Abstract
Background
Diabetes mellitus is a commonly occurring metabolic disorder accompanied by high morbidity and alarming mortality. Besides various available therapies, induction of pancreatic regeneration has emerged as a promising strategy for alleviating the damaging effect of diabetes. Honey, a potent antioxidative and anti-inflammatory agent, has been reported in the literature archive to exhibit favourable results in the regeneration process of several organ systems.
Design
The current research work was intended to explore the potential role of manuka honey in pancreatic regeneration in alloxan-induced diabetic rats by accessing the pancreatic histology and levels of relevant transcription factors, including MAFA, PDX-1, INS-1, INS-2, NEUROG3, NKX6-1, and NEUROD. An equal number of rats were allocated to all four experimental groups: normal, negative control, positive control, and treatment group. Diabetes was induced in all groups except normal through a single intraperitoneal dose of alloxan monohydrate. No subsequent treatment was given to the negative control group, while the positive control and treatment groups were supplemented with metformin (150 mg/kg/day) and manuka honey (3 g/kg/day), respectively.
Results
Statistical comparison of glucose and insulin levels, oxidative stress indicators, changes in the architecture of pancreatic islets, and expression levels of regeneration-associated transcription factors advocated the potential role of manuka honey in ameliorating the alloxan-induced hyperglycaemia, hyperinsulinemia, oxidative stress, and necrotic changes in islets along with significant upregulation of relevant transcription factors.
Conclusion
This suggests to us the auspicious role of antioxidants in honey in pancreatic regeneration and advocates the favourable role of manuka honey in combating diabetes mellitus.
Keywords: Alloxan, Oxidative stress, Pancreatic regeneration, Manuka honey, Diabetes
1. Introduction
Diabetes mellitus has metamorphosed into a multifactorial and gargantuan health challenge for developing and developed countries. It is defined as a combination of metabolic derangements caused by a problem with insulin secretion, action, or both, and is characterized by increased blood sugar levels for a sustained period resulting in problems with carbohydrate, lipid, and protein metabolism. Its common symptoms include hyperglycaemia, increased thirst, increased urination, increased hunger, slower wound healing, and a host of other phenomena [[1], [2], [3]].
Type 1 diabetes is predominantly linked with insulin shortage and is known to affect 5%–10% of people with diabetes. On the other hand, type 2 diabetes mellitus is linked to metabolic syndrome and insulin insensitivity; islet amyloid presents the typical histological finding in islet cells of patients with this form of diabetes [4]. Inadequate insulin production is a common feature of almost all types of diabetes which in turn depends on the number of functioning beta cells. An insufficient number of functional beta cells is the primary underlying factor in diabetes. Neither insulin administration nor medication can completely revert the impact of diabetes. Therefore, pancreatic regeneration has emerged as a new approach expected to help combat diabetes [[5], [6], [7], [8], [9]]. However, the regenerative capacity of the pancreatic beta cells is slower as compared to other tissues, including blood, skin, gut, etc., that possess well-developed adult stem cell functions [10].
To overcome the limitations of replacement therapy in pancreatic regeneration, there is growing interest in employing alternate approaches that cause restoration of islet mass through endogenous stimulation of regeneration in the pancreatic β-cell [11,12]. Due to their inexpensiveness, high effectiveness, and relatively lesser side effects, nature-oriented products offer a better option to meet the market's need for alternative treatment [13].
Honey constitutes a vast array of antioxidants, primarily including beneficial flavonoids and phenolic acids, which make it an efficient antidiabetic agent. These polyphenols are well-studied for boosting the process of pancreatic regeneration [14]. Quercetin, a flavonoid common to virtually all types of honey, is stated to have a profound effect on beta cell regeneration both in vitro and in vivo. The oral supplementation of quercetin to streptozotocin-generated diabetic rats caused elevated pancreatic insulin secretion, increased cell viability, decreased Ca2+-dependent cell death, and an expansion in the number of functional beta cells [15]. Gallic acid, a phenolic acid, has been demonstrated to enhance β-cell regeneration and reduce the levels of different biochemical parameters associated with the pathology of diabetes [16]. Likewise, honey at large has been reported to be effective in boosting the regeneration of many other vital tissues, including skin, bone, cartilage, liver, and corneal epithelium [17]. It has been extensively used in the tissue engineering industry because of its potent regenerative potential [18]. Though the healthful components of various types of honey have been well-documented in the literature archive for their potential role in various regenerative processes, there is a deficit of first-rate scientific study and research regarding the potential role of honey supplementation on pancreatic regeneration. Therefore, our research work was intended to assess the efficacy of Manuka honey in inducing pancreatic regeneration via assessing the changes in pancreatic histology and alteration in expression levels of principal transcription factors, including MAFA, PDX-1, Neurogenin-3, NEUROD, and NKX6.1.
2. Materials and methods
2.1. Chemicals
All materials were bought from Sigma Aldrich®, UK unless otherwise mentioned. Alloxan monohydrate used in the study was by Sigma-Aldrich®, St Louis, MO, USA (Catalog No: BCBR9198V), metformin (BP 500 mg, Martin Dow®, France) and all other routine chemicals were procured from an authentic local chemical supplier. All chemicals employed were of analytical quality.
2.2. Honey sample & dosage
The medical-grade Manuka honey (UMF 30+; MGO 400) was procured from Comvita™ Ltd., New Zealand. Upon arrival at the laboratory, the aliquots were kept in airtight glass vessels, stored in the dark at refrigerating temperature, and used within three months of receipt. Every time before administration, the Manuka honey sample was diluted with distilled water. The dosages were calculated at a rate of 3 g/kg of body weight and administered as a single dose using an oral cannula.
2.3. Experimental design & induction of diabetes
All twenty-four albino rats utilized in the study were procured from the Animal Research Station Facility of the Department of Physiology, Government College University, Faisalabad. All rats weighed around 180–200 g and had normal glucose levels. After selecting the rats for the current research, all dedicated rats were kept in new cages for acclimatization for about two weeks. They were kept in a well-ventilated Animal Research Station under a suitable temperature (23 ± 2 °C), with humidity (∼50%), and natural 12-h light/dark cycle conditions. The rats were provided with ad libitum chow maintenance diet (CMD) and water. All animals were reared, and research was performed taking into account the directions of the Animal Research Ethical Committee, Government College University Faisalabad, Pakistan (Ref. No. GCUF/AREC/133).
After completion of the acclimatization phase, all rats employed for the study were divided into four groups including normal, negative control, positive control, and treatment groups. Before the study began, all the animals were kept on fasting for 12 h but were given ad libitum availability of water. All the rats, except those in the normal control group, were given intraperitoneal injections of 150 mg/kg of alloxan dissolved in cold normal saline (0.9%) for induction of diabetes. Because alloxan can cause deadly hypoglycemia due to the rapid release of pancreatic insulin, rats were given a 20% glucose solution orally after 6 h. After three days of alloxan injection, blood glucose levels were evaluated for all of them, and those with higher than 200 mg/dl of blood glucose were classified as diabetic [19]. No subsequent treatment was given to the negative control group. In contrast, the positive control and treatment groups were supplemented with metformin (150 mg/kg/day) and manuka honey (3 g/kg/day), respectively, for consecutive 21 days post-diabetes induction. After the completion of the trial, all rats were sacrificed, and serum and tissue samples were stored for subsequent biochemical, histopathological, and gene analyses.
2.4. Measurement of blood glucose and serum insulin levels
The blood glucose was measured weekly until the end of the trial from the tails of conscious diabetic rats using a blood glucose meter (Accu-Chek® Instant Blood Glucose Monitor, Roche Diagnostics, Mannheim, Germany) that undergo test strips method to measure glucose oxidoreductase mediated dye reaction. After the completion of the trial period, insulin levels in serum samples were measured through the competitive ELISA method, using a commercially available rodent insulin ELISA kit (Sigma® Monoclonal Insulin Kit, St. Louis, Missouri, United States; SKU: EZRMI-13 K) according to the manufacturer's protocol.
2.5. Estimation of oxidative stress parameters
The total antioxidant capacity (TAC) was accessed using an assay described by Erel, 2004 [20]. We set a 660 nm monochromatic wavelength and permitted 5 min for the filter to get warm. A mixture of 5 μL sample and 200 μL of reagent-1 was used as blank. Thereafter, reagent 2 (20 μL) was mixed, and absorbance was measured after an incubation of 5 min at room temperature. After calibrating the standards of vitamin C, the results were measured. For the estimation of total oxidant status (TOS), a spectrophotometer (Biolab-310) employing the method explained by Erel, 2005 [21] was employed. In 35 μL of a serum sample, the 225 μL of reagent-1 was mixed. After mixing them, early absorbance was measured. Then 11 μL of reagent-2 was combined with the mixture of sample and reagent-1. After incubating at 25 °C for 4 min, the absorbance at biochromatic 560 nm wavelength and 800 nm differential wavelength were taken using a chemistry analyzer (Biosystem, BTS-330). The net absorbance variation was used to estimate definite concentration from the standard curve.
2.6. RNA extraction and real-time qRT-PCR assay
Trizol chemical was used to extract RNA from pancreatic tissues following the manufacturer's instructions (Ambion Trizol® by Thermo Scientific™, Massachusetts, USA; Catalogue No: 15596026). NanoDrop® ND-1000 Spectrophotometer was used to quantify the isolated RNA. The cDNA of all samples was prepared using RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific™, Massachusetts, USA; Catalogue No: K1621), and manufacturer protocol was followed. Relative quantification of mRNA levels of pancreatic Pdx-1, Nkx6.1, MafA, NEUROD, and Neurogenin-3 was conducted through quantitative real-time PCR (qRT-PCR) using Maxima SYBR Green/ROX qPCR Master Mix (Thermo Scientific™, Massachusetts, USA; Catalogue No: K0221). Table 1 indicates the primer sequence and size of the respective amplified PCR product. For normalization, the housekeeping gene β-actin was employed as a constitutive control. Finally, 2*(-ΔΔct) method was then used to analyze the threshold (Ct) values data obtained through qRT-PCR for the calculation of expression level.
Table 1.
Details of oligonucleotide used in the study along with product size and polymerase chain reaction conditions.
| Gene | NCBI Gene ID | RGD ID | Oligonucleotide Sequence |
GC% | Annealing Temperature | Product Size |
|---|---|---|---|---|---|---|
| MAFA | 366949 | 1562627 | Forward: GACCTGATGAAGTTCGAGGTG | 52.38 | 53.39 | 90 |
|
Reverse: GGGCGTCGAGGATAGCGA |
66.67 | 56.29 | ||||
| PDX-1 | 29535 | 62387 | Forward: CGTAGTAGCGGGACAACGAG | 60.00 | 55.25 | 71 |
| Reverse: CCCGAGGTTACGGCACAAT | 57.89 | 55.08 | ||||
| INS-1 | 16333 | 2915 | Forward: CTGGGAAATGAGGTGGAAAA | 45.00 | 56.65 | 98 |
|
Reverse: TCCACAAGCCACGCTTCTG |
57.89 | 56.89 | ||||
| INS-2 | 16334 | 2916 | Forward: AGAAAGGTTTGGTACCTGGAATAGAGC | 44.44 | 53.23 | 121 |
| Reverse: GTAGAAGAAGCTTCGCTCCCCACA | 54.16 | 53.76 | ||||
| NEUROG3 | 60329 | 631350 | Forward: CATAGCGGACCACAGCTTCT | 55.00 | 54.82 | 125 |
| Reverse: GGCTACCAGCTTGGGAAACT | 55.00 | 54.96 | ||||
| NKX6-1 | 65193 | 69318 | Forward: CGGAGAGTCAGGTCAAGGTC | 60.00 | 54.47 | 134 |
| Reverse: CTCGTTCTCCGAAGTCCCCT | 60.00 | 55.97 | ||||
| NEUROD | 29458 | 3165 | Forward: CATGTGTTCCACGTCAAGCC | 55.00 | 54.76 | 167 |
| Reverse: AACTCGGTGGATGGTTCGTG | 55.00 | 55.32 |
2.7. Histopathological analysis
The samples of the splenic region of the pancreas, preserved in 10% formaldehyde, were employed for histopathological analysis. The paraffin-embedded pancreas samples were sectioned using a semi-automated rotary microtome (149MULTI0C1, Leica Biosystems Nussloch GmbH) 5 μm thick slices, which were subsequently mounted on slides and then stained with H & E (hemotoxylin and eosin) stain. DPX was used as mounting media to mount the glass coverslip on stained tissue sections. Stained slides were examined under a light microscope at 40X for morphological studies. Morphometric analysis was performed using ImageJ®.
2.8. Statistical analysis
The data is presented as mean ± standard error (SE). One-way analysis of variance (ANOVA) was applied to compare all statistical comparisons except in the case of blood glucose levels, where values were compared using two-way ANOVA. The results were regarded as statistically significant if the p-values were less than 0.05. Differences among mean values of groups were determined via Duncan's multiple range test.
3. Results
3.1. Effect on blood glucose and serum insulin levels
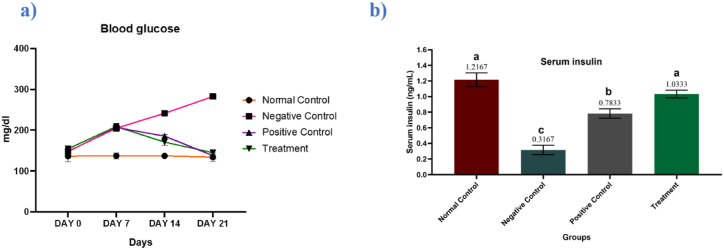
Significant differences were observed among the mean values of blood glucose (P ≤ 0.0001) and serum insulin (P ≤ 0.0001) of different experimental groups. At the beginning of the experiment, the blood glucose levels of all experimental rats were found around the normal range which progressively changed during the research trial. No significant change was seen in the blood glucose levels of the normal control group till the end of the experiment. In the negative control group, glucose levels kept on increasing throughout the experiment. In the positive control group, glucose levels decline during the first week, but as anticipated, the metformin treatment led to a gradual decline in blood glucose levels. Promisingly, a gradual decrease in glucose levels was observed in the group supplemented with Manuka honey that indicates the impactful potential of Manuka honey in lowering the blood glucose levels which follows other studies in the literature archive (Fig. 1a). Corresponding to the results of glucose levels, the levels of insulin found decreased in the negative control group because of hypoinsulinemia induced by alloxan. However, in groups supplemented with metformin and Manuka honey a significant alleviation of hypoinsulinemia resulted in the reduction of glucose levels in those groups (Fig. 1b).
Fig. 1.
Effects of metformin and manuka honey on (a) blood glucose (mg/dl) and (b) serum insulin (mg/ml) levels. Results are expressed as means ± SEM. This means sharing a significant difference of (P ≤ 0.05). Mean values not bearing a common superscript differ significantly.
3.2. Effect on oxidative stress parameters
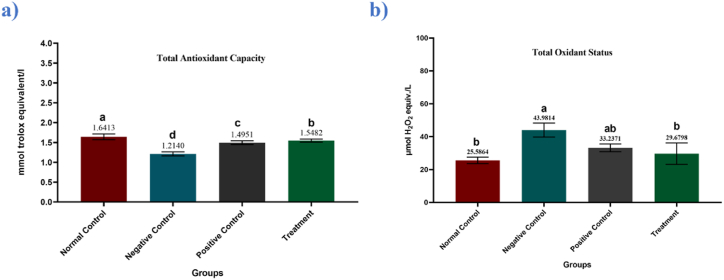
One-way analysis of the variance revealed significant differences among means of total antioxidant capacity (TAC) (P = 0.03) and total oxidant status (TOS) (P = 0.0001) of different groups revealed significant differences among groups. Administration of alloxan increased oxidative stress as indicated by TAC & TOS values of the negative control group. As anticipated, upon treatment with metformin, the level of TAC got significantly increased which resulted in a significant decrease in TOS levels. In groups supplemented with Manuka honey, the levels of TAC were found significantly increased as compared to the negative control group (Fig. 2a and b; Table 2).
Fig. 2.
Mean serum (a) total antioxidant capacity (mmol Trolox equivalent/L ± SEM) concentration and (b) total oxidant status (μmol H2O2 equivalent/L ± SEM) in control and treated groups. Mean values not bearing a common superscript differ significantly.
Table 2.
Mean values ± SEM of serum insulin, oxidative stress parameters and relative expression levels of corresponding transcriptional factors in control and treated groups. Mean values within a column, not bearing a common superscript differ significantly (P ≤ 0.05).
| Group | Insulin | Total oxidant status | Total antioxidant capacity | MAFA | NKX6.1 | NEUROD | NGN-3 | PDX-1 | INS-1 | INS-2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Normal control | 1.21 ± 0.0872a |
25.58 ± 1.993b | 1.64 ±0.073a |
3.23 ±0.071a |
3.34 ±0.072 a |
3.45 ±0.054 a |
2.77 ±0.19a |
2.56 ±0.09a |
2.33 ±0.071 a |
1.99 ±0.082 a |
| Negative control | 0.31 ± 0.0600c |
43.98 ±4.249a |
1.21 ±0.050d |
1.15 ±0.053d |
1.32 ±0.054 d |
1.78 ±0.083 d |
1.67 ±0.22c |
1.42 ±0.24c |
1.02 ±0.090 c |
0.94 ±0.072 d |
| Positive control | 0.78 ± 0.0600b |
33.23 ±2.333ab |
1.49 ±0.046c |
1.89 ±0.055b |
1.98 ±0.057 b |
2.47 ±0.088 b |
2.28 ±0.14b |
2.19 ±0.12b |
1.95 ±0.052 b |
1.59 ±0.082 b |
| Treatment | 1.03 ± 0.0494a |
29.67 ±6.533 b |
1.54 ±0.038b |
1.45 ±0.043c |
1.74 ±0.073 c |
2.15 ±0.082 c |
2.09 ±0.27b |
1.90 ±0.11b |
1.73 ±0.060 b |
1.24 ±0.056 c |
3.3. Effect on histopathology
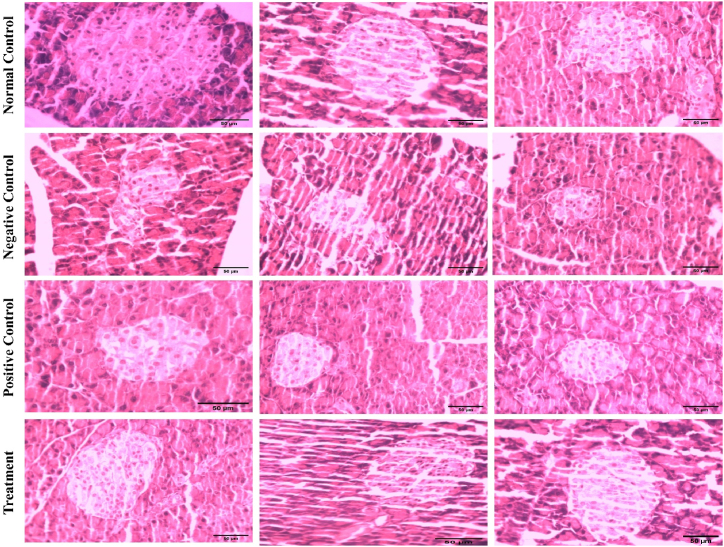
Histopathological examination and morphometric analysis indicated that in the normal control group, the islets of Langerhans exhibited a normal vesicular structure of beta cells. As anticipated, alloxan administration resulted in necrotic changes and marked disorganization of cellular components in islets and led to overall pancreatic degeneration as indicated by morphometric analysis. Noteworthily, both in metformin-treated and Manuka honey-supplemented groups, histological examination of the pancreas indicated a marked improvement in the vesicular structure of Langerhans islets as indicated by rounded vesicular nuclei with dispersed chromatin granules. Moreover, the vacuolation between the cells and capillaries which is observable in the negative control group is quite normalized in the metformin and Manuka honey groups. (Fig. 3; Table 2).
Fig. 3.
Histological examination of degenerative changes in pancreatic beta cells in diabetes and effect of metformin and manuka honey on the morphology of islets of Langerhans.
3.4. Effect on transcriptional machinery
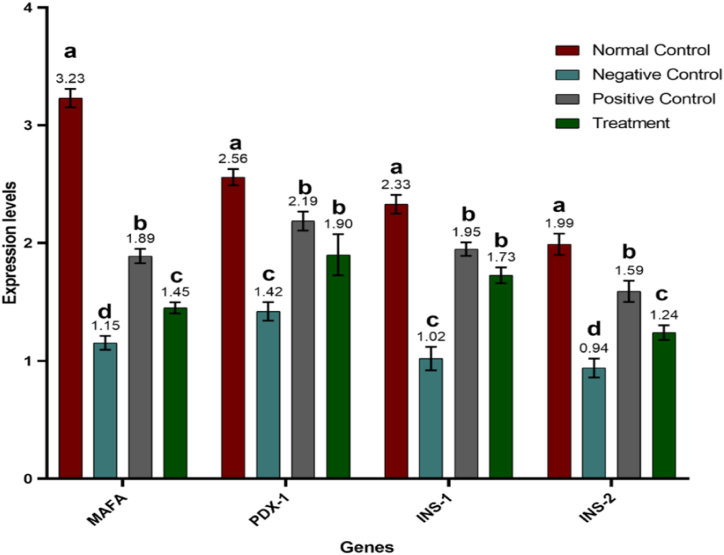
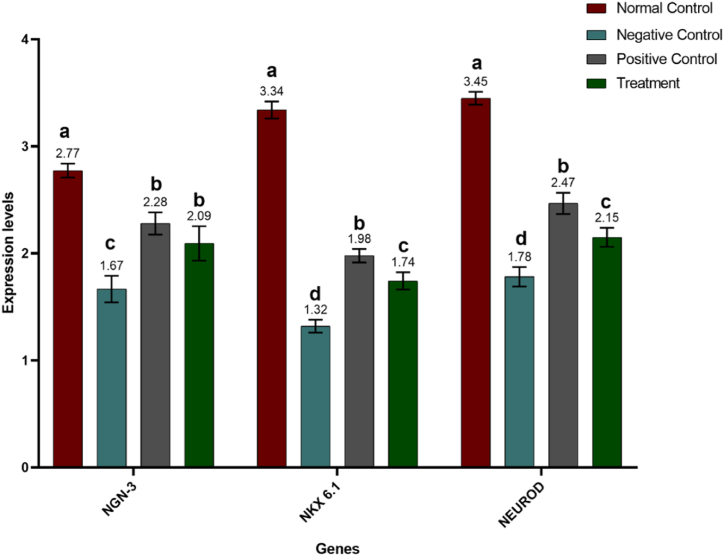
For further advocation of our hypothesis regarding the potent potential of Manuka honey in ameliorating the degenerative damage caused by alloxan and inducing regeneration of beta cells in islets of Langerhans of the diabetic-induced model, we investigated the levels of crucial transcription factors including MAFA, PDX-1, INS-1, INS-2, NEUROG3, NKX6-1, and NEUROD. Statistical analysis of results revealed that the expression of fundamental transcription factors varied significantly (P ≤ 0.0001) among different trial groups. MAFA expression level was highest in the normal control (non-diabetic) group whereas Alloxan supplementation resulted in decreased expression of MAFA in all groups other than the normal control. However, the administration of treatment caused an upregulation in the expression of MAFA levels which is demonstrated by higher levels of MAFA in the positive control (diabetic and metformin-treated) group and the treatment (diabetic and honey-treated) group A similar trend was observed in the expression levels of other transcription factors i.e., PDX-1, NGN-3, NKX6.1, and NEUROD. The expression level of NKX was 6.1 and was highest in the normal control group. However, the expression of both transcription factors i.e., NKX 6.1 and NEUROD was downregulated in the negative control group. Expression of these transcription factors was again upregulated in positive control and Manuka honey-supplemented groups. However, no significant difference was observed in the expression levels of NGN-3 in the positive control group and treatment group. Expression levels of insulin genes, INS-1 and INS-2 also exhibited similar trends among different experimental groups (Fig. 4, Fig. 5; Table 2).
Fig. 4.
Mean expression levels of MAFA, PDX-1, INS-1, and INS-2 in control and treated groups. Results are expressed as means ± SEM. This means sharing a significant difference of (P ≤ 0.05). Mean values not bearing a common superscript differ significantly.
Fig. 5.
Mean expression levels of NGN-3, NKX6.1, and NEUROD in control and treated groups. Results are expressed as means ± SEM. This means sharing a significant difference of (P ≤ 0.05). Mean values not bearing a common superscript differ significantly.
4. Discussion
Pancreatic regeneration is a prospective therapeutic approach for restoring β-cell loss in diabetic patients. It is a technique that can help compensate for the disease's burden by significantly replacing lost functional beta cell mass. Both endogenous regeneration and exogenous supplementation indicate the regeneration of β-cells that, in return, stimulates the restoration of islet mass by endogenous activation of pancreatic-cell regeneration ability [11,12,22]. The Manuka honey, abounding in beneficial flavonoids and phenolic acids, has been stated in the literature archive to exhibit potent antidiabetic effects. It has been indicated to lower blood glucose levels in diabetic patients and mitigate hyperglycemic conditions in both types of diabetes [23,24]. It also increases insulin concentration and decreases insulin resistance in diabetic individuals [25]. Honey supplementation raises serum insulin levels and improves islets in case of impaired pancreatic function and diabetes [26,27]. Besides other health benefits, honey possesses a strong ability to stimulate and enhance the regeneration process in many vital organs. It has been reported to exhibit a potential role in skin regeneration [28], liver regeneration [29], bone marrow regeneration [30], neural regeneration, epithelial regeneration [31,32], testicular regeneration [33], ovarian regeneration [34], and other tissues regeneration [18,35]. On this note, the present study was designed to explore the pancreatic regenerative potential of Manuka honey, the main element of apitherapy.
In the current study, alloxan was employed to induce diabetes and pancreatic degeneration since it is regarded as a potent diabetogenic agent that causes rapid degenerative changes in pancreatic β cells via enhanced generation of reactive oxygen species [36]. Degeneration in pancreatic β cells leads to reduced insulin secretion, decreased glucose consumption by body tissues, and ultimately enhanced glucose levels [37]. Consistently with previous studies, our study provides evidence that alloxan supplementation resulted in hyperglycemia and decreased insulin secretion [38]. However, all these adverse effects were reversed in metformin-treated and Manuka honey-supplemented groups. The honey treatment group, supplemented with Manuka honey for 21 days, exhibited significant elevation in serum insulin levels and decreased blood glucose levels. These effects can be ascribed to honey's strong antihyperglycemic and antidiabetic activity [39]. The precise mechanism underlying honey's antihyperglycemic action is quite complex and a topic of great concern among the research community. One proposed mechanism is the presence of fructose, oligosaccharides, and certain minerals such as zinc and copper in honey. Honey's fructose concentration is known to correlate negatively with the glycaemic index. Small quantities of fructose have been demonstrated to cause a reduction in blood glucose levels by improving hepatic glucose uptake by activating the glucokinase enzyme [40,41]. Numerous oligosaccharides found in honey are attributed to exhibit antihyperglycemic effects either through the regulation of gut microbiota or via the general effects of oligosaccharides [27,42]. Chromium, zinc, and copper, among other minerals found in honey, have been shown to lower blood sugar levels, regulate glucose tolerance, and enhance insulin secretion and sensitivity in pancreatic β -cells [[43], [44], [45]]. Besides, polyphenols present in manuka have been known to play role in controlling hyperglycemia via inhibition of α-amylase and α-glucosidase which are involved in carbohydrate breakdown. Quercetin efficiently hinders α-glucosidase enzymes and decreases maltose-induced postprandial hyperglycemia in diabetic individuals [46]. Another polyphenol luteolin is also shown to supress the activity of α-amylase and α-glucosidase thus ameliorating hyperglycemia [47].
Oxidative stress is known as a critical player in pancreatic dysfunction in diabetes. Reactive oxygen species serve as the key mediator in hyperglycemia-induced glucotoxicity. In our study, alloxan administration caused a significant increase in oxidative stress, as anticipated. This agreed with an earlier study by Ananthan et al. where alloxan administration induced oxidative stress and reduced the levels of antioxidative enzymes such as catalase (CAT), glutathione peroxidase (GPX), and superoxide dismutase (SOD) [48]. Various flavonoids and phenolic acids present in honey are regarded as rich sources of antioxidants, which can efficiently mitigate oxidative damage [49]. In the present study, the honey treatment caused a decrease in the level of oxidants and raised the total antioxidant capacity of diabetic rats. These findings are in accordance with a prior study by Omotayo et al. where honey supplementation, in conjunction with hypoglycemic drugs, exerted strong antioxidative effects and helped in the alleviation of oxidative damage in diabetic rats [50]. Another study by Wang et al. revealed the antidiabetic potential of apigenin, a commonly occurring flavonoid in manuka honey, where apigenin prevented beta cell damage and dysfunction via mitigating oxidative stress and boosting the activity of antioxidant enzymes [51].
Oxidative stress, induced by alloxan or hyperglycemia, leads to necrotic changes and dysfunction of pancreatic β-cells [52]. In the current study, the histopathological analysis of pancreatic beta cells further confirmed these findings. The alloxan-induced group showed a smaller number of islets and low islet density as compared to the normal group. Increased necrosis, degranulation of islets, and fibrosis were observed in alloxan treated group. However, interestingly, both metformin-treated and honey-supplemented groups exhibited improved architecture, increased islet size and density, and decreased necrotic changes in pancreatic beta cells. These results are consistent with previous studies regarding the regenerative effects of metformin and various types of honey; however, it's a new addition to the literature archive concerning Manuka honey supplementation. A study by Atanu et al. showed that metformin, combined with Aloe vera gel, helps diminish necrotic changes and promotes the healing of pancreatic β-cells in alloxan-induced diabetic rats [53]. Another study by El-Soud et al., studying the effects of honey and metformin supplementation, revealed that honey treatment showed more remarkable improvement in pancreatic histology than all other groups. Honey treatment triggered the regeneration of beta cells by reducing the shrinkage of islets, increasing the size of islets, and reducing fibrosis [54]. Polyphenolic compounds present in honey provide protection to beta cells against oxidative damage thus supressing degenerative changes in islets [55]. In the current study, the histopathological results obtained with Manuka honey supplementation strongly advocate the potent potential of Manuka honey in beta cell regeneration.
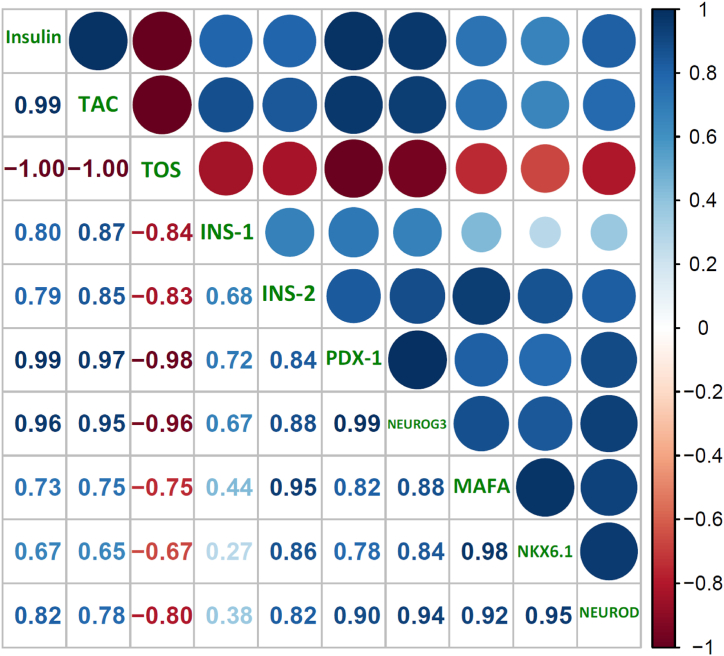
The beta cell cycle is greatly influenced by oxidative stress. In beta cells, accumulating ROS inhibits the expression of cyclins D1 and D2 and enhances cell-cycle inhibitors, including p21 and p27, resulting in a lower cell proliferation rate. In the meantime, ROS enhances FoxO1 nuclear translocation/activation, inhibiting PDX-1 and conceivably other cell-related gene transcriptions, hindering cell replication. Moreover, ROS inhibits transcription factors essential for beta cell proliferation, differentiation, insulin production, and secretion, such as PDX-1, MAFA, INS-1, INS-2, NKX6-1, and NEUROG3. The downregulated expression of these transcription factors indicates restricted beta cell replication and regeneration process [56]. MAFA regulates beta cell replication and development and is highly susceptible to oxidative stress [57]. MAFA is deactivated and translocated to the cytoplasm within 30 min of H2O2-induced oxidative stress. H2O2 administration resulted in the deactivation of NKX6-1 and PDX-1, two transcription factors necessary for differentiating beta cells. Further research utilizing transgenic mice with overexpression of antioxidant enzyme Gpx1 revealed that the levels of nuclear MAFA and NKX6-1 were recovered, validating that the loss of these critical cell transcription factors in diabetes is caused by oxidative stress [58]. PDX-1, the transactivator of the many genes including the insulin gene participating in glucose metabolism, is also translocated from the nuclei to the cytoplasm of pancreatic β-cells because of oxidative stress [59]. In coherence with this, in our study, the diabetic group showed downregulation in the expression levels of PDX-1, INS-1, INS-2, NEUROG3, MAFA, NKX6.1, and NEUROD, which led to the degeneration of pancreatic beta cells via increasing oxidative stress and ultimately resulted in hyperglycemia and hypoinsulinemia. However, manuka honey supplementation resulted in the upregulation of all these pancreatic transcription factors and improved insulin levels. This upregulation can be credited to the antioxidant property of honey, as antioxidant supplements have long been employed to reduce oxidative stress and boost pancreatic cell proliferation and function in individuals with diabetes [60]. These results are parallel with a previous study by Wang et al. where long-term supplementation of dietary selenate, an antioxidant nutrient, decreased blood glucose levels, improved islet architecture, and upregulated the genes that translate into proteins involved in differentiation and proliferation of beta cells [61]. Positive correlation between oxidative stress and transcription factors advocates the fact that regeneration of beta cells helped alleviate the oxidative stress caused by alloxan-induced diabetes (Fig. 6). As anticipated, insulin level was positively correlated, and glucose level was found negatively correlated with the expression of all transcriptional factor evaluated. Our results strongly endorse the potential role of Manuka honey supplementation in the regeneration of pancreatic beta cells and advocate the use of Manuka honey in ameliorating the damaging impact of diabetes mellitus.
Fig. 6.
Correlation among glycaemic, oxidative stress, and transcriptional control parameters.
5. Conclusion
The present study concluded that alloxan monohydrate induced degenerative changes in pancreatic islets, which led to increased oxidative stress, hyperglycemia, hypoinsulinemia, and decreased expression levels of MAFA, PDX-1, INS-1, INS-2, NEUROG3, NKX6-1, and NEUROD. Manuka honey supplementation efficiently improved the degenerative changes in pancreatic islets, increased insulin with a significant decrease in blood glucose levels, and significantly alleviated oxidative stress coupled with substantial upregulation in expression levels of transcription factors indicative of pancreatic beta cell regeneration. Thus, results obtained from our study suggest that manuka honey, an antidiabetic and antioxidative agent, could be recommended to boost the regeneration of pancreatic beta cells and help reduce some of the diabetes burdens.
6. Limitations and future recommendations
The current study presents the novel pancreatic regeneration potential of manuka honey. Our finding would open new horizons of future researches to explore other types of honey and natural products to explore their natural potential for diabetes prevention, management and treatment. Nevertheless, the smaller sample size and duration of study are a major limitation in our study. More experimental and clinical studies are needed to further confirm these results.
Ethical approval
The ethical approval for the current in-vivo research was obtained from Animal Research Ethical Committee, Government College University Faisalabad, Pakistan (Ref. No. GCUF/AREC/131).
Funding
The authors extend their appreciation to King Saud University for funding this work through research supporting project (RSP2023R376), Riyadh, Saudi Arabia.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Kitabchi A.E., Umpierrez G.E., Miles J.M., Fisher J.N. Hyperglycemic crises in adult patients with diabetes. Diabetes Care. 2009;32(7):1335–1343. doi: 10.2337/dc09-9032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kharroubi A.T., Darwish H.M. Diabetes mellitus: the epidemic of the century. World J. Diabetes. 2015;6(6):850. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Egan A.M., Dinneen S.F. What is diabetes? Medicine. 2019;47(1):1–4. [Google Scholar]
- 4.Bonner-Weir S., O'Brien T.D. Islets in type 2 diabetes: in honor of Dr. Robert C. Turner. Diabetes. 2008;57(11):2899–2904. doi: 10.2337/db07-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Risbud M.V., Bhonde R.R. Models of pancreatic regeneration in diabetes. Diabetes Res. Clin. Pract. 2002;58(3):155–165. doi: 10.1016/s0168-8227(02)00103-1. [DOI] [PubMed] [Google Scholar]
- 6.Domínguez-Bendala J., Inverardi L., Ricordi C. Regeneration of pancreatic beta-cell mass for the treatment of diabetes. Expet Opin. Biol. Ther. 2012;12(6):731–741. doi: 10.1517/14712598.2012.679654. [DOI] [PubMed] [Google Scholar]
- 7.Bouwens L., Houbracken I., Mfopou J.K. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat. Rev. Endocrinol. 2013;9(10):598–606. doi: 10.1038/nrendo.2013.145. [DOI] [PubMed] [Google Scholar]
- 8.Balaji S., Napolitano T., Silvano S., Friano M.E., Garrido-Utrilla A., Atlija J., Collombat P. Epigenetic control of pancreatic regeneration in diabetes. Genes. 2018;9(9):448. doi: 10.3390/genes9090448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Q., Melton D.A. Pancreas regeneration. Nature. 2018;557(7705):351–358. doi: 10.1038/s41586-018-0088-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montanya E., Nacher V., Biarnés M., Soler J. Linear correlation between beta-cell mass and body weight throughout the lifespan in Lewis rats: role of beta-cell hyperplasia and hypertrophy. Diabetes. 2000;49(8):1341–1346. doi: 10.2337/diabetes.49.8.1341. [DOI] [PubMed] [Google Scholar]
- 11.Bonner-Weir S., Baxter L.A., Schuppin G.T., Smith F.E. A second pathway for regeneration of adult exocrine and endocrine pancreas: a possible recapitulation of embryonic development. Diabetes. 1993;42(12):1715–1720. doi: 10.2337/diab.42.12.1715. [DOI] [PubMed] [Google Scholar]
- 12.Joglekar M.V., Parekh V.S., Hardikar A.A. New pancreas from old: microregulators of pancreas regeneration. Trends Endocrinol. Metabol. 2007;18(10):393–400. doi: 10.1016/j.tem.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Prabhakar P.K., Doble M. Mechanism of action of natural products used in the treatment of diabetes mellitus. Chin. J. Integr. Med. 2011;17(8):563–574. doi: 10.1007/s11655-011-0810-3. [DOI] [PubMed] [Google Scholar]
- 14.Pasupuleti V.R., Arigela C.S., Gan S.H., Salam S.K.N., Krishnan K.T., Rahman N.A., Jeffree M.S. A review on oxidative stress, diabetic complications, and the roles of honey polyphenols”, Oxidative Medicine and Cellular Longevity. 2020:2020. doi: 10.1155/2020/8878172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gurav M., Bhise S., Warghade S. Effect of quercetin on beta cell regeneration. Asian J. Pharm. Pharmacol. 2018;4:214–221. [Google Scholar]
- 16.Latha R.C.R., Daisy P. Insulin-secretagogue, antihyperlipidemic and other protective effects of gallic acid isolated from Terminalia bellerica Roxb. in streptozotocin-induced diabetic rats. Chem. Biol. Interact. 2011;189(1–2):112–118. doi: 10.1016/j.cbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Speer S.L., Schreyack G.E., Bowlin G.L. Manuka honey: a tissue engineering essential ingredient. J. Tissue and Sci. Eng. 2015;6(2):1. [Google Scholar]
- 18.Minden-Birkenmaier B.A., Bowlin G.L. Honey-based templates in wound healing and tissue engineering. Bioengineering. 2018;5(2):46. doi: 10.3390/bioengineering5020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Shamaony L., Al-Khazraji S.M., Twaij H.A. Hypoglycaemic effect of Artemisia herba alba. II. Effect of a valuable extract on some blood parameters in diabetic animals. J. Ethnopharmacol. 1994;43(3):167–171. doi: 10.1016/0378-8741(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 20.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004;37(4):277–285. doi: 10.1016/j.clinbiochem.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 21.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005;38(12):1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 22.Hardikar A.A., Karandikar M.S., Bhonde R.R. Effect of partial pancreatectomy on diabetic status in BALB/c mice. J. Endocrinol. 1999;162(2) doi: 10.1677/joe.0.1620189. [DOI] [PubMed] [Google Scholar]
- 23.Bahrami M., Ataie-Jafari A., Hosseini S., Foruzanfar M.H., Rahmani M., Pajouhi M. Effects of natural honey consumption in diabetic patients: an 8-week randomized clinical trial. Int. J. Food Sci. Nutr. 2009;60(7):618–626. doi: 10.3109/09637480801990389. [DOI] [PubMed] [Google Scholar]
- 24.Abdulrhman M., El-Hefnawy M., Hussein R., Abou El-Goud A. The glycemic and peak incremental indices of honey, sucrose and glucose in patients with type 1 diabetes mellitus: effects on C-peptide level—a pilot study. Acta Diabetol. 2011;48(2):89–94. doi: 10.1007/s00592-009-0167-7. [DOI] [PubMed] [Google Scholar]
- 25.Al-Waili N.S. Natural honey lowers plasma glucose, C-reactive protein, homocysteine, and blood lipids in healthy, diabetic, and hyperlipidemic subjects: comparison with dextrose and sucrose. J. Med. Food. 2004;7(1):100–107. doi: 10.1089/109662004322984789. [DOI] [PubMed] [Google Scholar]
- 26.Erejuwa O.O., Sulaiman S.A., Ab Wahab M.S., Sirajudeen K.N.S., M Salleh M.S., Gurtu S. Glibenclamide or metformin combined with honey improves glycemic control in streptozotocin-induced diabetic rats. Int. J. Biol. Sci. 2011;7(2):244. doi: 10.7150/ijbs.7.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Erejuwa O.O., Sulaiman S.A., Wahab M.S.A. Oligosaccharides might contribute to the antidiabetic effect of honey: a review of the literature. Molecules. 2011;17(1):248–266. doi: 10.3390/molecules17010248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukanc B., Potokar T., Erjavec V. Complete skin regeneration with medical honey after skin loss on the entire circumference of a leg in a cat. J. Tissue Viability. 2020;29(2):148–152. doi: 10.1016/j.jtv.2020.03.007. [DOI] [PubMed] [Google Scholar]
- 29.Prasetyo R.H., Hestianah E.P. Honey can repairing damage of liver tissue due to protein energy malnutrition through induction of endogenous stem cells. Vet. World. 2017;10(6):711. doi: 10.14202/vetworld.2017.711-715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niaz K., Maqbool F., Bahadar H., Abdollahi M. Health benefits of manuka honey as an essential constituent for tissue regeneration. Curr. Drug Metabol. 2017;18(10):881–892. doi: 10.2174/1389200218666170911152240. [DOI] [PubMed] [Google Scholar]
- 31.Nooh H.Z., Nour-Eldien N.M. The dual anti-inflammatory and antioxidant activities of natural honey promote cell proliferation and neural regeneration in a rat model of colitis. Acta Histochem. 2016;118(6):588–595. doi: 10.1016/j.acthis.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 32.Iftikhar A., Nausheen R., Mukhtar I., Iqbal R.K., Raza A., Yasin A., Anwar H. The regenerative potential of honey: a comprehensive literature review. J. Apicult. Res. 2022;1–16 [Google Scholar]
- 33.Safitri E., Utama S., Widiyatno T.V., Sandhika W., Prasetyo R.H. Auto-regeneration of mice testicle seminiferous tubules due to malnutrition based on stem cells mobilization using honey. Asian Pac, Reprod. 2016;5(1):31–35. [Google Scholar]
- 34.Prasetyo R.H., Safitri E. Effects of honey to mobilize endogenous stem cells in efforts intestinal and ovarian tissue regeneration in rats with protein energy malnutrition. Asian Pac, Reprod. 2016;5(3):198–203. [Google Scholar]
- 35.Iftikhar A., Nausheen R., Muzaffar H., Naeem M.A., Farooq M., Khurshid M.…Anwar H. Potential therapeutic benefits of honey in neurological disorders: the role of polyphenols. Molecules. 2022;27(10):3297. doi: 10.3390/molecules27103297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Szkudelski T. The mechanism of alloxan and streptozotocin action in B cells of the rat pancreas. Physiol. Res. 2001;50(6):537–546. [PubMed] [Google Scholar]
- 37.Dhanabal S.P., Raja M.M.M., Ramanathan M., Suresh B. Hypoglycemic activity of Nymphaea stellata leaves ethanolic extract in alloxan induced diabetic rats. Fitoterapia. 2007;78(4):288–291. doi: 10.1016/j.fitote.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 38.Pashapoor A., Mashhadyrafie S., Mortazavi P. Ameliorative effect of Myristica fragrans (Nutmeg) extract on oxidative status and histology of pancreas in Alloxan induced diabetic rats. Folia Morphol. 2020;79(1):113–119. doi: 10.5603/FM.a2019.0052. [DOI] [PubMed] [Google Scholar]
- 39.Erejuwa O.O., Sulaiman S.A., Wahab M.S., Sirajudeen K.N.S., Salleh M.M., Gurtu S. Antioxidant protection of Malaysian tualang honey in pancreas of normal and streptozotocin-induced diabetic rats. Ann. Endocrinol. 2010;71(4):291–296. doi: 10.1016/j.ando.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 40.Watfor M. Small amounts of dietary fructose dramatically increase hepatic glucose uptake through a novel mechanism of glucokinase activation. Nutr. Rev. 2002;60(8):253–257. doi: 10.1301/002966402320289377. [DOI] [PubMed] [Google Scholar]
- 41.Bogdanov S. Honey as nutrient and functional food. Proteins. 2012;1100:1400–2700. [Google Scholar]
- 42.Shin H.S., Ustunol Z. Carbohydrate composition of honey from different floral sources and their influence on growth of selected intestinal bacteria: an in vitro comparison. Food Res. Int. 2005;38(6):721–728. [Google Scholar]
- 43.Anderson R.A., Cheng N., Bryden N.A., Polansky M.M., Cheng N., Chi J., Feng J. Elevated intakes of supplemental chromium improve glucose and insulin variables in individuals with type 2 diabetes. Diabetes. 1997;46(11):1786–1791. doi: 10.2337/diab.46.11.1786. [DOI] [PubMed] [Google Scholar]
- 44.Sitasawad S., Deshpande M., Katdare M., Tirth S., Parab P. Beneficial effect of supplementation with copper sulfate on STZ-diabetic mice (IDDM) Diabetes Res. Clin. Pract. 2001;52(2):77–84. doi: 10.1016/s0168-8227(00)00249-7. [DOI] [PubMed] [Google Scholar]
- 45.Song M.K., Hwang I.K., Rosenthal M.J., Harris D.M., Yamaguchi D.T., Yip I., Go V.L. Antidiabetic actions of arachidonic acid and zinc in genetically diabetic Goto-Kakizaki rats. Metab. Clin. Exp. 2003;52(1):7–12. doi: 10.1053/meta.2003.50031. [DOI] [PubMed] [Google Scholar]
- 46.Hussain S.A., Ahmed Z.A., Mahwi T.O., Aziz T.A. Quercetin dampens postprandial hyperglycemia in type 2 diabetic patients challenged with carbohydrates load. Int. J. Diabetes Res. 2012;1(3):32–35. [Google Scholar]
- 47.Zulkhairi Amin F.A., Sabri S., Mohammad S.M., Ismail M., Chan K.W., Ismail N., Zawawi .N. Advances in Pharmacological and Pharmaceutical Sciences; 2018. Therapeutic Properties of Stingless Bee Honey in Comparison with European Bee Honey”. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ananthan R., Latha M., Ramkumar K.M., Pari L., Baskar C., Bai V.N. Modulatory effects of Gymnema montanum leaf extract on alloxan-induced oxidative stress in Wistar rats. Nutrition. 2004;20(3):280–285. doi: 10.1016/j.nut.2003.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Schramm D.D., Karim M., Schrader H.R., Holt R.R., Cardetti M., Keen C.L. Honey with high levels of antioxidants can provide protection to healthy human subjects. J. Agric. Food Chem. 2003;51(6):1732–1735. doi: 10.1021/jf025928k. [DOI] [PubMed] [Google Scholar]
- 50.Omotayo E.O., Gurtu S., Sulaiman S.A., Wahab M.S.A., Sirajudeen K.N.S., Salleh M.S.M. Hypoglycemic and antioxidant effects of honey supplementation in streptozotocin-induced diabetic rats. Int. J. Vitam. Nutr. Res. 2010;80(1):74. doi: 10.1024/0300-9831/a000008. [DOI] [PubMed] [Google Scholar]
- 51.Wang N., Yi W.J., Tan L., Zhang J.H., Xu J., Chen Y., Zhang .R. Apigenin attenuates streptozotocin-induced pancreatic β cell damage by its protective effects on cellular antioxidant defense. In Vitro Cell. Dev. Biol. Anim. 2017;53:554–563. doi: 10.1007/s11626-017-0135-4. [DOI] [PubMed] [Google Scholar]
- 52.Kajimoto Y., Kaneto H. vols. 168–176. Annals of the New York Academy of Sciences; 2004. Role of Oxidative Stress in Pancreatic β-cell Dysfunction”. [DOI] [PubMed] [Google Scholar]
- 53.Atanu F.O., Avwioroko O.J., Momoh S. Anti-diabetic effect of combined treatment with Aloe vera gel and Metformin on alloxan-induced diabetic rats. J. Ayurveda Holist. Med. 2018;4(1):1–5. [Google Scholar]
- 54.El-Soud N.H.A., El-Laithy N.A., Mohamed N., Youness E.R., Wasseif M.E., Yassen N., Abdel-Latif A.M. Honey versus metformin: effects on pancreatic beta-cells in streptozotocin induced diabetic rats. Der Pharma Chem. 2016;8:29–39. [Google Scholar]
- 55.Hossen M.S., Ali M.Y., Jahurul M.H.A., Abdel-Daim M.M., Gan S.H., Khalil M.I. Beneficial roles of honey polyphenols against some human degenerative diseases: a review. Pharmacol. Rep. 2017;69(6):1194–1205. doi: 10.1016/j.pharep.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 56.Kawamori D., Kajimoto Y., Kaneto H., Umayahara Y., Fujitani Y., Miyatsuka T., Hori .M. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH2-terminal kinase. Diabetes. 2003;52(12):2896–2904. doi: 10.2337/diabetes.52.12.2896. [DOI] [PubMed] [Google Scholar]
- 57.Nishimura W., Kondo T., Salameh T., El Khattabi I., Dodge R., Bonner-Weir S., Sharma A. A switch from MafB to MafA expression accompanies differentiation to pancreatic β-cells. Dev. Biol. 2006;293(2):526–539. doi: 10.1016/j.ydbio.2006.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo Guo S., Dai C., Guo M., Taylor B., Harmon J.S., Sander M., Stein R. Inactivation of specific β cell transcription factors in type 2 diabetes. J. Clin. Investig. 2013;123(8):3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhu Y., Liu Q., Zhou Z., Ikeda Y. PDX1, “Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration”. Stem Cell Res. Ther. 2017;8(1):1–7. doi: 10.1186/s13287-017-0694-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasri H., Shirzad H., Baradaran A., Rafieian-Kopaei M. Antioxidant plants and diabetes mellitus. J. Res. Med. Sci. 2015;20(5):491. doi: 10.4103/1735-1995.163977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang C., Yang S., Zhan N., Mu Y., Ren H., Wang Y., Li K. Long-term supranutritional supplementation with selenate decreases hyperglycemia and promotes fatty liver degeneration by inducing hyperinsulinemia in diabetic db/db mice. PLoS One. 2014;9(7) doi: 10.1371/journal.pone.0101315. [DOI] [PMC free article] [PubMed] [Google Scholar]