Abstract
Despite the beneficial aspect of a natural drainage system, increasing human-induced activities, which include urbanization and growth in industrialization, degrade the ecosystem in terms of trace metal contamination. In response, given the great importance of the south-eastern drainage system in Bangladesh, a detailed evaluation of the human health risk as well as the potential ecological risk of trace metals (Be, Cd, Co, Cr, Cu, Hg, Ni, Pb, Se, V, Zn, and As) in Karnaphuli riverbed sediment was conducted. Mean levels of the elements in mg/kg were As (5.62 ± 1.47); Se (0.84 ± 0.61); Hg (0.37 ± 0.23); Be (1.17 ± 0.49); Pb (15.62 ± 8.42); Cd (0.24 ± 0.33); Co (11.59 ± 4.49); Cr (112.75 ± 40.09); Cu (192.67 ± 49.71); V (27.49 ± 10.95); Zn (366.83 ± 62.82); Ni (75.83 ± 25.87). Pollution indicators, specifically contamination factor (CF), pollution load index (PLI), degree of contamination (Cd), enrichment factor (EF), geo-accumulation index (Igeo), and potential ecological risk index (RI), were computed to assess sediment quality. For the first observation of health risk, chronic daily intake (CDI), hazard quotient (HQ), hazard index (HI), carcinogenic risk (CR) and total carcinogenic risk (TCR) indices were calculated. According to the results, CDI values through the ingestion route of both the adult and child groups were organized in the following descending mode respectively: Zn > Cu > Cr > Ni > V > Pb > Co > As > Se > Be > Cd > Hg. The non-carcinogenic risks were generally low for all routes of exposure, except HQingestion was slightly higher for both adults and children. The calculated hazard index (HI) was, nevertheless, within the permitted range (HI < 1). Similarly, none of the metals exhibited any carcinogenic risks, as all CR values were within the 10−4-10−6 range. The need for authoritative efforts and water policy for the sake of the surrounding ecosystem and human health in the vicinity of the examined watershed is strongly felt as an outcome of this study. The purpose of this study is to protect public health by identifying trace metal sources and reducing industrial and domestic discharge into this natural drainage system.
Keywords: Sediment, Trace metals, Ecological risk, Human health risk, Non-carcinogenic risk, Carcinogenic risk, Bangladesh
Highlights
-
•
Twelve metals have been evaluated in the south-eastern drainage system of Bangladesh.
-
•
Se, Cr, Cu, Zn, and Ni levels were higher than average shale values.
-
•
Geological indices were used to estimate the severity of contamination.
-
•
The HI values were less than 1.
-
•
CR demonstrated no carcinogenic risk.
-
•
The main potential sources were anthropogenic discharges with surface runoff.
1. Introduction
Hundred thousand tons of wastewater were drained into the Karnaphuli river (originating from the Saithah village of Mamit district in Mizoram considered as the drainage system of the south-western part of Mizoram, India), only natural drainage system of Chittagong city, has a serious health crisis running amok [1,2] in the south-eastern people of Bangladesh. Compared to many other pollutants, trace element poisoning especially in riverine ecosystems has received attention on a global scale because of its ecotoxicity, quantity and persistence [[3], [4], [5]]. Heavy metal buildup in aquatic environments is a result of both human-caused factors (rapid urbanization, industrialization, agricultural expansion, economic channelization, land-use changes, smelting, vehicle exhaust emissions, etc.) [[6], [7], [8], [9]] and natural factors (volcanic eruptions, forest fires, rock weathering, soil erosion, etc.) [10,11]. Low concentrations of heavy metals in aquatic environments are commonly linked with natural or geological sources, but high concentrations of heavy metals in sediments may indicate anthropogenic sources [8,12,13]. Heavy metals no longer remain dissolved in water; they are especially adsorbed on backside sediments, which they stockpile [8]. On one side, the sediments can act as storehouses and the other as capacity sources of contaminants to the water column and may adversely affect sediment-dwelling habitats, aquatic-dependent creatures, and eventually human health [14]. Therefore, sediments may be utilized in investigations of sources of long-term pollution, environmental circumstances [7,15,16] as well as human health consequences.
Bangladesh, a developing nation, has experienced unplanned urban growth and rapid industrial development in recent years [17,18], but the pollutants these activities produce are causing environmental issues on a never-before-seen scale, primarily because of arsenic and other heavy metal pollution [[19], [20], [21], [22]]. The streams of Chittagong and Chittagong slope tracts are not associated with the other waterway frameworks of the nation. The fundamental waterway of this area is Karnaphuli. It moves through the district of Chittagong and the Chittagong Hills [23], cuts over the slopes and runs quickly downhill toward the west and southwest lastly to the Bay of Bengal. Not only Karnaphuli Reservoir and Dam but also Chittagong Port is situated on the bank of the Karnaphuli River. Though the Karnaphuli River, the lifeline of Chittagong, is not enlisted as a transboundary in the list of Bangladesh India Joint Rivers Commission (JRC), a new research publication identifies it as a cross-border river. These rivers are particularly important both hydrologically and politically since they transport a lot of sediments that aid in the accretion of land in the estuary zone. Karnaphuli River also known as the “Southeastern Drainage System of Bangladesh”, receives huge amounts of untreated effluents from medical and industries such as spinning mills, dying, cotton, textile, steel mills, oil refineries and others through 36 canals of the Chattogram city and primary source of cross-border water pollution.
According to Ref. [24], the presence of toxic and hazardous compounds in rivers, estuaries, and marine habitats poses an existential crisis to Bangladeshi people's health and welfare in addition to impacting the ecological integrity [25,26]. In general, human health hangs in the balance due to the intake of excess metal through three pathways viz. oral ingestion, inhalation, and dermal absorption [27]. Ingestion and dermal absorption are regarded as major pathways for the accumulation of those toxic metals into the human body among the three adsorption routes [28]. The majority of heavy metals are carcinogenic, detrimental to the nervous and immune systems, and have toxic effects on all living things, even at low concentrations [29]. Literature has also shown that trace metals, especially Zn and Cu, build up in human body tissues, which can affect the immune, cardiovascular, nervous, and urogenital systems in ways that range from short-term reactions to long-term diseases [30]. Cadmium (Cd) is one of the trace metals in the environment with widespread distribution. Human encounters with Cd can occur through ingesting food cultivated in contaminated fields, dust inhalation, and dermal contact with water and sediment. This trace metal is able to enter the bloodstream and bind to blood cells via the lungs and gastrointestinal tract [31]. Lead (Pb), which is regarded as a non-essential metal, has negative health effects that can harm the liver and kidneys and, in the end, cause death [32]. Mercury (Hg) is regarded as a fatal element for both people and other living due to its extreme toxicity [33]. Although ingesting some metals, such as Hg, As, Cd, and Pb, is highly hazardous for human health even at a low concentration, some metals, such as Cu, Co, Zn, Fe, Se, Ni, and Mn, are helpful to humans at low quantities [[34], [35], [36]]. According to Ref. [37], chromium (Cr) plays a critical role in the metabolism of lipids and insulin. Excessive consumption of Cr can result in hepatic and renal dysfunction as well as pulmonary diseases [38,39]. Analyzing the anthropogenic impacts and ecological risks of trace elements can give an idea of how polluted aquatic ecosystems are caused by contaminated sediments [[40], [41], [42]]. As a consequence, it is essential to measure the concentration of trace metals in sediments from contaminated river systems. Trace metal studies in Karnaphuli River and estuary sediments of the Bengal Basin, Bangladesh, have investigated only the occurrence and toxicity profile of selective trace metals (As, Cd, Cr, Pb) in the sediment [6,17,43,44]. To the best of our knowledge, this is the first time our group assessed the human health risk of trace metals in south-eastern drainage. The main objectives of the present study concentrated on 1) the potential trends in the environmental level of important trace metals like Zn, Cr, Ni, Cu, V, Pb, Co, As, Cd, Be, Hg, and Se in the sediment; 2) evaluation of potential noncarcinogenic and carcinogenic risk (human health risk) due to inhalation, dermal contact, and ingestion exposure pathways.
2. Materials and methods
2.1. Study area description
The research described here was conducted near the Kalurghat bridge of the Karnaphuli River (Latitude 22° 23′ 43.01″ N and Longitude 91° 53′ 21″ E), which flows through Chittagong City, Bangladesh, close to the Bay of Bengal (Fig. 1). Karnaphuli, which originates in the Lushai hills, connects Mizoram to the port city of Chittagong on the coast of the Bay of Bengal. It is a 667-m-wide river in Bangladesh's south-eastern region that flows 270 km southwest into the Bay of Bengal via the Chittagong Hill Tracts and Chittagong. Kalurghat Bridge is an older bridge that was previously the only link between the southern portion of the Chittagong division on the bank of the river Karnaphuli and the rest of the country. In 1930, this 239-m-long steel-structured rail bridge was built between Janalihut and Gomdandi railway stations.
Fig. 1.
Map of the study area, Karnaphuli River, Bangladesh.
2.2. Sample collection and preparation
Based on direction as well as the impact of potential pollution sources (cities, industrial areas, rivers), sediment and water samples were collected from four sites, including south-west, north-west, south-east, and north-east of the Karnaphuli River, with three replications. The southwest station has the Kalurghat BSCIC industrial and shipyard area, is situated near the Kalurghat Bridge, downstream of the Karnaphuli River, and has become hostile due to untreated waste discharge. The northwest station, Madhunaghat (Chittagong), where Halda meets the Karnphuli River near Madhunaghat, is the world's only natural carp breeding ground. Domestic and industrial effluents carried by the river have an impact on this site. The southeast zone of the Kalurghat bridge contains CNC cutting zones, paper mills, and textile mills. The northeast station, known as the city discharge area, generally releases domestic waste. Sampling was started from Madhunaghat (22° 40′ 53″ N and 91° 89′ 21″ E) and ended at Char Khidirpur (22° 38′ 79″ N and 91° 88′ 85″ E).
In Bangladesh, during the summer, river water levels rise due to significant downpours; during the winter, there is no rainfall, thus river water levels fall, causing metal concentrations in water and sediment to fluctuate. For the study, highly impacted areas considered to be contaminated by agricultural, residential, and industrial waste products, such as cement factories, pharmaceutical factories, textile industries, and paper mills, were identified through a preliminary survey. Sampling was done during the dry season. A total of twelve sediment samples were collected from four sampling zones during December 2021 for the study. For water, the same number of samples were also studied. Using an Ekman grab sampler, approximately 100 g of sediment was extracted from the Karnaphuli river bed at a depth of 0–5 cm and put into a plastic zipper bag. Water samples were collected from sampling stations along the river below 0.5 m below the water surface and stored in PVC bottles. After collection samples were brought to the Soil and Environment Section, Biological Research Division, Dhaka Laboratory, Bangladesh Council of Scientific and Industrial Research, Dhaka, Bangladesh.
The collected sediment samples were transferred to porcelain dishes from the plastic zipper bags for air drying. Each air-dried sample was pulverized with an agate mortar and pestle and sieved through a 2.0 mm (10 US mesh) sieve to get a uniform mixture and fine grain size. Before being analyzed in the lab, water samples were properly stored at −4 °C.
2.3. Reagents
Analytical grade reagents and chemicals were used throughout the experiment (Sigma-Aldrich, Merck, Switzerland). De-ionized water (resistivity & gt; 18 MΩcm, manufactured using an E-pure system, Thermo Scientific, USA), hydrogen peroxide (30%, Sigma Aldrich), nitric acid (67%, BDH), and certified reference material (CRM) for As (1000 mg±4 mg/L; Cica-Reagent; Cat. No. 01805-1B; Lot. No. 301U9529), Cu (1000 mg±4 mg/L; Cica-Reagent; Cat. No. 08046-2B; Lot. No. 212U9521), Ni (1000 ± 3 mg/kg; Cica-Reagent; Cat. No. 28577-2B; Lot. No. 301U9512), for Pb, Cd, Cr, and Se (1000 mg/L±4 mg/L; SIGMA-ALDRICH, Switzerland), for Zn (1000 mg±4 mg/L; SIGMA-ALDRICH, Lot: BCBR0978V, Switzerland) were used in this study. All chemicals and reagents were employed after further purification, and working standards were generated from stock CRM adopting the standard technique.
2.4. Sample digestion
In a pre-acid-washed 100-mL beaker, 10 mL of HNO3 acid was added to 1 g of the dried and finely ground sediment sample and pre-digested overnight. Then followed 5–6 h heating at 100–180 °C on a hot plate until the solution becomes clear. For complete digestion, 5 mL of perchloric acid (HClO4) was added and further heated until only 2–3 mL remained in the beaker. After cooling at room temperature, the sample solution was shifted into a 50-mL calibrated volumetric flask by washing 2–3 times with deionized water, and the volumetric flask was then filled to the mark. The sample solution was well mixed before being filtered through Whatman no. 41 filter paper into a previously washed and labelled 50-mL opaque plastic bottle. Water samples were digested with 2 mL of concentrated HNO3 acid, filtered, filled up to 25 mL, and collected in prewashed plastic bottles. For quality control, blank samples for both sediment and water (without sediment and water) were also generated using the same digestion method, respectively.
2.5. Analytical technique and accuracy check
To avoid contamination, the samples were handled with clean lab coats and clear, powder-free latex gloves. The identification and quantification of metals (As, Se, Hg, Be, Pb, Cd, Co, and V) were performed using an inductively coupled plasma mass spectroscopy (ICP-MS, NexION 2000, PerkinElmer, USA). An atomic absorption spectrophotometer (Shimadzu AAS-7000, Japan) was used to test other metals (Cr, Cu, Zn, and Ni). The instruments were calibrated before use, to ensure the operation of the instruments within acceptable ranges. Certified reference materials (CRM) from Sigma-Aldrich Chemie (GmbH) (Switzerland) were used for the accuracy and precision of data. The percentages of recovery were found between 93% and 106%. When the calibration curves had an R2 value > 0.999, the trace metal concentration was calculated. Deionized water was used for all analytical operations, including sample preparation, and analytical-grade reagents were used. Before usage, all of the lab's equipment and materials underwent a thorough cleaning by soaking in 20% nitric acid for at least 24 h. The precision was determined by analyzing duplicate samples and the accuracy by analyzing standard samples.
2.6. Ecological assessment of sediment
In order to assess trace metal contamination in sedimentary deposits of rivers, several indices have been established. Pollution indicators including contamination factor (CF), pollution load index (PLI), degree of contamination (Cd), enrichment factor (EF), geo-accumulation index (Igeo), and potential ecological risk index (RI) were taken into consideration to determine the level of pollution in the sediments of the Karnaphuli River. Table S1 provides more information on the pollution indices that were used.
2.7. Assessment of human health risk
The threat to human health from exposure to trace metals found in soil and sediment was evaluated using chronic daily intake (CDI) [27]. Since humans can absorb metal substances through three different routes (ingestion, skin contact, and inhalation), CDIs can be examined for the following routes using Equations (1)–(3) [[45], [46], [47], [48]]. Details of the equations are illustrated in Table S2.
| (1) |
| (2) |
| (3) |
2.7.1. Assessment of non-carcinogenic risk
The hazard quotient (HQ) was obtained for a specific component due to various exposures to the metal contents of a particular person in order to assess the hazard index (HI) as the non-carcinogenic risk. According to Ref. [45], HQ is the ratio of CDI (mg/kg/d) to reference dosage (RfD) (mg/kg/d). For each trace metal, HI represents the sum of the three exposure pathways of HQ. HQ and HI can be evaluated by the following Equations (4) and (5) [49].
| (4) |
| (5) |
Non-carcinogenic effects are expected to be associated with an increase in the HI value. There is no significant threat of non-carcinogenic effects if the HI value is less than one (HQ < 1). Humans may be subjected to non-carcinogenic effects when the HI value is larger than one (HQ > 1), and the effects may worsen due to the lack of potential remediation [50,51]. Table S3 lists the RfD values (mg/kg/d) for the various elements.
2.7.2. Assessment of carcinogenic risk
For each pathway, the carcinogenic risk (CR) exposure for lifetime encounters was calculated using the cancer risk factor (CSF) of the individual metal load [52,53]. The CSF of As is 1.5 mg/kg/d [49], 15 mg/kg/d for Cd [54], 0.5 mg/kg/d for Cr [55], and Pb and Ni are 0.38 and 1.7 mg/kg/d, respectively [56]. Using the following Equations (6) and (7), CR and TCR can be estimated:
| (6) |
| (7) |
where TCR stands for total carcinogenic risk. Lifetime CR permissible limits vary from 10−6 to 10−4 [57]. An individual's cancer progression will be greater than 1 in 100,000 if a value is greater than 10−6 [39,58].
2.8. Statistical analysis
SPSS software (IBM, Version: 25.0, USA) was used for statistical analysis. MS Excel 2013 was used to create the graphs. The calculated HMs concentrations in water and sediment are presented as mean ± standard deviation, in mg/L and mg/kg-ww respectively. Tukey's post hoc tests (ANOVA, p < 0.05) and one-way ANOVA for the multivariate analysis used multiple comparisons with a 5% threshold of significance. To investigate the relationship between metals, Pearson's correlation test was conducted to check the association among metals. R performed principal component analysis (PCA) and cluster analysis (CA) to confirm the distribution of the metals, assisting in the identification of the metals' source.
3. Results and discussion
3.1. Trace metals concentration in sediment
Excess levels of metals in river sediment are generated by agricultural runoff and untreated domestic sewage waste from residential and industrial areas in urban areas [[59], [60], [61], [62]]. The concentration of trace elements in the sediment samples of the study area was shown in (Table 2). The Zn, Cr, Ni, Cu, V, Pb, Co, As, Cd, Be, Hg, and Se concentrations were 336.83 ± 62.82 mg/kg; 112.75 ± 40.09 mg/kg; 75.83 ± 25.87 mg/kg; 192.67 ± 49.71 mg/kg; 27.49 ± 10.95 mg/kg; 15.62 ± 8.42 mg/kg; 11.59 ± 4.49 mg/kg; 5.62 ± 1.47 mg/kg; 0.24 ± 0.33 mg/kg; Be 1.17 ± 0.49 mg/kg; 0.37 ± 0.23 mg/kg; Se 0.84 ± 0.61 mg/kg, respectively. This study demonstrated that the mean concentrations (mg/kg) of trace elements were found in the decreasing order as Zn > Cu > Cr > Ni > V > Pb > Co > As > Se > Be > Hg > Cd. However, it has been stated that human-induced processes may be responsible for the elevated levels of Zn, Cu, and Cr [63].
Table 2.
Comparison of trace metal concentrations in sediment (mg/kg) and water (μg/L) with different other studies in the world.
| Location | As | Se | Hg | Be | Pb | Cd | Co | Cr | Cu | V | Zn | Ni | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sediment | |||||||||||||
| Rupsha River, Bangladesh | 5.18 | – | – | – | 29.21 | 1.8 | – | 43.2 | – | – | – | – | [47] |
| Brahmaputra River watershed, Bangladesh | 4.21 | – | – | – | 20.25 | – | – | 54.10 | 7.59 | 67.60 | 48.20 | 22.28 | [43] |
| Salt marsh ecosystem, Bangladesh | < MDL | – | – | – | 5.48 | – | – | < MDL | 42.90 | – | 41.72 | – | [62] |
| Buriganga River, Bangladesh | 2.15 | 110.64 | 0.281 | 22.4 | 168.9 | 222.3 | 21.04 | [66] | |||||
| Karnaphuli River, bangladesh | – | – | – | – | 16.57 | – | – | 1.13 | – | – | 53.84 | 25.30 | [67] |
| Karnaphuli River Coast, Bangladesh | – | – | – | – | 26.70 | 0.43 | – | – | 45.79 | – | 105.00 | – | [44] |
| Karnaphuli River, Bangladesh | 81.09 | – | – | – | 43.69 | 2.01 | – | 20.3 | – | – | – | – | [6] |
| Karnaphuli River estuary, Bangladesh | – | – | – | – | 23.66–25.05 | – | – | 77.70–99.08 | 20.34–33.06 | – | 59.69–74.32 | 34.10–41.27 | [17] |
| Hooghly River, India | 4 | – | 0.02 | – | 10.7 | 0.16 | – | 31.8 | – | – | – | 19.8 | [68] |
| Yangtze River Estuary, China | – | – | – | – | 25.8 | 0.13 | – | 34.4 | – | – | – | – | [69] |
| Yangtze, China | 13.51 | – | 0.67 | – | 70.36 | 0.73 | – | 47.49 | – | – | – | – | [70] |
| Karnaphuli River, Bangladesh | 5.62 ± 1.47 | 0.838 ± 0.61 | 0.375 ± 0.23 | 1.171 ± 0.49 | 15.62 ± 8.42 | 0.24 ± 0.33 | 11.59 ± 4.49 | 112.75 ± 40.09 | 192.67 ± 49.71 | 27.49 ± 10.95 | 366.83 ± 65.89 | 75.83 ± 25.87 | Our study |
| Shale Value | 13 | 0.6 | 0.4 | 3 | 20 | 0.3 | 19 | 90 | 45 | 130 | 95 | 68 | [71] |
| Water | |||||||||||||
| Buriganga River, Bangladesh | 134 | – | – | - | 119 | 59 | 199 | 114 | 239 | – | 332 | 150 | [72] |
| Bhairab River, Bangladesh | 3.6775 | – | – | - | 23.815 | 1.44 | – | 31.7385 | – | – | – | – | [48] |
| Indus Drainage System, Pakistan | – | – | 0.33 | - | 1.2 | 0.98 | 0.3 | 2.6 | 1.7 | – | 14 | 3.8 | [73] |
| Karnaphuli River, Bangladesh | 4.35 | 0.81 | 3.54 | - | 21.43 | 0.026 | 0.124 | 8.44 | 17.87 | 1.47 | 24.2 | 5.31 | Our Study |
| WHO | 10 | 40 | 6 | - | 10 | 3 | – | 50 | 2000 | – | 3000 | 70 | [74] |
| EU | 10 | 10 | 1 | - | 10 | 5 | – | 50 | 2000 | – | – | 20 | [75] |
3.1.1. Arsenic
Arsenic (As), a substance that naturally exists in Bangladesh, poisons people over a short exposure period. Congenital flaws, problems during reproduction, and skin and vascular disorders are long-term consequences on the human body that might result in cancer [64,65].
The highest concentration of As was found at 7.03 ± 1.18 mg/kg at the south-west part of Karnaphuli riverbed sediment, and the lowest level was observed at 3.62 ± 0.61 mg/kg at the north-west part of Karnaphuli riverbed sediment (Table 1). The mean concentration of arsenic was 5.6241 mg/kg and was lower than the shale value. The average value of As in Karnaphuli River water was 4.349 μg/L. The inorganic form of the potentially toxic element arsenic causes arsenicosis disease which is lethal to human health. The observed mean metal concentration (5.6241 mg/kg) in the study region was almost 15 times lower than prior studies of Karnaphuli (81.09 mg/kg) investigated by Ref. [6] and nearly equal to the Rupsha River (5.18 mg/kg) studied by Ref. [47] (Table 2).
Table 1.
Measured concentrations (mg/kg) of studied trace metals in sediment from all sampling sites of the Karnaphuli River.
| Sl | Metals | Trace Metal Concentration (mg/kg) |
Significance value (p-value) | |||
|---|---|---|---|---|---|---|
| South-West (Mean ± SD) | North-West (Mean ± SD) | South-East (Mean ± SD) | North-East (Mean ± SD) | |||
| 1 | As | 7.03 ± 1.18a | 3.62 ± 0.61b | 6.12 ± 0.43a | 5.72 ± 0.80a | 0.005 |
| 2 | Se | 1.58 ± 0.49a | 0.14 ± 0.09c | 0.85 ± 0.13b | 0.78 ± 0.47bc | 0.007 |
| 3 | Hg | 0.69 ± 0.04a | 0.20 ± 0.01c | 0.45 ± 0.05b | 0.15 ± 0.02c | 0.000 |
| 4 | Be | 1.52 ± 0.35a | 0.46 ± 0.14b | 1.51 ± 0.23b | 1.19 ± 0.16a | 0.002 |
| 5 | Pb | 18.61 ± 1.92a | 6.22 ± 4.00b | 23.16 ± 9.57a | 14.49 ± 6.74ab | 0.053 |
| 6 | Cd | 0.79 ± 0.11a | 0.05 ± 0.02b | 0.07 ± 0.02b | 0.07 ± 0.02b | 0.000 |
| 7 | Co | 15.33 ± 1.57a | 5.03 ± 1.42c | 14.65 ± 1.91a | 11.35 ± 1.83b | 0.000 |
| 8 | Cr | 166.0 ± 7.55b | 68.33 ± 4.04d | 130.0 ± 3.00b | 86.67 ± 8.74c | 0.000 |
| 9 | Cu | 244.67 ± 62.74a | 155.00 ± 19.52b | 205.33 ± 34.82ab | 165.67 ± 23.29b | 0.078 |
| 10 | V | 36.06 ± 3.24a | 11.27 ± 4.24c | 35.10 ± 4.67ab | 27.54 ± 4.21b | 0.000 |
| 11 | Zn | 445.33 ± 38.37a | 290.67 ± 16.17c | 388.67 ± 15.63ab | 342.67 ± 13.80bc | 0.000 |
| 12 | Ni | 112.67 ± 5.69a | 47.67 ± 5.03d | 81.33 ± 5.51b | 61.67 ± 5.41c | 0.000 |
* Means containing the same letter do not differ significantly at 5% level of significance.
* Comparisons are column-column.
3.1.2. Selenium
Selenium (Se) is an essential nutrient for humans and vertebrates; however, it is only needed in trace amounts, with a very limited range between insufficient and dangerous levels [76]. Selenium is also known to participate in a variety of critical metabolic interactions with a number of potentially hazardous elements, including mercury, cadmium, and arsenic, which may be crucial to public health [77]. Selenium may detoxify mercury in humans by forming 1:1 Hg–Se compounds [78]. Additionally, there is a hostile interaction between arsenic and selenium. Arsenic toxicity is enhanced if the selenium level is also very high. However, at lower concentrations of selenium, it produces an As–Se compound, which reduces the toxicity of arsenic. In contrast, excess selenium is extremely toxic and can lead to selenium poisoning (also known as selenosis) in human beings as well as animals. Animals that consume feed containing more than 5 mg of selenium per kilogram become poisoned by selenium [79].
The highest concentration of Se was found at 1.58 ± 0.49 mg/kg at the southeast station and the lowest level was observed at 0.14 ± 0.088 mg/kg at the northwest station, respectively (Table 1). The mean concentration of selenium was 0.838 mg/kg and was higher than the shale value (Table 2). No comparison was found with other studies as our study revealed selenium value for the first time. The average concentration of Se was observed at 0.81 μg/L in Karnaphuli River water (Table 2).
3.1.3. Mercury
According to Ref. [33], mercury (Hg) is one of the most dangerous and lethal substances for both human health and other living things. Chronic exposure to all Hg compounds can damage the growing fetus, the kidneys, and the brain. Additionally, increased exposure to mercury's toxicity may disrupt how the brain functions and tends to tremors, irritation, and alterations in vision or hearing [36].
Hg concentrations in the studied region range from 0.15 to 0.69 mg/kg (Table 1), with a significant mean value of 0.37 mg/kg that was lower than the shale value (Table 2). The highest concentration of Hg was found at 0.69 ± 0.04 mg/kg at the southwest station and the lowest level was observed at 0.15 ± 0.018 mg/kg at the northeast station, respectively (Table 1). Anthropogenic activities like industrial effluents, agricultural practices, municipal wastewater, and incineration can all bring this higher concentration to river water [80,81]. The lower level of Hg in the Hooghly River, India (0.02 mg/kg) was found by Ref. [68] (Table 2). Karnaphuli River water carries on an average of 3.54 μg/L of Hg (Table 2).
3.1.4. Beryllium
Beryllium (Be) naturally remains in small amounts in soils; however, human activities have also raised these beryllium levels. Beryllium will be added to the air and water via industrial emissions and wastewater discharge. It usually settles in sediment. The uptake of beryllium has consequences mainly for human health.
Be concentration ranged from 0.46 ± 0.14 mg/kg at the northeast station to 1.52 ± 0.35 mg/kg at the southwest station, with the southwest station having the greatest concentration. Compared to the shale value of 3 mg/kg, the average beryllium concentration was lower at 1.1714 mg/kg (Table 2).
3.1.5. Lead
Lead (Pb) is a non-essential hazardous element that causes nephrotoxicity, neurotoxicity, and anaemia in humans (both adults and children) [82]. Due to its ability to accumulate not just in individual organisms but also in entire food chains, lead is a highly toxic chemical.
The mean Pb concentration was 21.43 μg/L in water (Table 2) and 15.62 mg/kg in sediment, respectively. The lowest concentration of 6.22 ± 3.99 mg/kg was recorded at the northwest station (Table 1) and the highest concentration of 23.16 ± 9.57 mg/kg was recorded at the southeast station. The mean metal concentration was lower than the shale value (20 mg/kg) (Table 2). About 29.21 mg/kg, 20.25 mg/kg, 5.48 mg/kg, 16.57 mg/kg, 26.70 mg/kg, and 23.66–25.05 mg/kg were found in the Rupsha River, Brahmaputra River, Salt marsh Ecosystem, Karnaphuli River, Karnaphuli River Coast, and Karnaphuli River estuary respectively, by Refs. [17,43,44,47,62,67]. These results were higher than those found in the current study except salt marsh ecosystem [68]. found reduced Pb levels in the Hooghly River, India [69,70]. found a greater concentration of Pb in the Yangtze River Estuary, China than in the current research (Table 2). Many sectors, including dyeing, printing, oil refineries, and textiles, are now dumping untreated contaminants into river water, which is thought to be a potential source of the element [83].
3.1.6. Cadmium
Cadmium (Cd) is a toxic non-essential transition metal that poses a health risk for both humans and animals. It is a hazardous metal, and according to Ref. [84], its concentration in unpolluted ambient water can be as low as ng/ml. Compared to other potentially hazardous elements, cadmium is rather soluble. Anthropogenic Cd emissions into a riverine environment are derived from industry operations (batteries and plastic industries) and agricultural operations (mineral fertilizer) [39,85]. It has been designated as a group 1 carcinogenic element for humans due to its lethal consequences such as lung cancer, proteinuria, and osteomalacia [86].
The average concentration of Cd was 0.24 mg/kg and was lower than the shale value (Table 2). The highest concentration of Cd was found at 0.79 ± 0.11 mg/kg at the south-west and the lowest level was observed at 0.05 ± 0.02 mg/kg at the north-west position of riverbed sediment, respectively (Table 1). The mean record of the metal concentration (0.24 mg/kg) was lower than in previous investigations of the Rupsha River (1.8 mg/kg) and Karnaphuli River (2.01 mg/kg), but higher than in the Hooghly River (0.16 mg/kg) and Yangtze River (0.13 mg/kg) (Table 2). The average concentration of Cd in river water was 0.026 μg/L (Table 2).
3.1.7. Cobalt
Cobalt (Co) is a naturally occurring element in the environment. The average cobalt level in the soil is 8 ppm. Cobalt is beneficial to humans since it is a component of vitamin B12, which is essential for human health. Once in the environment, cobalt cannot be destroyed.
The highest concentration of Co was found at 15.33 ± 1.57 mg/kg at the south-west station and the lowest level was observed at 5.03 ± 1.42 mg/kg at the north-west station, respectively (Table 1). The mean concentration of cobalt was 11.59 mg/kg and was lower than the shale value (19 mg/kg). A very low content of Co (0.12 μg/L) was found in the southeastern drainage system of Bangladesh.
3.1.8. Chromium
Chromium (Cr) compounds are potent oxidizers that are both irritating and corrosive, giving them a hazardous appearance [32]. Hexavalent chromium rapidly penetrates biological membranes and is more toxic than Cr+3 due to its high oxidizing capacity.
Considering four sites along the Karnaphuli River, average Cr concentrations ranged from 68.33 ± 4.04 to 166 ± 7.55 mg/kg. The mean metal concentration (112.75 mg/kg) was higher than the shale value (90 mg/kg) (Table 2). [6,17,67], found a much lower level of chromium concentration in the Karnaphuli River (Table 2). 43.2 mg/kg of Cr was found at a lower level in the Rupsha riverbed sediment than in our studied Karnaphuli riverbed sediment. The lower level of Cr was also found in the Hooghly River, India [68] (Table 2). Chromium present in the Karnaphuli River sediment could have been anthropogenic: industrial activities such as textile factories are discharging Cr-based oxidants (chromate, dichromate, etc.). On the other hand, the average concentration of Cr in Karnaphuli River water was 8.44 μg/L.
3.1.9. Copper
Copper (Cu) is a minor element found in the earth's crust, ranking 25th in abundance and approximately 25 mg/kg [87]. Copper is an essential nutrient that is abundant in food and water. Zinc influenced both the absorption and the metabolism of copper negatively [88].
In our study, Cu concentrations ranged from 155 ± 19.52 mg/kg to 244.67 ± 62.74 mg/kg, with a mean value of 192.67 mg/kg (Table 1). The metal concentration levels at all of the sites were higher than the shale value (45 mg/kg) (Table 2). The highest concentration of Cu was found at 244.67 ± 62.74 mg/kg at the south-west station and the lowest level was observed at 155 ± 19.52 mg/kg at the north-west station, respectively (Table 1). The south-west zone consists of heavy industrial areas, which are the major causes of high metal loading. Other studies of the Karnaphuli River found a much lower level of copper concentration. The lower level of Cu in the Karnaphuli River Coast (45.79 mg/kg) was found by Ref. [44] (Table 2). A lower level of Cu was observed in the salt marsh ecosystem (42.90 mg/kg) than in our study. The average content of copper was found 17.87 μg/L in Karnaphuli River water (Table 2).
3.1.10. Vanadium
Algae, plants, insects, fish, and several other species in the environment contain the element vanadium (V). Vanadium strongly bioaccumulates in mussels and crabs, reaching concentrations 105 to 106 times greater than those in seawater.
The highest concentration of V was found at 36.06 ± 3.24 mg/kg at the south-west station and the lowest level was observed at 11.27 ± 4.24 mg/kg at the north-west station, respectively (Table 1). The mean concentration of vanadium was 27.49 mg/kg and was 4 times lower than the shale value (Table 2). Compared to other studies, the observed mean metal concentration (27.49 mg/kg) in the study area was about 2.5 times lower than Brahmaputra River (67.60 mg/kg) [43] (Table 2). A minimum V concentration (1.47 μg/L) was found in the Karnaphuli River water.
3.1.11. Zinc
Zinc (Zn) is an essential trace metal with very low toxicity to humans. Zinc levels that are too high can harm the pancreas, mess with protein metabolism, and lead to arteriosclerosis. Fish residing in zinc-contaminated streams can build up zinc in their bodies, allowing Zn to bio-magnify the food chain. The absorption and utilization of iron and copper in animals are hampered by excessive intake of zinc. Even at relatively low doses, continuous oral zinc supplementation in humans may have negative clinical consequences [89]. An increase in dietary zinc causes copper insufficiency in sheep [90].
The highest concentration of Zn was found at 445.33 ± 38.37 mg/kg at the south-west station and the lowest level was observed at 290.67 ± 16.17 mg/kg at the north-west station, respectively (Table 1). The mean concentration of zinc was 366.83 mg/kg and was 4 times higher than the shale value (Table 2). In comparison to prior studies of Karnaphuli, the measured average metal concentration (366.83 mg/kg) in the investigated area was greater than [17] (59.69–74.32 mg/kg) and [44] (105 mg/kg), respectively (Table 2). Industrial activities (solid waste dumpling, industrial exhaust) and agricultural practices (applications of fertilizers and pesticides) were regarded as the main reasons for higher Zn concentrations. Karnaphuli River water contains 24.2 μg/L Zn.
3.1.12. Nickel
In the aquatic environment, nickel (Ni) is one of the most transportable heavy metals. Nickel is a necessary diet for animals in small doses. Nickel, on the other hand, is not just beneficial as a vital element; if the upper limit of the tolerated amount is crossed, it can be hazardous.
The highest concentration of Ni was found at 112.67 ± 5.69 mg/kg at the south-west station and the lowest level was observed at 47.67 ± 5.03 mg/kg at the north-west station, respectively (Table 1). The mean concentration of nickel was 75.84 mg/kg and was higher than the shale value (Table 2). Compared to other studies, the measured mean content (75.84 mg/kg) in the research area was approximately three times greater than other studies' (25.30 mg/kg) evaluated by Ref. [67]. In the Hooghly River, India found decreased levels of nickel [68] (Table 2). A higher level of Ni was documented at the stations near the river's industrial and urban zones, which indicates a higher input of Ni in sediment that might originate from urban and industrial wastes. However, the average content of Ni in Karnaphuli River water was found 5.31 μg/L (Table 2).
3.2. Statistical results and source identification
Statistical investigations were performed with the goal of generating some related possibilities utilizing the correlation matrix (CM), principal component analysis (PCA), and cluster analysis (CA). Metal interactions in water and sediment in the aquatic realm provide critical details on hazardous metal origins and accumulation paths in water basins [6,48]. The CM was created to evaluate the interrelationship as well as identify the whereabouts of the origin and element distribution pattern [36,91].
3.2.1. Correlation matrix
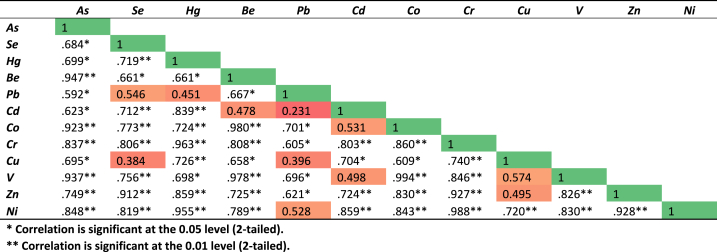
The mathematical association among the heavy metals in correlation coefficients (r) is demonstrated by the correlation table. The significance level for two-tailed t-tests is indicated by an asterisk (*). In sediment, very significant and positive linear connections in As vs Be (0.947), As vs Co (0.923), As vs V (0.937), Se vs Zn (0.912), Hg vs Cr (0.963), Hg vs Ni (0.955), Be vs Co (0.980), Be vs V (0.978), Co vs V (0.994), Co vs V (0.994), Cr vs Zn (0.927), Cr vs Ni (0.988), Ni vs Zn (0.928), and they are significant at the 0.01 level of significance (Fig. 9). As and Se (0.684), As and Hg (0.699), As and Pb (0.592), As and Cd (0.623), As and Cu (0.695), Se and Be (0.661), Hg and Be (0.661), Hg and V (0.698), Be and Pb (0.667), Be and Cu (0.658), Pb and Co (0.701), Pb and Cr (0.605), Pb and V (0.696), Pb and Zn (0.621), Cd and Cu (0.704), Co and Cu (0.609) have been identified to have high correlations that are positive at the 0.05 level of significance (Fig. 9).
Fig. 9.
Pearson's correlation of heavy metals and metalloids in sediment.
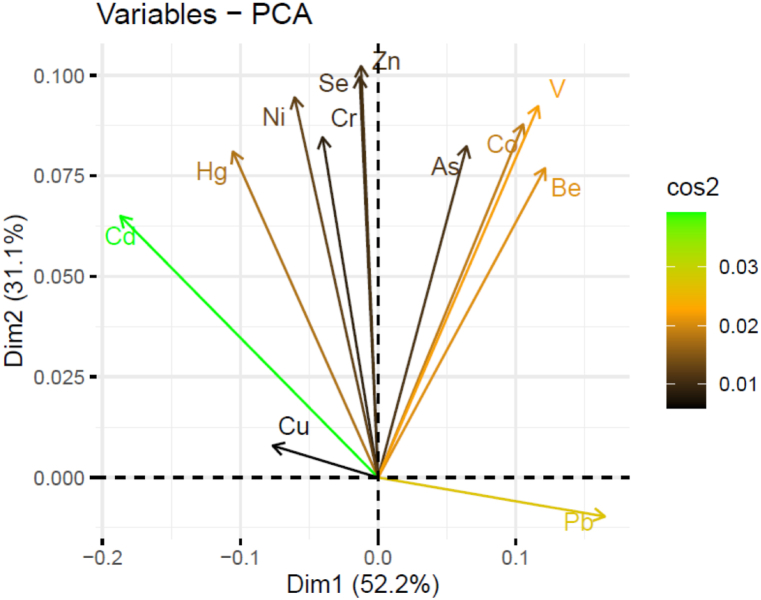
3.2.2. Principal component analysis
When creating predictive models, multicollinearity results from high levels of correlation among the independent variables. In this situation, the estimation of the model is highly unstable, and the predictions will not be precise. PCA was done to manage the situation. To identify hypothetical sources of potentially harmful components (natural or anthropogenic) in sediments of the South-Eastern Coast of Bangladesh, a PCA (Fig. 10) was carried out using the methodology of [92]. This analysis demonstrates the clustering of the trace elements into various groups, where the trace elements in one group are connected with the trace elements in another group. The first four principal components were 99.5% of cumulative input. In sediments, dimensions-1 and 2 accounted for 52.2% and 31.1% of the variability, respectively. Two categories of trace elements were found in the sediments overall, according to the PCA. Strong positive loadings on the first principal component (PC1) were predominant on As, Co, V, Be, and Pb. In principal component 2 (PC2), loads of Cu, Cd, Hg, Ni, Cr, Se, and Zn predominated. From the biplot above:
-
•
Highest cos2 attributes are colored in green: Cd
-
•
High cos2 attributes are colored in orange-green: Pb.
-
•
Mid cos2 attributes have an orange color: Be, V, Hg, and Co.
-
•
Finally, low cos2 attributes have a black color: Se, Zn, As, Ni, Cu, and Cr.
Fig. 10.
Principal Component Analysis (PCA) of heavy metals and metalloids in sediment.
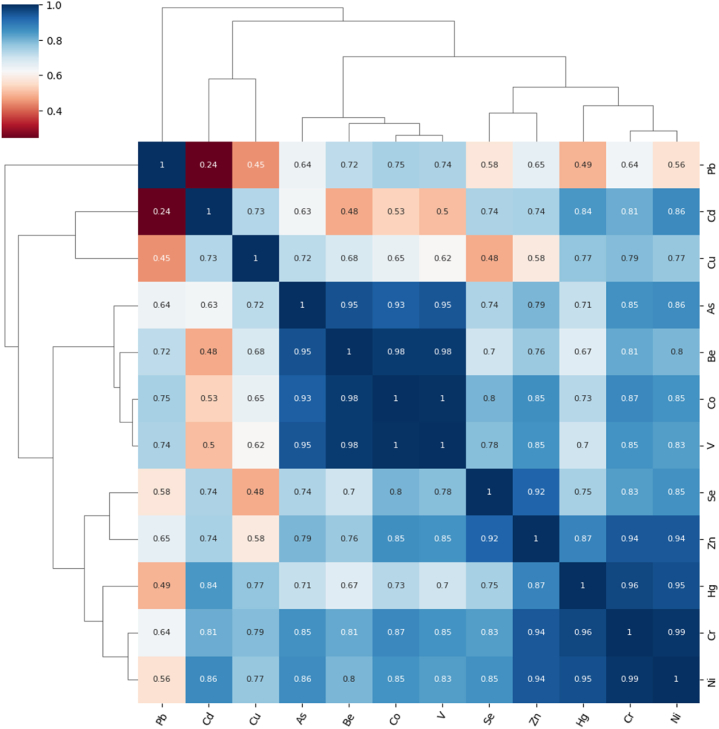
3.2.3. Cluster analysis
The findings of the cluster analysis (CA) (Fig. 11) represent that the metals in the surface sediment of the studied drainage system of Bangladesh were divided into four groups. The findings of the CA are similar to the findings of the PCA. The cluster which is the closest to the origin (blue) presents individuals with the best results in soil stations. The Violet cluster shows the individuals with average results, whereas the remaining clusters (green and red) correspond to the worst.
Fig. 11.
k-means clustering method of heavy metals and metalloids in sediment.
Based on PCA and CA analyses, Cu, Zn, and Cd are likely to be derived from similar sources. The sediments of the coast contain these elements at different levels. There is evidence of Cu and Zn loading in sediment, caused by agricultural activities such as chemical herbicides and fertilizers used on farmlands [[93], [94], [95], [96]]. Additionally, correlation analysis showed that As and Cr are strongly correlated (0.969) (P < 0.01). It is possible that agricultural practices in the study area may provide Cr and As in sediment as pesticides, chemical fertilizers, and weedicides are rich in Cr and As [97,98]. Several chemicals are released during tanning, including arsenic sulfide [20,99], and copper arsenate is released from timber treatment [100,101]. It is also possible that Pb is produced naturally [102,103] due to its widespread presence in crustal base resources. The metals in this study were derived from anthropogenic and natural sources simultaneously.
3.3. Contamination level and ecological risk assessment
Trace elements contamination in sediment is assessed and classified using contamination indices. In this study, the contamination of trace elements As, Se, Hg, Be, Pb, Cd, Co, Cr, Cu, V, Zn, and Ni in sediments from the Karnaphuli riverbed was evaluated using the contamination factor (CF) in Table 3, pollution load index (PLI) and degree of contamination (Cd) in Table 4, enrichment factor (EF) in Table 5, accumulation index (Igeo) in Table 6, and potential ecological risk index (RI) in Table 7.
Table 3.
Contamination factor (CF) of trace metals in river sediments.
| Stations | CFAs | CFSe | CFHg | CFBe | CFPb | CFCd | CFCo | CFCr | CFCu | CFV | CFZn | CFNi | CF Standard classes [104] |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Value | Contamination status | ||||||||||||||
| South-West | A1 | 0.436 | 3.458 | 1.655 | 0.375 | 0.848 | 2.3 | 0.720 | 1.77 | 3.911 | 0.249 | 5.15 | 1.59 | CF < 1 | Low contamination |
| A2 | 0.591 | 2.612 | 1.703 | 0.587 | 1.036 | 3.03 | 0.884 | 1.83 | 6.644 | 0.296 | 4.39 | 1.63 | 1 ≤ CF < 3 | Moderate contamination | |
| A3 | 0.595 | 1.834 | 1.848 | 0.561 | 0.908 | 2.57 | 0.815 | 1.93 | 5.755 | 0.288 | 4.53 | 1.75 | 3 ≤ CF ≤ 6 | Considerable contamination | |
| North-West | B1 | 0.249 | 0.357 | 0.48 | 0.122 | 0.183 | 0.17 | 0.200 | 0.73 | 3.422 | 0.062 | 2.91 | 0.63 | CF > 6 | Very high contamination |
| B2 | 0.255 | 0.066 | 0.507 | 0.132 | 0.209 | 0.2 | 0.247 | 0.73 | 3.889 | 0.075 | 3.03 | 0.69 | |||
| B3 | 0.333 | 0.255 | 0.523 | 0.208 | 0.542 | 0.1 | 0.347 | 0.81 | 3.022 | 0.124 | 3.24 | 0.78 | |||
| South-East | C1 | 0.439 | 1.309 | 0.993 | 0.416 | 1.710 | 0.23 | 0.656 | 1.41 | 4.755 | 0.231 | 4.12 | 1.12 | ||
| C2 | 0.470 | 1.273 | 1.158 | 0.553 | 0.874 | 0.17 | 0.814 | 1.44 | 5.222 | 0.277 | 3.92 | 1.19 | |||
| C3 | 0.504 | 1.680 | 1.230 | 0.539 | 0.889 | 0.27 | 0.843 | 1.48 | 3.711 | 0.302 | 4.24 | 1.28 | |||
| North-East | D1 | 0.389 | 0.455 | 0.333 | 0.336 | 0.470 | 0.3 | 0.497 | 0.86 | 4.044 | 0.174 | 3.44 | 0.84 | ||
| D2 | 0.424 | 2.007 | 0.395 | 0.439 | 1.107 | 0.17 | 0.689 | 0.99 | 3.088 | 0.232 | 3.72 | 0.91 | |||
| D3 | 0.507 | 1.455 | 0.418 | 0.417 | 0.597 | 0.27 | 0.603 | 1.04 | 3.911 | 0.229 | 3.66 | 0.97 | |||
Table 4.
Pollution load index (PLI) and Degree of contamination (Cd) of trace metals in river sediments.
| Stations | PLI | PLI Standard classes [105] |
Cd | Cd Standard classes [48] |
|||
|---|---|---|---|---|---|---|---|
| Value | Pollution load status | Value | Contamination status | ||||
| South-West | A1 | 1.28 | PLI <1 | No pollution | 22.46 | Cd < 6 | low degree of contamination |
| A2 | 1.48 | PLI >1 | Polluted | 25.23 | 6 < Cd < 12 | moderate degree of contamination | |
| A3 | 1.40 | 23.38 | 12 < Cd < 24 | significant degree of contamination | |||
| North-West | B1 | 0.38 | 9.52 | Cd > 24 | high degree of contamination | ||
| B2 | 0.36 | 10.03 | |||||
| B3 | 0.48 | 10.28 | |||||
| South-East | C1 | 0.93 | 17.39 | ||||
| C2 | 0.94 | 17.36 | |||||
| C3 | 1.00 | 16.97 | |||||
| North-East | D1 | 0.61 | 12.14 | ||||
| D2 | 0.77 | 14.17 | |||||
| D3 | 0.77 | 14.08 | |||||
Table 5.
Enrichment Factor (EF) of studied trace metals in river sediments.
| Stations | EF values |
EF standard classes [106] |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Se | Hg | Be | Pb | Cd | Co | Cr | Cu | V | Zn | Ni | Value | Enrichment status | ||
| South-West | A1 | 0.23 | 1.84 | 0.88 | 0.20 | 0.45 | 1.14 | 0.38 | 1.43 | 2.09 | 0.13 | 2.82 | 0.87 | EF < 1 | no enrichment |
| A2 | 0.29 | 1.30 | 0.91 | 0.29 | 0.51 | 1.65 | 0.44 | 1.30 | 3.30 | 0.15 | 3.11 | 1.16 | 1 < EF < 3 | no enrichment | |
| A3 | 0.30 | 0.94 | 0.92 | 0.29 | 0.47 | 1.37 | 0.42 | 1.04 | 2.95 | 0.15 | 2.32 | 0.90 | 3 < EF < 5 | mild enrichment | |
| North-West | B1 | 0.20 | 0.29 | 0.26 | 0.10 | 0.15 | 0.08 | 0.16 | 0.36 | 2.77 | 0.05 | 1.46 | 0.32 | 5 < EF < 10 | moderately severe enrichment |
| B2 | 0.18 | 0.05 | 0.28 | 0.09 | 0.15 | 0.11 | 0.17 | 0.39 | 2.75 | 0.05 | 1.68 | 0.39 | 10 < EF < 25 | severe enrichment | |
| B3 | 0.18 | 0.14 | 0.28 | 0.11 | 0.29 | 0.05 | 0.19 | 0.42 | 1.62 | 0.07 | 1.73 | 0.42 | 25 < EF < 50 | very severe enrichment | |
| South-East | C1 | 0.24 | 0.72 | 0.80 | 0.23 | 0.94 | 0.12 | 0.36 | 0.77 | 2.61 | 0.13 | 2.21 | 0.60 | EF > 50 | extremely severe enrichment |
| C2 | 0.24 | 0.64 | 0.82 | 0.28 | 0.44 | 0.09 | 0.41 | 0.79 | 2.63 | 0.14 | 2.15 | 0.65 | |||
| C3 | 0.27 | 0.91 | 0.62 | 0.29 | 0.48 | 0.14 | 0.46 | 0.74 | 2.02 | 0.16 | 2.11 | 0.64 | |||
| North-East | D1 | 0.22 | 0.25 | 0.18 | 0.19 | 0.26 | 0.24 | 0.28 | 0.48 | 2.25 | 0.09 | 2.79 | 0.68 | ||
| D2 | 0.23 | 1.08 | 0.20 | 0.24 | 0.59 | 0.12 | 0.37 | 0.53 | 1.66 | 0.12 | 2.02 | 0.50 | |||
| D3 | 0.28 | 0.80 | 0.23 | 0.23 | 0.33 | 0.15 | 0.33 | 0.57 | 2.14 | 0.13 | 1.97 | 0.52 | |||
Table 6.
Geo-accumulation index (Igeo) of trace metals in river sediments.
| Stations | As | Se | Hg | Be | Pb | Cd | Co | Cr | Cu | V | Zn | Ni | Igeo Standard classes [62] |
||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Class | Sediment quality | ||||||||||||||
| South-West | A1 | −1.78 | 1.20 | 0.14 | −2.00 | −0.82 | 0.62 | −1.06 | 0.24 | 1.38 | −2.59 | 1.78 | 0.08 | <0 | Practically unpolluted |
| A2 | −1.34 | 0.80 | 0.18 | −1.35 | −0.53 | 1.02 | −0.76 | 0.29 | 2.15 | −2.34 | 1.55 | 0.12 | 0–1 | Unpolluted to moderately polluted | |
| A3 | −1.33 | 0.29 | 0.30 | −1.42 | −0.72 | 0.77 | −0.88 | 0.37 | 1.94 | −2.38 | 1.59 | 0.22 | 2–3 | Moderately to strongly polluted | |
| North-West | B1 | −2.59 | −2.07 | −1.64 | −3.62 | −3.03 | −3.17 | −2.90 | −1.03 | 1.19 | −4.60 | 0.95 | −1.25 | 3–4 | Strongly polluted |
| B2 | −2.56 | −4.51 | −1.56 | −3.50 | −2.85 | −2.91 | −2.60 | −1.03 | 1.37 | −4.32 | 1.02 | −1.12 | 4–5 | Strongly to extremely polluted | |
| B3 | −2.17 | −2.56 | −1.52 | −2.85 | −1.47 | −3.91 | −2.11 | −0.89 | 1.01 | −3.60 | 1.11 | −0.94 | >5 | Extremely polluted | |
| South-East | C1 | −1.77 | −0.19 | −0.59 | −1.85 | 0.19 | −2.68 | −1.19 | −0.09 | 1.66 | −2.70 | 1.46 | −0.43 | ||
| C2 | −1.67 | −0.24 | −0.37 | −1.44 | −0.78 | −3.17 | −0.88 | −0.05 | 1.80 | −2.44 | 1.38 | −0.33 | |||
| C3 | −1.57 | 0.16 | −0.29 | −1.48 | −0.75 | −2.49 | −0.83 | −0.02 | 1.30 | −2.31 | 1.50 | −0.23 | |||
| North-East | D1 | −1.95 | −1.72 | −2.17 | −2.16 | −1.68 | −2.32 | −1.59 | −0.81 | 1.43 | −3.10 | 1.20 | −0.84 | ||
| D2 | −1.82 | 0.42 | −1.93 | −1.77 | −0.44 | −3.17 | −1.12 | −0.60 | 1.04 | −2.69 | 1.31 | −0.72 | |||
| D3 | −1.56 | −0.04 | −1.85 | −1.85 | −1.33 | −2.49 | −1.31 | −0.52 | 1.38 | −2.70 | 1.29 | −0.63 | |||
Table 7.
Potential ecological risk indexes of trace elements in river sediments.
| Stations | Ecological Risk for Single Metal |
RI | RI Standard Classes [104] |
Standard Classes [7] |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| As | Hg | Pb | Cd | Cr | Cu | Zn | Ni | Value | Pollution status | Value | Ecological risk status | |||
| South-West | A1 | 4.36 | 66.2 | 4.24 | 69 | 3.53 | 19.56 | 5.15 | 7.94 | 179.98 | RI < 150 | Low pollution | Er < 40 | Low ecological risk |
| A2 | 5.91 | 68.1 | 5.18 | 91 | 3.67 | 33.22 | 4.39 | 8.16 | 219.63 | 150 < RI < 300 | Considerable pollution | Er ≤ 80 | Moderate ecological risk | |
| A3 | 5.95 | 73.9 | 4.54 | 77 | 3.87 | 28.78 | 4.53 | 8.75 | 207.32 | 300 < RI < 600 | High pollution | 80 < Er ≤ 160 | Appreciable ecological risk | |
| North-West | B1 | 2.49 | 19.2 | 0.92 | 5 | 1.47 | 17.11 | 2.91 | 3.16 | 52.26 | RI ≥ 600 | Very high pollution | 160 < Er ≤ 320 | High ecological risk |
| B2 | 2.55 | 20.3 | 1.05 | 6 | 1.47 | 19.45 | 3.03 | 3.46 | 57.3 | Er > 320 | Serious ecological risk | |||
| B3 | 3.33 | 20.9 | 2.71 | 3 | 1.62 | 15.11 | 3.24 | 3.90 | 53.81 | |||||
| South-East | C1 | 4.39 | 39.7 | 8.55 | 7 | 2.82 | 23.78 | 4.12 | 5.59 | 95.95 | ||||
| C2 | 4.70 | 46.3 | 4.37 | 5 | 2.89 | 26.11 | 3.92 | 5.96 | 99.24 | |||||
| C3 | 5.04 | 49.2 | 4.45 | 8 | 2.96 | 18.56 | 4.24 | 6.40 | 98.84 | |||||
| North-East | D1 | 3.89 | 13.3 | 2.35 | 9 | 1.71 | 20.22 | 3.44 | 4.19 | 58.1 | ||||
| D2 | 4.24 | 15.8 | 5.54 | 5 | 1.98 | 15.44 | 3.72 | 4.56 | 56.28 | |||||
| D3 | 5.07 | 16.7 | 2.99 | 8 | 2.09 | 19.56 | 3.66 | 4.85 | 62.91 | |||||
3.3.1. Contamination factor (CF), degree of contamination (Cd) and pollution load index (PLI)
The degree of heavy metal pollution is estimated using CF [104]. The estimated values of CF < 1: low contamination; 1 ≤ CF < 3: moderate contamination; 3 ≤ CF ≤ 6: considerable contamination; CF > 6: very high contamination (Table 3). The CF values along the Karnaphuli River followed the descending order of Cu (4.28) > Zn (3.86) > Se (1.40) > Cr (1.25) > Ni (1.12) > Hg (0.94) > Cd (0.82) > Pb (0.78) > Co (0.61) > As (0.43) > Be (0.39) > V (0.21) (Fig. 2a and Table 3). Specifically, the CF for As, Be, Co, and V was below 1 at all sampling stations, suggesting their low enrichment and contamination [71]. The order of CF in four stations is as follows southwest > south-east > north-east > north-west (Fig. 2b).
Fig. 2.
Contamination factor (CF) (a) trace metals in Karnaphuli sediment, (b) trace metals in four different stations of Karnaphuli sediment.
Further, Cd was calculated to estimate the degree of contamination of each location. According to the value of Cd, the studied stations followed the decreasing order of south-west > south-east > north-east > north-west (Fig. 3 and Table 4). The Cd values found in this study indicated that the studied stations had a significant to high degree of contamination, mostly; because of Zn and Cd concentration.
Fig. 3.
Degree of Contamination (Cd) of studied trace metals from all sampling sites of the Karnaphuli river.
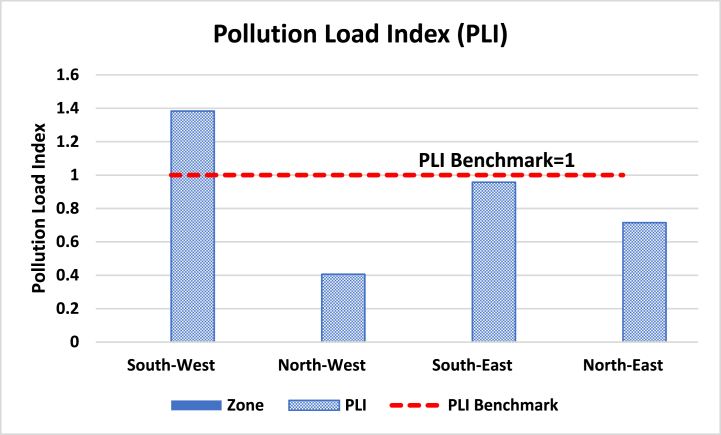
A PLI value of zero signifies excellence, a value of one indicates the presence of just baseline levels of pollutants, and values above one indicate a progressive deterioration of the site and estuarine quality [107,105]. The PLI, however, gives insight into the sample's overall level of toxicity. This provides important information on the level of pollution in the area for decision-makers as well [91]. In this study, the PLI values followed the order of south-west (1.38) > south-east (0.96) > north-east (0.72) > north-west (0.41) (Fig. 4 and Table 4). The value of PLI of the south-west station (1.38) was found to be above 1, indicating that the river is facing progressive deterioration (Table 4).
Fig. 4.
Pollution load index (PLI) of trace metals from four zones south-eastern drainage area.
3.3.2. Enrichment factor (EF)
EF measures the influence of anthropogenic activities [108]. In this study, the mean EF values of As, Ba, Be, Cd, Co, Cr, Cu, Hg, Ni, Pb, Se, Sr, V, and Zn followed the increasing order of V (0.11) < Be (0.21) < As (0.24) < Co (0.33) < Pb (0.42) < Cd (0.44) < Hg (0.53) < Ni (0.64) < Cr (0.74) < Se (0.75) < Zn (2.20) < Cu (2.40) (Fig. 5a and Table 5). This study also revealed that the EF of Cr was the highest in the south-west zone. The order of EF in four stations is as follows southwest > south-east > north-west > north-east (Fig. 5b).
Fig. 5.
Enrichment factor (EF) (a) trace metals in Karnaphuli River sediment, (b) trace metals in four different stations of Karnaphuli River sediment.
3.3.3. Geo-accumulation index (Igeo)
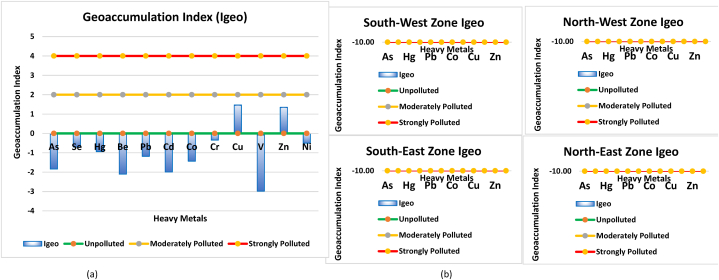
Igeo evaluates the contamination levels of metals in comparison to background values [109]. The values of Igeo have been used to estimate sediment quality [110]. In this experiment, the values of Igeo for trace elements ranged from −2.98 (V) to 1.47 (Cu). The average values of Igeo for each metal decreased as per the following order: Cu (1.47) > Zn (1.35) > Cr (−0.34) > Ni (−0.50) > Se (−0.71) > Hg (−0.94) > Pb (−1.18) > Co (−1.44) > As (−1.84) > Cd (−1.99) > Be (−2.10) > V (−2.98) (Fig. 6a and Table 6). According to the classification, the calculated values of Igeo for Co, As, Be, and V in the sediments belonged to class zero, indicating that the sediment in all the sites was unpolluted by these metals. The highest value showed for Cu indicating that it belonged to the class moderately polluted [111]. showed that the studied area was uncontaminated to moderately contaminated with Pb, Co, Cd, Hg, and Zn. The order of Igeo in four stations is as follows south-west > south-east > north-west > north-east (Fig. 6b).
Fig. 6.
Geoaccumulation Index (a) trace metals in Karnaphuli River sediment, (b) trace metals in four different stations of Karnaphuli River sediment.
3.3.4. Potential ecological risk factor (Er) and risk index (RI)
The ecological risk index identifies the individual ecological threat of each metal and the combined ecological and toxicological repercussions of the increased pollution by diving into the ecological risk levels of soil contamination [67]. The average monomial risk factor of metals in sediment samples from the Karnaphuli River was rated in the following order: Hg (37.47) > Cd (24.42) > Cu (21.41) > Ni (5.58) > As (4.33) > Pb (3.91) > Zn (3.86) > Cr (2.51) (Fig. 7a and Table 7). All the values of ecological risk for trace metals found below 40 indicate that these metals pose a low risk in the surrounding ecosystem. The order of Er in the four stations is as follows: south-west > south-east > north-east > north-west (Fig. 7b).
Fig. 7.
Ecological risk (a) trace metals in Karnaphuli River sediment, (b) trace metals in four different stations of Karnaphuli River sediment.
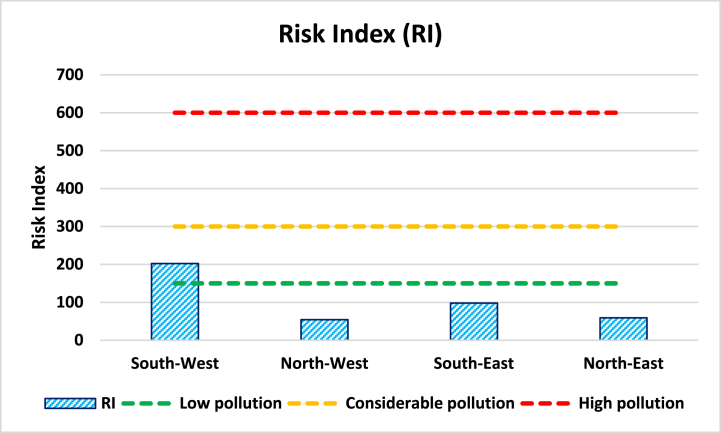
The calculated potential ecological risk index (RI) for trace metals in this study is shown in Table 7. Samples from different stations followed the order of: south-west (202.31) > south-east (98.01) > north-east (59.10) > north-west (54.46) (Fig. 8). The RI values of the south-west (202.31) posed the highest values indicating considerable pollution (RI ≥ 300). The lowest value (54.46) for the north-west indicates considerable pollution (RI < 150).
Fig. 8.
Risk indexes of trace elements in sediments of Karnaphuli River, Bangladesh.
3.4. Assessment of human health risk
The human health risk was considered because the river basin's residents were directly involved in activities such as farming. The majority of inhabitants utilized the riverbank sediment for agricultural purposes. As a result, they came into direct contact with the sediment. For understanding the undesirable human health impacts linked with environmental hazard exposure, one of the significant characterizations and critical techniques is risk assessment [39,112]. The health risks of trace metals for adults (men and women) and child were estimated by a deterministic method for three considerable exposure paths, i.e., dermal contact, ingestion, and inhalation, using an equation.
3.4.1. Estimation of chronic daily intake (CDI)
Tables S6–S9 lists the CDIs of the metals from the sites that were investigated for adults and children. The table demonstrated that CDIs were greater in children than in adults via the following pathways, in declining order: ingestion > dermal contact > inhalation. The south-west point had the highest CDI value for twelve metals for the targeted groups of participants across all exposure paths of the four stations.
CDI values through the ingestion route of men, women, and child groups of the south-west station have been placed in the following downward trend: Zn > Cu > Cr > Ni > V > Pb > Co > As > Se > Be > Cd > Hg (Table S6, S7 and S8). The result revealed that Zn was the most consumed and absorbed metal, with 0.00061 mg/kg/day, 0.000657 mg/kg/day, and 0.002847 mg/kg/day through the ingestion route for men, women, and child, respectively (Tables S6 and S7, and S8). Children living near the riverbank had higher metal content than adults. This is due to the fact that children have an elevated exposure rate and a higher consumption limit based on what they weigh than adults [113].
3.4.2. Hazard quotient (HQ) and hazard index (HI) assessment (non-carcinogenic risk)
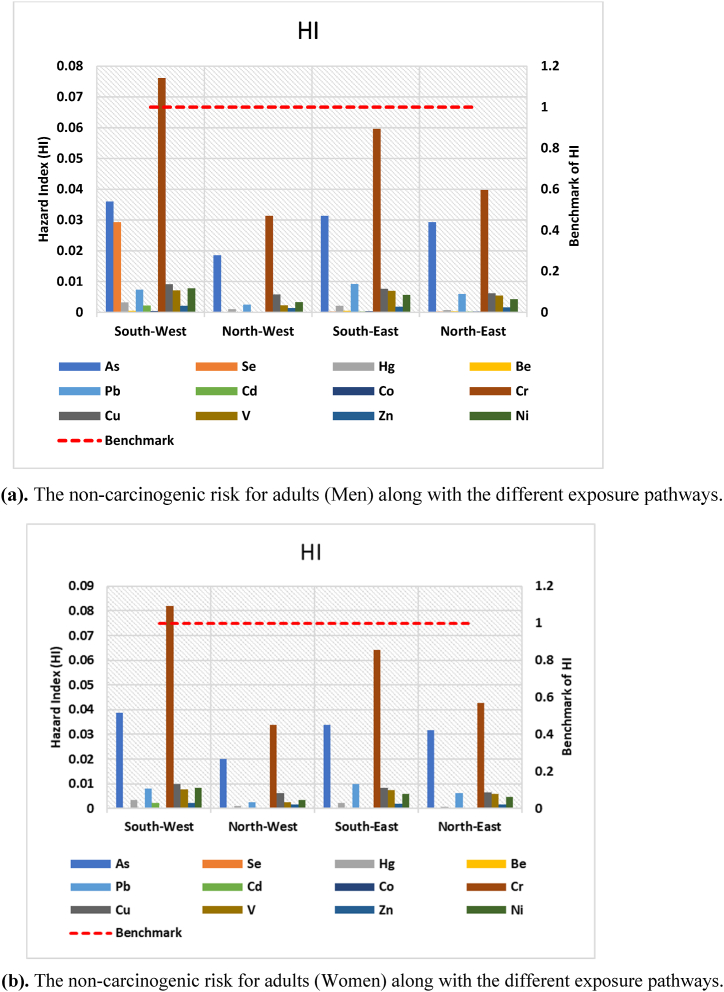
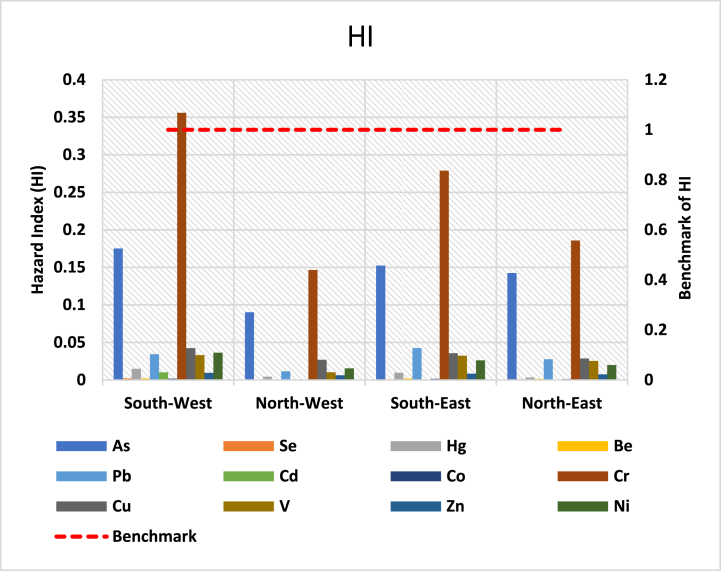
Average CDI values were used to assess the non-carcinogenic risk. Tables S6, S7, and S8 show the calculated hazard quotient (HQ) of trace metals for men, women, and children. The highest HQ value for Cr content via ingestion for men, women and child is 0.76 × 10−1, 0.82 × 10−1, and 3.54 × 10−1, respectively. As was the most popular dermal contact channel for each age category of people (adult: 3.84 × 10−3 and 4.14 × 10−3 for men and women, respectively, and Child: 2.52 × 10−2). In comparison with ingestion and dermal pathways, the HQ due to inhalation was much lower, and the risk of trace metals exposure through inhalation is almost negligible when compared to the other two paths. Overall, exposure through different pathways was in the order of ingestion > dermal absorption > inhalation. According to the results, the HI values of the south-west zone for adult (men and Women) and child groups were organized in the following trend, respectively: Cr > As > Se > Cu > Ni > Pb > V > Hg > Cd > Zn > Be > Co; Cr > As > Cu > Ni > Pb > V > Hg > Cd > Zn > Se > Be > Co; and Cr > As > Cu > Ni > Pb > V > Hg > Cd > Zn > Se > Be > Co (Fig. 12a, Fig. 12b, and Fig. 13; Table S6, S7 and S8). The cumulative results, however, were less than one, suggesting that there were no substantial non-carcinogenic risk effects in the research area and that the locals were protected from high risk.
Fig. 12.
(a). The non-carcinogenic risk for adults (Men) along with the different exposure pathways. (b) The non-carcinogenic risk for adults (Women) along with the different exposure pathways.
Fig. 13.
The non-carcinogenic risk for children along with the different exposure pathways.
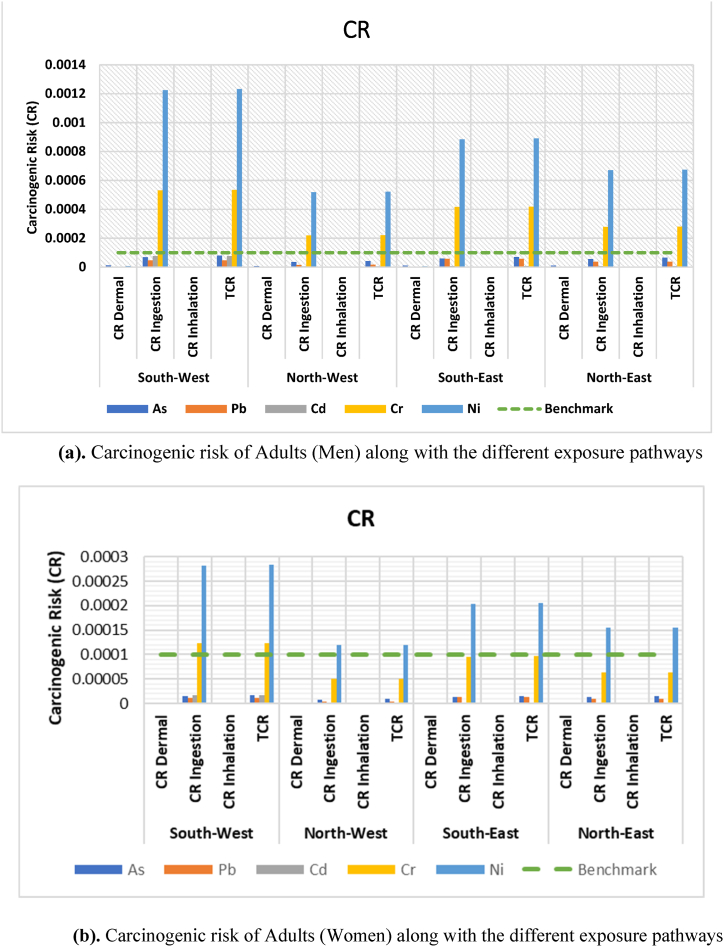
3.4.3. Carcinogenic risk (CR) evaluation
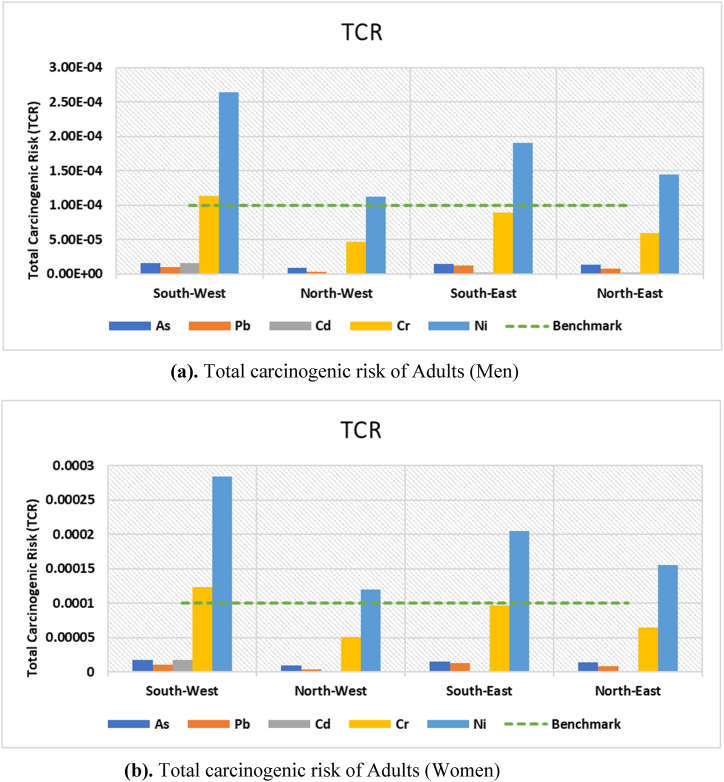
The carcinogenic risk (CR) for As, Pb, Cd, Cr, and Ni was presented in Tables S9, S10, and S11. Among the three exposure routes, ingestion exposure was the highest for both adults (men and women) and children. Surprisingly, the CRs of various metals for different age groups may differ (Table S12, S13 and S14). The average values of total carcinogenic risk (TCR) for each metal in men, women, and child groups were organized in the following descending trend, respectively: Ni (0.00018) > Cr (7.8 × 10−5) > As (1.3 × 10−5) > Pb (8.2 × 10−6) > Cd (5.1 × 10−6), Ni (0.00019) > Cr (8.35 × 10−5) > As (1.39 × 10−5) > Pb (8.86 × 10−6) > Cd (5.4 × 10−6), and Ni (0.00083) > Cr (0.00036) > As (6.3 × 10−5) > Pb (3.8 × 10−5) > Cd (2.4 × 10−5). According to Fig. 14a and b, and Fig. 16, children have greater CR values than adults. Additionally, children were more strongly affected by higher CR when it comes to Ni (0.001224) and Cr (0.000531) through the ingestion route than by any other element [30,114]. also showed that children had higher CR values than adults. All As, Cd, Cr, and Ni risk levels were observed to be above 1 × 10−5, proving that the research area had negative impacts from CR values in both children and adults (Tables S12, S13, and S14). Total carcinogenic risk (TCR) for men, women and child were illustrated in Fig. 15a and b, and Fig. 17. Although, the combined CR value fell inside the 10−6 to 10−4 threshold range.
Fig. 14.
(a) Carcinogenic risk of Adults (Men) along with the different exposure pathways. (b) Carcinogenic risk of Adults (Women) along with the different exposure pathways.
Fig. 16.
Carcinogenic risk of Children along with the different exposure pathways.
Fig. 15.
(a) Total carcinogenic risk of Adults (Men). (b) Total carcinogenic risk of Adults (Women).
Fig. 17.
Total Carcinogenic risk of Children.
4. Conclusions
An investigational study was carried out to analyze trace metal levels in Karnaphuli, a natural drainage system in Bangladesh's south-eastern region. The findings of the present study revealed evidence of trace metal contamination. Except for Se, Cr, Cu, Zn, and Ni, the average concentrations of the majority of the trace elements in the sediment were lower than the shale values. All the computed Igeo values for As, Be, Pb, Co, and V were assigned to class zero, revealing that none of the sites were contaminated with As, Be, Pb, Co, or V. The average ecological risk factors of metals in sediment samples of the Karnaphuli River were ranked in the following order: Hg (37.47) > Cd (24.42) > Cu (21.41) > Ni (5.58) > As (4.33) > Zn (3.86) > Pb (3.91) > Cr (2.51). Most of the time, Cd readings obtained in this study suggested that the analyzed stations had a significant degree of contamination because of Cu and Zn concentrations. According to the CF, Cd, PLI, and RI values, there is considerable sediment contamination in the studied area. Industrial wastewater, which is found in the textile industries in the area under study, is one of the main potential sources of trace metals. Ingestion > dermal contact > inhalation are generally the three exposure modes that pose the most non-carcinogenic health concerns. For the targeted populations through all exposure routes, Zn showed a substantially higher CDI value among the metals. According to the findings of this study, the investigated area continues to encounter high levels of trace metal pollutants from diverse sources, and if their concentrations stay elevated, their toxicity will rise as well, disrupting every link in the food chain in the ecosystem. Last but not least, to protect public health, point sources of trace metals should be constantly observed, unlawful industrial effluent and domestic sewage discharge should be reduced, and effluent should be processed before being discharged into the Karnaphuli River. It can assist in identifying places that require cleaning and ensuring compliance with rules and regulations.
Author contribution statement
Fahima Islam: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Afroza Parvin: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Afsana Parvin: Performed the experiments; Analyzed and interpreted the data. Umme Sarmeen Akhtar: Performed the experiments and interpreted data. Md Aftab Ali Shaikh: Conceived and designed the experiments. Md Nashir Uddin: Analyzed and interpreted the data.
Mohammad Moniruzzaman: Contributed reagents, materials, analysis tools or data. Badhan Saha: Performed the experiments. Juliya Khanom: Performed the experiments. Priyanka Dey Suchi: Performed the experiments. Md Anwer Hossain: Analyzed and interpreted the data Md Kamal Hossain: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
There are no financial and any other benefits of declaration of interest.
Acknowledgments
The authors highly acknowledge the authorities of the Bangladesh Council of Scientific and Industrial Research for approved Research and Development (R & D) work, and the Ministry of Science and Technology in Bangladesh for providing partial funds through granted special allocation (2022–23); sincerely acknowledge to Mr. Md Shanewaz Khan, Barisal University for drawing GIS Map of the sample location.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20040.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Ali M.M., Ahmed M.K. Effect of a bone mill effluents on the river karnafully: a case study. Dhaka Univ. J. Biol. Sci. 2009;18(1):75–77. [Google Scholar]
- 2.Ali M.M., Ali M.L., Proshad R., Islam M.S., Rahman M.Z., Kormoker T. Assessment of trace elements in the demersal fishes of a coastal River in Bangladesh: a public health concern. Thalas An Int. J. Mar. Sci. 2020;36(2):641–655. doi: 10.1007/s41208-020-00227-7. [DOI] [Google Scholar]
- 3.Ali M.M., Ali M.L., Islam M.S., Rahman M.Z. Assessment of toxic metals in water and sediment of Pasur River in Bangladesh. Water Sci. Technol. 2018;77(5):1418–1430. doi: 10.2166/wst.2018.016. [DOI] [PubMed] [Google Scholar]
- 4.Pandey L.K., Park J., Son D.H., Kim W., Islam M.S., Choi S., Lee H., Han T. Assessment of metal contamination in water and sediments from major rivers in South Korea from 2008 to 2015. Sci. Total Environ. 2019;651:323–333. doi: 10.1016/j.scitotenv.2018.09.057. [DOI] [PubMed] [Google Scholar]
- 5.Proshad R., Kormoker T., Islam M.S. Distribution, source identification, ecological and health risks of heavy metals in surface sediments of the Rupsa River, Bangladesh. Toxin Rev. 2019;40:1–25. [Google Scholar]
- 6.Ali M.M., Ali M.L., Islam M.S., Rahman M.Z. Preliminary assessment of heavy metals in water and sediment of Karnaphuli River, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2016;5:27–35. doi: 10.1016/j.enmm.2016.01.002. [DOI] [Google Scholar]
- 7.Hossain H.M.Z., Hossain Q.H., Islam M.S. Spatial distribution of heavy metals in surface sediments from the Ganges River basin, Bangladesh. Arabian J. Geosci. 2019;12(22):676. doi: 10.1007/s12517-019-4841-y. [DOI] [Google Scholar]
- 8.Algül F., Beyhan M. Concentrations and sources of heavy metals in shallow sediments in Lake Bafa, Turkey. Sci. Rep. 2020;10 doi: 10.1038/s41598-020-68833-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shorna S., Quraishi S.B., Hosen M.M., Hossain M.K., Saha B., Hossain A., Habibullah-Al-Mamun M. Ecological risk assessment of trace metals in sediment from the Old Brahmaputra River in Bangladesh. Chem. Ecol. 2021;(37):809–826. doi: 10.1080/02757540.2021.1989422. [DOI] [PubMed] [Google Scholar]
- 10.Huang P., Li T.G., Li A.C., Yu X.K., Hu N.J. Distribution, enrichment and sources of heavy metals in surface sediments of the North Yellow Sea. Continent. Shelf Res. 2014;73:1–13. [Google Scholar]
- 11.Saroop S., Tamchos S. Heavy Metals in the Environment. Elsevier; 2021. Monitoring and impact assessment approaches for heavy metals; pp. 57–86. [Google Scholar]
- 12.Guo R., He X. Spatial variations and ecological risk assessment of heavy metals in surface sediments on the upper reaches of Hun River, Northeast China. Environ. Earth Sci. 2013;70:1083–1090. doi: 10.1007/s12665-012-2196-8. [DOI] [Google Scholar]
- 13.Hossain M.A., Ali N.M., Islam M.S., Hossain H.M.Z. Spatial distribution and source apportionment of heavy metals in soils of Gebeng industrial city, Malaysia. Environ. Earth Sci. 2015;73:115–126. [Google Scholar]
- 14.U.S. EPA. (Environmental Protection Agency . National Center for Environmental Assessment; Washington, DC: 2005. Predicting Toxicity to Amphipods from Sediment Chemistry.http://www.epa.gov/ncea EPA/600/R-04/030. Available from: National Technical Information Service, Springfield, VA, and online at. [Google Scholar]
- 15.Hsu L.C., Huang C.Y., Chuang Y.H., Chen H.W., Chan Y.T., Teah H.Y., Chen T.Y., Chang C.F., Liu Y.T., Tzou Y.M. Accumulation of heavy metals and trace elements in fluvial sediments received effluents from traditional and semiconductor industries. Sci. Rep. 2016;6(1):1–12. doi: 10.1038/srep34250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiang R., Lin C., Zhou K., Liu Y., Chen J., Wang S., Pan Z., Sun X., Wang W., Lin H. Pollution, ecological risk, and source identification of potentially toxic elements in sediments of a landscape urban lagoon, China. Mar. Pollut. Bull. 2022;174 doi: 10.1016/j.marpolbul.2021.113192. [DOI] [PubMed] [Google Scholar]
- 17.Wang A.J., Kawser A., Xu Y.H., Ye X., Rani S., Chen K.L. Heavy metal accumulation during the last 30 years in the Karnaphuli River estuary, Chittagong, Bangladesh. SpringerPlus. 2016;5(1):1–14. doi: 10.1186/s40064-016-3749-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mia M.A., Nasrin S., Zhang M., Rasiah R. Chittagong, Bangladesh. Cities. 2015;48:31–41. [Google Scholar]
- 19.Tareq S.M., Safiullah S., Anawar H.M., Rahman M.M., Ishizuka T. Arsenic pollution in groundwater: a self-organizing complex geochemical process in the deltaic sedimentary environment, Bangladesh. Sci. Total Environ. 2003;313:213–226. doi: 10.1016/S0048-9697(03)00266-3. [DOI] [PubMed] [Google Scholar]
- 20.Bhuiyan M.A.H., Suruvi N.I., Dampare S.B., Islam M.A., Quraishi S.B., Ganyaglo S., Suzuki S. Investigation of the possible sources of heavy metal contamination in lagoon and canal water in the tannery industrial area in Dhaka, Bangladesh. Environ. Monit. Assess. 2011;175:633–649. doi: 10.1007/s10661-010-1557-6. [DOI] [PubMed] [Google Scholar]
- 21.Islam M.S., Ahmed M.K., Habibullah-Al-Mum M., Hoque M.F. Preliminary assessment of heavy metal contamination in surface sediments from a river in Bangladesh. Environ. Earth Sci. 2015;73:1837–1848. doi: 10.1007/s12665-014-3538-5. [DOI] [Google Scholar]
- 22.Sharifuzzaman S.M., Rahman H., Ashekuzzaman S.M., Islam M.M., Chowdhury S.R., Hossain M.S. In: Environmental Remediation Technologies for Metal-Contaminated Soils. Hasegawa H., Rahman I.M.M., Rahman M.A., editors. Springer; Japan: 2016. Heavy metals accumulation in coastal sediments; pp. 21–42. [Google Scholar]
- 23.En.wikipedia.org. Drainage. 2019. online] Available at: https://en.wikipedia.org/wiki/Drainage. [Google Scholar]
- 24.Rahman M.S., Saha N., Molla A.H. Potential ecological risk assessment of heavy metal contamination in sediment and water body around Dhaka export processing zone, Bangladesh. Environ. Earth Sci. 2014;71(5):2293–2308. doi: 10.1007/s12665-013-2631-5. [DOI] [Google Scholar]
- 25.Alam M.G.M., Snow E.T., Tanaka A. Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. Sci. Total Environ. 2003;308:83–96. doi: 10.1016/S0048-9697(02)00651-4. [DOI] [PubMed] [Google Scholar]
- 26.Islam M.S., Ahmed M.K., Habibullah-Al-Mamun M., Islam K.N., Ibrahim M., Masunaga S. Arsenic and lead in foods: a potential threat to human health in Bangladesh. Food Addit. Contam. 2014;31(12):1982–1992. doi: 10.1080/19440049.2014.974686. [DOI] [PubMed] [Google Scholar]
- 27.Hu B., Jia X., Hu J., Xu D., Xia F., Li Y. Assessment of heavy metal pollution and health risks in the soil-plant-human system in the Yangtze River Delta, China. Int. J. Environ. Res. Publ. Health. 2017;14(9):1042. doi: 10.3390/ijerph14091042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu B., Zhao D.Y., Jia H.Y., Zhang Y., Zhang X.X., Cheng S.P. Preliminary risk assessment of trace metal pollution in surface water from Yangtze River in Nanjing Section, China. Bull. Environ. Contam. Toxicol. 2009;82(4):405–409. doi: 10.1007/s00128-008-9497-3. [DOI] [PubMed] [Google Scholar]
- 29.Nour H.E., Alshehri F., Sahour H., El-Sorogy A.S. Evaluation of sediment and water quality of Ismailia Canal for heavy metal contamination, Eastern Nile Delta, Egypt. Reg. Stud. Mar. Sci. 2022;56 doi: 10.1016/j.rsma.2022.102714. [DOI] [Google Scholar]
- 30.Zafarzadeh A., Taghani J.M., Toomaj M.A., Ramavandi B., Bonyadi Z., Sillanpää M. Assessment of the health risk and geo-accumulation of toxic metals in agricultural soil and wheat, northern Iran. Environ. Monit. Assess. 2021;193:1–10. doi: 10.1007/s10661-021-09530-z. [DOI] [PubMed] [Google Scholar]
- 31.Zafarzadeh A., Bonyadi Z., Feyzi K. Health risk assessment related to cadmium in dairy products in Gorgan, Iran. Int. J. Environ. Anal. Chem. 2020;102(16):4058–4066. doi: 10.1080/03067319.2020.1779244. [DOI] [Google Scholar]
- 32.Rahman M.S., Molla A.H., Saha N., Rahman A. Study on heavy metals levels and its risk assessment in some edible fishes from Bangshi River, Savar, Dhaka, Bangladesh. Food Chem. 2012;134(4):1847–1854. doi: 10.1016/j.foodchem.2012.03.099. [DOI] [PubMed] [Google Scholar]
- 33.Rana S.V.S. Perspectives in endocrine toxicity of heavy metals—a review. Biol. Trace Elem. Res. 2014;160(1):1–14. doi: 10.1007/s12011-014-0023-7. [DOI] [PubMed] [Google Scholar]
- 34.Saha N., Zaman M.R. Evaluation of possible health risks of heavy metals by consumption of foodstuffs available in the central market of Rajshahi City, Bangladesh. Environ. Monit. Assess. 2013;185(5):3867–3878. doi: 10.1007/s10661-012-2835-2. [DOI] [PubMed] [Google Scholar]
- 35.Bosch A.C., O'Neill B., Sigge G.O., Kerwath S.E., Hoffman L.C. Heavy metals in marine fish meat and consumer health: a review. J. Sci. Food Agric. 2016;96(1):32–48. doi: 10.1002/jsfa.7360. [DOI] [PubMed] [Google Scholar]
- 36.Ahmed A.S.S., Hossain M.B., Babu S.M.O.F., Rahman M.M., Sarker M.S.I. Human health risk assessment of heavy metals in water from the subtropical river, Gomti, Bangladesh. Environ. Nanotechnol. Monit. Manag. 2021;15 doi: 10.1016/j.enmm.2020.100416. [DOI] [Google Scholar]
- 37.Ahmed M.K., Baki M.A., Islam M.S., Kundu G.K., Habibullah-Al-Mamun M., Sarkar S.K., Hossain M.M. Human health risk assessment of heavy metals in tropical fish and shellfish collected from the river Buriganga, Bangladesh. Environ. Sci. Pollut. Res. 2015;22(20):15880–15890. doi: 10.1007/s11356-015-4813-z. [DOI] [PubMed] [Google Scholar]
- 38.Velusamy A., Kumar P.S., Ram A., Chinnadurai S. Bioaccumulation of heavy metals in commercially important marine fishes from Mumbai Harbor, India. Mar. Pollut. Bull. 2014;81(1):218–224. doi: 10.1016/j.marpolbul.2014.01.049. [DOI] [PubMed] [Google Scholar]
- 39.Ahmed A.S.S., Hossain M.B., Babu S.M.O.F., Rahman M., Sun J., Sarkar M.S.I. Spatial distribution, sources, and associated risks of trace metals (As, Pb, Cr, Cd, and Hg) from a subtropical river, Gomti, Bangladesh. Int. J. Sediment Res. 2021;37(1):83–96. [Google Scholar]
- 40.Abadi M., Zamani A., Parizanganeh A., Khosravi Y., Badiee H. Distribution pattern and pollution status by analysis of selected heavy metal amounts in coastal sediments from the southern Caspian Sea. Environ. Monit. Assess. 2019;191(3):144. doi: 10.1007/s10661-019-7261-2. [DOI] [PubMed] [Google Scholar]
- 41.Saleem M., Iqbal J., Shah M.H. Geochemical speciation, anthropogenic contamination, risk assessment and source identification of selected metals in fresh water sediments – a case study from Mangla Lake, Pakistan. Environ. Nanotechnol. Monit. Manag. 2015;4:27–36. [Google Scholar]
- 42.Yi Y., Yang Z., Zhang S. Ecological risk assessment of heavy metals in sediment and human health risk assessment of heavy metals in fishes in the middle and lower reaches of the Yangtze River basin. Environ. Pollut. 2011;159:2575–2585. doi: 10.1016/j.envpol.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 43.Hossain H.M. Spatial distribution and pollution assessment of heavy metals in sediments from the Brahmaputra River watershed in Bangladesh. Environ. Sci. Pollut. Res. 2022;29(54):81557–81570. doi: 10.1007/s11356-022-21522-1. [DOI] [PubMed] [Google Scholar]
- 44.Siddique M.A.M., Aktar M. Heavy metals in salt marsh sediments of porteresia bed along the Karnafully River coast, Chittagong. Soil Water Res. 2012;7(3):117–123. [Google Scholar]
- 45.USEPA . 2011. Exposure Factors Handbook 2011 Edition (Final)http://cfpub.epa.gov/ Retrieved from: [Google Scholar]
- 46.Kusin F.M., Azani N.N.M., Hasan S.N.M., Sulong N.A. Distribution of heavy metals and metalloid in surface sediments of heavily-mined area for bauxite ore in Pengerang, Malaysia and associated risk assessment. Catena. 2018;165:454–464. doi: 10.1016/j.catena.2018.02.029. [DOI] [Google Scholar]
- 47.Kubra K., Mondol A.H., Ali M.M., Palash M., Ullah A., Islam M., Islam A.R.M., Bhuyan M., Ahmed A.S., Rahman M. Pollution level of trace metals (As, Pb, Cr and Cd) in the sediment of Rupsha River, Bangladesh: assessment of ecological and human health risks. Front. Environ. Sci. 2022:1260. [Google Scholar]
- 48.Ali M.M., Rahman S., Islam M.S., Rakib M.R.J., Hossen S., Rahman M.Z. Distribution of heavy metals in water and sediment of an urban river in a developing country: a probabilistic risk assessment. Int. J. Sediment Res. 2022;37(2):173–187. doi: 10.1016/j.ijsrc.2021.09.002. [DOI] [Google Scholar]
- 49.USEPA . 2002. Supplemental Guidance for Developing Soil Screening Levels for Superfund Sites OSWER 9355. Washington, DC, USA. [Google Scholar]
- 50.Hossain M.B., Ahmed A.S.S., Sarker M.S.I. Human health risks of Hg, As, Mn, and Cr through consumption of fish, Ticto barb (Puntius ticto) from a tropical river. Bangladesh. Environ. Sci. Pollut. Res. 2018;25(31):31727–31736. doi: 10.1007/s11356-018-3158-9. [DOI] [PubMed] [Google Scholar]
- 51.Zhao X.M., Yao L.A., Ma Q.L., Zhou G.J., Wang L., Fang Q.L. Distribution and ecological risk assessment of cadmium in water and sediment in Longjiang River, China: implication on water quality management after pollution accident. Chemosphere. 2018;194:107–116. doi: 10.1016/j.chemosphere.2017.11.127. [DOI] [PubMed] [Google Scholar]
- 52.USEPA . USEPA; Washington, DC: 2000. Risk-based Concentration Table. [Google Scholar]
- 53.Fantke P., Friedrich R., Jolliet O. Health impact and damage cost assessment of pesticides in Europe. Environ. Int. 2012;49:9–17. doi: 10.1016/j.envint.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Mo Z., Qin J., Li Q., Wei Y., Ma S., Xiong Y., Zou Y. Change of water sources reduces health risks from heavy metals via ingestion of water, soil, and rice in a riverine area, South China. Sci. Total Environ. 2015;530:163–170. doi: 10.1016/j.scitotenv.2015.05.100. [DOI] [PubMed] [Google Scholar]
- 55.Papillomaviruses H. IARC; Lyon, France: 2011. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. [Google Scholar]
- 56.Alsafran M., Usman K., Rizwan M., Ahmed T., Al Jabri H. The carcinogenic and non-carcinogenic health risks of metal (oid) s bioaccumulation in leafy vegetables: a consumption advisory. Front. Environ. Sci. 2021:380. [Google Scholar]
- 57.Yin S., Feng C., Li Y., Yin L., Shen Z. Heavy metal pollution in the surface water of the Yangtze estuary: a 5-year follow-up study. Chemosphere. 2015;138:718–725. doi: 10.1016/j.chemosphere.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 58.Traina A., Bono G., Bonsignore M., Falco F., Giuga M., Quinci E.M., Sprovieri M. Heavy metals concentrations in some commercially key species from Sicilian coasts (Mediterranean Sea): potential human health risk estimation. Ecotoxicol. Environ. Saf. 2019;168:466–478. doi: 10.1016/j.ecoenv.2018.10.056. [DOI] [PubMed] [Google Scholar]
- 59.Islam M.S., Hossain M.B., Matin A., Sarker M.S.I. Assessment of heavy metal pollution, distribution and source apportionment in the sediment from Feni River estuary, Bangladesh. Chemosphere. 2018;202:25–32. doi: 10.1016/j.chemosphere.2018.03.077. [DOI] [PubMed] [Google Scholar]
- 60.Kormoker T., Proshad R., Islam M.S., Shamsuzzoha M., Akter A., Tusher T.R. Concentrations, source apportionment and potential health risk of toxic metals in foodstuffs of Bangladesh. Toxin Rev. 2020:1–14. doi: 10.1080/15569543.2020.1731551. [DOI] [Google Scholar]
- 61.Tusher T.R., Sarker M.E., Nasrin S., Kormoker T., Proshad R., Islam M.S., Al-Mamun S., Tareq A.R.M. Contamination of toxic metals and polycyclic aromatic hydrocarbons (PAHs) in rooftop vegetables and human health risks in Bangladesh. Toxin Rev. 2020;1–16 doi: 10.1080/15569543.2020.1767650. [DOI] [Google Scholar]
- 62.Rakib R.J., Hossain M.B., Jolly Y.N., Akther S., Islam S. EDXRF detection of trace elements in salt marsh sediment of Bangladesh and probabilistic ecological risk assessment. Soil Sediment Contam. 2021;31(2):220–239. doi: 10.1080/15320383.2021.1923644. [DOI] [Google Scholar]
- 63.Islam M.S., Proshad R., Ahmed S. Ecological risk of heavy metals in sediment of an urban river in Bangladesh. Hum. Ecol. Risk Assess. 2018;24(3):699–720. [Google Scholar]
- 64.Bhuyan M.S., Bakar M.A., Rashed-Un-Nabi M., Senapathi V., Chung S.Y., Islam M.S. Monitoring and assessment of heavy metal contamination in surface water and sediment of the Old Brahmaputra River, Bangladesh. Appl. Water Sci. 2019;9(5):125. doi: 10.1007/s13201-019-1004-y. [DOI] [Google Scholar]
- 65.Siddique M.A.B., Alam M.K., Islam S., Diganta M.T.M., Akbor M.A., Bithi U.H., Chowdhury A.I., Ullah A.A. Apportionment of some chemical elements in soils around the coal mining area in northern Bangladesh and associated health risk assessment. Environ. Nanotechnol. Monit. Manag. 2020;14 doi: 10.1016/j.enmm.2020.100366. [DOI] [Google Scholar]
- 66.Akbor M.A., Rahman M.M., Bodrud-Doza M., Haque M.M., Siddique M.A.B., Ahsan M.A., Bondad S.E.C., Uddin M.K. Metal pollution in water and sediment of the Buriganga River, Bangladesh: an ecological risk perspective. Desalination Water Treat. 2020;193:284–301. [Google Scholar]
- 67.Hossain M.B., Rahman M.A., Hossain M.K., Nur A.A.U., Sultana S., Semme S., Albeshr M.F., Arai T., Yu J. Contamination status and associated ecological risk assessment of heavy metals in different wetland sediments from an urbanized estuarine ecosystem. Mar. Pollut. Bull. 2022;185 doi: 10.1016/j.marpolbul.2022.114246. [DOI] [PubMed] [Google Scholar]
- 68.Mondal P., Schintu M., Marras B. Geochemical fractionation and risk assessment of trace elements in sediments from tide-dominated Hooghly (Ganges) river Estuary, India. Chem. Geol. 2020;532 doi: 10.1016/j.chemgeo.2019.119373. [DOI] [Google Scholar]
- 69.Fan H., Chen S., Li Z., Liu P., Xu C., Yang X. Assessment of heavy metals in water, sediment and shellfish organisms in typical areas of the Yangtze River Estuary, China. Mar. Pollut. Bull. 2020;151 doi: 10.1016/j.marpolbul.2019.110864. [DOI] [PubMed] [Google Scholar]
- 70.Liu X., Song Q., Tang Y., Li W., Xu J., Wu J., Brookes P.C. Human health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysis. Sci. Total Environ. 2013;463:530–540. doi: 10.1016/j.scitotenv.2013.06.064. [DOI] [PubMed] [Google Scholar]
- 71.Turekian K.K., Wedepohl K.H. Distribution of the elements in some major units of the earth's crust. Geol. Soc. Am. Bull. 1961;72(2):175–192. [Google Scholar]
- 72.Bhuiyan M.A.H., Dampare S.B., Islam M.A., Suzuki S. Source apportionment and pollution evaluation of heavy metals in water and sediments of Buriganga River, Bangladesh, using multivariate analysis and pollution evaluation indices. Environ. Monit. Assess. 2015;187:4075. doi: 10.1007/s10661-014-4075-0. [DOI] [PubMed] [Google Scholar]
- 73.Khan K., Younas M., Sharif H.M.A., Wang C., Yaseen M., Cao X., Zhou Y., Ibrahim S.M., Yvette B., Lu Y. Heavy metals contamination, potential pathways and risks along the Indus Drainage System of Pakistan. Sci. Total Environ. 2022;809 doi: 10.1016/j.scitotenv.2021.151994. [DOI] [PubMed] [Google Scholar]
- 74.WHO . fourth ed. World Health Organization; Geneva, Switzerland: 2011. Guidelines for Drinking-Water Quality. [Google Scholar]
- 75.EU, EU’s Drinking Water Standards . 1998. Council Directive 98/83/EC on the Quality of Water Indented for Human Consumption. [Google Scholar]
- 76.National Research Council . Revised edition. National Academy Press; Washington DC: 1983. Selenium in Nutrition. 24. [Google Scholar]
- 77.Levander O.A., Cheng L. Micronutrient interactions: vitamins, minerals, and hazardous elements. Ann. N. Y. Acad. Sci. 1980;355:1–372. [Google Scholar]
- 78.Kosta L., Byrne A.R., Zelenko V. Correlation between selenium and mercury in man following exposure to inorganic mercury. Nature. 1975;254:238–239. doi: 10.1038/254238a0. [DOI] [PubMed] [Google Scholar]
- 79.National Research Council . National Academy Press; Washington DC: 1980. Mineral Tolerance of Domestic Animals: Selenium; pp. 302–420. [Google Scholar]
- 80.Chen B., Liang X., Xu W., Huang X., Li X. The changes in trace metal contamination over the last decade in surface sediments of the Pearl River Estuary, South China. Sci. Total Environ. 2012;439:141–149. doi: 10.1016/j.scitotenv.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 81.Chen C.W., Chen C.F., Dong C.D. Distribution and accumulation of mercury in sediments of Kaohsiung river Mouth, Taiwan. APCBEE Procedia. 2012;1:153–158. [Google Scholar]
- 82.García-Lestón J., Méndez J., Pásaro E., Laffon B. Genotoxic effects of lead: an updated review. Environ. Int. 2010;36(6):623–636. doi: 10.1016/j.envint.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 83.Saha N., Rahman M.S., Ahmed M.B., Zhou J.L., Ngo H.H., Guo W. Industrial metal pollution in water and probabilistic assessment of human health risk. J. Environ. Manag. 2017;185:70–78. doi: 10.1016/j.jenvman.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 84.Ferreira A.C., Costa A.C., das Ga Korn M. Preliminary evaluation of the cadmium concentration in seawater of the Salvador City, Brazil. Microchem. J. 2004;78(1):77–83. [Google Scholar]
- 85.Saha N., Mollah M.Z.I., Alam M.F., Rahman M.S. Seasonal investigation of heavy metals in marine fishes captured from the Bay of Bengal and the implications for human health risk assessment. Food Control. 2016;70:110–118. [Google Scholar]
- 86.Henson M.C., Chedrese P.J. Endocrine disruption by cadmium, a common environmental toxicant with paradoxical effects on reproduction. Exp. Biol. Med. 2004;229(5):383–392. doi: 10.1177/153537020422900506. [DOI] [PubMed] [Google Scholar]
- 87.Wedepohl K.H. The composition of continental crust. Geochem. Cosmochim. Acta. 1995;59:1217–1232. [Google Scholar]
- 88.Reinstein N.H., Lönnerdal B., Keen C.L., Hurley L.S. Zinc-copper interactions in the pregnant rat: fetal outcome and maternal and fetal zinc, copper and iron. J. Nutr. 1984;114:1266–1279. doi: 10.1093/jn/114.7.1266. PubMed: 6737088. [DOI] [PubMed] [Google Scholar]
- 89.Prasad A.S., Brewer G.J., Schoomaker E.B., Rabbani P. Hypocupremia induced by zinc therapy in adults. J. Am. Med. Assoc. 1978;240:2166–2168. [PubMed: 359844. [PubMed] [Google Scholar]
- 90.Campbell J.K., Mills C.F. The toxicity of zinc to pregnant sheep. Environ. Res. 1979;20:1–13. doi: 10.1016/0013-9351(79)90080-x. PubMed: 499163. [DOI] [PubMed] [Google Scholar]
- 91.Suresh G., Ramasamy V., Meenakshisundaram V., Venkatachalapathy R., Ponnusamy V. Influence of mineralogical and heavy metal composition on natural radionuclide concentrations in the river sediments. Appl. Radiat. Isot. 2011;69(10):1466–1474. doi: 10.1016/j.apradiso.2011.05.020. [DOI] [PubMed] [Google Scholar]
- 92.Franco-Uria A., Lopez-Mateo C., Roca E., Fernandez-Marcos M.L. Source identification of heavy metals in pasture land by multivariate analysis in NW Spain. J. Hazard Mater. 2009;165:1008–1015. doi: 10.1016/j.jhazmat.2008.10.118. [DOI] [PubMed] [Google Scholar]
- 93.Manzoor S., Shah M.H., Shaheen N., Khalique A., Jaffar M. Multivariate analysis of trace metals in textile effluents in relation to soil and groundwater. J. Hazard Mater. 2006;137:31–37. doi: 10.1016/j.jhazmat.2006.01.077. [DOI] [PubMed] [Google Scholar]
- 94.Shah M.H., Shaheen N. Annual TSP and trace metal distribution in urban atmosphere of Islamabad in comparison with mega-cities of the world. Human Ecol. Risk Assess. 2007;13:884–899. [Google Scholar]
- 95.Bing H., Zhou J., Wu Y., Wang X., Sun H., Li R. Current state, sources, and potential risk of heavy metals in sediments of Three Gorges Reservoir, China. Environ. Pollut. 2016;214:485–496. doi: 10.1016/j.envpol.2016.04.062. [DOI] [PubMed] [Google Scholar]
- 96.Deng M., Yang X., Dai X., Zhang Q., Malik A., Sadeghpour A. Heavy metal pollution risk assessments and their transportation in sediment and overlay water for the typical Chinese reservoirs. Ecol. Indicat. 2020;112 doi: 10.1016/j.ecolind.2020.106166. [DOI] [Google Scholar]
- 97.Fu J., Zhao C., Luo Y., Liu C., Kyzas G.Z., Luo Y., Zhao D., An S., Zhu H. Heavy metals in surface sediments of the Jialu River, China: their relations to environmental factors. J. Hazard Mater. 2014;270:102–109. doi: 10.1016/j.jhazmat.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 98.Renner R. Arsenic and lead leach out of popular fertilizer. Environ. Sci. Technol. 2004;38:382A. doi: 10.1021/es040642c. [DOI] [PubMed] [Google Scholar]
- 99.Asaduzzaman A.T.M., Nury S.N., Hoque S., Sultana S. Water and soil contamination from tannery waste: potential impact on public health in Hazaribag and surroundings, Dhaka, Bangladesh. Atlas Urban Geol. 2002;14:415–443. [Google Scholar]
- 100.Baeyens W., De Brauwere A., Brion N., De Gieter M., Leermakers M. Arsenic speciation in the river Zenne, Belgium. Sci. Total Environ. 2007;384:409–419. doi: 10.1016/j.scitotenv.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 101.Pravin U.S., Trivedi P., Ravindra M.M. Sediment heavy metal contaminants in Vasai Creek of Mumbai: pollution impacts. Am. J. Chem. 2012;2:171–180. [Google Scholar]
- 102.Pan H., Lu X., Lei K. A comprehensive analysis of heavy metals in urban road dust of Xi’an, China: contamination, source apportionment and spatial distribution. Sci. Total Environ. 2017;609:1361–1369. doi: 10.1016/j.scitotenv.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 103.Iqbal J., Shah M.H. Distribution, correlation and risk assessment of selected metals in urban soils from Islamabad, Pakistan. J. Hazard Mater. 2011;192:887–898. doi: 10.1016/j.jhazmat.2011.05.105. [DOI] [PubMed] [Google Scholar]
- 104.Hakanson L. An ecological risk index for aquatic pollution control. A sedimentological approach. Water Res. 1980;14(8):975–1001. doi: 10.1016/0043-1354(80)90143-8. [DOI] [Google Scholar]
- 105.Tomlinson D.L., Wilson J.G., Harris C.R., Jeffrey D.W. Problems in the assessment of heavy-metal levels in estuaries and the formation of a pollution index. Helgoländer Meeresun. 1980;33(1):566. doi: 10.1007/BF02414780. [DOI] [Google Scholar]
- 106.Birch G.F., Olmos M.A. Sediment-bound heavy metals as indicators of human influence and biological risk in coastal water bodies. ICES J. Mar. Sci. 2008;65(8):1407–1413. [Google Scholar]
- 107.Maanan M., Saddik M., Maanan M., Chaibi M., Assobhei O., Zourarah B. Environmental and ecological risk assessment of heavy metals in sediments of Nador lagoon, Morocco. Ecol. Indicat. 2015;48:616–626. [Google Scholar]
- 108.Sayadi M.H., Sayyed M.R., Kumar S. Short-term accumulative signatures of heavy metals in river bed sediments in the industrial area, Tehran. Iran. Environ. Monit. Assess. 2010;162(1):465–473. doi: 10.1007/s10661-009-0810-3. [DOI] [PubMed] [Google Scholar]
- 109.Muller G. Index of geoaccumulation in sediments of the Rhine river. Geojournal. 1969;2:108–118. [Google Scholar]
- 110.Karbassi A.R., Monavari S.M., Bidhendi G.R.N., Nouri J., Nematpour K. Metal pollution assessment of sediment and water in the Shur River. Environ. Monit. Assess. 2008;147(1–3):107. doi: 10.1007/s10661-007-0102-8. [DOI] [PubMed] [Google Scholar]
- 111.Nour H.E.S., Ramadan F., Aita S., Zahran H. Assessment of sediment quality of the Qalubiya drain and adjoining soils, Eastern Nile Delta, Egypt. Arabian J. Geosci. 2021;14:1–13. doi: 10.1007/s12517-021-06891-0. [DOI] [Google Scholar]
- 112.El-Alfy M.A., Darwish D.H., El-Amier Y.A. Land use Land cover of the Burullus Lake shoreline (Egypt) and health risk assessment of metal contaminated sediments. Hum. Ecol. Risk Assess. 2021;27(4):898–920. [Google Scholar]
- 113.Horton L.M., Mortensen M.E., Iossifova Y., Wald M.M., Burgess P. What do we know of childhood exposures to metals (arsenic, cadmium, lead, and mercury) in emerging market countries? Int. J. Pediatr. 2013 doi: 10.1155/2013/872596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Alharbi T., Al-Kahtany K., Nour H.E., Giacobbe S., El-Sorogy A.S. Contamination and health risk assessment of arsenic and chromium in coastal sediments of Al-Khobar area, Arabian Gulf, Saudi Arabia. Mar. Pollut. Bull. 2022;185 doi: 10.1016/j.marpolbul.2022.114255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.