Abstract
Podocarpus is the most dominant genus of Podocarpaceae, with higher taxonomical proximity to the Taxaceae, having numerous pharmaceutical applications, however, scarce studies dealing with the physiological and metabolic criteria of Podocarpus in Egypt were reported. Thus, the objective of this work was to assess the physiological and metabolical patterns of the different species of Podocarpus; P. gracilior, P. elongates, P. macrophyllus and P. neriifolius. The highest terpenoids contents were reported in P. neriifolius, followed by P. elongatus, and P. macrophyllus. P. gracilior had the highest antioxidants amount, followed by P. macrophyllus, P. neriifolius and P. elongatus. From the GC/MS metabolic profiling, caryophyllene, β-cadinene, β-cuvebene, vitispirane, β-cadinene and amorphene were the most dominant metabolites in P. gracilior. β-Caryophyllene was the common in P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius with an obvious fluctuation. The plant methanolic extracts have an obvious activity against the multidrug resistant bacteria; E. coli, P. aeruginosa, S. pyogenes and S. aureus, and fungi; A. fumigatus, A. flavus, A. niger and C. albicans in a concentration-dependent manner. The highest Taxol yield was assessed in the extracts of P. elongatus (16.4 μg/gdw), followed by P. macrophyllus, and P. neriifolius. The chemical identity of Taxol derived from P. elongatus was resolved by LC/MS, with molecular mass 854.6 m/z, and similar structural fragmentation pattern of the authentic one. The highest antitumor activity of P. elongatus extracted Taxol was assessed towards HCT-116 (30.2 μg/ml), HepG-2 (53.7 μg/ml) and MCF-7 (71.8 μg/ml). The ITS sequence of P. elongatus “as potent Taxol producer” was deposited on Genbank with accession #ON540734.1, that is the first record of Podocarpus species on Genbank.
Keywords: Podocarpaceae, Taxol, Metabolomic profiling, ITS region, GC/MS profile, Bioactivity
1. Introduction
Medicinal plants are the frequent traditional natural source of diverse bioactive secondary metabolites especially terpenoids, flavonoids, alkaloids, saponins, tannins, sterols, and antioxidants. Among the medicinal plants, Podocarpaceae is one of the most recognized plant families with various valuable therapeutic activities such as anti-inflammatory, anticancer, antimicrobial, antioxidant and antidiuretic [1,2]. The water extract of the red seaweed Kappaphycus alvarezii displayed a powerful antioxidant, antibacterial activity against various pathogenic bacteria [3]. The medicinal plants Centella asiatica and Allium sativum have been used as source of bioactive compounds against various pathogenic microbes such as Candida albicans, Aspergillus niger and Penicillium sp [4,5]. Podocarpaceae (Yellow Wood) is one of the largest coniferous family after Pinaceae [6], including seven genera namely Podocarpus, Dacrydium, Phyllocladus, Acmopyle, Microcachrys, and Saxegothaea. The genus Podocarpus is usually with evergreen shrub or tree habit, dioecious, simple linear-lanceolate or linear-elliptic foliage with alternate, opposite or spiral arrangement, solitary ovulate cones with swollen, and fleshy seeds [7,8]. Podocarpus elongatus has been morphologically characterized with spirally arranged, narrowly elliptic, light green-blue colored entire simple leaves. Podocarpus macrophyllus (Thunb.) has a broad leaf with congested and spirally arranged strap-shaped entire simple leaves, dark green on top, grayish underneath, and tapered at the ends. Podocarpus neriifolius has a spiral arranged leaves, slightly curved lanceolate deep green simple broad leaves, and obtuse at tips [6].
The genera of Podocarpaceae have been implemented in various technological applications including dyes, waxes and tanning leather manufacturing [1]. Nageia nagi has been used as herbal dietary supplement, fleshy receptacle fruits of P. dacrydioides, P. nivalis, P.totara, and P. salignus have been eaten [1]. Young leaves and stems of P. nagi are eaten [9], their seeds were used in oil industry [10]. Species of Podocarpaceae have been used as an herbal remedy in traditional medicine for treatment of various human and animal diseases [11]. The stem and bark extracts of P. macrophyllus are used in treatment of blood disorders and ringworms [12]. A decoction of P. neriifolius leaves has been used in treatment of painful joints and rheumatism [13]. Overall, the extracts of Podocarpus spp are used in treatment of venereal diseases, fevers, asthma, coughs, cholera, distemper and chest complaints [1]. From the phytochemical profiles, myriad of bioactive chemical constituents were identified from the leaves and receptacles of Podocarpus spp, especially diterpenoids, flavonoids and antioxidants with useful cytotoxic properties and biological activities [11,14]. Among these bioactive compounds, Taxol is one of the most successful anticancer diterpenoid that was isolated from stems of P. gracilior [15], consequently, use of the medicinal plant extracts were reported to be an alternative to the traditional antibiotics [16]. The current study was an extension to our previous work [[17], [18], [19]], to assess the metabolic traits especially Taxol contents of the different Podocarpus species inhabiting Egyptian botanical gardens. With the myriads of predicted bioactive compounds from Podocarpus spp, these plants receive less attention in Egypt. Thus, the objective of this work was to explore the physiological and metabolic traits of different Podocarpus species, to assess their potential antimicrobial activity against the multidrug resistant microorganisms, extraction of Taxol and evaluating their anticancer activity.
2. Materials and methods
2.1. Chemicals
Authentic Taxol (Cat. #T7402), linalool (Cat. #L2602), quercetin (Cat. #Q3001), gallic acid (Cat. #G7384), l-ascorbic acid (Cat. #A92902) and bovine serum albumin (Cat. # A9056) were obtained from Sigma-Aldrich (MO, USA). All other chemicals and reagents were of analytical grades.
2.2. Collection of the Podocarpus samples
Four species of the genus Podocarpus inhabiting Egyptian botanical gardens were collected from different localities in Egypt in June/2021. Fresh and healthy leaves sample of Podocarpus gracilior (Pilg.) and P. elongatus (Aiton) L'Hér. ex Pers., were collected from Botanic Garden of the Faculty of Science, Alexandria University and Al-Zohriya garden, Giza, Egypt, respectively. The leaves of P. macrophyllus (Thunb.) D. and P. neriifolius D. were obtained from Aswan Botanical Garden, Aswan, Egypt. The plants were identified according to their taxonomic characteristic features [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30]].
2.3. Phytochemical analyses
2.3.1. Preparation of methanolic crude extracts
Fresh leaves of the experimented Podocarpus spp were washed, air-dried indoors at room temperature on sterile paper for 20 days. After drying, the plant samples were individually pulverized into fine powder, then stored in sterile vials to avoid microbial contamination, till used for extraction. Methanolic extracts of the leaves were prepared [31], with slight modifications. Briefly, 10 g of the air-dried powdered leaves of each Podocarpus species were cold macerated by soaking in 80 ml of absolute methanol with occasional shaking at 30 °C for 24 h, using a rotary shaker. The crude methanolic extracts were filtered by sterile cheesecloth, centrifuged at 5000 g for 15 min, and the supernatant was collected. The extracts from the three consecutive extractions were combined and concentrated by rotary evaporator at 40 °C, and the obtained oily residues were suspended in 10 ml absolute methanol. The extraction yield of methanolic crude extracts was quantified [32,33] according to the following equation:
| (1) |
The crude methanolic extracts were stored at −20 °C, for the ongoing analysis.
2.3.2. Phytochemical analyses
The total alkaloids, flavonoids, phenolic compounds, carbohydrate and proteins were estimated as follow.
2.3.3. Qualitative analyses
The methanolic extracts of the leaves of P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius were analyzed to identify their active constituents; terpenoids, phytosterols, flavonoids, alkaloids, saponins, tannins, phlobatannins, glycosides, anthraquinone glycosides, anthraquinone, carbohydrates, proteins, fats and oils [[34], [35], [36], [37], [38], [39], [40]].
The total alkaloids of the plant extracts were determined by Wagner's reagent. The total carbohydrate in the plant extracts was assessed by Molish Reagent (3.75 g of α-naphthol dissolved in 25 ml of Ethanol 99%). The total reducing sugars were determined by Fehling's solution. Monosaccharides were determined by Barfoed's reagent (copper acetate in distilled water and glacial acetic acid). The amino acids contents of the methanolic extract of the tested plants were determined by Ninhydrin reagent [41] and total protein content was assessed by Folin-Ciocalteau [42]. The terpenoids were assessed by chloroform: sulfuric acid assay, the plant extract was mixed with chloroform, sulfuric acid, and the reddish brown color formation at the interface between the upper chloroform layer and lower sulfuric acid layer confirms the presence of terpenoids. The phytosterols of the plant extract were determined by Liebermann-Burchard's assay. The assay contains methanolic extract of plant (0.5 ml), chloroform (1 ml), acetic acid (1 ml), followed by addition of sulfuric acid. Appearance of pink color which gradually turns into deep green color indicates the presence of sterols.
The flavonoids contents of the plants were detected by ammonia test. The reaction contains plant methanolic extract (0.5 ml) boiled with 10 ml ethylacetate for 3 min, the reaction was filtered, the filtrate was amended with 1 ml of 1% ammonia. Observation of yellowish brown coloration at lower layer of ammonia reveals the flavonoids. The flavonoids were determined by Lead acetate test. The reaction contains plant methanolic extract (1 ml), with drops of lead acetate (10%), the developed yellow precipitate confirmed the flavonoids contents. Also, the flavonoids were detected by Shinoda's test: Magnesium turnings and drops of hydrochloric acid were added to 0.5 ml of the methanolic extract of each plant species. Appearance of crimson red color after 10 min reveals the presence of flavonoids.
The alkaloids were determined by Wagner's test: The reaction contains plant methanolic extract (0.5 ml) warmed with sulfuric acid (2%) for 2 min, followed by addition of Wagner's reagent. The positive result of alkaloid test was confirmed by formation of reddish-brown colored precipitate.
Saponins concentration of the plants were assessed by Foam Test. The reaction contains plant methanolic extract (0.5 ml) combined with distilled water, shaken for 30 min. Formation of a stable honeycomb shaped foam layer persisted for at least 30 min indicates saponins presence.
The tannins titers were quantitatively determined by Braymer's test (Ferric chloride Test). The reaction mixture contains the plant methanolic extract (0.5 ml) with 4 ml of 5% ferric chloride, the development of deep blue color refers to tannins. The phlobatannins concentrations were determined by HCl test. The reaction contains plant methanolic extract (0.5 ml) boiled with 2 ml of conc. HCl for 2 min, and the red precipitate confirms the presence of phlobatannins.
The glycosides concentrations were detected by Keller-Kiliani Test. The methanolic extract of each plant (2 ml) was mixed with glacial acetic acid, followed by addition of 5 ml of 5% ferric chloride, then sulfuric acid (1 ml) was added. Formation of reddish brown ring, with bluish green at the upper surface, indicates the presence of glycosides. The anthraquinone glycosides were determined by hydroxyanthraquinone: Two mls of the methanolic extract of each plant were amended with 5 ml of potassium hydroxide solution (10%), the appeared red color indicates presence of anthraquinone glycosides.
The anthraquinone were determined by Borntrager's test: The assay contains 1 ml of methanolic extract of each plant, 10 ml of diluted ammonia (10%), shaken vigorously for 30 s. The appearance of red solution confirms the presence of anthraquinone. Phenolic compounds were determined by Ferric chloride assay; the methanolic extract of each plant (0.5 ml) was diluted by distilled water, followed by 3 drops of 10% ferric chloride. The appearance of black blue color reveals the presence of phenol compounds. The soluble starch of the tested plants was determined by 5% KOH (1 ml) to the methanolic extract of plant, the reaction was cooled, and then acidified with concentrated sulfuric acid, and the yellow coloration reveals the presence of soluble starch.
The fats and fixed oils were determined by sodium hydroxide test: The extract of each plant (5 drops) was mixed with 2 ml copper sulphate (1%), and sodium hydroxide (10%). Appearance of a clear blue solution indicates the presence of fats and oils. The resins were determined by acetic anhydride test: The methanolic extract for each plant (0.5 ml) was combined with 1 ml of acetic anhydride solution and sulfuric acid, the developed orange to yellow color indicates the resins presence.
The concentrations of coumarins were determined by NaOH test: The plant extract (0.5 ml) was mixed with 1 ml of 10% sodium hydroxide and 1 ml chloroform. Appearance of yellow color confirms the coumarins. Emodins concentration was determined by ammonium hydroxide/benzene test: The plant extract was mixed with 2 ml ammonium hydroxide and 3 ml benzene, and the appearance of a red color confirms the presence of emodins.
The anthocyanin concentration was determined by HCl test: The methanolic extract (0.5 ml) of the plant was mixed with 2 ml of 2 N HCl, followed by 1 ml of ammonia solution. Development of a Pink-red solution which turns blue-violet after addition of ammonia confirms the presence of anthocyanins. The leuconthocyanins concentration was determined by isoamyl alcohol test. The extract (0.5 ml) of each plant was mixed with 1 ml of isoamyl alcohol, the appearance of red upper layer indicates the presence of leuconthocyanins.
The gums and mucilage were qualitatively assessed by alcohol test: The methanolic extract (25 mg) of each plant was dissolved in distilled water (2.5 ml), followed by 2.5 ml of alcohol. The appearance of cloudy precipitate shows the presence of gums and mucilages. The volatile oils were determined by the Fluorescence test: The methanolic extract (2 ml) of each plant was exposed to UV light, as revealed from the development of Bright pinkish fluorescence.
2.4. Quantitative phytochemical analysis
The methanolic extracts of the leaves of P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius were used for the quantitative phytochemical assays of their secondary metabolites; terpenoids, flavonoids, phenolic compounds and total antioxidants.
2.4.1. Total terpenoids content (TTC)
The total terpenoids were assessed by sulfuric acid assay [43]. Dried plant extract (0.5 g) was frozen in liquid nitrogen, homogenized in pre-chilled absolute methanol (3.5 ml) with a homogenizer (JRJ300-SH) at 30 Hz for 5 min. After homogenization, the samples were incubated at 30 °C for 48 h in the dark, centrifugation at 4000g for 15 min, 0.2 ml of the supernatant were mixed with 1.5 ml chloroform, vortex and kept for 3 min. Sulfuric acid (0.1 ml) was added, and the tubes were incubated at room temperature for 2 h in dark, except the standard solution that was incubated for 5 min. The reddish brown precipitate was collected, and the precipitate was partially soluble in absolute methanol and measured at λ538 nm by UV-VIS Spectrophotometer with methanol as blank. The total terpenoids was calculated from the linalool authentic concentrations (Cat. #L2602).
2.4.2. Total flavonoids content (TFC)
Total flavonoids were assessed by aluminum chloride [44,45], the methanolic extract was mixed well with methanol (1.5 ml), aluminum chloride (0.1 ml), potassium acetate (0.1 ml) and water (2 ml), incubated at 30 °C for 30 min. Sample and blank of all extracts were prepared and their absorbance was measured at λ415 nm. The flavonoids content was determined regarding to the authentic concentrations of quercetin (Cat. #Q3001).
2.4.3. Total phenolic content (TPC)
The contents of phenolic compounds were assessed by Folin-Ciocalteu assay [[46], [47], [48]], with some modification. Methanolic plant extract was mixed well with 1 ml of Folin-Ciocalteau reagent. After 5 min, 10 ml of sodium carbonate solution (7.5%) was added, mixed, the solution mixture was diluted with water (25 ml) thoroughly, incubation for 90 min at ambient temperature. After incubation, the absorbance of sample was measured at λ750 nm. The total phenolic content of the plant samples was measured by the authentic gallic acid (Sigma-Aldrich, Cat. #G7384).
2.4.4. Antioxidant activities
The total antioxidant contents was determined by 2,2-diphenyl-1-picrylhydrazyl (DPPH) assay [[49], [50], [51]]. The reagent was prepared by dispensing 24 mg of DPPH in absolute methanol (100 ml). The reaction mixture contains 100 μl of the plant methanolic extract to DPPH solution (3 ml). The sample absorbance and the reference compound (ascorbic acid) were measured at λ517 nm. The ratio of free radical scavenging activity (RSA) was determined by the formula:
| (2) |
The inhibitory concentration (IC50) was expressed by the sample concentration inhibiting 50% DPPH radical scavenging according to the graphic plot of the dose response curve.
2.5. Antimicrobial activity of the plant extract
The biological activity of the methanolic extract of the experimented plants was evaluated against various antibiotic resistant bacteria such as E. coli, P. aeruginosa, S. pyogenes and S. aureus [52,53]. The bacterial isolates were identified regarding to their biochemical properties by Bergey's manual [54]. In addition, the antifungal activity of the different plant extracts was assessed against various pathogenic fungi; Aspergillus flavus, A. fumigatus, A. niger and C. albicans. The antimicrobial activity of the different extracts was assessed by disc diffusion method [[52], [53], [54], [55]]. The bacterial suspension of 24 h old culture was seeded to the nutrient agar medium, and poured to the sterilized plates. The inoculated plates were incubated for 4 h, then different concentrations of the plant methanolic extracts (15, 30, 45 and 60 μl) were loaded to the filter paper discs to the plate surface. The bacterial cultures were incubated for 37 °C for 24 h, and the fungal cultures was incubated for 5 days at 30 °C. Negative control of methanol was used by the same volumes. Amoxicillin and fluconazole were used as positive controls for bacteria and fungi. The diameters of the inhibition zones were measured in millimeters, and calculated using Image J software portal [52].
2.6. Metabolic profiling of the plant extracts
2.6.1. GC/MS analysis
The metabolic identity of the tested plants methanolic extracts was assessed by GC/MS interfaced with mass selective-detector with polar Agilent HP-5 ms (5%-Phenyl methyl polysiloxane) column (30 m × 0.25 mm × 0.25 μm thickness) and carrier gas “helium”, at linear velocity rate 1 ml/min, injector temperature 200 °C and detector temperature 250 °C. The sample (100 μl) was injected, ionization potential 70 eV, interface 250 °C, and acquisition mass range 50–800 m/z. The chemical identity of the phytoconstituents was identified according to their spectral fragmentation pattern, and retention times by NIST mass spectral library.
2.6.2. Taxol extraction
Taxol was extracted from the leaves and twigs of Podocarpus spp [15]. Parts of the four Podocarpus spp (leaves and twigs) were washed and dried at 30 °C, grinded in liquid nitrogen to fine powder. Taxol was extracted according to Whiterup et al. [56], with slight modification. Fifty grams (50 g) of the dried powdered plant tissues was amended with 400 ml n-Hexane for 48 h, centrifuged for 10 min at 5000 rpm to remove the undesirable non-polar compounds. The hexane supernatant solution was carefully discarded, the resulting defatted pellets were frozen at −20 °C overnight, and the pellets were soaked in 200 ml methanol: methylene chloride (1:1) with shaking at room temperature for 24 h, filtered by sterile cheesecloth, and centrifuged at 5000 rpm. The pellets were dried in oven at 60 °C till constant weight, and the combined supernatant extracts from the consecutive extractions were evaporated using rotary vacuum evaporator to separate the solvent from the extract, then kept at −20 °C overnight. To extract Taxol, the dried crude extracts were dissolved in 50 ml methylene chloride and partitioned with 50 ml of distilled water, the two fractions were separated using a separation funnel, the lower layer of methylene chloride fractions (d: 1.3 g/mL) were collected, evaporated till dryness, followed by re-dissolving the dried residues in 5 ml absolute methanol.
2.6.3. Taxol quantification, HPLC and LC/MS analyses
The extracted Taxol was quantified by UV-absorption at wavelength λ227 nm [17] using UV-VIS Spectrophotometer compared to authentic one (Cat. #T7402). Methanol was used as negative control for zeroing the spectrophotometer. Standard curve of different authentic Taxol was plotted at λ227 nm. The extracted Taxol from each individual Podocarpus species was checked by TLC [17],[57]. Ten μl of authentic Taxol and 10 μl of each Taxol extract was spotted on Merck 1 mm, pre-coated silica gel plates with Chloroform: Methanol: Dimethyl formamide (90:9:1 v/v/v) as mobile phase, and the spots of Taxol was detected by illumination at λ254 nm.
The proposed spots of Taxol containing silica were scraped-off and suspended in methanol, for Taxol extraction [17]. The amount of Taxol was assessed by HPLC (YOUNG In.) of RP-C18 column (Cat.#959963-902) with methanol: acetonitrile: water (25:35:40, v/v/v) as an isocratic mobile phase at flow rate 1.0 ml/min for 20 min [17]. The identity and concentration of extracted Taxol was confirmed from the retention time and absorption peak at 227 nm [17],[56]. The chemical structure of Taxol was resolved from the LC/MS analysis coupled with a UV and quadruple detector. The mobile phase was water: acetonitrile (10/90) with flow rate of 0.2 ml/min [58,59].
2.6.4. Cytotoxicity of the purified Podocarpus Taxol
The anticancer activity of the putative Taxol sample was assessed towards the liver carcinoma (HepG-2), breast carcinoma (MCF-7) and colon carcinoma (HCT-116), compared to the normal healthy VERO cells, by the MTT assay [60]. The 96-wells plate was seeded with 103 cells/well, incubated at 12 h at 37 °C in CO2 incubator, then adding of different Taxol concentrations, and the plates were incubated for 24 h. The MTT reagent was added, and the intracellular formazan complex was dissolved in DMSO, and the resulted complex was measured at λ570 nm. The cell viability was expressed by the sample absorbance per control x100. The IC50 value was expressed by Taxol concentration reducing 50% of the initial number of tumor cells compared to saline as baseline.
2.7. Molecular identification of the experimented plants
2.7.1. DNA extraction
The plant genomic DNA was isolated from the dried healthy leaves of P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius by Cetyltrimethylammonium bromide (CTAB) [[61], [62], [63]], with minor modification. The dried healthy leaves (0.25 g) were minced to fine powder in liquid nitrogen, transferred to tubes of 500 μl freshly prepared CTAB-β-mercaptoethanol lysis buffer (2x CTAB isolation buffer: 2% CTAB, Tris HCl (100 mM, pH 8.0), EDTA (20 mM), NaCl (1.4 M), PVP40 (2%) and β-mercaptoethanol (0.2%)). The tubes were vortexed, incubated in water bath at 65 °C for 60 min, then centrifuged at 10000 rpm for 10 min at 4 °C, supernatant was mixed with chloroform: isoamylalcohol (24:1), vortex, and centrifuged. The upper aqueous phase was carefully withdrawn and mixed with two volumes of chilled absolute isopropanol and inverted gently several times, then kept at −20 °C for 1 h for DNA precipitation. After sample centrifugation, the DNA pellets were washed by 70% ethanol, the supernatant was discarded, and dried at 30 °C, and 50 μl of TAE buffer was added, and pellets of DNA was kept at −20 °C till further use. The extracted genomic DNA from the Podocarpus spp was checked by 1.0% agarose gel (1% agarose) in 1 x TAE (Cat. # AM9864), normalized to 100 bp DNA ladder (Cat. #. DM003-R500 Gene Direx, Inc.). The DNA was visualized by gel documentation system.
2.7.2. PCR amplification and sequencing of the ITS region
The internal transcribed spacer (ITS) region has been used as informative region for phylogenetic comparisons of related species [64,65]. The PCR reaction was performed using the 2 × PCR master mixture (Cat. # 25027) according to the manufacturer's instructions. The amplicons were checked by the 2.0% agarose gel and sequenced by Applied Biosystem Sequencer [18]. The recovered sequence was non-redundantly BLAST searched on NCBI database by ClustalW muscle [66], the phylogenetic relationships were conducted by MEGA version X [67].
2.8. Statistical analysis
The experiments were conducted in biological triplicates, and the results were expressed by the mean ± SD. The significance and F-test were calculated using one-way ANOVA with Fisher's Least Significant Difference of post hoc test.
3. Results and discussion
3.1. Extraction and qualitative phytochemical properties of the different species of Podocarpus
The phytochemical properties of the experimented Podocarpus species namely; P. gracillior, P. elongates, P. macrophyllus and P. neriifolius were determined. The air-dried plant leaves were pulverized, and the methanolic extracts were prepared. The total weight of the crude extract, dried powder extract and total methanolic extract was shown in Fig. S1. The morphological appearance of the experimented plants were varied from yellow-green (P. gracillior), olive green (P. elongates) and dark green (P. macrophyllus and P. neriifolius). The yield of the methanolic extract of P. gracillior, P. elongates, P. macrophyllus and P. neriifolius were 41.5% (4.15 g), 11% (1.10 g), 18.7% (1.87 g) and 26.9% (2.69 g), respectively (Fig. S1). The bioactive secondary phytoconstituents were analyzed in the methanolic extracts of the experimented Podocarpus species. The total terpenoids, phytosterols, flavonoids, alkaloids, saponins, tannins, phlobatannins, glycosides, anthraquinone glycosides, anthraquinone, carbohydrates, proteins, amino acids, fats and oils were qualitatively estimated. The qualitative detection of the phytochemicals was conducted based on the visual coloration of the reaction. The appearance and intensities of the evolved colored solutions and precipitates, reveals the chemical constituents of leaf methanolic extracts of the investigated plants, as shown on Table 1, and Fig. S2. The methanolic extracts of the plant leaves displayed a fluctuated coloration, revealing the different intrinsic concentrations of the terpenoids, steroids, flavonoids, alkaloids, saponins, tannins, phlobatannins, glycosides, phenolic compounds, monosaccharides, free and combined reducing sugars, fats, oils, coumarins, quinones and volatile oils. But anthraquinine, anthraquinone glycosides, soluble starch, proteins, amino acids, resins, emodins, anthocyanins, leuconthocyanins, gums and mucilages are absent. From the qualitative analysis of bioactive metabolites, P. neriifolius had the highest constituents of terpenoids, phytosterols, flavonoids, saponins, tennins, phlobatannins, phenolic compounds, carbohydrates, fats and fixed oils, coumarins, quinones, and volatile oils, followed by P. macrophyllus and P. elongatus. However, P. gracilior exhibited a lower frequency of the tested phytochemical constituents, comparing to the other three experimented plants.
Table 1.
Comparative quantification of total phytochemicals of methanol extract of the four Podocarpus spp. The methanolic extracts of the tested plants were prepared and their total terpenoids, flavonoids, phenolic compounds and antioxidant activity were determined.
| Phytoconstituents | Selected Plant species |
|||
|---|---|---|---|---|
| P. gracilior | P. elongatus | P. macrophyllus | P. neriifolius | |
| Total Terpenoids (mg/g) | 3.13 ± 0.79 | 4.19 ± 0.95 | 3.98 ± 0.64 | 4.53 ± 0.71 |
| Total Flavonoids (mg/g) | 15.36 ± 0.62 | 22.67 ± 1.45 | 15.31 ± 0.95 | 23.54 ± 1.42 |
| Total Phenolic compounds (mg/g) | 140.92 ± 3.64 | 243.71 ± 5.93 | 250.56 ± 6.72 | 274.38 ± 8.19 |
| Total antioxidant activity (IC50, μg/ml) | 20.75 ± 3.94 | 7.90 ± 1.65 | 12.73 ± 2.19 | 10.20 ± 1.98 |
3.2. Quantitative analyses of the phytochemical contents of the tested Podocarpus species
The total bioactive terpenoids, flavonoids, and phenolic compounds were estimated on the experimented Podocarpus species. From the profile of phytochemical analysis (Table 1, Fig. 1A,B,C), the highest terpenoids contents were measured for P. neriifolius (4.53 mg/g), followed by P. elongatus (4.19 mg/g), P. macrophyllus (3.98 mg/g), and P. gracilior (3.13 mg/g). P. neriifolius had the highest concentrations of total flavonoids (23.5 mg/g) and total phenolic compounds (274.3 mg/g), followed by P. elongatus, P. macrophyllus and P. gracilior. The highest contents of total antioxidants activity was reported for P. gracilior (20.7 μg/ml), followed by P. macrophyllus (12.7 μg/ml), P. neriifolius (10.2 μg/ml) and P. elongatus (7.9 μg/ml). From the profile of the phytochemical constituents, P. neriifolius had the highest total terpenoids, flavonoids, phenolic compounds and antioxidants activity, followed by P. elongatus and P. macrophyllus. The fluctuation of the phytochemical constituents of the experimented plants, might be correlated to the environmental and geographical circumstances regulating the molecular expression of the bioactive encoding-gene cluster.
Fig. 1.
Quantitative assay of Terpenoids (A), flavonoids (B) and total phenolic compound (C) and total antioxidants (D,E) of P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius. The data were represented by means ± STDEV.
The total antioxidant activities including proteineous and non-proteineous compounds were determined on the methanolic extracts of the tested plants by DPPH. From the profile of antioxidant activity (Fig. 1D and E), the concentration of the antioxidants was increased gradually with the increasing of the plant concentration on a concentration-dependent pattern, with the highest antioxidant activity for P. gracilior (20.7 μg/ml), followed by P. macrophyllus (12.7 μg/ml), P. neriifolius (10.2 μg/ml) and P. elongatus (7.9 μg/ml). The higher frequency of the antioxidant activity of P. gracilior comparing to other experimented species of Podocarpus, authenticate the molecular expression of the antioxidant compounds on the environmental conditions.
3.3. GC/MS metabolic profiling
The metabolic profiling of the experimental plants; P. gracilior, P. macrophyllus, P. neriifolius, and P. elongatus were assessed by the GC/MS. Recently, the GC/MS was used as an authenticating technological tool for metabolic outlining, phytochemical constituents and taxonomical purposes [68]. The methanolic extracts of the experimented Podocarpus were investigated for their bioactive volatile phyto-constituents by GC-MS. The overall ion current chromatograms of GC–MS results was shown in Fig. 2, Fig. 3, suggesting the presence of various compounds of corresponding peaks at different retention times (Table 2). From the incidence of secondary metabolites as revealed from the metabolic profiling of the experimented plants, P. gracilior have the highest frequency of diverse bioactive metabolites (99%), followed by P. elongatus (75%), P. macrophyllus (60%) and P. neriifolius (50%). Total fifty eight compounds were detected in the methanolic extract of the Podocarpus species, as revealed the GC/MS profile. From the GC-MS profiling (Table 2, Fig. 2A), the most dominant compound of P. gracilior were caryophyllene (17.10%), β-cadinene (4.22%), β-cuvebene (9.16%), vitispirane (8.25%), δ-amorphene or cadina-1(10),4-diene (3.93%) and α-cedrene (3.81%). The most frequent metabolites of P. elongatus was lepidozene (26.52%), germacrene (18.27%), spathulenol (10.29%), caryophyllene (7.26%), 9-octadecenoic acid (3.94%) and 1-heptatria-cotanol (3.71%) (Fig. 2B). The most dominant secondary metabolites by P. macrophyllus was caryophyllene (36.41%), Kaur-16-ene (18.85%), vitispirane (14.63%), cis-α-bisabolenen (6.45%), selinene (5.19%), caryophylene oxide (2.86%). As revealed from the GC-MS results (Fig. 3A), the most incident metabolites of P. neriifolius, was Kaur-16-ene (70.95%), labda-8(20),12,14-triene (7.00%), elemene (3.88%), β-caryophyllene (3.66%), atis-16-ene (3.51%), and retinal (1.26%). From the GC/MS profiling, β-caryophyllene was the only compound shared in all of the experimented plants P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius (Fig. 3B). Practically, among the recovered terpenoids, cubebol, β-cadinene, β-cubebene and δ-amorphene were the most dominant sesquiterpens of P. gracilior, unlike to the absence of these compounds on the other Podocarpus spp. The unique presence of these metabolites on P. gracilior, revealing the molecular manipulation and expression of the sesquiterpens bioactive encoding genes cluster that might be related to the environmental conditions. Similar results for active compounds, antioxidant, podocarpic acid, and other flavonoids, and diterpenoids were extracted from leaves of Podocarpaceae [1]. Many of these active compounds displayed beneficial biological activities, cytotoxic properties, antimicrobial activities [14]. Podocarpus macrophyllus bark has been used for treatment of blood disorders, tonic for heart, lungs, kidneys and stomach disorders [12]. The metabolic profiling was used extensively for various taxonomic purposes of the family Taxaceae and Podocarpaceae. The recovered compounds were designated to numerous chemical classes; esters, fatty acids, fatty acid esters, phenolic compounds, alcohols and vitamin E. So, from this study, the phytochemical ingredients of leaves of the experimented species of Podocarpus collected from different localities in Egypt were explored. The compound 1,2-benzenedicarboxylicacid, bis(2-ethylhexyl) ester isolated from twigs of Thevetia peruviana had a strong anticancer activity on PC3, and MCF-7 [69].
Fig. 2.
GC-MS metabolic profiling of the methanolic extracts of P. gracilor and P. elongatus. The total ion chromatogram (TIC) of the methanolic extract of P. gracilor (A) and P. elongatus (B). The mass spectra of the most common six metabolites were illustrated under the TIC chromatogram of each plant.
Fig. 3.
GC-MS metabolic profiling of the methanolic extracts of P. macrophyllus and P. neriifolius. The total ion chromatogram (TIC) of the methanolic extract of P. macrophyllus (A) and P. elongatus (B). The mass spectra of the most common six metabolites were illustrated under the TIC chromatogram of each plant.
Table 2.
Comparative GC-MS metabolic profiling of the methanolic extracts of the experimented Podocarpus species, showing the predicted compound name, molecular formula and their identities.
| RT (min) | Compound | Nature of Compound | Molecular formula | Selected Plant species (Peak area %) |
||||
|---|---|---|---|---|---|---|---|---|
| P. gracilior | P. elongatus | P. macrophyllus | P. neriifolius | |||||
| 1 | 4.09 | Linoleic Acid Chloride | Omega-6 fatty acid | C18H31ClO | 0.96 | – | – | – |
| 2 | 4.16 | 2-(7-Heptadecynyloxy) tetrahydro-2H-pyran | Flavonoid | C22H40O2 | 0.87 | – | – | – |
| 3 | 4.26 | Oleic acid | Omega-9 fatty acid | C18H34O2 | 1.22 | – | – | – |
| 4 | 7.45 | Oxamyl | Carbamate ester | C7H13N3O3S | 1.18 | – | – | – |
| 5 | 7.82 | 2,5,5,8A-tetramethyl −3,5,8,8 A- tetrahydro-2H-chromene |
C13-apocarotenoids or Norisoprenoids | C13H20O | 1.94 | – | – | – |
| 6 | 8.26 | Vitispirane | C13-apocarotenoids or Norisoprenoids | C13H20O | 8.25 | – | 14.63 | 1.99 |
| 7 | 9.39 | ç-Elemene | Sesquiterpenoid | C15H24 | – | 1.98 | 5.19 | 3.88 |
| 8 | 9.60 | Farnesol (3,7,11-trimethyl-2,6,10-dodecatrien-1-ol) | Acyclic sesquiterpene alcohol | C15H26O | 1.46 | – | – | – |
| 9 | 9.61 | α-copaene | Sesquiterpenoid | C15H24 | – | – | 1.91 | – |
| 10 | 10.13 | α-cedrene | Sesquiterpenoid | C15H24 | 3.81 | |||
| 11 | 10.80 | 2-Methyl-4-(2,6,6-trimethyl-1-cyclohexen-1-yl)-2-butenal | Aldehyde | C14H22O | – | 1.14 | – | – |
| 12 | 10.81 | 2,3-Dehydro-4-oxo-α-ionol | Sesquiterpenoid | C13H18O2 | 0.65 | – | – | – |
| 13 | 11.01 | β- Caryophyllene | Bicyclic sesquiterpenoid | C15H24 | 17.10 | 7.26 | 36.41 | 3.66 |
| 14 | 11.71 | Cis-α-Bisabolene | Sesquiterpenoid | C15H24 | 3.21 | – | 6.45 | – |
| 15 | 12.28 | β-Cuvebene or β –Cubebene | Sesquiterpenoid | C15H24 | 9.16 | 1.12 | – | – |
| 16 | 12.58 | α –Selinene | Sesquiterpenoid | C15H24 | – | – | 5.19 | 3.88 |
| 17 | 12.59 | Lepidozene | bicyclogermacrene sesquiterpenoid |
C15H24 | – | 26.52 | – | 3.88 |
| 18 | 12.70 | β-Cadinene | Sesquiterpenoid | C15H24 | 4.22 | – | – | – |
| 19 | 13.03 | α-ylangene | Sesquiterpenoid | C15H24 | 1.62 | 1.51 | 2.12 | – |
| 20 | 13.16 | δ-Amorphene or cadina-1(10),4-diene | Sesquiterpenoid | C15H24 | 3.93 | 3.64 | – | – |
| 21 | 13.31 | Cubebol | Sesquiterpenoid | C15H26O | 3.49 | – | – | – |
| 22 | 14.28 | Spathulenol | Sesquiterpenoid | C15H24O | – | 10.29 | – | – |
| 23 | 14.29 | Caryophylene oxide | sesquiterpenoid alcohol | C15H24O | 0.75 | – | 2.86 | – |
| 24 | 15.37 | α-acorenol | Sesquiterpenoid | C15H26O | 0.63 | 1.79 | – | – |
| 25 | 15.49 | Cedrane-8,13-diol | Sesquiterpenoid | C15H26O2 | 3.53 | – | – | |
| 26 | 15.54 | Longifolene or Junipene | Tricyclic sesquiterpenoid | C15H24 | 1.52 | – | – | – |
| 27 | 15.78 | 2,5-Octadecadiynoic acid, methyl ester | Fatty acid | C19H30O2 | 0.74 | – | 2.94 | – |
| 28 | 15.85 | 2-[4-Methyl-6-(2,6,6-trimethylcyclohex-1-enyl)hexa-1,3,5-trienyl]cyclohex-1-en-1-carboxaldehyde | Aldehyde | C23H32O | 0.75 | – | – | – |
| 29 | 15.89 | 10,12-Pentacosadiynoic acid methyl ester | Fatty acid | C26H44O2 | – | 1.33 | – | – |
| 30 | 15.95 | Methyl 4,7,10,13-hexadecatetraenoate | Fatty Acid | C17H26O2 | 1.66 | – | – | – |
| 31 | 16.43 | Retinal (Vitamin A aldehyde) | Vitamin | C20H28O | 3.18 | – | – | 1.26 |
| 32 | 18.92 | 9-octadecenoic acid (Z) | Fatty Acid | C18H34O2 | – | 3.94 | 3.67 | 2.49 |
| 33 | 18.94 | 12-Methyl-E,E−2,13-octadecadien-1-ol | Fatty alcohol | C19H36O | 2.05 | – | – | – |
| 34 | 19.65 | Vincadifformine | Alkaloid | C21H26N2O2 | – | – | 1.80 | – |
| 35 | 19.66 | 1-Heptatriacotanol | Fatty alcohol | C37H76O | – | 1.33 | – | – |
| 36 | 20.36 | Atis-16-ene, (5á,8à,9á,10à,12à | Diterpenoid | C20H32 | – | – | – | 3.51 |
| 37 | 20.52 | Palmitic acid, methyl ester | Fatty acid | C17H34O2 | – | 3.50 | ||
| 38 | 20.54 | Tetraacetyl-d-xylonic nitrile | Sugar acid | C14H17NO9 | 1.11 | – | – | – |
| 39 | 20.83 | Biformene or Labda-8(20),12,14-triene | Diterpenoid | C20H32 | – | – | – | 7.00 |
| 40 | 20.99 | Desulphosinigrin | Carbohydrate | C10H17NO6S | 2.31 | – | – | – |
| 41 | 21.06 | Melezitose | Carbohydrate | C18H32O16 | 2.01 | – | – | – |
| 42 | 21.15 | 3-O-Methylhexose (3-O-Methyl-d-glucose) | Carbohydrate | C7H14O6 | 4.31 | – | – | – |
| 43 | 21.99 | Kaur-16-ene or α-Podocarpene | Diterpenoid | C20H32 | – | – | 18.85 | 70.95 |
| 44 | 22.09 | 2,3-Dihydroxypropyl palmitate | Fatty acid ester | C19H38O4 | 1.48 | – | – | – |
| 45 | 22.18 | Dodecanoic acid, 2,3-bis(acetyloxy)propyl ester | Fatty acid ester | C19H34O6 | 1.03 | – | – | – |
| 46 | 23.26 | Pentadecanoic acid | Fatty acid | C15H30O2 | 0.61 | – | – | – |
| 47 | 23.30 | cis-Oxiraneundecanoic acid | Fatty acid | C19H36O3 | 1.01 | – | – | – |
| 48 | 23.42 | 3-Oxo-20-methyl-11-à- hydroxyconanine-1,4-diene | Steroid alkaloid | C22H31NO2 | 0.80 | – | – | – |
| 49 | 23.61 | Methyl stearate | Fatty acid | C19H38O | – | 1.70 | – | – |
| 50 | 24.68 | Methyl-9,9,10,10-D4-octadecanoate | Fatty acid | C19H34D4O2 | 0.99 | – | – | – |
| 51 | 26.73 | 9,10 dideutero octadecanoic acid | Fatty acid | C18H34D2O2 | 1.45 | – | – | – |
| 52 | 29.36 | 10-Methoxy-N(b)-à –methylcorynantheol | Alkaloid | C21H29N2O2 | 1.46 | 1.72 | – | – |
| 53 | 34.19 | 2-Monolinolenin 2TMS derivative |
Fatty acid | C27H52O4Si2 | 0.35 | – | – | – |
| 54 | 34.34 | Calcitriol or 1,25-Dihydroxyvitamin D3, TMS derivative | Vitamin | C30H52O3Si | 1.29 | 1.02 | – | 0.83 |
| 55 | 34.58 | Vitamin E, alpha-tochopherol | Vitamin | C29H50O2 | – | – | 3.18 | – |
| 56 | 34.66 | Quercetin 7,3′,4′-trimethyl ether | Flavenoid | C18H16O7 | 0.76 | 3.03 | 1.58 | |
| 57 | 35.48 | 1-Heptatriacotanol | Fatty alcohol | C37H76O | – | 3.71 | – | – |
| 58 | 35.50 | Ergosta-5,22-dien-3-ol, acetate, (3á,22E)- | Steroid | C30H48O2 | 1.05 | – | – | – |
3.4. Antimicrobial activity of the methanolic extracts of the Podocarpus species
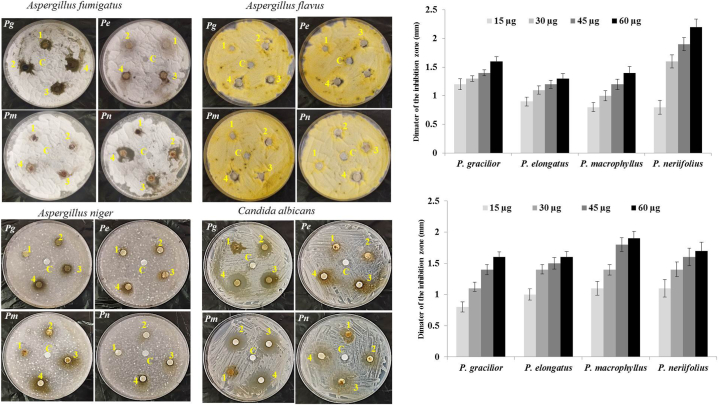
The efficacy of the different methanolic extracts of P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius was assessed by disc diffusion assay, against the multidrug resistant bacteria; E. coli, P. aeruginosa, S. pyogenes and S. aureus, as well as, against fungal isolates Aspergillus fumigatus, A. niger and C. albicans. From the antibacterial profile (Fig. 4A), the methanolic extracts of the plants displayed an obvious activity towards the tested bacterial isolates in a concentration-dependent manner, comparing to methanol as negative control. The inhibition zone diameter of P. gracilior methanolic extract (60 μl) for E. coli, P. aeruginosa, S. pyogenes and S. aureus were ranged between 20 mm and 25 mm. As well as, the antibacterial activity of P. neriifolius was maximally reported towards E. coli, P. aeruginosa, S. pyogenes and S. aureus with inhibition zone ranged between 20 and 28 mm, comparing to methanol as negative control. The antimicrobial efficiency of the tested extracts was assessed from the IC50 values towards the different bacterial isolate (Fig. 4B). The antifungal activity of the methanolic extract of the experimented plants was assessed towards A. fumigatus, A. niger and C. albicans. From the antifungal activity profile (Fig. 5A), the extracts of Podocarpus species displayed a strong activity towards A. fumigatus, A. flavus and A. niger. The highest antifungal was reported for the extracts of P. neriifolius towards A. fumigatus, A. niger and C. albicans with average inhibition zone 20–25 mm, at 60 μl of the plant extract (Fig. 5B). Remarkably, the antimicrobial activity of the plant extract towards the tested fungal isolates was increased in a concentration-dependent manner, revealing the significant impact of the bioactive constituents of the plant extracts.
Fig. 4.
Antibacterial activity of the methanolic leaf extracts of Podocarpus spp towards the multiple drug resistant bacteria; E. coli, Pseudomonas aeruginosa, Streptococcus pyogenes and Staphylococcus aureus by disc diffusion assay. P. gracillior (Pg); P. elongatus (Pe); P. macrophyllus (Pm) and P. neriifolius (Pn). Different concentrations of the plant methanolic extracts were used (1 (15 μl), 2 (30 μl), 3 (45 μl) and 4 (60 μl)), injected to the Whatman Filter paper disk, uniformly placed on the surface of bacterial plate culture and incubated at 3 days at 37 °C. B, The diameter of the inhibition zones the different bacterial isolates in response to the response to plant extracts. The data were represented by means ± STDE, with p-value ≤0.05 (ONE Way ANOVA test).
Fig. 5.
Antifungal activity of the methanolic leaf extracts of Podocarpus spp against the different phytopathogens Aspergillus fumigatus, A. flavus, A. niger and C. albicans P. gracillior (Pg); P. elongatus (Pe); P. macrophyllus (Pm) and P. neriifolius (Pn). Different concentrations of the plant methanolic extracts were used (1 (15 μl), 2 (30 μl), 3 (45 μl) and 4 (60 μl)), injected to the Whatman Filter paper disk, uniformly placed on the surface of 5 h old fungal plate culture, incubated for 5 days at 30 C. B, The diameter of the inhibition zones the tested fungal isolates in response to the response to plant extracts were measured. The data were represented by means ± STDE, with p-value ≤0.05 (ONE Way ANOVA test).
3.5. Taxol extraction, chemical identification and antiproliferative activity from the Podocarpus species
Taxol as powerful bioactive compound was extracted and determined from the experimented Podocarpus species. The plant samples were grinded in liquid nitrogen, amended with n-hexane, dissolved in methylene chloride, and Taxol was extracted by the solvent systems (Fig. 6A). From the TLC profile, a noticeable fluctuation on the yield of Taxol was reported from the different Podocarpus species (Fig. 6B). The yield of Taxol was chemically verified from the HPLC chromatogram (Fig. 6C, Fig. S3), regarding to the concentration and retention time of authentic Taxol at 27–2.9 min. The maximum Taxol yield was detected on the extracts of P. elongatus (16.4 μg/g dry weight), P. macrophyllus (11.2 μg/g dry weight), and P. neriifolius (8.6 μg/g dry weight). Based on the productivity, the Taxol sample extracted from P. elongatus has been used for further chemical verification analyses. The crude Taxol extracts from P. elongatus has been further purified by the preparative-TLC. The structural identity of the extracted Taxol from P. elongatus was confirmed by LC/MS analysis. Taxol of P. elongatus had the same molecular mass to charge ratio (854.6 m/z), and the same molecular fragmentation pattern of the authentic one, as revealed from the LC-MS/MS analysis (Fig. 7A). Similarly, LC/MS has been used for verification of the chemical structure of the small bioactive metabolites of microbial and plant origins [41]. Consistently, Taxol has been structurally authenticated from Ozonium sp [70] by the LC/MS approach. Similar results validate the chemical identity of Taxol from plant and fungal sources implementing the identical approaches of chromatography and spectroscopy [17,18].
Fig. 6.
Taxol extraction and quantification from the tested Podocarpus species. A, Overall view of plant maceration and Taxol extraction from the tested plants. B, TLC profile of Taxol from the experimented plants. C, HPLC chromatogram of Taxol extracted from the different plants and the authentic one. D, Taxol concentration based on the HPLC chromatogram compared to the authentic concentration of Taxol. The data were represented by means ± STDE, with p-value ≤0.05 (ONE Way ANOVA test).
Fig. 7.
LC/MS chromatogram and antiproliferative activity of the extracted Taxol from P. elongatus. A, LC/MS chromatogram of authentic Taxol and putative sample. B, antiproliferative activity of extracted Taxol from P. elongatus towards different tumor cell lines HepG-2, MCF-7 and HCT-116. C, IC50 values of Taxol for the tested cell lines.
3.6. The antiproliferative activity of extracted Taxol from the selected Podocarpus species
The anticancer activity of the purified Taxol from P. elongatus was verified towards various cell lines namely liver carcinoma (HepG-2), breast carcinoma (MCF-7), and colon carcinoma HCT-116 by MTT assay (Fig. 7B). The antitumor activity of the Taxol was evaluated based on viability of tumor cells, as well as, the IC50 values. From the anticancer activity, the extracted Taxol from P. elongatus had the highest activity towards the HCT-116 (IC50 value 30.2 μg/ml), followed by HepG-2 (53.7 μg/ml) and MCF-7 (71.8 μg/ml). The antitumor efficiency of the purified Taxol from P. elongatus was very consistent to Taxol from Taxus brevifolia [70], and from Aspergillus terreus [18], and A. flavipes [52]. As well as, the antitumor efficiency of the purified Taxol from P. elongatus being consistent with Taxol from N. sylviforme and C. oxysporum extracted Taxol [71]. The cytotoxic activity of A. terreus Taxol against HEPG2 and MCF7 tumor cells was higher than Taxol from T. brevifolia (590–762 nM).
3.7. Molecular identification of the potent Taxol producing Podocarpus species
The ITS sequence region has been used as a molecular marker for identification of the plant species [68]. Using the genomic DNA as PCR template for amplification of the ITS regions, the size of PCR product was about 650 bp (Fig. 8A and B). The PCR products were sequenced and non-redundantly BLAST searched on NCBI database. From the multiple alignment analysis, the ITS sequence of P. elongatus EFBL-NZM was deposited on Genbank with accession # ON540734.1, that is the first record for Podocarpus species on Genbank. The phylogenetic analysis of the ITS sequence of P. elongatus has been constructed by MEGA-X software package with the Maximum Likelihood method. From the phylogenetic analysis of ITS region of P. elongatus, two clades were evolved; Clade I “Bauhinia group” and Clade II “Taxus group” (Fig. 8C). The ITS sequence of P. elongatus displayed 99.5% similarity with the ITS regions of Bauhinia blakeana AF3879701, B. variegate AY258378.1, B. purpurea KX057836.1, B. acuminata JX856404.1, B. monandra KX057835.1, B. faberi AF390195.1, B. brachycarpa FJ432276.1, B. ungulata FJ009818.1, B. cheilantha DQ787410.1, B. jennigsii AY258411.1, B. macranthera JN942381.1, B. rufescens KX057837.1, and C. chingii JQ425130 with zero E-value and 100% query coverage. While, the sequence of the ITS region of P. elongatus displayed a 60% similarity with the ITS sequence of different Taxus species; Taxus walichiana MH711827.1, T. cuspidata MK123471.1, T. brevifolia MK1234701.1, T. canadensis MK211148.1, T. x media MK123472.1, T. canadensis MK272738.1, T. cuspidata MK168616.1 and MK123473.1.
Fig. 8.
Morphological view and molecular identification of the potent Taxol producer plant “Podocarpus elongatus”. A, Morphological view of Podocarpus elongatus, showing the entire tree, and leaves. The plant was molecularly identified based on the sequence of the ITS region of the rDNA. The genomic DNA was extracted and used as PCR template. B, Plant genomic DNA and PCR amplicons of the ITS region with molecular size about 650 bp. C, The phylogenetic tree of the ITS region of P. elongatus using the MEGAX software package with Maximum likelihood.
4. Conclusion
Although the myriad pharmaceutical applications of the bioactive metabolites of the different species of Podocarpaceae, a few studies reporting the phytochemical constituents, and metabolic profiling of the Podocarpus species inhabiting Egypt. Thus, different species of Podocarpus “P. gracilior, P. elongatus, P. macrophyllus and P. neriifolius” were collected from the different geographical locations in Egypt, and their metabolic profiling and phytochemical constitutes were analyzed comparatively. Podocarpus gracilior being the reservoir of the bioactive metabolites especially terpenoids, followed by P. elongatus, P. neriifolius and P. macrophyllus. The methanolic extracts of P. elongatus gave the highest activity against E. coli, P. aeruginosa, S. pyogenes and S. aureus, as well as the Aspergillus fumigatus, A. niger and C. albicans. The bioactive diterpenoids “Taxol” has been extracted and quantified from the experimented Podocarpus species, P. elongatus have the highest concentration of Taxol, followed by P. macrophyllus, P. neriifolius and P. gracilior. The anticancer activity of Taxol derived from P. elongatus was authenticated on different tumor cell lines (HepG-2, MCF-7 and HCT-116), that being similar to commercial Taxol from T. brevifolia. The highest Taxol encompassing P. elongatus has been molecularly identified as the first record and deposited on Genbank with accession number ON540734.1. However, the main limitation of this study was the tiny yield of Taxol, as well as the availability of these plants. Thus, further experimental trials on using the tissue culture approaches of P. elongatus of Taxol production are ongoing by our research group.
5. Author contributions statements
1 - Conceived and designed the experiments;
2 - Performed the experiments;
3 - Analyzed and interpreted the data;
4 - Contributed reagents, materials, analysis tools or data;
5 - Wrote the paper.
Ethical standards
This article does not contain any studies with human participants or animals.
Availability of data and materials
All the data are available in the manuscript.
Declaration of competing interest
The authors declare that they have no competing of interests.
Acknowledgement
The authors are also very much thankful and grateful to Trease Labib, a plant taxonomist at Orman Garden, Giza, Egypt, who shared her knowledge on identification of the plant materials, and confirmation of the Podocarpus species.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20034.
Contributor Information
Lamis Shaaban, Email: lamisshaaban@yahoo.com.
Samia Safan, Email: samiasaffan@zu.edu.eg.
Ashraf S.A. El-Sayed, Email: ash.elsayed@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
figs2.
References
- 1.Abdillahi H.S., Stafford G.I., Finnie J.F., Staden J.V. Ethnobotany, phytochemistry and pharmacology of Podocarpus sensus latissimo (S.I) South Afr. J. Bot. 2010;76(1):1–24. [Google Scholar]
- 2.Bhuyar Garboui S.S., Borg-Karlson A.K., Palsson K. Tick repellent properties of three Libyan plants. J. Med. Entomol. 2009;46(6):1415–1419. doi: 10.1603/033.046.0623. [DOI] [PubMed] [Google Scholar]
- 3.Bhuyar P., Rahim M., Sundararaju S., Maniam G., Govindan N. Antioxidant and antibacterial activity of red seaweed Kappaphycus alvarezii against pathogenic bacteria. Global J EnvironSci Manag. 2020;6(1):47–58. [Google Scholar]
- 4.Bhuyar P., Hasbi M., Rahim A., Pragas, Maniam G., Govindan N. Isolation and characterization of bioactive compounds in medicinal plant Centella asiatica and study the effects on fungal activities. J. Microbiol. Biotechnol. Food Sci. 2021;10(4):631–635. [Google Scholar]
- 5.El-Sayed A.S.A., Khalaf S.A., Azez H.A., Hussein H.A., EL-Moslamy S.H., Sitohy B., El-Baz A.F. Production, bioprocess optimization and anticancer activity of Camptothecin from Aspergillus terreus and Aspergillus flavus, endophytes of Ficus elastica. Process Biochem. 2021;107:59–73. [Google Scholar]
- 6.Mill R.R. A monographic revision of the genus Podocarpus (Podocarpaceae): I. Historical review. Edinb. J. Bot. 2014;71:309–360. [Google Scholar]
- 7.Dallimore W., Jackson A.B., Harrison S.G. fourth ed. Edward Arnold; London: 1966. Taxaceae. A Handbook of Coniferae and Ginkgoaceae; pp. 37–106. [Google Scholar]
- 8.Fu L., Li Y., Mill R.R. In: Flora of China. Wu Z.Y., Raven P.H., editors. vol. 4. Science Press, Beijing, and Missouri BotanicalGarden Press; St. Louis: 1999. Podocarpus. (Cycadaceae through Fagaceae)). [Google Scholar]
- 9.Cribb A.B., Cribb J.W. William Collins Pty Ltd; Sidney: 1981. Useful Wild Plants in Australia. ISBN: 0-00-216441-8. [Google Scholar]
- 10.Facciola S. Kampong Publications; 1990. Cornucopia A Source Book of Edible Plants. ISBN: 0-9628087-0-9. [Google Scholar]
- 11.Abdillahi H.S., Stafford G.I., Finnie J.F., Van Staden J. Antimicrobial activity of South African Podocarpus species. J. Ethnopharmacol. 2008;119:191–194. doi: 10.1016/j.jep.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 12.Duke J.A., Ayensu E.S. Reference Publications Inc.; Algonac, MI: 1985. Medicinal Plants of China; p. 381. [Google Scholar]
- 13.Chopra R.N., Nayar S.L., Chopra I.C. Council of Scientific and Industrial Research; New Delhi, India: 1986. Glossary of Indian Medicinal Plants (Including the Supplement) [Google Scholar]
- 14.Kuo Y.J., Hwang S.Y., Wu M.D., Liao C.C., Liang Y.H., Kuo Y.H., Ho H.O. Cytotoxic constituents from Podocarpus fasciculus. Chem. Pharm. Bull. 2008;56:585–588. doi: 10.1248/cpb.56.585. [DOI] [PubMed] [Google Scholar]
- 15.Stahlhut R., Park G., Petersen R., Ma W., Hylands P. The occurrence of the anti-cancer diterpene taxol in Podocarpus gracilior Pilger (Podocarpaceae) Biochem. Systemat. Ecol. 1999;27(6):613–622. [Google Scholar]
- 16.El-Sayed A.S.A., Yassin M.A., Ibrahim H. Coimmobilization of l -methioninase and glutamate dehydrogenase: novel approach for l -homoalanine synthesis. Biotechnol. Appl. Biochem. 2015;62:514–522. doi: 10.1002/bab.1299. [DOI] [PubMed] [Google Scholar]
- 17.El-Sayed A.S.A., Safan S., Mohamed N.Z., Shaban L., Ali G.S., Sitohy M.Z. Induction of Taxol biosynthesis by Aspergillus terreus, endophyte of Podocarpus gracilior Pilger, upon intimate interaction with the plant endogenous microbes. Process Biochem. 2018;71:31–40. [Google Scholar]
- 18.El-Sayed A.S.A., Mohamed N.Z., Safan S., et al. Restoring the Taxol biosynthetic machinery of Aspergillus terreus by Podocarpus gracilior Pilger microbiome, with retrieving the ribosome biogenesis proteins of WD40 superfamily. Sci. Rep. 2019;9 doi: 10.1038/s41598-019-47816-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mohamed N.Z., Shaaban L., Safan S., El-Sayed A.S.A. Physiological and metabolic traits of Taxol biosynthesis of endophytic fungi inhabiting plants: plant-microbial crosstalk, and epigenetic regulators. Microbiol. Res. 2023;272 doi: 10.1016/j.micres.2023.127385. [DOI] [PubMed] [Google Scholar]
- 20.Bailey L.H. Macmillan Co; New York: 1949. Manual of Cultivated Plants Most Commonly Grown in the Continental United States and Canada. [Google Scholar]
- 21.Bailey L.H., Bailey E.Z. The Macmillan Publishing Company; New York: 1976. A Concise Dictionary of Plants Cultivated in the U.S. And Canada. Hortus Third “Revised by Staff of the L. H. Bailey Hortium”. [Google Scholar]
- 22.Barker N.P., Muller E.M., Mill R.R. A yellowwood by any other name: molecular systematics and the taxonomy of Podocarpus and the Podocarpaceae in southern Africa. South Afr. J. Sci. 2004;100(11):629–632. [Google Scholar]
- 23.Buchholz J.T., Gray N.E. A taxonomic revision of Podocarpus I. The sections of the genus and their subdivisions with special reference to leaf anatomy. Journal of the Arnold Arboretum. 1948;29(1):49–63. [Google Scholar]
- 24.Buchholz J.T., Gray N.E. A taxonomic revision of Podocarpus: II. The American species of Podocarpus: section Stachycarpus. Journal of the Arnold Arboretum. 1948;29(1):64–76. [Google Scholar]
- 25.Buchholz J.T., Gray N.E. A taxonomic revision of Podocarpus: III. The American species of Podocarpus: section polypodiopsis. Journal of the Arnold Arboretum. 1948;29:117–122. [Google Scholar]
- 26.Buchholz J.T., Gray N.E. A taxonomic revision of Podocarpus: VI. The south pacific species of Podocarpus: section sundacarpus. Journal of the Arnold Arboretum. 1951;32(1):93–97. [Google Scholar]
- 27.De Laubenfels D.J. A taxonomic revision of the genus Podocarpus. Blumea: Biodiversity, Evolution and Biogeography of Plants. 1985;30(2):251–278. [Google Scholar]
- 28.Gray N.E. A taxonomic revision of Podocarpus: VII. The african species of Podocarpus: section: afrocarpus. Journal of the Arnold Arboretum. 1953;34(1):67–76. [Google Scholar]
- 29.Gray N.E. A taxonomic revision of Podocarpus VIII. The African species of section Eupodocarpus, subsections A and E. Journal of the Arnold Arboretum. 1953;34:163–175. [Google Scholar]
- 30.Gray N.E. A taxonomic revision of Podocarpus X. The south pacific species of section eupodocarpus, subsection D. Journal of the Arnold Arboretum. 1956;37(2):160–172. [Google Scholar]
- 31.Dhawan D., Gupta J. Comparison of different solvents for phytochemical extraction potential from Datura metel plant leaves. Int. J. Biol. Chem. 2017;11:17–22. [Google Scholar]
- 32.El-Sayed A.S.A., El-Sayed M.T. Biosynthesis and anti-mycotoxigenic activity of Zingiber officinale roscoe-derived metal nanoparticles. Molecules. 2021;26(8) doi: 10.3390/molecules26082290. art. no. 2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed M., Ji M., Sikandar A., Iram A., Qin P., et al. Phytochemical analysis, biochemical and mineral composition and GC-MS profiling of methanolic extract of Chinese Arrowhead Sagittaria trifolia L. from Northeast China Molecules. Molecules. 2019;24(17):3025. doi: 10.3390/molecules24173025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.El-Sayed A.S.A.F.E., Fujimoto S., Yamada C., Suzuki H. Enzymatic synthesis of γ-glutamylglutamine, a stable glutamine analogue, by γ-glutamyltranspeptidase from Escherichia coli K-12. Biotechnol. Lett. 2010;32:1877–1881. doi: 10.1007/s10529-010-0364-z. [DOI] [PubMed] [Google Scholar]
- 35.Harborne J.B. second ed. Chapman and Hall; London: 1984. Phytochemical Methods: a Guide to Modern Techniques of Plant Analysis. [Google Scholar]
- 36.Farnsworth N.R., Akerele O., Bingel A.S., Soejarto D.D., Guo Z. Medicinal plants in therapy. Bull. World Health Organ. 1985;63:965–981. [PMC free article] [PubMed] [Google Scholar]
- 37.El-Sayed A.S.A., Fathalla M., Yassin M.A., Zein N., Morsy S., Sitohy M., Sitohy B. Conjugation of Aspergillus flavipes taxol with porphyrin increases the anticancer activity of taxol and ameliorates its cytotoxic effects. Molecules. 2020;25:263. doi: 10.3390/molecules25020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sofowora A. Research on medicinal plants and traditional medicine in Africa. J. Alternative Compl. Med. 1996;2:365–372. doi: 10.1089/acm.1996.2.365. [DOI] [PubMed] [Google Scholar]
- 39.Harborne J.B. third ed. Chapman and Hall; London: 1998. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis; pp. 107–108. [Google Scholar]
- 40.Kokate C.K. fifth ed. 2005. A Text Book of Practical Pharmacognosy; pp. 107–111. Vallabh Prakashan New Delhi. [Google Scholar]
- 41.Yassin M.A., Shindia A., Labib M., Soud M., El-Sayed A.S.A. Thermostable Chitosan-L-Asparaginase conjugate from Aspergillus fumigatus is a novel structurally stable composite for abolishing acrylamide formation in French fried potatoes. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2022;62 [Google Scholar]
- 42.Lowry O.H., Rosebrough N.J., Farr A.L., Randall R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 43.Ghorai N., Chakraborty S., Gucchait S., Saha S.K., Biswas S. 2012. Estimation of Total Terpenoids Concentration in Plant Tissues Using a Monoterpene, Linalool as Standard Reagent. Research Square Protocol Exchange. 10.1038/protex.2012.055. [Google Scholar]
- 44.Chang C.C., Yang M.H., Wen H.M., Chern J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002;10:178–182. [Google Scholar]
- 45.Kalita P., Tapan B., Pal T., Kalita R. Estimation of total flavonoids content (TFC) and antioxidant activities of methanolic whole plant extract of Biophytum sensitivum Linn. J. Drug Deliv. Therapeut. 2013;3(4):33–37. [Google Scholar]
- 46.Singleton V., Rossi J.A. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965;16:144–158. [Google Scholar]
- 47.Kim D.O., Jeongb S.W., Lee C.Y. Antioxidant capacity of phenolic phytochemicals from various cultivars of plums. Food Chem. 2003;81:321–326. [Google Scholar]
- 48.Baliyan S., Mukherjee R., Priyadarshini A., Vibhuti A., Gupta A., Pandey R.P., Chang C.M. Determination of antioxidants by DPPH radical scavenging activity and quantitative phytochemical analysis of Ficus religiosa. Molecules. 2022;27:1326. doi: 10.3390/molecules27041326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yen G.C., Duh P.D. Scavenging effect of methanolic extracts of peanut hulls on free radical and active oxygen species. J. Agric. Food Chem. 1994;42:629–632. [Google Scholar]
- 50.Brand-Williams W., Cuvelier M.E., Berset C. Use of a free radical method to evaluate antioxidant activity. Lebenson Wiss Technol. 1995;28:25–30. [Google Scholar]
- 51.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 52.El-Sayed A.S.A., Ali G.S. Aspergillus flavipes is a novel efficient biocontrol agent of Phytophthora parasitica. Biol. Control. 2020;140 [Google Scholar]
- 53.El Sayed M.T., El-Sayed A.S.A. Tolerance and mycoremediation of silver ions by Fusarium solani. Heliyon. 2020;6(5) doi: 10.1016/j.heliyon.2020.e03866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bergey D.H., Holt J.G. ninth ed. Lippincott Williams & Wilkins; Philadelphia: 2000. Bergey's Manual of Determinative Bacteriology. [Google Scholar]
- 55.El-Sayed A.S.A., George N.M., Abou-Elnour A., El-Mekkawy R.M., El-Demerdash M.M. Production and bioprocessing of camptothecin from Aspergillus terreus, an endophyte of Cestrum parqui, restoring their biosynthetic potency by Citrus limonum peel extracts. Microb. Cell Factories. 2023;22(1) doi: 10.1186/s12934-022-02012-y. 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Whiterup K.M., Look S.A., Stasko M.W., Guiorzi T.J., Muschik G.M., Cragg G.M. Taxus spp. needles contain amounts of taxol comparable to the bark of Taxus brevifolia: analysis and isolation. J. Nat. Prod. 1990;53:1249–1255. doi: 10.1021/np50071a017. [DOI] [PubMed] [Google Scholar]
- 57.Li J.Y., Strobel G., Sidhu R., Hess W.M., Ford E.J. Endophytic taxol-producing fungi from bald cypress, Taxodium distichum. Microbiology. 1996;142:2223–2226. doi: 10.1099/13500872-142-8-2223. [DOI] [PubMed] [Google Scholar]
- 58.Bitsch F., Ma W., Macdonald F., Nieder M., Shackleton C.H. Analysis of Taxol and related diterpenoids from cell cultures by liquid chromatography-electrospray mass spectrometry. J. Chromatogr. A. 1993;615:273–280. doi: 10.1016/0378-4347(93)80341-z. [DOI] [PubMed] [Google Scholar]
- 59.Liu J., Volk K.J., Mata M.J., Kerns E.H., Lee M.S. Miniaturized HPLC and ion spray mass spectrometry applied to the analysis of paclitaxel and taxanes. J. Pharmaceut. Biomed. Anal. 1997;15:1729–1739. doi: 10.1016/s0731-7085(96)01969-3. [DOI] [PubMed] [Google Scholar]
- 60.Cory A.H., Owen T.C., Barltrop J.A., Cory J.G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991;3:207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- 61.El-Sayed A.S.A., Yassin M.A., Ibrahim H. Coimmobilization of l -methioninase and glutamate dehydrogenase: novel approach for l -homoalanine synthesis. Biotechnol. Appl. Biochem. 2015;62:514–522. doi: 10.1002/bab.1299. [DOI] [PubMed] [Google Scholar]
- 62.El Sayed M.T., El-Sayed A.S.A. Tolerance and mycoremediation of silver ions by Fusarium solani. Heliyon. 2020;6(5) doi: 10.1016/j.heliyon.2020.e03866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rawat S., Joshi G., Annapurna D., Arunkumar A.N., Karaba N.N. Standardization of DNA extraction method from mature dried leaves and ISSR-PCR conditions for Melia dubia cav.-A fast growing multipurpose tree species. Am. J. Plant Sci. 2016;7:437–445. [Google Scholar]
- 64.Bachmann K. Molecular markers in plant ecology. New Phytol. 1994;126(3):403–418. doi: 10.1111/j.1469-8137.1994.tb04242.x. [DOI] [PubMed] [Google Scholar]
- 65.El-Sayed A.S.A., Shindia A.A., Ali G.S., Yassin M.A., Hussein H., Awad S.A., Ammar H.A. Production and bioprocess optimization of antitumor Epothilone B analogue from Aspergillus fumigatus, endophyte of Catharanthus roseus, with response surface methodology. Enzym. Microb. Technol. 2021;143 doi: 10.1016/j.enzmictec.2020.109718. [DOI] [PubMed] [Google Scholar]
- 66.Edgar R.C. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinf. 2004;5:113–132. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.El-Demerdash M.M., El-Sayed A.S.A., Hussein H.A., Teleb S.S., Shehata R.S. Molecular and metabolic traits of some Egyptian species of Cassia L. and Senna Mill (Fabaceae-Caesalpinioideae) BMC Plant Biol. 2022;22(1):205. doi: 10.1186/s12870-022-03543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.El-Sayed A.S., Khalaf S.A., Abdel-Hamid G., El-Batrik M.I. Screening, morphological and molecular characterization of fungi producing cystathionine γ-lyase. Acta Biol. Hung. 2015;66(1):119–132. doi: 10.1556/ABiol.66.2015.1.10. [DOI] [PubMed] [Google Scholar]
- 70.Stierle A., Strobel G., Stierle D., Grothaus P., Bignami G. The search for a taxol-producing microorganism among the endophytic fungi of the Pacific yew, Taxus brevifolia. J. Nat. Prod. 1995;58(9):1315–1324. doi: 10.1021/np50123a002. [DOI] [PubMed] [Google Scholar]
- 71.Abdel-Fatah S.S., El-Batal A.I., El-Sherbiny G.M., Khalaf M.A., El-Sayed A.S. Production, bioprocess optimization and γ-irradiation of Penicillium polonicum, as a new Taxol producing endophyte from Ginko biloba. Biotechnology Reports. 2021;30 doi: 10.1016/j.btre.2021.e00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data are available in the manuscript.