Abstract
Objective
Infection with Helicobacter pylori (H. pylori) may increase atherosclerosis, which can lead to carotid plaque formation. Our study examined the relationship between H. pylori infection and carotid plaque formation, and its underlying mechanisms.
Methods
A total of 36,470 people who underwent physical examination in Taizhou Hospital Health Examination Center from June 2017 to June 2022 were included in this study. All people participated in the urease test, neck ultrasound, blood pressure detection, anthropometric measurement and biochemical laboratory examination. In addition, the GSE27411 and GSE28829 datasets in the Gene Expression Omnibus (GEO) database were used to analyze the mechanism of H. pylori infection and atherosclerosis progression.
Results
H. pylori infection, sex, age, blood lipids, blood pressure, fasting blood glucose, glycated hemoglobin and body mass index were risk factors for carotid plaque formation. An independent risk factor was still evident in the multivariate logistic regression analysis, indicating H. pylori infection. Furthermore, after weighted gene coexpression network analysis (WGCNA), we discovered 555 genes linked to both H. pylori infection and the advancement of atherosclerosis. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses revealed a strong correlation between these genes and immunity, infection, and immune disorders. SsGSEA analysis showed that H. pylori infection and atherosclerosis included changes in the immune microenvironment. Finally, three genes MS4A6A, ADAMDEC1 and AQP9 were identified to be involved in the formation of atherosclerosis after H. pylori infection. Conclusion: Our research affirms that H. pylori is a unique contributor to the formation of carotid plaque, examines the immune microenvironment associated with H. pylori infection and advanced carotid atherosclerosis, and offers fresh perspectives on how H. pylori infection leads to atherosclerosis.
Keywords: Helicobacter pylori, Carotid plaque, Bioinformatics analysis, Immune microenvironment, Gene expression
1. Introduction
The prevalence of Helicobacter pylori (H. pylori) infection is extensive on a global scale, impacting around 50% of the adult populace. This infection mainly resides in the gastrointestinal system and is notably more prevalent in developing countries [1]. It can colonize the mucosal surface and glands of the human stomach, produce a variety of adhesins and deliver virulence factors, which lead to changes in host signaling pathways and induce proinflammatory responses [2]. H. pylori has a close relationship with peptic ulcers, chronic gastritis, and even gastric cancer [3,4]. Increasing evidence indicates that infection with H. pylori is linked to diseases outside the stomach, including liver diseases, metabolic disorders, respiratory disorders, cardiovascular conditions, and even hyperemesis gravidarum [[5], [6], [7]].
Atherosclerosis is a pathological state marked by inflammation in the walls of arteries, resulting from abnormal lipid metabolism within the body [8]. It causes intimal thickening, arteriosclerosis and lumen stenosis, as well as pathological plaque formation [9]. Infection with H. pylori could potentially enhance the secretion of cytotoxin related gene A (Cag A), and further promote atherosclerosis and plaque formation through inflammatory reactions and immune responses [10,11]. In addition, H. pylori infection may aggravate disorders of lipid and lipoprotein metabolism and further aggravate atherosclerosis [10]. Nevertheless, the primary focus of these investigations was the examination of clinical data, with limited exploration of the genetic aspect regarding the association between H. pylori infection and carotid plaque formation.
The advancement of bioinformatics analysis technology using microarrays in recent times has facilitated a deeper comprehension of the genetic aspects of diseases [12]. Weighted gene coexpression network analysis (WGCNA), a technique for identifying crucial genes, involves partitioning genes into distinct modules based on their similarity in expression. It helps facilitate a systematic understanding of the function of genes at the molecular level [13,14]. In this research, our objective was to examine the association between H. pylori and carotid plaque formation, while also exploring the underlying mechanism using clinical samples and genomic data obtained from publicly available databases.
2. Materials and methods
2.1. Data collection
Individuals who received physical examinations at Taizhou Hospital's health examination center from June 2017 to June 2022 were included as participants in this study. Patients were included according to the following criteria: patients with complete data on age, sex, laboratory parameters, urea breath test results, blood pressure, body mass index (BMI) and neck ultrasound data. Laboratory parameters included triglycerides (TGs), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total cholesterol (TC), fasting blood glucose (FBG), and glycated hemoglobin A1c (HbA1c). Patients with thyroid function problems, gastrointestinal surgery, malignancies, pregnancy, minors or incomplete clinical information were excluded from this research. A total of 36,470 patients were retrospectively analyzed as part of this study. The Ethics Committee of Taizhou Hospital (K20220790) has granted approval for this study. This study was a retrospective analysis of clinical data obtained during the course of the study, and the written informed consent requirement was waived by the ethics committee of Taizhou Hospital. In addition, we obtained datasets GSE27411 and GSE28829 from the Gene Expression Omnibus (GEO) database. The GSE27411 dataset included transcriptome sequencing data of 6 H. pylori positive patients and 6 H. pylori negative patients, and the GSE28829 dataset contained transcriptome sequencing data of 13 early carotid atherosclerotic (EA) plaque samples and 16 advanced carotid atherosclerotic (AA) plaque samples.
2.2. Measurement of carotid plaque
The subjects were instructed to lie in a low occipital supine position, with their heads tilted back and inclined to the non-examination side to fully expose the neck. The intima-media thickness (IMT) in the neck vessels was measured by an experienced sonographer using a color Doppler ultrasound instrument. Plaques were defined as distinct areas with an IMT >50% of that of surrounding areas.
2.3. Collection of clinical indicators
Trained nurses gathered information regarding the patient's age and sex, and performed measurements of height and weight. Additionally, sitting diastolic blood pressure (DBP) and systolic blood pressure (SBP) were recorded. After a period of 8 h of fasting during the night, the individuals undergoing the physical examination had their venous blood samples taken and then sent to Taizhou Hospital's laboratory for additional analysis. The laboratory testing involved the use of an automatic biochemical analyzer to determine parameters such as TG, TC, HDL, LDL, and FBG. The level of HbA1c was measured using a glycosylated hemoglobin analyzer.
2.3.1. Identifying Helicobacter pylori
The detection of H. pylori was established through the utilization of either a13C or 14C urease breath examination. The process for the 13C breath test involved the following steps: (a) collecting the first breath sample from the patient while they were fasting, (b) ingesting 13C labeled capsules with warm boiled water, (c) waiting for a duration of 30 min, (d) gathering respiratory samples subsequent to the ingestion of the medication, and (e) analyzing the two samples using an instrument. The process for the 14C breath test consisted of the following steps: (a) taking a14C urea capsule orally, (b) following by a wait for 15–20 min, (c) exhaling evenly into the device for 3 min, and (d) placing a collection bottle into the apparatus to extract the results.
2.3.2. Building the network of coexpression
The WGCNA package in R was utilized to conduct coexpression network analysis [15]. WGCNA aims to discover the relationship between gene modules and phenotypes. The hierarchical clustering tree is constructed using the correlation matrix, which consists of correlation coefficients between gene pairs, and is based on Pearson coefficients. Different branches on the tree represent distinct gene modules, with each module assigned a specific color. The heatmap displays the outcomes of the correlation analysis between gene modules and disease traits. The module demonstrating the highest correlation with the traits of interest is then selected for further analysis.
2.4. Analysis of functional enrichment
The Gene Ontology (GO) project serves as an extensive source for functional genomics. GO annotations are categorized into three categories, namely, molecular function (MF), biological process (BP) and cellular component (CC) [16]. The Kyoto Encyclopedia of Genes and Genomes (KEGG) is an extensive repository that combines genomic, chemical, and systemic functional data [17]. In particular, the KEGG pathway functions as a storage for genetic pathway data across various organisms. To clarify the biological roles of the target genes, we performed enrichment analyses for both GO and KEGG.
2.4.1. Gene differential expression analysis
The R package “limma” was utilized to detect dissimilarly expressed mRNAs among distinct groups [18]. The screening threshold was established as |log2-fold change (FC)| > 1 and P < 0.05.
2.4.2. Estimating the abundance of immune cells
Single sample gene set enrichment analysis (ssGSEA) was used to quantitatively estimate the infiltration of 28 immune cell types [19]. The relative infiltration abundance of immune cells in different groups was estimated using ssGSEA algorithm in the GSVA package of R software.
2.5. Statistical analysis
The t-test was used to express data on continuous variables that followed a normal distribution as the mean ± standard deviation (SD). Chi-square tests were used to express categorical variables as counts and percentages. After adjusting for confounding factors by multicollinearity tests, the relationship between H. pylori infection and carotid plaque formation was analyzed by multivariate logistic regression. The correlation between gene expression and immune cells was tested by the Spearman correlation test. Statistical analysis was performed in SPSS software (Windows Version 25.0, Armonk, NY, USA), and R (4.1.2) software, and P < 0.05 was considered significant.
3. Result
3.1. Clinical analysis
Table 1 displayed the demographic and laboratory features of all populations. Univariate analysis showed that gender, age, TC, HDL, LDL, H. pylori infection, DBP, SDP, FBG, HbA1c and BMI were risk factors for carotid plaque (Table 2). In the multicollinearity test, lipids, blood pressure values, FBG, and HbA1c were filtered out, and these variables were not included in the adjusted analysis except for lipids judged according to clinical significance, Table 3.
Table 1.
Baseline characteristics of all physical examination populations.
| Variables | H. pylori-negative (n = 22718) | H. pylori-positive (n = 13752) | P value |
|---|---|---|---|
| Gender (n, %) | 0.022 | ||
| Female | 7606 (33.5) | 4444 (32.3) | |
| Male | 15112 (66.5) | 9308 (67.7) | |
| Age (year) | 51.25 ± 9.57 | 52.20 ± 9.25 | <0.001 |
| Triglycerides (mmol/L) | 1.95 ± 1.68 | 2.01 ± 1.73 | 0.004 |
| Total cholesterol (mmol/L) | 5.14 ± 0.98 | 5.13 ± 0.97 | 0.631 |
| High density lipoprotein (mmol/L) | 1.40 ± 0.32 | 1.39 ± 0.31 | <0.001 |
| Low density lipoprotein (mmol/L) | 2.83 ± 0.75 | 2.79 ± 0.74 | <0.001 |
| Arterial plaque (n, %) | <0.001 | ||
| No | 14589 (64.2) | 8572 (62.3) | |
| Yes | 8129 (35.8) | 5180 (37.7) | |
| Diastolic blood pressure (mmHg) | 77.38 ± 11.72 | 78.01 ± 11.85 | <0.001 |
| Systolic blood pressure (mmHg) | 128.40 ± 17.54 | 129.42 ± 18.12 | <0.001 |
| Fasting blood glucose (mmol/L) | 5.59 ± 1.55 | 5.65 ± 1.70 | <0.001 |
| Glycated hemoglobin A1c (%) | 5.93 ± 0.94 | 6.01 ± 1.03 | <0.001 |
| Body mass index (kg/m2) | 24.65 ± 3.15 | 24.83 ± 3.11 | <0.001 |
Table 2.
Univariate analysis of risk factors for carotid plaque.
| Variables | OR (95%CI) | P value |
|---|---|---|
| Male (%) | 1.579 (1.507–1.655) | <0.001 |
| Age (>50) | 5.372 (5.117–5.640) | <0.001 |
| Triglycerides (mmol/L) | 1.012 (1.000–1.025) | 0.060 |
| Total cholesterol (mmol/L) | 1.216 (1.190–1.243) | <0.001 |
| High density lipoprotein (mmol/L) | 0.879 (0.821–0.941) | <0.001 |
| Low density lipoprotein (mmol/L) | 1.394 (1.354–1.435) | <0.001 |
| H. pylori (+) | 1.085 (1.038–1.133) | <0.001 |
| Diastolic blood pressure (mmHg) | 1.025 (1.024–1.027) | <0.001 |
| Systolic blood pressure (mmHg) | 1.031 (1.029–1.032) | <0.001 |
| Fasting blood glucose (mmol/L) | 1.244 (1.225–1.263) | <0.001 |
| Glycated hemoglobin A1c (%) | 1.555 (1.516–1.595) | <0.001 |
| Body mass index (kg/m2) | 1.041 (1.034–1.048) | <0.001 |
Table 3.
Multivariate logistic analysis of risk factors for carotid plaque.
| Variables | OR (95%CI) | P value |
|---|---|---|
| Male (%) | 1.659 (1.573–1.749) | <0.001 |
| Age (>50) | 5.57 (5.298–5.855) | <0.001 |
| Triglycerides (mmol/L) | 1.042 (1.015–1.070) | 0.002 |
| Total cholesterol (mmol/L) | 0.831 (0.756–0.914) | <0.001 |
| Low density lipoprotein (mmol/L) | 1.678 (1.505–1.870) | <0.001 |
| Body mass index (kg/m2) | 1.017 (1.009–1.025) | <0.001 |
| H. pylori (+) | 1.055 (1.006–1.107) | 0.029 |
3.1.1. WGCNA analysis
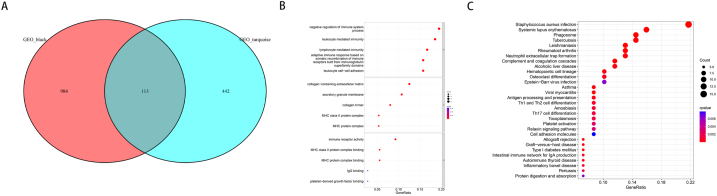
The GSE27411 and GSE28829 date were analyzed by WGCNA to build a coexpression network, and the genes associated with H. pylori infection and arterial plaque formation were identified. In GSE27411, the optimal soft threshold was set to 9 to construct a scale-free network (Fig. 1A). Using the dynamic clustering tree cutting approach, a grand total of 16 modules were identified (Fig. 1C). Among them, the black module gene was most significantly associated with H. pylori infection, including 1097 genes, with a correlation coefficient of 0.9, P < 0.01 (Fig. 1E). Similarly, in the GSE28829 dataset, the optimal soft threshold was set to 14 (Fig. 1B), and 11 modules were identified (Fig. 1D). Among them, the turquoise module genes were significantly correlated with plaque progression, including 555 genes, with a correlation coefficient of 0.83, P < 0.01 (Fig. 1F).
Fig. 1.
Construction of coexpression network of H. pylori infection and arterial plaque progression. (A, B) Selection of the optimal soft threshold in GSE27411 and GSE28829 datasets. (C, D) Division of different modules in GSE27411 and GSE28829 datasets. (E, F) Correlation between modules and diseases in GSE27411 and GSE28829 datasets.
3.1.2. Enrichment analysis
Gene enrichment analysis was performed on the module genes that were significantly related to H. pylori infection and plaque progression (Fig. 2A). GO enrichment analysis indicated a strong association between these genes and immune regulation, immune activation of various cells, extracellular matrix formation and immune receptor activity (Fig. 2B). Fig. 2C showed that the KEGG enrichment analysis revealed a strong correlation between these genes and both bacterial infection as well as various immune disorders.
Fig. 2.
Enrichment analysis of genes related to H. pylori infection and plaque progression. (A) Intersection gene of black module and turquoise module. (B) Go enrichment analysis of intersection genes. (C) KEGG enrichment analysis of intersection genes.
Identification of genes between differentially expressed genes and coexpression modules.
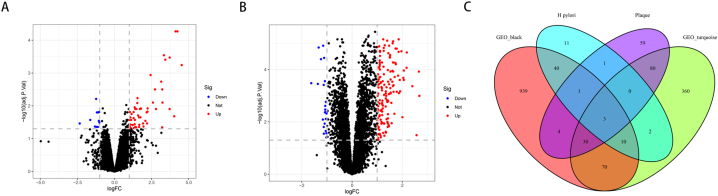
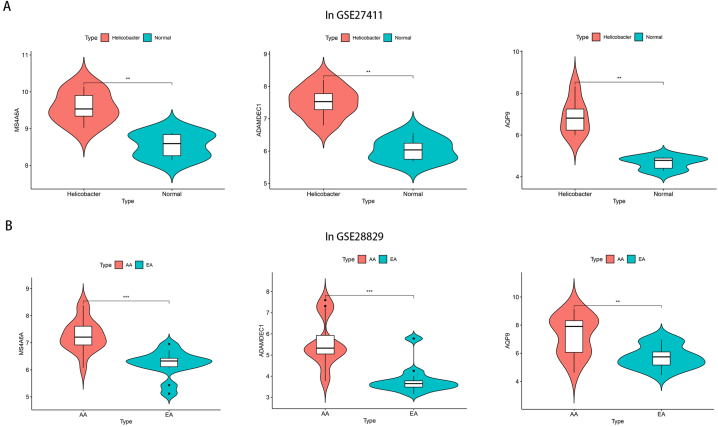
According to the screening threshold, in the GSE27411 dataset, a total of 67 differential genes were screened for differential expression in normal samples and H. pylori infected samples. Out of these genes, 11 were found to be downregulated while 56 were upregulated (Fig. 3A). In the dataset GSE28829, there were 177 genes that showed differential expression between EA and AA. Among these genes, 23 were found to be downregulated while 154 were upregulated, as depicted in Fig. 3B. The overlapping genes between the differentially expressed genes and the coexpression module genes were assumed to be the hub genes, and three hub genes, MS4A6A, ADAMDEC1 and AQP9, were identified (Fig. 3C). The expression of these genes was significantly elevated in patients infected with H. pylori and patients diagnosed with AA (Fig. 4A–B).
Fig. 3.
Identification of differential genes and hub genes. (A) Differential genes in GSE27411 dataset. (B) Differential genes in GSE28829 dataset. (C) Overlapping genes of modular genes and differential genes.
Fig. 4.
Expression of hub gene in H. pylori infected patients and patients with advanced plaque formation. (A) Expression of hub gene in GSE27411 dataset. (B) Expression of hub gene in GSE28829 dataset.
3.1.3. Immune infiltration analysis
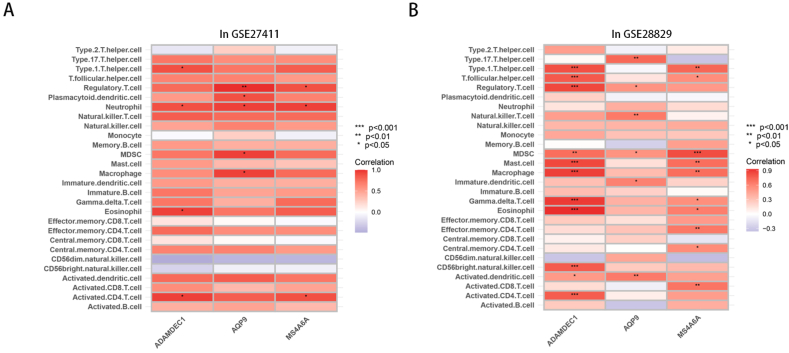
Based on the GSE27411 dataset, we used the ssGSEA method to compare 28 immune cell subsets in H. pylori infected and -uninfected patients. We found that there were differences in 10 immune cells, encompassing activated dendritic cells, activated CD4 T cells and gamma delta T cells (Fig. 5A–B). In the GSE28829 dataset, we observed significant differences in the expression of 23 immune cells, including activated CD8 T cells, activated CD4 T cells and activated B cells (Fig. 5C–D). Among them, 8 types of immune cells, including activated CD4 T cells, activated dendritic cells, gamma delta T cells, immature dendritic cells, myeloid-derived suppressor cells (MDSCs), macrophages, monocytes, and regulatory T cells, were differentially expressed in H. pylori infection and plaque progression.
Fig. 5.
Immune cell infiltration among different samples. (A) Heatmap of distribution of different immune cells in GSE27411 dataset. (B) Difference of immune cells between H. pylori infected patients and normal people. (C) Heatmap of distribution of different immune cells in GSE28829 dataset. (D) Difference of immune cells between EA patients and AA patients.
3.1.4. The correlation between hub genes and immune infiltration
We used the Spearman correlation test to compare the relationship between hub genes and various immune cells during H. pylori infection and plaque progression. In the GSE27411 dataset, MS4A6A, ADAMDEC1 and AQP9 were significantly correlated with a variety of immune cells, among which neutrophils were significantly correlated with the three hub genes (Fig. 6A). Similarly, in the GSE28829 dataset, MS4A6A, ADAMDEC1 and AQP9 were also related to a variety of immune cells, among which MDSCs and hub genes were closely related (Fig. 6B). In the process of H. pylori infection and plaque progression, ADAMDEC1 was significantly correlated with differentially expressed activated CD4 T cells, and AQP9 was closely correlated with differentially expressed regulatory T cells and MDSCs.
Fig. 6.
Relationship between hub gene and immune cells. (A) Relationship between hub gene and immune cells in GSE27411 dataset. (B) Relationship between hub gene and immune cells in GSE28829 dataset.
4. Discussion
To date, an increasing number of studies have confirmed that H. pylori infection is closely related to cardiovascular events. According to reports, infection with H. pylori may elevate the likelihood of recurring cardiovascular incidents in individuals diagnosed with acute coronary syndrome (ACS) [20]. In a meta-analysis involving 230,288 participants, it was discovered that the presence of H. pylori was associated with an elevated risk of cardiovascular disease [21]. In addition, infection with H. pylori can elevate the risk of cardiovascular events by 3–4 times [22]. However, the relationship between H. pylori and arterial plaque formation remains controversial. Some studies have verified that infection with H. pylori can elevate the likelihood of carotid atherosclerosis in individuals below the age of 50 [23]. In another study involving 14588 healthy people, H. pylori was not associated with increased carotid intima thickness [24]. However, in most studies, H. pylori infection was consistent with increased CIMT [25,26]. In our study, it was further confirmed that H. pylori infection increased the risk of carotid plaque formation.
Dyslipidemia, such as elevated TC and LDL, is an important risk factor for arterial plaque formation [27,28]. In our study, TC, LDL and H. pylori infection were observed as independent risk factors associated with the formation of carotid plaques. In a study of male subjects in Japan, H. pylori infection was significantly correlated with low HDL and high LDL [29]. Our study confirmed that in all populations, the HDL level of H. pylori infected patients was reduced, but LDL was also reduced. This result indicates that H. pylori infection may further affect the changes in lipid mass spectra [30].
Nevertheless, additional investigation is required to understand the process through which H. pylori infection contributes to the formation of atherosclerosis. Research has shown that H. pylori infection leads to chronic inflammation, triggering the release of various chemical mediators, such as tumor necrosis factor (TNF-α) and a variety of interleukins [31,32]. The activation of these inflammatory agents can trigger inflammatory reactions, either directly or indirectly causing harm to the blood vessel lining, ultimately resulting in the development of atherosclerosis. Furthermore, H. pylori additionally triggers an immune response through the activation of cyclooxygenase-2 (COX-2), leading to an elevation in the synthesis of prostaglandins and nitric oxide (NO), consequently enhancing the likelihood of developing atherosclerosis [33]. Infection with H. pylori that is positive for CagA can enhance the development of foam cells and accelerate the growth of arterial plaques [34]. In our study, we found that H. pylori infection and advanced arteriosclerosis were related to immunity, inflammation and bacterial infection.
There are different immune microenvironments in patients with H. pylori infection and advanced arteriosclerosis. Infection with H. pylori can cause a variety of T-cell immune responses, such as gamma delta T cells, regulatory T cells, activated CD4 T cells [[35], [36], [37]]. Furthermore, the activation of macrophages, dendritic cells and B cells contributes to the production of proinflammatory cytokines and chemokines [38]. Likewise, in atherosclerosis, there are also a variety of T cells, macrophages, dendritic cells and monocytes, which may participate in the formation of atherosclerotic plaques [[39], [40], [41]]. The findings of our research were in line with this. We further screened three genes related to H. pylori infection and advanced atherosclerosis, MS4A6A, ADAMDEC1 and AQP9. In patients with Alzheimer's disease, MS4A6A was confirmed to be associated with dysregulation of lipid metabolism [42]. In addition, ADAMDEC1 has been reported to be associated with susceptibility to atherosclerosis [43]. Based on whole genome sequencing, AQP9 was confirmed as a candidate gene for atherosclerosis [44]. Similarly, these genes were related to immunity, inflammation and bacterial infection [[45], [46], [47]]. Nevertheless, additional verification is required to establish the connection between these genes and H. pylori infection.
Our research has certain limitations. This study was retrospective, and it is possible that there may be some bias. In addition, the immune microenvironment and genes analyzed by bioinformatics still require further exploration by experimental research. The study also lacked other risk factors that may influence carotid plaque, such as socioeconomic status, dietary intakes, obesity, and insulin resistance [[48], [49], [50]]. H. pylori infection may further influence risk factors such as insulin resistance and obesity [51]. In the future, more well-designed intervention trials will be conducted to further exclude the influence of confounding factors on the study.
5. Conclusion
The findings of our research validated that the presence of H. pylori increased the risk of carotid plaque formation. Furthermore, we further explored the potential mechanism by which H. pylori infection causes carotid plaques, and screened three genes that may be involved in its regulation. This study provides new insight into the advanced carotid atherosclerosis caused by H. pylori infection.
Funding
None.
Data availability statement
The public data set of this study can be found in the GEO database (https://www.ncbi.nlm.nih.gov/geo/) (Accessions: GSE27411, GSE28829). Other detailed data may be obtained from the corresponding author upon reasonable request.
Authorship
Jinshun Zhang: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data. Yi Chen: Performed the experiments, Analyzed and interpreted the data, Wrote the paper. Ningning You: Analyzed and interpreted the data, Wrote the paper. Chaoyu Yang: Wrote the paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Hooi J.K.Y., Lai W.Y., Ng W.K., et al. Global prevalence of Helicobacter pylori infection: systematic review and meta-analysis. Gastroenterology. 2017;153(2):420–429. doi: 10.1053/j.gastro.2017.04.022. [DOI] [PubMed] [Google Scholar]
- 2.Keilberg D., Ottemann K.M. How Helicobacter pylori senses, targets and interacts with the gastric epithelium. Environ. Microbiol. 2016;18(3):791–806. doi: 10.1111/1462-2920.13222. [DOI] [PubMed] [Google Scholar]
- 3.White J.R., Winter J.A., Robinson K. Differential inflammatory response to Helicobacter pylori infection: etiology and clinical outcomes. J. Inflamm. Res. 2015;8:137–147. doi: 10.2147/jir.S64888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uemura N., Okamoto S., Yamamoto S., et al. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001;345(11):784–789. doi: 10.1056/NEJMoa001999. [DOI] [PubMed] [Google Scholar]
- 5.Abdel-Razik A., Mousa N., Shabana W., et al. Helicobacter pylori and non-alcoholic fatty liver disease: a new enigma? Helicobacter. 2018;23(6) doi: 10.1111/hel.12537. [DOI] [PubMed] [Google Scholar]
- 6.Pellicano R., Ianiro G., Fagoonee S., et al. Review: extragastric diseases and Helicobacter pylori. Helicobacter. 2020;25(Suppl 1) doi: 10.1111/hel.12741. [DOI] [PubMed] [Google Scholar]
- 7.Ng Q.X., Venkatanarayanan N., De Deyn M., et al. A meta-analysis of the association between Helicobacter pylori (H. pylori) infection and hyperemesis gravidarum. Helicobacter. 2018;23(1) doi: 10.1111/hel.12455. [DOI] [PubMed] [Google Scholar]
- 8.Gaudio E., Carpino G., Grassi M., Musca A. [Morphological aspects of atherosclerosis lesion: past and present] Clin. Ter. 2006;157(2):135–142. [PubMed] [Google Scholar]
- 9.Ziegler T., Abdel Rahman F., Jurisch V., Kupatt C. Atherosclerosis and the capillary network; pathophysiology and potential therapeutic strategies. Cells. 2019;9(1) doi: 10.3390/cells9010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jamkhande P.G., Gattani S.G., Farhat S.A. Helicobacter pylori and cardiovascular complications: a mechanism based review on role of Helicobacter pylori in cardiovascular diseases. Integr Med Res. 2016;5(4):244–249. doi: 10.1016/j.imr.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mladenova I. Helicobacter pylori and cardiovascular disease: update 2019. Minerva Cardioangiol. 2019;67(5):425–432. doi: 10.23736/s0026-4725.19.04986-7. [DOI] [PubMed] [Google Scholar]
- 12.Liang W., Sun F. Weighted gene co-expression network analysis to define pivotal modules and genes in diabetic heart failure. Biosci. Rep. 2020;40(7) doi: 10.1042/bsr20200507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang W., Sun F., Zhao Y., et al. Identification of susceptibility modules and genes for cardiovascular disease in diabetic patients using WGCNA analysis. J. Diabetes Res. 2020;2020 doi: 10.1155/2020/4178639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao M., Zhang C., Gao C., et al. Exploration of the shared gene signatures and molecular mechanisms between systemic lupus erythematosus and pulmonary arterial hypertension: evidence from transcriptome data. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.658341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Langfelder P., Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gene Ontology Consortium Going forward. Nucleic Acids Res. 2015;43:D1049–D1056. doi: 10.1093/nar/gku1179. Database issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kanehisa M., Sato Y., Kawashima M., et al. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44(D1):D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritchie M.E., Phipson B., Wu D., et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015;43(7):e47. doi: 10.1093/nar/gkv007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bindea G., Mlecnik B., Tosolini M., et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39(4):782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 20.Eskandarian R., Ghorbani R., Shiyasi M., et al. Prognostic role of Helicobacter pylori infection in acute coronary syndrome: a prospective cohort study. Cardiovasc J Afr. 2012;23(3):131–135. doi: 10.5830/cvja-2011-016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sun L., Zheng H., Qiu M., et al. Helicobacter pylori infection and risk of cardiovascular disease. Helicobacter. 2023 doi: 10.1111/hel.12967. [DOI] [PubMed] [Google Scholar]
- 22.Nam S.Y., Ryu K.H., Park B.J., Park S. Effects of Helicobacter pylori infection and its eradication on lipid profiles and cardiovascular diseases. Helicobacter. 2015;20(2):125–132. doi: 10.1111/hel.12182. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L., Chen Z., Xia X., et al. Helicobacter pylori infection selectively increases the risk for carotid atherosclerosis in young males. Atherosclerosis. 2019;291:71–77. doi: 10.1016/j.atherosclerosis.2019.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Y., Zhou W., Luo L., Xu W. Helicobacter pylori infection is not related to increased carotid intima-media thickness in general population. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-32465-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shan J., Bai X., Han L., et al. Association between atherosclerosis and gastric biomarkers concerning Helicobacter pylori infection in a Chinese healthy population. Exp. Gerontol. 2018;112:97–102. doi: 10.1016/j.exger.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 26.Rožanković P.B., Huzjan A.L., Cupić H., et al. Influence of CagA-positive Helicobacter pylori strains on atherosclerotic carotid disease. J. Neurol. 2011;258(5):753–761. doi: 10.1007/s00415-010-5824-9. [DOI] [PubMed] [Google Scholar]
- 27.Chen Z., Ichetovkin M., Kurtz M., et al. Cholesterol in human atherosclerotic plaque is a marker for underlying disease state and plaque vulnerability. Lipids Health Dis. 2010;9:61. doi: 10.1186/1476-511x-9-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Khatana C., Saini N.K., Chakrabarti S., et al. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxid. Med. Cell. Longev. 2020;2020 doi: 10.1155/2020/5245308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh H., Saijo Y., Yoshioka E., Tsutsui H. Helicobacter Pylori infection is a significant risk for modified lipid profile in Japanese male subjects. J. Atherosclerosis Thromb. 2010;17(10):1041–1048. doi: 10.5551/jat.5157. [DOI] [PubMed] [Google Scholar]
- 30.Haeri M., Parham M., Habibi N., Vafaeimanesh J. Effect of Helicobacter pylori infection on serum lipid profile. J Lipids. 2018;2018 doi: 10.1155/2018/6734809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Consolazio A., Borgia M.C., Ferro D., et al. Increased thrombin generation and circulating levels of tumour necrosis factor-alpha in patients with chronic Helicobacter pylori-positive gastritis. Aliment. Pharmacol. Ther. 2004;20(3):289–294. doi: 10.1111/j.1365-2036.2004.02074.x. [DOI] [PubMed] [Google Scholar]
- 32.Russo F., Jirillo E., Clemente C., et al. Circulating cytokines and gastrin levels in asymptomatic subjects infected by Helicobacter pylori (H. pylori) Immunopharmacol. Immunotoxicol. 2001;23(1):13–24. doi: 10.1081/iph-100102563. [DOI] [PubMed] [Google Scholar]
- 33.Furuto Y., Kawamura M., Yamashita J., et al. Relationship between Helicobacter pylori infection and arteriosclerosis. Int. J. Gen. Med. 2021;14:1533–1540. doi: 10.2147/ijgm.S303071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang S., Xia Y.P., Luo X.Y., et al. Exosomal CagA derived from Helicobacter pylori-infected gastric epithelial cells induces macrophage foam cell formation and promotes atherosclerosis. J. Mol. Cell. Cardiol. 2019;135:40–51. doi: 10.1016/j.yjmcc.2019.07.011. [DOI] [PubMed] [Google Scholar]
- 35.Nurgalieva Z.Z., Conner M.E., Opekun A.R., et al. B-cell and T-cell immune responses to experimental Helicobacter pylori infection in humans. Infect. Immun. 2005;73(5):2999–3006. doi: 10.1128/iai.73.5.2999-3006.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kandulski A., Malfertheiner P., Wex T. Role of regulatory T-cells in H. pylori-induced gastritis and gastric cancer. Anticancer Res. 2010;30(4):1093–1103. [PubMed] [Google Scholar]
- 37.Futagami S., Hiratsuka T., Suzuki K., et al. Gammadelta T cells increase with gastric mucosal interleukin (IL)-7, IL-1beta, and Helicobacter pylori urease specific immunoglobulin levels via CCR2 upregulation in Helicobacter pylori gastritis. J. Gastroenterol. Hepatol. 2006;21(1 Pt 1):32–40. doi: 10.1111/j.1440-1746.2005.04148.x. [DOI] [PubMed] [Google Scholar]
- 38.Reyes V.E., Peniche A.G. Helicobacter pylori deregulates T and B cell signaling to trigger immune evasion. Curr. Top. Microbiol. Immunol. 2019;421:229–265. doi: 10.1007/978-3-030-15138-6_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gisterå A., Hansson G.K. The immunology of atherosclerosis. Nat. Rev. Nephrol. 2017;13(6):368–380. doi: 10.1038/nrneph.2017.51. [DOI] [PubMed] [Google Scholar]
- 40.Fernandez D.M., Rahman A.H., Fernandez N.F., et al. Single-cell immune landscape of human atherosclerotic plaques. Nat. Med. 2019;25(10):1576–1588. doi: 10.1038/s41591-019-0590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Munjal A., Khandia R. Atherosclerosis: orchestrating cells and biomolecules involved in its activation and inhibition. Adv Protein Chem Struct Biol. 2020;120:85–122. doi: 10.1016/bs.apcsb.2019.11.002. [DOI] [PubMed] [Google Scholar]
- 42.Liu Y., Thalamuthu A., Mather K.A., et al. Plasma lipidome is dysregulated in Alzheimer's disease and is associated with disease risk genes. Transl. Psychiatry. 2021;11(1):344. doi: 10.1038/s41398-021-01362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goncharova I.A., Makeeva O.A., Golubenko M.V., et al. [Genes for fibrogenesis in the determination of susceptibility to myocardial infarction] Mol Biol (Mosk) 2016;50(1):94–105. doi: 10.7868/s0026898415060099. [DOI] [PubMed] [Google Scholar]
- 44.Inouye M., Ripatti S., Kettunen J., et al. Novel Loci for metabolic networks and multi-tissue expression studies reveal genes for atherosclerosis. PLoS Genet. 2012;8(8) doi: 10.1371/journal.pgen.1002907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gölz L., Buerfent B.C., Hofmann A., et al. Genome-wide transcriptome induced by nickel in human monocytes. Acta Biomater. 2016;43:369–382. doi: 10.1016/j.actbio.2016.07.047. [DOI] [PubMed] [Google Scholar]
- 46.Plaza-Díaz J., Robles-Sánchez C., Abadía-Molina F., et al. Adamdec1, Ednrb and Ptgs1/Cox1, inflammation genes upregulated in the intestinal mucosa of obese rats, are downregulated by three probiotic strains. Sci. Rep. 2017;7(1):1939. doi: 10.1038/s41598-017-02203-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Holm A., Magnusson K.E., Vikström E. Pseudomonas aeruginosa N-3-oxo-dodecanoyl-homoserine lactone elicits changes in cell volume, morphology, and AQP9 characteristics in macrophages. Front. Cell. Infect. Microbiol. 2016;6:32. doi: 10.3389/fcimb.2016.00032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woolsey A.B., Arsang-Jang S., Spence J.D., et al. The impact of socioeconomic status on the burden of atherosclerosis, and the effect of intensive preventive therapy on its progression: a retrospective cohort study. Atherosclerosis. 2022;358:29–33. doi: 10.1016/j.atherosclerosis.2022.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Muñoz-Cabrejas A., Laclaustra M., Guallar-Castillón P., et al. Association of beverage consumption with subclinical atherosclerosis in a Spanish working population. Sci. Rep. 2023;13(1):6509. doi: 10.1038/s41598-023-33456-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rovella V., Anemona L., Cardellini M., et al. The role of obesity in carotid plaque instability: interaction with age, gender, and cardiovascular risk factors. Cardiovasc. Diabetol. 2018;17(1):46. doi: 10.1186/s12933-018-0685-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen L.W., Kuo S.F., Chen C.H., et al. A community-based study on the association between Helicobacter pylori Infection and obesity. Sci. Rep. 2018;8(1) doi: 10.1038/s41598-018-28792-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The public data set of this study can be found in the GEO database (https://www.ncbi.nlm.nih.gov/geo/) (Accessions: GSE27411, GSE28829). Other detailed data may be obtained from the corresponding author upon reasonable request.