.34 | 1.17 | 0.4186 | 0.0485 |
| CD | −9.70 | −4.85 | 0.4186 | 0.0003 |
| ABC | 1.70 | 0.85 | 0.4186 | 0.1121 |
| ABD | −0.68 | −0.34 | 0.4186 | 0.4653 |
| ACD | 2.02 | 1.01 | 0.4186 | 0.0729 |
| BCD | −1.50 | −0.75 | 0.4186 | 0.1477 |
Otherwise, Table 4 shows that for pH and contact time, the side effect related to their interaction is higher than that of each of them taken individually. Thus, the effect of pH cannot be interpreted without taking into account the contact time and vice versa [39]. The effects of third-order interactions (ABC and ABD) are not significant (p > 0.05). When modeling the migration phenomenon, they may not be considered. High values of second-order interactions show how fundamental it is to add them to the model.
3.4. Conditions for the migration of phthalates from paint bucket to food
Table 3 shows the test matrices of the face-centered composite design. The validation criteria are standard, therefore the model can be considered as valid (Table 2). The estimated quadratic model is:
| (10) |
where A is the pH, B the temperature and C the contact time. These are the coded factors.
Eq. (10) shows the dependence on the quadratic effect of pH. The effect of pH is therefore non linear. On the other hand, the main effects of contact time and temperature are very high. The interaction coefficients are high, it is the same for the quadratic effects. The variation of the response as a function of temperature or contact time is therefore not always linear. This is in agreement with the results of the full factorial design. The variation of the response as a function of the different factors can be represented on the response surface curves (data not shown). The response surface curves are often difficult to interpret, that is why isoresponse curves are preferred. In the latter case, a factor is set and the variation of the response is drawn as a function of two other factors.
Fig. 2 (A to F) shows the isoresponse curves of the variation of total phthalate content as a function of pH, temperature and time. Fig. 2 (A and B) shows that the effect of temperature on the migration of phthalates from paint buckets is greater at extreme pH (pH < 4 and pH > 10). Similar results were obtained by Ref. [46]. They reported that the rates of phthalates degradation were higher at both limits of pH range, i.e. ≥10 and ≤ 3. Phthatales are therefore more easily removed from the structure of containers at these two pH values. In fact, at extreme pH, temperature increases molecular agitation by weakening the polypropylene structure. This allows acidic food to come in contact with plastic formulation additives such as fillers (kaolin, talc or carbonate calcium etc) and destroys them [4]. Basic food may affect UV stabilizers or antioxidants. Amines and phenols are the most used chemical compounds as UV stabilizers [47]. At temperatures below 4 °C, the migration of phthalates is very low.
Fig. 2.
Isoresponse curves of temperature versus pH at times (A) 35.5 h and (B) 72 h; isoresponse curves of time versus pH at temperatures (C) 4 °C and (D) 90 °C; isoresponse curves of time versus temperature at pHs (E) 4 and (F) 7.
Otherwise, Fig. 2 (C and D) shows the isoresponse curves of the effect of contact time on the migration of phthalates from paint buckets to food at different values of pH. Like temperature, contact time increases the migration of phthalates from buckets to food at extreme pH [39]. observed that storage time increased the migration of phthates from bottles to acidic fruit juices (vinegars, orange juice, etc). They showed that migration of PAEs increases with juice's acidity [42]. also revealed that the extent of PAEs' migration was greater under acidic conditions and occurred faster. This finding points out the stimulating effect of an acidic medium on PAEs' migration from plastic containers.
At neutral pH (pH = 7), the effect of temperature remains high, but less than what is observed at extreme pH, that is due to the interactions between these two factors (Fig. 2A and B). Fig. 2 (E and F) shows that when pH is neutral, migration is lower in the first moments (≤1 h) of contact between food and paint bucket. Whatever the contact time (≤72 h), the level of phthalates released from the buckets is higher than 50 μg/L if temperature exceeds 70 °C [22]. showed that the migration of phthalates from lunch boxes stored for 10 days is significantly greater at 60 °C than at 40 °C. The acidic foods promote the hydrolysis of the esters, and the higher the temperature, the faster the rate of hydrolysis [21]. Thus, temperature increase the effect of pH.
Fig. 2 (E and F) shows that at low temperature (≤10 °C), the migration of phthalates is low (≤10 μg/L). The minimum condition for phthalate migration is obtained for neutral pH (pH ~ 7), low temperature (≤4 °C) and a contact time of 1 h. These conditions are not in agreement with the use of paint buckets in the shops of donut/bean/pap in Cameroon [2]. Therefore, it is recommended to no longer use plastic paint buckets for preservation of hot pap. Water at neutral pH can be stored in these buckets for a period not exceeding 51 h (Fig. 2 (E and F)).
3.5. Migration kinetics of phthalates from paint buckets to food
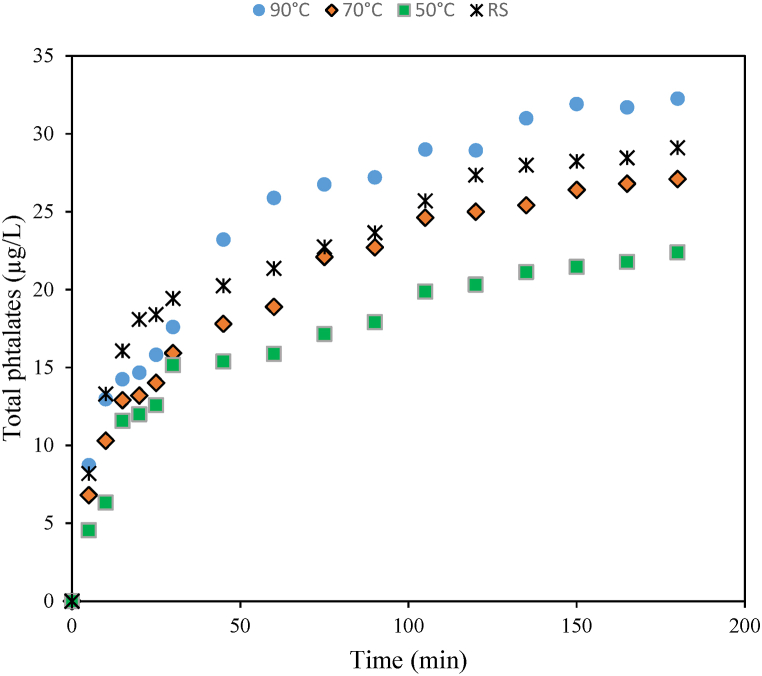
Fig. 3 shows the migration kinetics of phthalates from plastic paint buckets to pap (starch 10 g/L). The phthalate content increases rapidly in hot pap for the first 40 min, then increases very slightly. This high migration during the first minutes is related to phthalates from the surface of the bucket, that diffuse rapidly in contact with food [40]. The low migration speed after 1 h could be related to the structure of the plastic polymer (formulation additives) [31]. observed that the migration of phthalate from PET bottles increases slightly for the first 50 days, then exponentially increases to 365 days. In the present work, the maximum of contact time is 72 h, to get into the conditions of use of the paint buckets in Cameroon households.
Fig. 3.
Migration kinetic of total phthalates from plastic paint buckets to pap at different temperatures (50, 70 and 90 °C) and different study conditions (Pieces of bucket and whole bucket (RS)).
The results of kinetic study were fitted to the pseudo-first order, pseudo-second order and intra-particle diffusion models. Table 5 summarizes the results obtained for this modeling and the validation criteria. Based on the coefficient of determination R2 and the adjusted R2, the best model is the pseudo-second order, followed by the pseudo-first order. The results of these models are comparable, but the pseudo-second order model gives the best values of the equilibrium concentration. These values at temperature of 50, 70 and 90 °C were 24.8 μg/L, 30.5 μg/L and 36.4 μg/L respectively. The latter are very close to the experimental values, (23 μg/L, 28 μg/L and 33 μg/L respectively), with a maximum bias of 10%, while with the pseudo-first order model, it reaches 24.9%. However the rate constant of the pseudo-second order model decreases with temperature. Therefore, the rate (V≈ k2 [phthalate]2) is more swayed by the initial phthalate concentration in the bucket, than by the rate constant (k2). High temperatures decrease the rate constant, whereas the equilibrium concentration of phthalates increases. It can be deduced that at higher temperature, there is a destruction of the polymer structure. The kinetic study in real situation (pap poured in the bucket), allows to get similar results. The values obtained are close to those of the kinetics at 70 °C (Table 5). The results in real situation therefore confirm those of the kinetic study at different temperatures.
Table 5.
Characteristics of kinetic models.
| Parameters |
50 °C |
70 °C |
90 °C |
RS |
Validation criteria |
|---|---|---|---|---|---|
| Ce (exp) (μg/L) | 23.0 | 28.0 | 33.0 | 30.0 | |
| Pseudo-first order model | |||||
| k1 (min−1) x 102 | 3.800 | 3.938 | 4.352 | 3.685 | |
| Ce (calculated) (μg/L) | 17.27 | 21.74 | 26.19 | 19.87 | ≈ Ce (exp) |
| Ajusted R2 | 0.920 | 0.960 | 0.976 | 0.913 | >0.85 |
| AAD | −0.029 | 0.002 | 0.004 | −0.040 | ≈0.0 |
| BF | 1.016 | 0.996 | 0.995 | 1.003 | 0.75–1.2 |
| Af | 1.102 | 1.041 | 1.038 | 1.068 | 0.75–1.2 |
| Pseudo-second order model | |||||
| k2 (L μg−1 min−1) x103 | 1.586 | 1.264 | 1.077 | 1.689 | |
| Ce (calculated) (μg/L) | 24.81 | 30.49 | 36.36 | 31.25 | ≈ Ce (exp) |
| Ajusted R2 | 0.938 | 0.977 | 0.988 | 0.926 | >0.85 |
| AAD | 0.011 | 0.016 | 0.013 | 0.023 | ≈0.0 |
| BF | 0.985 | 0.979 | 0.980 | 0.972 | 0.75–1.2 |
| Af | 1.067 | 1.071 | 1.076 | 1.081 | 0.75–1.2 |
| Intra-particle diffusion model | |||||
| kind (μg L−1 min−0.5) | 1.333 | 1.668 | 2.012 | 1.501 | |
| C | 4.916 | 5.57 | 6.61 | 9.323 | |
| Ajusted R2 | 0.928 | 0.390 | 0.923 | 0.946 | >0.85 |
| AAD | −0.023 | 0.006 | 0.005 | −0.002 | ≈0.0 |
| BF | 1.004 | 0.989 | 0.990 | 0.993 | 0.75–1.2 |
| Af | 1.122 | 1.063 | 1.066 | 1.073 | 0.75–1.2 |
Ce (exp): equilibrium concentration obtained during experiment; Ce (Calculated): equilibrium concentration calculated by the model; RS: Real situation.
Table 6 presents the status of work carried out on the migration of phthalates from plastic materials to food. The most influential factors of phthalate's migration from plastics to food are temperature, contact time, pH, nature of food (type of food simulant), and microwave conditions [23,38,21]. The microwave conditions were not used in this study because plastic paint buckets are not heated in the microwave [2]. In this work, the influence of starch content, %NaCl and % sucrose were studies based to the real composition of food studied (pap) [32]. Likewise, the influence of the number of washes was studied based on real use of plastic paint bucket in households and donut shops [2]. All these added factors allowed to emerge the most influential parameters of the migration of phthalates from plastic containers to food. Before the present work, no study was focused on the interaction between the main factors that affect the migration of PAEs.
Otherwise, Table 6 shows that the number of PAEs studied in the literature ranged from 1 to 18 [38,17]. In the present work, a LC-MS/MS method was developed to study total phthalates after derivatization. The study of individual migration of phthalates is fastidious since there is more than 50 phthalates. The study of total phthalates is based to the fact that all phthalates have similar chemical structure, so they are all potentially dangerous.
Four types of phthalate migration modeling are recorded in the literature (Table 6). Some authors showed that the best model was exponential fit [[38], [39], [42]]. This modeling is used when the storage time is long (40–365 days) such as canned food or bottled food. When pap or water is stored in re-used plastic paint buckets, the storage time does not exceed 3 h or 7 day respectively [2]. Otherwise, other authors used Fick's second law equation or the artificial neural network model to describe the migration of PAEs from plastic to food [23,16,38]. However, this equation may not described the migration that occur in more than one step. This is the case when the material is thick, the migration implies a diffusion of the migrating compound into the material, before its contact and diffusion through food. Moreover, the exponential fit model, the Fick's equation and the artificial neural network model could predict the amount of migrated PAEs, but may not describe the mechanism of transfer of PAEs like reaction order models.
In the same trend, the results obtained in this work are different from those of other authors who observed that the migration kinetic of phthalates is best described by pseudo-first order model [22,21]. The difference is related to the nature of plastic material. The paint buckets have a greater thickness than the lunch boxes or wrap film (Table 6). In fact, the pseudo-first order model is commonly observed when migration occurs mainly through diffusion from the interface; While the pseudo-second order model is based on the assumption that the rate limiting step is reaction is chemical desorption of the migrant, that may occurs if the plastic materials is thick [49].
4. Study limitation
This work studied the migration of phthalates from plastic paint buckets to food. The polypropylene that constitutes the polymer of these buckets was not characterized, in order to correlate the increase in the content of PAEs into the pap with the content of PAEs that would leave the material (polymer of the paint plastic buckets).
5. Conclusion
The research work described in the present paper is the first attempt to study the mechanism and the conditions of the migration of phthalates from non-food containers to food. For this purpose, the migration of total phthalates from plastic paint buckets to pap was investigated though experimental design modeling and kinetic study. The screening of factors via Plackett-burman design showed that the three most influential factors of the migration are pH, temperature and contact time. The full factorial design revealed that the effects of these factors are not linear, and therefore, it is necessary to take into account their interactions to understand the migration of phthalates from plastic paint buckets to liquid food. The minimum conditions for phthalates migration from these buckets to food ware defined at neutral pH, a contact time ≤ 1 h, and a temperature ≤4 °C. These conditions are different from the actual use of plastic paint buckets for hot pap preservation in donut shops of Cameroon. The study shows that above 70 °C, at pH ≤ 4 or pH ≤ 10, for a contact time of more than 2 h, the amount of phthalates released becomes greater than 50 μg/L. Therefore, it is recommended to avoid the preservation of hot food and acidic or alkaline food in these containers. The kinetic study revealed that the migration of total phthalates from plastic paint buckets to pap is best described by a pseudo-second order model. The future works should focus on determining the composition and structure of non-food containers during phthalate migration in order to better understand their transfer mechanism.
Author contribution statement
Olivier SONGUE SAME: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Pierre NOBOSSE; Guillaume Legrand NGOLONG NGEA; Mohamed LEMDANI: Analyzed and interpreted the data; Wrote the paper.
Catherine PIVETEAU: Performed the experiments.
Richard KAMGA: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Benoît DEPREZ: Conceived and designed the experiments; Interpreted the data and wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to thank Centre Pasteur of Cameroon, especially Dr. Mirdad KAZANJI, Dr. Sara EYANGOH and entire team of Hygiene and Environment department (SHEpc). Our thanks also go to the French Embassy in Cameroon that funded mobility to France through SCAC grant. Institute Pasteur of Lille and ADME platform of U1177 for the providing access to analytical equipment.
References
- 1.Marsh K., Bugusu B. Food Packaging?Roles, materials, and environmental issues. J. Food Sci. 2007;72(3) doi: 10.1111/j.1750-3841.2007.00301.x. R39-R55. [DOI] [PubMed] [Google Scholar]
- 2.Songue S.O., Catherine P., Alexandre B., Richard K., Benoit D. Evaluation of the exposure to bisphenols from baby bottles and non-food containers used for food preservation in Cameroon. Journal of Hazardous Materials Advances. 2023;9 doi: 10.1016/j.hazadv.2022.100212. [DOI] [Google Scholar]
- 3.Pouokam G.B., Ajaezi G.C., Mantovani A., Orisakwe O.E., Frazzoli C. Use of Bisphenol A-containing baby bottles in Cameroon and Nigeria and possible risk management and mitigation measures : community as milestone for prevention. Sci. Total Environ. 2014;481:296–302. doi: 10.1016/j.scitotenv.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 4.Hahladakis J.N., Velis C.A., Weber R., Iacovidou E., Purnell P. An overview of chemical additives present in plastics : migration, release, fate and environmental impact during their use, disposal and recycling. J. Hazard Mater. 2018;344:179–199. doi: 10.1016/j.jhazmat.2017.10.014. [DOI] [PubMed] [Google Scholar]
- 5.Giuliani A., Zuccarini M., Cichelli A., Khan H., Reale M. Critical review on the presence of phthalates in food and evidence of their biological impact. Int. J. Environ. Res. Publ. Health. 2020;17(16):5655. doi: 10.3390/ijerph17165655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson K., Lindh C.H., Jönsson B.A., Giovanoulis G., Bibi M., Bottai M., Bergström A., Berglund M. Phthalates, non-phthalate plasticizers and bisphenols in Swedish preschool dust in relation to children's exposure. Environ. Int. 2017;102:114–124. doi: 10.1016/j.envint.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 7.Meeker J.D., Sathyanarayana S., Swan S.H. Phthalates and other additives in plastics : human exposure and associated health outcomes. Phil. Trans. Biol. Sci. 2009;364(1526):2097–2113. doi: 10.1098/rstb.2008.0268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moreira M., André L., Cardeal Z. Analysis of phthalate migration to food simulants in plastic containers during microwave operations. Int. J. Environ. Res. Publ. Health. 2013;11(1):507–526. doi: 10.3390/ijerph110100507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fierens T., Van Holderbeke M., Willems H., De Henauw S., Sioen I. Phthalates in Belgian cow's milk and the role of feed and other contamination pathways at farm level. Food Chem. Toxicol. 2012;50(8):2945–2953. doi: 10.1016/j.fct.2012.05.036. [DOI] [PubMed] [Google Scholar]
- 10.Dobaradaran S., Akhbarizadeh R., Javad Mohammadi M., Izadi A., Keshtkar M., Tangestani M., Moazzen M., Shariatifar N., Mahmoodi M. Determination of phthalates in bottled milk by a modified nano adsorbent : presence, effects of fat and storage time, and implications for human health. Microchem. J. 2020;159 doi: 10.1016/j.microc.2020.105516. [DOI] [Google Scholar]
- 11.European Food Safety Authorities Opinion of the scientific panel on food additives, flavourings, processing aids and material in contact with food (AFC) on a request from the commission related to di-butylphthalate (DBP) for use in food contact materials. EFSA J. 2005;242:1–17. http://www.efsa.europa.eu/en/efsajournal/doc/242.pdf [Google Scholar]
- 12.European Food Safety Authorities Opinion of the scientific panel on food additives, flavourings, processing aids and materials in contact with food (AFC) on a request from the commission related to bis(2-ethylhexyl) phthalate (DEHP) for use in food contact materials. EFSA J. 2005;243:1–20. www.efsa.europa.eu/en/efsa journal/doc/243.pdf [Google Scholar]
- 13.Kim Min-Seon, Shim, Won Joon O., Jae-Ryong, Park Jongman. Simultaneous gas chromatography-mass spectrometric determination of total and individual phthalic esters utilizing alkaline hydrolysis and silyl derivatization technique. Bull. Kor. Chem. Soc. 2007;28(3):432–438. doi: 10.5012/BKCS.2007.28.3.432. [DOI] [Google Scholar]
- 14.Xie Q., Sun D., Han Y., Jia L., Hou B., Liu S., Li D. Determination of total phthalates in edible oils by high-performance liquid chromatography coupled with photodiode array detection : liquid Chromatography. J. Separ. Sci. 2016;39(5):857–863. doi: 10.1002/jssc.201501169. [DOI] [PubMed] [Google Scholar]
- 15.Zaater M.F., Tahboub Y.R., Al Sayyed A.N. Determination of phthalates in Jordanian bottled water using GC–MS and HPLC–UV : environmental study. Journal of Chromatographic Science. 2014;52(5):447–452. doi: 10.1093/chromsci/bmt059. [DOI] [PubMed] [Google Scholar]
- 16.Yuan H., Hao Q., Su R., Qi W., He Z. Migration of phthalates from polyvinyl chloride film to fatty food simulants : experimental studies and model application. Journal of Consumer Protection and Food Safety. 2020;15(2):135–143. doi: 10.1007/s00003-019-01249-x. [DOI] [Google Scholar]
- 17.Yang J., Song W., Wang X., Li Y., Sun J., Gong W., Sun C. Migration of phthalates from plastic packages to convenience foods and its cumulative health risk assessments. Food Addit. Contam. B. 2019;12(3):151–158. doi: 10.1080/19393210.2019.1574909. [DOI] [PubMed] [Google Scholar]
- 18.Fang H., Wang J., Lynch R.A. Migration of di(2-ethylhexyl)phthalate (DEHP) and di-n-butylphthalate (DBP) from polypropylene food containers. Food Control. 2017;73:1298–1302. doi: 10.1016/j.foodcont.2016.10.050. [DOI] [Google Scholar]
- 19.Fei F., Liu Z., Chen Q., Liu F. Kinetic migration of diethylhexyl phthalate in functional PVC films. Plasma Sci. Technol. 2012;14(2):152–156. doi: 10.1088/1009-0630/14/2/13. [DOI] [Google Scholar]
- 20.Ayamba A.A.-M., Agyekum A.A., Derick C., Dontoh D. Assessment of phthalate migration in polyethylene food contact materials sold on the Ghanaian market. Cogent Environmental Science. 2020;6(1) doi: 10.1080/23311843.2020.1794242. [DOI] [Google Scholar]
- 21.Liu J., Li C., Yang F., Zhao N., Lv S., Liu J., Chen L., He Z., Zhang Y., Wang S. Assessment of migration regularity of phthalates from food packaging materials. Food Sci. Nutr. 2020;8(10):5738–5747. doi: 10.1002/fsn3.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu J., Li C., Zhao N., Wang Z., Lv S., Liu J., Chen L., Wang J., Zhang Y., Wang S. Migration regularity of phthalates in polyethylene wrap film of food packaging. J. Food Sci. 2020;85(7):2105–2113. doi: 10.1111/1750-3841.15181. [DOI] [PubMed] [Google Scholar]
- 23.Farhoodi M., Emam-Djomeh Z., Reza Ehsani M., Onomiehie A. Effect of environmental conditions on the migration of Di(2-Ethylhexyl)phthalate) from PET bottles into yagurt drinks : influence of time, temperature and food simulant. Arabian J. Sci. Eng. 2008:279–287. [Google Scholar]
- 24.Maragou N.C., Makri A., Lampi E.N., Thomaidis N.S., Koupparis M.A. Migration of bisphenol A from polycarbonate baby bottles under real use conditions. Food Addit. Contam. 2008;25(3):373–383. doi: 10.1080/02652030701509998. [DOI] [PubMed] [Google Scholar]
- 25.Mostafa A.R., Soliman E.A., Mohy El-Din M.S., Abd El-Naeem G. 2012. Survey of Bispheniol A in Infant Feeding Baby Bottles in the Local Market of egypt.http://www.researchgate.net/publication/259873145 [Google Scholar]
- 26.Brede C., Fjeldal P., Skjevrak I., Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003;20(7):684–689. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 27.Moazzen M., Mahvi A.H., Shariatifar N., Jahed Khaniki G., Nazmara S., Alimohammadi M., Ahmadkhaniha R., Rastkari N., Ahmadloo M., Akbarzadeh A., Dobaradaran S., Norouzian Baghani A. Determination of phthalate acid esters (PAEs) in carbonated soft drinks with MSPE/GC–MS method. Toxin Rev. 2018;37(4):319–326. doi: 10.1080/15569543.2017.1378234. [DOI] [Google Scholar]
- 28.Inna S., Alvine E.E.C., Henriette A.Z., Joseph S. Enhancing of copper (II) adsorption efficiency by mixing plantain and orange peelings as bio-sorbent support. Am. J. Appl. Sci. 2020;17(1):218–230. doi: 10.3844/ajassp.2020.218.230. [DOI] [Google Scholar]
- 29.Bhunia K., Sablani S.S., Tang J., Rasco B. Migration of chemical compounds from packaging polymers during microwave, conventional heat treatment, and storage : migration of chemical compounds. Compr. Rev. Food Sci. Food Saf. 2013;12(5):523–545. doi: 10.1111/1541-4337.12028. [DOI] [PubMed] [Google Scholar]
- 30.Starker C., Welle F. Migration of bisphenol A from can coatings into beverages at the end of shelf life compared to regulated test conditions. Beverages. 2019;5(1):3. doi: 10.3390/beverages5010003. [DOI] [Google Scholar]
- 31.Luo Q., Liu Z., Yin H., Dang Z., Wu P., Zhu N., Lin Z., Liu Y. Migration and potential risk of trace phthalates in bottled water : a global situation. Water Res. 2018;147:362–372. doi: 10.1016/j.watres.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 32.Ponka R., Bouba A., Fokou E., Beaucher E., Piot M., Leonil J., Gaucheron F. Nutritional composition of five varieties of pap commonly consumed in maroua (far- north, Cameroon) Pol. J. Food Nutr. Sci. 2015;65(3):183–190. doi: 10.1515/pjfns-2015-0016. [DOI] [Google Scholar]
- 33.Belaid K.D., Kacha S. Étude cinétique et thermodynamique de l’adsorption d’un colorant basique sur la sciure de bois. Rev. Sci. Eau. 2011;24(2):131–144. doi: 10.7202/1006107ar. [DOI] [Google Scholar]
- 34.Ho Y.S., McKay G. Pseudo-second order model for sorption processes. Process Biochemistry. 1999;34(5):451–465. doi: 10.1016/S0032-9592(98)00112-5. [DOI] [Google Scholar]
- 35.Gao G., Chen H., Chai Y., Jin L., Liu X., Lu C. A method based on precolumn derivatization and ultra high performance liquid chromatography with high-resolution mass spectrometry for the simultaneous determination of phthalimide and phthalic acid in tea. J. Separ. Sci. 2019;42(7):1304–1311. doi: 10.1002/jssc.201801128. [DOI] [PubMed] [Google Scholar]
- 36.Ibrahim N., Osman R., Abdullah A., Saim N. Determination of phthalate plasticisers in palm oil using online solid phase extraction-liquid chromatography (SPE-LC) J. Chem. 2014;1–9 doi: 10.1155/2014/682975. 2014. [DOI] [Google Scholar]
- 37.Notardonato I., Protano C., Vitali M., Bhattacharya B., Avino P. A method validation for simultaneous determination of phthalates and bisphenol A released from plastic water containers. Appl. Sci. 2019;9(14):2945. doi: 10.3390/app9142945. [DOI] [Google Scholar]
- 38.Wang C., Huang P., Qiu C., Li J., Hu S., Sun L., Bai Y., Gao F., Li C., Liu N., Wang D., Wang S. Occurrence, migration and health risk of phthalates in tap water, barreled water and bottled water in Tianjin, China. J. Hazard Mater. 2021;408 doi: 10.1016/j.jhazmat.2020.124891. [DOI] [PubMed] [Google Scholar]
- 39.Arfaeinia L., Dobaradaran S., Nasrzadeh F., Shamsi S., Poureshgh Y., Arfaeinia H. Phthalate acid esters (PAEs) in highly acidic juice packaged in polyethylene terephthalate (PET) container : occurrence, migration and estrogenic activity-associated risk assessment. Microchem. J. 2020;155 doi: 10.1016/j.microc.2020.104719. [DOI] [Google Scholar]
- 40.Grinbaum M., Camponovo A., Desseigne J.-M., Poupault P., Meisterman E., Chatelet B., Davaux F., Lempereur V. Phthalates : potential sources and control measures. BIO Web of Conferences. 2019;12 doi: 10.1051/bioconf/20191204008. [DOI] [Google Scholar]
- 41.Zhou Q., Fang Z., Liao X. Determination of phthalate esters from environmental water samples by micro-solid-phase extraction using TiO 2 nanotube arrays before high-performance liquid chromatography : sample Preparation. J. Separ. Sci. 2015;38(14):2526–2531. doi: 10.1002/jssc.201500361. [DOI] [PubMed] [Google Scholar]
- 42.Cheshmazar E., Arfaeinia L., Vasseghian Y., Ramavandi B., Moradi M., Hashemi S.E., Asgari E., Arfaeinia H., Dragoi E.-N., Mousavi Khaneghah A. Phthalate acid esters in pickled vegetables packaged in polyethylene terephthalate container : occurrence, migration, and estrogenic activity-associated risk assessment. J. Food Compos. Anal. 2021;99 doi: 10.1016/j.jfca.2021.103880. [DOI] [Google Scholar]
- 43.Rastkari N., Zare Jeddi M. vol. 55. Center for Air Pollution Research, Institute for Environmental Research, Tehran University of Medical Sciences; Tehran, Iran, Ahmadkhaniha, R: 2017. The effect of storage time, temperature and type of packaging on release of phthalate ester into packed acidic juice. Food technology and biotechnology. (Department of Human Ecology, School of Public Health, Tehran University of Medical Sciences, Tehran, Iran). Tehran, Iran, Yunesian, M., Department of Environmental Health Engineering, School of Public Health, Tehran University of Medical Sciences. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Keresztes S., Tatár E., Czégény Z., Záray G., Mihucz V.G. vols. 458–460. Science of The Total Environment; 2013. pp. 451–458. (Study on the Leaching of Phthalates from Polyethylene Terephthalate Bottles into Mineral Water). [DOI] [PubMed] [Google Scholar]
- 45.Sungur S., Koroglu M., Ozkan A. Determinatıon of bisphenol a migrating from canned food and beverages in markets. Food Chem. 2014;142:87–91. doi: 10.1016/j.foodchem.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 46.Lau T.K., Chu W., Graham N. The degradation of endocrine disruptor di-n-butyl phthalate by UV irradiation : a photolysis and product study. Chemosphere. 2005;60(8):1045–1053. doi: 10.1016/j.chemosphere.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 47.Walsh A.N., Reddy C.M., Niles S.F., McKenna A.M., Hansel C.M., Ward C.P. Plastic formulation is an emerging control of its photochemical fate in the ocean. Environmental Science & Technology. 2021;55(18):12383–12392. doi: 10.1021/acs.est.1c02272. [DOI] [PubMed] [Google Scholar]
- 48.Wang X., Song M., Liu S., Wu S., Thu A.M. Analysis of phthalate plasticizer migration from PVDC packaging materials to food simulants using molecular dynamics simulations and artificial neural network. Food Chem. 2020;317:126465. doi: 10.1016/j.foodchem.2020.126465. [DOI] [PubMed] [Google Scholar]
- 49.Sahoo T.R., Prelot B. Nanomaterials for the Detection and Removal of Wastewater Pollutants. Elsevier; 2020. Adsorption processes for the removal of contaminants from wastewater; pp. 161–222. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.