Abstract
Objectives
Dengue infection is spreading worldwide. The clinical spectrum is broad and includes asymptomatic infections. This review provides an overview of the different proportions of asymptomatic infections described in epidemiological studies according to definitions, study designs, and detection methods.
Methods
Medline and Embase databases were searched without restriction of date or language. Studies were included if they reported data on the incidence or prevalence of asymptomatic dengue infections. The data were summarized and classified according to the definitions of the term 'asymptomatic'.
Results
A total of 74 studies were included. The mean proportion of asymptomatic infections among dengue-infected persons was 54% in 50 included studies. The prevalence of dengue infections detected in healthy persons was 0.2% in 24 included studies. The term ‘asymptomatic’ has been used to refer to ‘clinically undetectable infection’, but also to ‘undiagnosed infection’ or ‘mild infection’. Only 8% were clinically undetectable laboratory-confirmed dengue infections.
Conclusion
The proportion of asymptomatic dengue infections varied greatly. Studies proving data on clinically undetectable laboratory-confirmed dengue infections were very few, but provided consistent results of low proportions of asymptomatic infections. These data challenge the assumption that the majority of dengue cases are asymptomatic.
Keywords: Dengue, Asymptomatic infection, Subclinical infection
1. Introduction
Dengue is the most globally prevalent arboviral disease [1]. Up to 3.97 billion people is at risk of contracting dengue virus infection [2]. Incidence of dengue has increased thirty times during the last fifty years. 50 to 100 million new infections are estimated to occur annually [1,3,4]. This increasing trend is expected to continue due to the increase in urbanization, population size, air traffic and climate change [5,6].
The symptomatic disease spectrum ranges from mild fever to severe and deadly haemorrhagic fever and shock syndrome [1]. The dengue-like syndrome is defined, according to the World Health Organization, as an acute fever disease with two or more of the following signs or symptoms: nausea, vomiting, rash, headache, retro-orbital pain, myalgia, arthralgia, and haemorrhagic signs [1].
A dengue virus infection can also present without clinically detectable symptoms. Duong et al. in 2015 demonstrated that symptomless infected humans transmitted the virus to Aedes aegypti [7], which could have a significant impact in spreading the disease. It is commonly accepted that the higher proportion of dengue infections are ‘asymptomatic’. The factsheet about dengue of the European Centre for Disease Prevention and Control mentioned: “up to 40–80% of all dengue infections are asymptomatic” [8]. However, data on the proportion of dengue infection referred to as ‘asymptomatic’ are highly heterogeneous in literature, which could be partly explained by a lack of clarity in the wording and on the meaning of the word ‘asymptomatic’. As illustrated by the WHO Key facts of the May 10, 2021 [8] “A vast majority of cases are asymptomatic or mild and self-managed, and hence the actual numbers of dengue cases are under-reported”, the clinical term ‘asymptomatic’ is frequently associated to ‘mild’ infections or ‘under-reported’ infections. Clinical terms such as ‘asymptomatic’ defined as the absence of symptoms, or ‘mild’ presentations are often gathered with the epidemiological consideration of ‘under-reported’ or ‘unapparent’ infections which correspond to undetected cases regardless of the presence or absence of symptoms. In fact, there are many reasons why dengue infection remains ‘unapparent’ for health authorities, apart from infections without symptoms, including mild symptoms, easily self-managed symptoms by an accustomed population, a lack of access to health care or misdiagnoses with other febrile diseases.
For epidemiological needs, such as mathematical modelling, infections presenting no symptoms are frequently pooled with ‘unapparent symptomatic’ infections as in Clapham et al. 2017 meta-analysis [9], where the word ‘asymptomatic’ gathers ‘clinically asymptomatic’ plus ‘unapparent symptomatic’ resulting in a proportion of 82% ‘asymptomatic’ for primary dengue infections and 59% for secondary infections in cohort studies and 78% and 43% respectively in cluster studies. These data are used for a mathematical modelling study [10] that concludes, “more than 80% of dengue virus infections are attributable to individuals with mild to no symptoms who do not seek treatment from a physician”. As described in these studies, for epidemiological and modelling purposes, dengue infections could be divided into infections with no symptoms, detected symptomatic infections and undetected symptomatic infections. Grange et al., in 2014 [11] published a comprehensive review of the epidemiological factors associated with the frequency of unapparent dengue virus infections. They concluded that the epidemiological evidence suggested that the majority of infections were unapparent in endemic regions and highlighted the important role of short-term immunity. In their review, they grouped together under the term “unapparent” infections that were clinically undetectable, mild and not detected by surveillance systems. In this review, we aimed to provide an update as many studies have been published since 2014, but also to offer a different perspective by trying to demonstrate that part of the large heterogeneity in rates of asymptomatic dengue between studies is due to a lack of standardisation of terminology and study designs, which jeopardises the assessment of associated risk factors.
The proportion of infections with no symptoms needs to be known and clearly distinguished from ‘unapparent’ grouped data, because a symptomatic infection, even a ‘mild’ one, can be detected by the patient him/herself or by a healthcare practitioner, using a questionnaire, whereas infections without any symptoms are completely hidden. So, when deciding on screening policies for blood donors or at the start of new outbreaks and on the preventive message to be communicated to the population, it is essential to distinguish between infections whose symptoms are not clinically detectable and ‘unapparent’ infections, both of which are described as ‘asymptomatic’.
The authors of this review recently conducted an observational study in the Southwest Indian Ocean islands [12], where dengue is emerging, to estimate the proportion of the infections with no symptoms through active screening for dengue infections in the community and, surprisingly, none could be found. Given the wide variation in data and the lack of clarity in clinical and epidemiological formulation in the literature, we decided to undertake this literature review to extract the proportions of ‘asymptomatic’ infections in epidemiological studies and to provide data with a higher granularity based on a specific definition of ‘asymptomatic’ and the detection method used.
2. Methods
2.1. Eligibility criteria
All observational studies reporting proportion, prevalence or incidence of asymptomatic dengue infections were included. No language, publication date, or publication status restrictions were imposed. Participants with any age with a diagnosis of dengue were considered.
A confirmed infection of dengue was defined by the detection of the virus with one of the following assays: virus culture and isolation, real-time polymerase chain reaction (RT-PCR), transcription-mediated amplification (TMA), detection of non-structural protein 1 antigen (NS1) by ELISA or rapid diagnostic tests (RDT); or, detection of an antibody response as a seroconversion or a 4-fold increase in total antibodies with one of the following assays: IgM/IgG ELISA or RDT, haemagglutination inhibition assays, plaque reduction neutralisation test (PRNT).
We did not restrict to a pre-defined definition of ‘asymptomatic infection’ nor to a pre-defined term used.
2.2. Information sources
Studies were identified by searching electronic databases and scanning references lists of published literature reviews on the topic. No limits were applied for language and other than English or French languages' paper were translated. This search was applied to the National Library of MEDicine's MEDLINE (1966-Present) by PubMed and Embase (1980-present) by ODS and NB. The last search was run on December 01, 2020. ODS and NB conducted the search in blind each other. In addition to searching databases, authors used i) https://connectedpapers.com/, ii) search in Google Scholar and iii) backward citation tracking to identify articles not retrieved by electronic searches.
2.3. Search
The following search strategies were used: Pubmed: ((dengue[MeSH Terms]) AND ((asymptomatic infection[MeSH Terms]) OR (asymptomatic disease[MeSH Terms]) OR (infection, subclinical[MeSH Terms]))); Embase: ‘dengue’ AND (‘asymptomatic infection’ OR ‘asymptomatic disease’).
Observational studies found through backward citation tracking were included if they contained data on the proportion of dengue asymptomatic infections.
2.4. Study selection
First, eligibility assessment was performed independently in an unblinded standardized manner by two reviewers (ODS and NB) by screening first title and abstract. If title and/or abstract provided insufficient information to assess the relevance or if a final decision could not be made, the full article was assessed. Second, full texts of articles selected in the first stage were independently reviewed for final inclusion. Disagreements were resolved by discussion between the two reviewers (ODS and NB). All duplicates’ articles were removed. When more than one published manuscript concerned the same study, these manuscripts were pooled, and data were extracted only once.
2.5. Data collection process
We developed a data extraction sheet containing the data items listed below. ODS extracted the data from included studies and NB checked the extracted data. Disagreements were resolved by discussion.
2.6. Data items
Data extracted concerned the following items: publication year, study site, recruitment design, age of participants, term used to describe ‘asymptomatic infections’, definition of ‘asymptomatic infection’, dengue diagnostic test, proportion of asymptomatic infections on total dengue infection, asymptomatic/symptomatic ratio, percent of dengue infection among asymptomatic participants, follow-up of dengue infection if any.
2.7. Risk of bias in individual studies
Risk of bias in individual studies were discussed considering the recruitment design and the diagnostic test used. We attempted to minimize selection, publication, and language bias through a comprehensive search strategy without language restrictions and by employing a transparent methodology. However, some biases remain due to the lack of standardization regarding the definition of “asymptomatic” infections and variations in recruitment designs among the included studies. The main bias encountered in this review, as explained later, is recall bias in serosurveys, particularly in children. In fact, as discussed later, the clinical presentation of dengue is similar to that of other common viral diseases, making it highly likely that children and parents may not remember the specific occurrence of a dengue infection.
2.8. Data analysis
R Core Team (2021) was used to calculate the summary statistics. The estimated proportions of asymptomatic infections and confidence intervals were obtained by compiling the frequencies of asymptomatic infections and the sample sizes of the populations of the different selected studies.
3. Results
3.1. Search strategy and PRISMA flow diagram
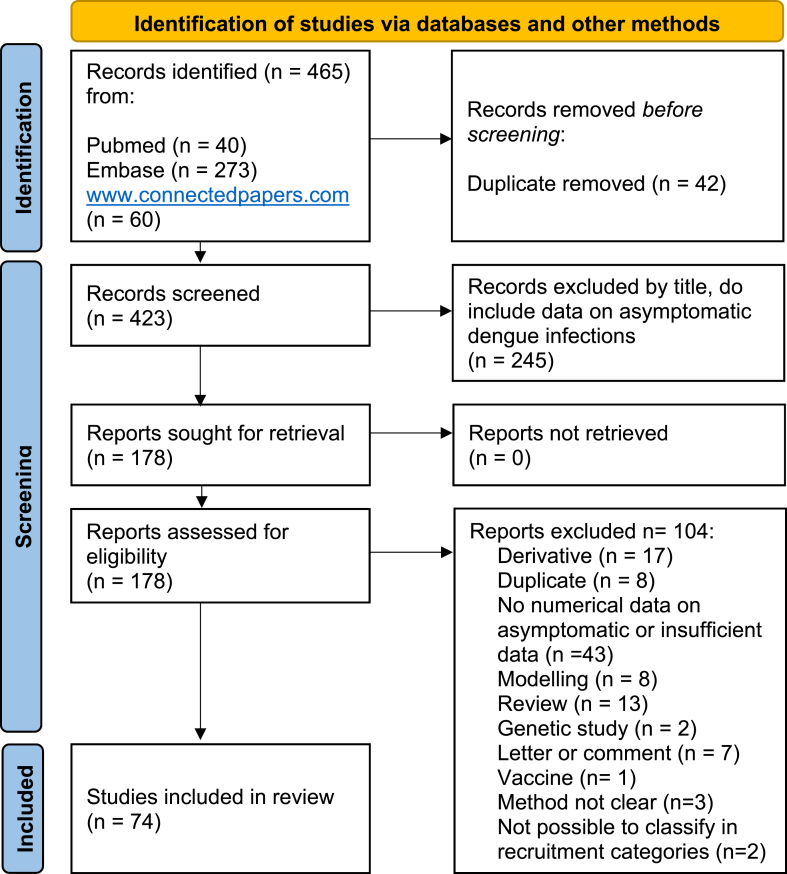
We identified 465 papers. After an automatic removal of 42 duplicates by a reference management tool (Endnote), 423 papers were screened by title, of which 178 were selected, retrieved and assessed for eligibility, with abstract and full text reading. One hundred and four papers were excluded (reasons listed in Fig. 1: PRISMA flow diagram), and 74 were included, listed in Table S1 (supplementary material). Table 1 lists the 50 studies that provided asymptomatic rates of dengue infections by calculating the number of asymptomatic infection among the total dengue infections detected and the associated study characteristics and epidemiological risk factors. The 24 remaining studies, presented in Supplementary Table S1, provided the prevalence of dengue infections among asymptomatic populations.
Fig. 1.
PRISMA Flow diagram.
From: Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372:n71. https://doi.org/10.1136/bmj.n71. For more information, visit: http://www.prisma-statement.org/
Table 1.
List of 50 observational studies providing data on the asymptomatic rate of dengue infections.
| Definition | Reference | Publication Year | Study Type |
Region | Age (years) | Diagnostic Test | Serotype | Sero-prevalence | Incidence* |
Dengue infections N |
Asympto-matic Rate % (N) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| No symptom | [29] | 2020 | Cluster | South-East Asia | 11–63 | RT-PCR | Dengue virus 1 (1/13) Dengue virus 2 (2/13) Dengue virus 3 (7/13) Dengue virus 4 (5/13) | 9% | NA | 175 | 7.4% (13) |
| No symptom | [38] | 2000 | Travellers | America, Caribbean | ≥ 14 | IgM/IgG ELISA, PRNT | NA | 69% | NA | 22 | 0% (0) |
| No symptoms | [31,39,40] | 2008 | Cluster | South-East Asia | 0.5–15 | RT-PCR, IgM/IgG ELISA | All, mainly Dengue virus 1, Dengue virus 4 | 8% | NA | 119 | 20% (24) |
| No symptoms | [41] | 2010 | Cluster | Latin America | All | IgM/IgG ELISA, HI titersx4, RT-PCR | Dengue virus 2 | 4% | NA | 12 | 42% (5) |
| No symptoms | [14] | 2018 | Cluster | Latin America | All | RT-PCR, NS1 | NA | 13% | NA | 50 | 32% (16) |
| No symptoms | [30,42] | 2019 | Cluster | South-East Asia | 0.5–40 | RT-PCR | Dengue virus 1 (80.8%) Dengue virus 2 (7.7%) Dengue virus 4 (11.5%) Dengue virus 3 (1.1%) |
4% | NA | 346 | 7.5% (26) |
| Subclinical | [43] | 2015 | Cluster | South-East Asia | All (mainly <15) | IgM/IgG ELISA | NA | 6% | NA | 113 | 92% (104) |
| Subclinical | [44] | 2005 | Cluster | South-East Asia | All | HI titersx4, RT-PCR | Dengue virus 1, Dengue virus 2 | 2% | NA | 17 | 47% (8) |
| Subclinical | [45] | 2012 | Cluster | South-East Asa, Latin America | >2 | RT-PCR | Dengue virus 1, Dengue virus 2, Dengue virus 3 | 10% | NA | 101 | 29% (29) |
| Subclinical | [46] | 2016 | Cluster | South Asia | All | RDT NS1,IgM/IgG | NA | 11% | NA | 226 | 63% (142) |
| Subclinical | [17] | 2015 | Cluster | Latin America | ≥ 5 | IgM/IgG ELISA seronconversion or ab titers x4 | NA | 22% | NA | 253 | 60% (151) |
| Subclinical | [47] | 2015 | Cluster | East Asia | All | IgM/igG ELISA | NA | 5% | NA | 41 | 68% (28) |
| Subclinical | [48] | 2011 | Cohort | South-East Asia | 0–8 | PRNTx4 | NA | NA | 11% | NA | 75% (NA) |
| Subclinical | [15] | 2016 | Cohort | South-East Asia | All | HI titersx4 | NA | NA | 9% | 77 | 79% (61) |
| Subclinical | [35] | 2009 | Cohort | South-East Asia | <1 | IgM/IgG ELISA | NA | NA | 1% | 10 | 90% (9) |
| Subclinical | [49] | 2010 | Cohort | South-East Asia | 2–15 | IgG ELISA | Dengue virus 2, Dengue virus 1 | NA | 3% | 953 | 80% (764) |
| Subclinical | [50] | 2013 | Cohort | Latin America | ≥ 5 | IgM/IgG ELISA, PRNT | Dengue virus 3, Dengue virus 4 | NA | 11% | 2286 | 90% (2074) |
| Subclinical | [51] | 1973 | Serosurvey | Latin America | All | HI titersx4 | NA | 45% | NA | 148 | 43% (63) |
| Subclinical | [52] | 1967 | Serosurvey | Latin America | >4 | HI titers x4 | NA | 38% | NA | 25 | 16% (4) |
| Subclinical | [53] | 1985 | Serosurvey | Latin America | All | HI titersx4 | Dengue virus 4 | 7% | NA | 56 | 45% (25) |
| Subclinical | [54] | 2009 | Serosurvey | Latin America | 1–79 | IgM/IgG ELISA | NA | 10% | NA | 33 | 70% (23) |
| Subclinical | [55] | 1998 | Serosurvey | Latin America | NA | HI | Dengue virus 2 | 44% | NA | 588 | 41% (243) |
| Subclinical | [56] | 1998 | Serosurvey | Oceania | 14–50 | HI, IgG ELISA, PRNT | Dengue virus 2 | 26% | NA | 139 | 11.5% (16) |
| Subclinical | [57] | 1990 | Serosurvey | Latin America | All | PRNT | Dengue virus 1, Dengue virus 2 | 17% | NA | 219 | 76% (167) |
| Subclinical | [58] | 2013 | Serosurvey | South-East Asia | 7–85 | IgM/IgG ELISA | NA | 7% | NA | NA | 78% (NA) |
| Subclinical | [59] | 2006 | Serosurvey | East Asia | ≥ 18 | IgG ELISA | Dengue virus 2 | NA | NA | 55 | 78% (43) |
| Subclinical | [60] | 2006 | Serosurvey | Latin America | 7–20 | Viral isolation | Dengue virus 1, Dengue virus 2, Dengue virus 3, Dengue virus 4 | 7% | NA | 215 | 86% (185) |
| Subclinical | [61] | 2002 | Serosurvey | Latin America | ≥ 14 | IgM/IgG ELISA | Dengue virus 1 | 21% | NA | 42 | 33% (14) |
| Subclinical | [62] | 2011 | Travellers | America, Caribbean | ≥ 18 | RT-PCR, IgM ELISA | Dengue virus 1 | 33% | NA | 7 | 0% (0) |
| Subclinical | [63] | 2011 | Travellers | NA | ≥ 18 | IgM/IgG ELISA | NA | 1% | NA | 14 | 64% (9) |
| Subclinical | [64] | 2002 | Travellers | NA | ≥ 18 | IgM/IgG ELISA | NA | 3% | NA | NA | 77% (NA) |
| Subclinical | [65] | 1999 | Travellers | NA | ≥ 18 | IgM/IgG ELISA | NA | 7% | NA | 7 | 42% (3) |
| Subclinical | [21] | 2012 | Travellers | Asia | ≥ 16 | IgG ELISA | NA | 1% | NA | 4 | 100% (4) |
| Subclinical | [66] | 2005 | Travellers | South-East Asia | ≥ 18 | Serology | NA | 10% | NA | 27 | 11% (3) |
| Subclinical | [26] | 1969 | Travellers | South-East Asia | All | Serology | NA | NA | NA | NA | 0% (NA) |
| Subclinical | [67] | 1995 | Travellers | Horn of Africa | ≥ 18 | IgM ELISA | NA | 9% | NA | 44 | 16% (7) |
| Unapparent | [68] | 1988 | Cohort | South-East Asia | 4–16 | HI titers x4 | Dengue virus 1, Dengue virus 2, Dengue virus 4 | NA | 12% | 103 | 87% (90) |
| Unapparent | [69,70] | 2005 | Cohort | South-East Asia | 18–66 | HI titersx4 | All, mainly Dengue virus 4 | NA | 1% | NA | 72% (NA) |
| Unapparent | [16,34,71,72] | 2002 | Cohort | South-East Asia | 7–16 | HI titers x4 | All, mainly Dengue virus 3 | NA | 7% | 615 | 66% (406) |
| Unapparent | [73] | 2006 | Cohort | Latin America | 4–16 | HI titersx5 | Dengue virus 1, Dengue virus 2 | NA | 5% | NA | 85–92% (NA) |
| Unapparent | [74,75] | 2010 | Cohort | Latin America | 2–14 | Total antibodies Inhibition ELISA titersx4 | Dengue virus 1, Dengue virus 2 | NA | 1% | NA | 60–95% (NA) |
| Unapparent | [76] | 2010 | Cohort | South-East Asia | 6 and 18 weeks | HI titersx4 | NA | NA | 1% | NA | 85% (NA) |
| Unapparent | [77] | 2015 | Cohort | Latin America | 10–18 | IgG ELISA | NA | NA | 6% | 19 | 61% (10) |
| Unapparent | [78] | 2010 | Cohort | Latin America | 5–60 | PRNT | Dengue virus 1, Dengue virus 2, Dengue virus 3 | NA | 7% | NA | 50–84% (NA) |
| Unapparent | [79] | 2014 | Cohort | South-East Asia | ≤ 12 | IgG ELISA | NA | NA | 4% | 67 | 60% (40) |
| Unapparent | [80] | 1995 | Serosurvey | Latin America | 5–19 | HI | Dengue virus 1, Dengue virus 2 | 62% | NA | 277 | 58% (160) |
| Unapparent | [81] | 1995 | Serosurvey | Latin America | All | IgM/IgG ELISA | NA | 17% | NA | 59 | 53% (28) |
| Unapparent | [82] | 2000 | Serosurvey | Latin America | All | IgM ELISA, PRNT | Dengue virus 1, Dengue virus 2 | 41% | NA | NA | 97% (NA) |
| Unapparent | [83] | 2006 | Serosurvey | South-East Asia | All (mainly >18) | IgM ELISA | NA | NA | 21% | NA | 82% (NA) |
| Unapparent | [84] | 2009 | Serosurvey | South-East Asia | 18–74 | IgM/IgG ELISA | NA | 3% | NA | NA | 96% (NA) |
3.2. Study sites
Excluding three studies in travellers in multiples regions, 96% of studies took place in Asia and Latin America, half in each continent. Only one study was conducted in Africa. In supplementary material. As presented in Table 1, the Asymptomatic Rate (AR) per region ranged from 16 to 97% (19 studies, median 58%) for Latin America; 0–96% (19 studies, median 75%) for South-east Asia; 63–100% (4 studies) for East Asia; 0% (2 studies) for America, Caribbean; 16% (1 study) for the Horn of Africa and 11.5% (1 study) for Oceania.
3.3. Publication dates
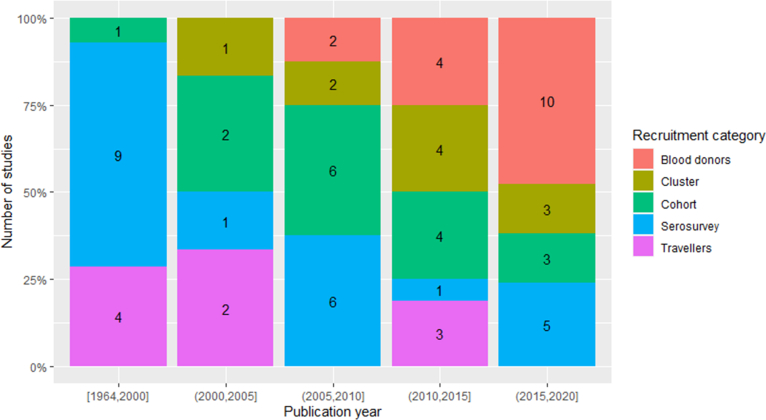
Most of the studies (73%) were published since 2006. Before 2000, only some serosurveys were conducted in endemic areas or after outbreaks, and some studies on travellers returning from endemic countries. In the last fifteen years, cohort or cluster studies were mostly conducted. In the last five years, many studies on blood donors were published. (See Fig. S1 in Supplementary material).
Different meanings of ‘asymptomatic’ dengue infection.
We defined the three following categories to classify with more precision the group of dengue infections referred to as ‘asymptomatic’ detected by the included studies.
-
-
the “no symptoms”: detected laboratory confirmed infections with absolutely no symptoms declared during a follow-up; 6 studies corresponds to this definition and 3/6 were published after the Grange et al., 2014 [11], previous review. The AR ranged from 0 to 42% (median 7.5%)
-
-
the “subclinical”: mild or aspecific infections: presence of symptoms but that do not fit with the WHO definition of clinical dengue; 30 studies included with this definition. The AR ranged form 0–100% (median 64%)
-
-
the “unapparent”: infections not detected by the health care system or by any surveillance system regardless of symptomatology; 14 studies. The AR ranged from 50 to 97% (median 72%)
The term ‘asymptomatic’ is kept as a container including all the above definitions and corresponds to the proportions of cases extracted in the studies because referred to as ‘asymptomatic’.
3.4. Proportion of asymptomatic participants among dengue infections (asymptomatic rate) versus prevalence of dengue infections among asymptomatic participants
In the majority of the included studies (50), the results extracted for this review were the proportions of asymptomatic participants among dengue infections. Studies were classified according to the definitions of ‘asymptomatic’, the diagnostic tests and the age group as shown in the summary Table 2. In the remaining 24 studies, including all the studies on blood donors (16), 2 cohorts and 6 serosurveys, the results presented were the prevalence of dengue infections among healthy participants. We presented these results separately in Table 2, classified according to the viral or antibody detection method. Indeed, in these 24 studies, the denominator is a population of healthy persons and not dengue infected cases. The 6 serosurveys studies presented in Table 2 presented a proportion of participants with traces of old dengue infections (i.e. presence of IgG in blood) but no dengue history. We decided to include these studies in the review, as they are another way to detect the presence of possible dengue infections with no symptoms, in the population. The studies on blood donors are important to evaluate the risk of dengue transmission through blood transfusion.
Table 2.
Prevalence of dengue infection among asymptomatic participants per study category and detection method.
| Recruitment method | Virus detectionb | Antibody detection | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number of studies | References | I/Aa | Prevalence of dengue infection (CI) | Range | Number of studies | References | I/Aa | Prevalence of dengue infections (CI) | Range | |
| Blood donors | 14 | [13,[85], [86], [87], [88], [89], [90], [91], [92], [93], [94], [95], [96], [97]] | 125/54333 | 0,2% (0-0,7%) | [0–5.5%] | 2 | [98,99] | 15/573 | 2,60% (0–6,8%) | [0–4.2%] |
| Cohort | 1 | [100] | NA | 12,70% | 1 | [32] | NA | 5–20% | ||
| Serosurveyc | 6 | [33,[101], [102], [103], [104], [105]] | NA | 28% | [7–48%] | |||||
| Total | 15 | 9 | ||||||||
I/A: number of dengue infections (I) among healthy asymptomatic people (A).
Virus detection thourgh RT-PCR, Transcription Mediated Amplification (TMA) or NS1.
Presence of IgG and no history of dengue.
3.5. Asymptomatic rate according to the categories of recruitment and detection methods
Five categories according to the participants’ recruitment design were identified among the included studies: 1) Cluster studies; 2) Cohort studies; 3) Serosurveys; 4) Studies on travellers; 5) Studies on blood donors. Median (and interquartile range) proportions of asymptomatic infections according to the categories of recruitment for 50 studies providing data on the proportion of asymptomatic infection among dengue infections were calculated. Studies on travellers show the lower proportions of asymptomatic infections but with a high variability and cohort studies the higher with the shorter.
Each recruitment design presented different characteristics, risk of bias and distribution of dengue infections prevalence or incidence. Table 3 present the results of proportion of asymptomatic infections according to the clinical definition of ‘asymptomatic’ and to the detection method (viral or antibody). In cluster studies (number of studies = 11), the recruitment took place in geographical areas of a predefined radius, among people living in the neighbourhood of a dengue index case. Dengue prevalence in this kind of studies ranged from 2.2% to 21.5% (median 7.9%) and the AR ranged 7.4–92% (median 42%). Five studies provided proportions of asymptomatic infections corresponding to a strict clinical definition of ‘No symptom’ and detected with virus detection methods (RT-PCR or viral isolation), the mean proportion was of 8%.
Table 3.
Summary table providing the proportions expressed in % of asymptomatic infections among dengue infections classified by the definition of ‘asymptomatic’ and the diagnostic test (the detailed tables are provided in supplementary material).
| Recruitment category | Asymptomatic definition | Virus detection | Antibody detection | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of studies | References | A/Id | Proportion of ‘asymptomatic’ infections (CI) | Number of studies | References | A/Id | Proportion of ‘asymptomatic’ infections (CI) | ||
| Cluster | No symptoms | 5a | [14,29,31,41,42] | 59/702 | 8% (5–12%) | 2a | [31,41] | 25/131 | 19% (10–28%) |
| Subclinical | 2a | [44,45] | 31/118 | 26% (17–35%) | 5a | [17,43,44,46,47] | 433/650 | 67% (63–71%) | |
| Unapparent | |||||||||
| Cohort | No symptoms | ||||||||
| Subclinical | 5 | [15,35,[48], [49], [50]] | 2908/3326 | 87% (86–89%) | |||||
| Unapparent | 9 | [68,69,71,73,74,[76], [77], [78], [79]] | 546/804b | 68% (64–71%) | |||||
| Serosurvey | No symptoms | ||||||||
| Subclinical | 1 | [60] | 185/215 | 86% (79–93%) | 10 | [[51], [52], [53], [54], [55], [56], [57], [58], [59],61] | 598/1305 | 46% (43–49%) | |
| Unapparent | 5 | [[80], [81], [82], [83], [84]] | 188/336c | 56% (50–61%) | |||||
| Travellers | No symptoms | 1 | [38] | 0/22 | 0% (0–21%) | ||||
| Subclinical | 1 | [62] | 0/7 | 0% (0–38%) | 7 | [21,26,[63], [64], [65], [66], [67]] | 26/96 | 27% (17–37%) | |
| Unapparent | |||||||||
Ref 39, 47 and 53 used both detection methods: virus or antibody detection, results have been splitted for the table.
Data extracted from 4/9 studies that provided detailed data.
Data extracted from 2/5 studies that provied detailed data.
Number of 'asymptomatic' dengue infection/total number of dengue infections, aggregated results extracted from the publications.
In cohort studies (n = 14), the recruitment took place in a predefined group of persons, as children of a primary school. Participants gave a blood sample at inclusion, and were followed by annual blood samples for serology. Detection of clinical apparent dengue infection was possible thanks to school absenteeism surveillance and with the collaboration of surrounding health centres. Dengue incidence ranged from 0.8% to 12.7% (median 6%) with an AR that ranged from 60 to 92% (median 78%). All cohort studies used antibody detection methods. By refining the results according to asymptomatic definition, only subclinical or unapparent infections were described with high proportion of 87 and 68% respectively (Table 2). The risk of recall bias was high mainly in children who might present aspecific clinical forms and would not have consulted a general practitioner for mild symptoms.
Serosurveys (n = 22) recruited participants in general population of an administrative region to know the attack rate and herd immunity of the population. Dengue prevalence showed a large distribution ranging from 2.6% to 61.6% (median 19.3%) with an AR range of 6.6–97% (median 53%). Sixteen serosurveys provided data on asymptomatic infections among dengue infections presented in Table 2. The proportion of asymptomatic infections according to clinical definition varies among 46–86%, only subclinical and unapparent infections were considered. Six serosurveys provided a mean of 28% of healthy participants who had IgG positive but no history of dengue infections (Table 1).The studies were retrospective with a high risk of recall bias. Interpretation of lab results differed among studies and the WHO recommendation to collect paired sera was not always respected.
Studies on travellers or migrants (n = 9) included adult participants, not immune for dengue virus, and not used to this disease. Sample sizes were small. Dengue prevalence showed also a large distribution among studies, between 1% and 68.8% (median 7.8%), the AR was of 0% for one study using virus detection method and between 0 and 27% for studies using antibody detection methods. The risk of recall bias was low as a symptomatic episode during a short holiday or mission was generally a key fact for participants. Moreover, the small size of the sample sizes and the very aggregated and defined group of participants allowed a better follow-up and accurate clinical data. Studies on travellers included almost only adult participants (one study >14 years old and another >16 years old), only one included also children. On seven studies including adult participants with available data, we gathered 125 adult participants. Twenty-six participants had subclinical symptoms. Thus 99/125 (79%) of adult travellers had typically dengue-like syndrome. Travellers all came from countries without dengue. In all but one study, the diagnostic was based on a seroconversion, which mean that all were primary infections.
Studies on blood donors (n = 16) only included adult ‘asymptomatic’ participants due to the eligibility criteria for blood donation. The prevalence of asymptomatic infections was among all the included study population and was equivalent to the prevalence of dengue infections that ranged between 0 and 5.5% (median 0.07%). As presented in Table 1, most of the studies (14/16) used viral detection methods and the prevalence of dengue were very low for all except for one study (5.5%) [13]. These studies were undertaken to assess the risk of dengue transmission through blood donation during outbreaks or in endemic countries but the methodology was not elaborated to detected and evaluate asymptomatic infections. Indeed, no questionnaire on signs and symptoms was submitted to the participants besides the basic eligibility criteria for blood donation. As no follow-up was undertaken, it was not possible to determine if a dengue-positive participant had an asymptomatic or a pre-symptomatic infection.
3.6. Asymptomatic rate according to the age group
By classifying the studies according to the age group (the age is mentioned in Table 1), it resulted that the AR for adults defined as, more than 14 years or more than 18 years old, depending on studies, ranged 0–96% (13 studies, median 33%). The AR for the children group ranged 20–87% (11 studies, median 66%) and two studies concerned infants (less than one year old) with an AR of 85–90%. The AR in 23 studies including participants of all ages ranged 0–97% (median 63%). The AR seems then be higher for children than for adults but with a high variability.
3.7. Asymptomatic rate according to the serotype
In Table 1 are listed the serotypes detected during the studies. Data were not available for 26 on 50 studies and when available, the data were mostly aggregated and did not provide sufficient detail to be able to calculate any association between the AR and the serotype circulated. Moreover, for many studies many or even all four serotypes were circulating concomitantly. We extracted the AR for the studies were one serotype was dominant: Dengue virus 1: AR 0–33% (4 studies); Dengue virus 2: AR 11.5–78% (4 studies); Dengue virus 3: AR 7.4–66% (2 studies); Dengue virus 4: AR 20–72% (3 studies). But here again, the variability is high and the number of studies low.
3.8. Asymptomatic rate according to primary versus secondary infections
Since the Grange et al. review [11], some new studies provided information concerning the association between the severity of symptoms and the immunity. Most of the recent studies suggest that primary infections are more likely to be overt symptomatic and milder or asymptomatic in secondary or repeated infections especially if the time between the infections is short [[14], [15], [16], [17]]. Inversely, Sun et Luo, China, 2018 [18], suggested that secondary or repeated infections are less likely to be asymptomatic.
4. Discussion
4.1. Main results
The mean proportion of ‘asymptomatic’ in the broad sense, including mild and unapparent dengue infections among identified dengue infections, was 54% overall in the 50 studies included in this review. By extracting data from studies with a precise definition of the word ‘asymptomatic’, meaning a clinical absence of symptoms, this proportion is equal to 18%. A proportion of 8% is obtained by combining the definition of no symptoms with a viral detection method using molecular detection, antigen detection or viral isolation. What is sometimes referred to as the ‘majority’ [19] or ‘40–80%’ [8,20] of asymptomatic dengue infections includes both purely no symptoms infections and subclinical or unapparent infections which could be confirmed infections detected by molecular biology but also suspected infection diagnosed by antibody detection.
The prevalence of dengue infections detected in apparent healthy participants in endemic countries was 14% in the 24 studies included. Including only blood donors and using viral detection methods yielded 0.2% of detected infections. If we exclude one study which presented particularly high results of 5.5% [13], the prevalence of dengue infections among blood donors, using the viral detection method, is 0.1%.
4.2. High heterogeneity in data
Our results showed that there was considerable heterogeneity in the proportion of dengue infections classified as ‘asymptomatic’ in the studies, which ranged from 0% to 100%. Methodological differences could explain this heterogeneity. The following parameters differed from one study to another: recruitment designs, the definitions considered for ‘asymptomatic’ infections, the age of the participants included and the diagnostic tests used to detect dengue infection. The extreme differences between 0 and 100% observed in the studies of travellers are also due to the small sample sizes and different interpretations of what was considered an ‘asymptomatic’ infection. For example, the study with 100% asymptomatic infections [21] included four dengue-infected travellers whose symptoms did not meet the WHO clinical definition of a dengue-like syndrome and who were therefore classified as ‘asymptomatic. In attempting to identify epidemiological risk factors – such as age, serotype, immunity, study location - associated to the AR of dengue infections, we were confronted with considerable heterogeneity in the results preventing to identify trends. This heterogeneity can be explained by study parameters that are not standardized, such as the definition considered for ‘asymptomatic’, the study recruitment design or the detection method. To be able to evaluate the risk factors more accurately, these parameter would have to be fixed, but there have not been enough studies published to have a reliable sample with fixed parameters. Up to now, only five studies shared the same definition of “no symptoms”, used a cluster recruitment design and an RT-PCR detection method.
4.3. Study sites
The study sites of almost all the included studies are located in Asia and in Latin America. Historically, the burden of dengue concerned essentially these two continents, sharing the same vector, A. aegypti. However, the epidemiology of dengue has changed, partly because of the spread of a second vector A. albopictus due to international trade [22,23]. A. albopictus invaded Africa since 1989 and was responsible for several dengue outbreaks [24]. Unfortunately, research on dengue has been completely neglected in this continent [25]. Although several studies were published on the epidemiology of dengue, the resulting knowledge is partial due to the exclusivity of study sites, which share a high level of endemicity and the same vector.
4.4. History of recruitment designs and asymptomatic detection throughout the years
This review includes 74 studies published between 1964 and 2020. Most of the studies (80%) were published in the last 20 years with an increasing trend over the last 5 years. Five categories of recruitment methods were identified: Serosurveys in general population, Cohort studies, Cluster studies, Surveys on travellers or migrants and Surveys on blood donors. Before 2001, all studies were serological surveys carried out in endemic countries or following outbreaks. Some were carried out on travellers returning from tropical area in European or US countries. The design of the studies subsequently changed to adapt and keep pace with the growing threat of dengue. The need for precise data on disease transmission and clinical presentations, led researchers to refine their methodologies by adopting cohort and cluster studies. Finally, recent years have seen an increase in studies of blood donors, reflecting the fear of this emerging disease and the need for data for policy makers.
The hypothesis that infections with no symptoms could play a role in the transmission of the disease appeared in the literature around 2000. Prior to this, a few sporadic detections of “not overt diseases” [26,27] had been described in large serosurveys. The increasing spread of the disease and the development of new vaccines [28] compelling to a better knowledge of the prevalence and herd immunity may explain the growing interest in studying infections without clinical presentation or not detected by surveillance systems.
4.5. Definitions of ‘asymptomatic’ in literature
No consensus has been reached on a standard definition or terminology for ‘asymptomatic’ dengue infections. The literature provided different terms such as: “asymptomatic”; “subclinical”; “unapparent” or “inapparent”; “mild”; “not overt disease”. These terms were not always defined in the same way and sometimes were used as synonyms. By refining the definitions and classifying the studies as no symptoms, subclinical and unapparent, the proportions of asymptomatic fall into opposite trends (18, 55 and 75% respectively).
The studies estimating the proportion of dengue infections with no symptoms detected by the presence of the virus (RT-PCR, TMA, virus isolation or NS1) were only five in number and resulted in a proportion of 8% of infections with no symptoms by aggregating the data. All five used a cluster recruitment design, which appears to be the most suitable for finding infections without any clinical presentation. The two studies with the largest sample sizes [7,29] were from Asia (Cambodia and Thailand) and both reached a same result of 7.5%.
4.6. Clinical presentation according to age
The high and prolonged endemicity meant that it was mainly children who fell victims to dengue and that by adulthood, they had already acquired partial or complete immunity. Most dengue infections were mild and rarely led to a medical consultation. The WHO has provided a clinical definition for a dengue-like syndrome but no definition for a “mild” dengue infection. In this review, the publications considered mild infections as infections without fever [30,31], or presenting low-grade dengue symptoms (headache, muscular and articular pain, rash, pruritus, fatigue) [32,33] or, mainly in children, as undifferentiated fevers presenting as other febrile childhood diseases [[34], [35], [36], [37]]. No typical symptoms that would help diagnose mild dengue infection emerged from the papers included in this review.
If we consider the studies carried out on travellers, 80% of these adult travellers with a primary dengue infection presented with a typical dengue-like syndrome. Twenty percent were not compatible with the WHO definition of dengue, which does not mean that they presented no symptoms. Only one study of travellers also included children and concluded that these children did not have typical dengue symptoms. In addition to travellers, most studies included participants with multiple dengue infections.
4.7. Parameters that contribute to explain the extent of asymptomatic infection in studies
By classifying the studies and extracting the data according to the following parameters: recruitment design - definition of ‘asymptomatic’ - age of participants - diagnostic tests - it emerged that certain parameters appeared to influence the proportion of asymptomatic infections (see Table 2). This interpretation must be taken with caution, as the results could not be perfectly compared due to the lack of standardization in the methods used to collect the data. Nevertheless, it is possible to identify a trend in these factors that is medically and epidemiologically plausible. The following parameters appear to increase the proportion of asymptomatic infections: “children”, “serosurveys” and “cohort studies”, “dengue diagnosed with antibodies detection”. Rather, “adults”, “cluster studies”, “studies on travellers” and “dengue diagnosed with viral detection methods” would decrease this proportion.
The multiplication of study parameters such as methods of detection, study designs, and the clinical definition of “asymptomatic” makes it difficult or impossible to interpret the impact of other variables such as serotype or age on the rate of asymptomatic infections. However, the number of studies using a strict definition of the absence of symptoms, a similar design and a similar method of detection can be counted on the fingers of one hand (5). Further studies with fixed parameters are needed to determine the association of serotype, primary or secondary infection, and age on the proportion of asymptomatic cases.
4.8. Limitations and futures prospects
While the systematic review provides an overview of the proportions of asymptomatic dengue infections, it is subject to limitations mainly as heterogeneity of definitions, and variability in study designs. The lack of standardized criteria for defining asymptomatic dengue infections makes it challenging to compare and synthesize the results accurately. To address these limitations, future research should focus on establishing standardized definitions, conducting large-scale prospective studies, and strengthening global surveillance efforts. These steps will contribute to a more robust understanding of asymptomatic dengue infections and inform public health strategies to control and prevent the spread of the disease.
5. Conclusion
This literature review provides a more detailed understanding of the proportion of asymptomatic dengue infections. By carefully examining the available data and considering the context and design of the studies, it becomes evident that infections with no symptoms were rare. Most of the so-called ‘asymptomatic’ dengue infections were actually mild or nonspecific infections that could easily go undetected by public health surveillance. These considerations are particularly valuable for areas with high endemicity, as there is very little data available for geographic areas where dengue is emerging.
Dengue is rapidly spreading worldwide, reaching countries without herd immunity and where the population has limited knowledge about the disease. The transition to endemicity can occur swiftly, as demonstrated by the sustained transmission of the virus on La Reunion since 2016. Therefore, there is a critical need for data to model the disease and forecast its evolution; interpret surveillance data with caution, and discuss the possibility of introducing a vaccination. In areas recently affected by dengue, the proportion of infections with no symptoms appears to be quite low, as well as the proportion of unapparent cases, assuming adequate surveillance systems and the access to healthcare. However, if the disease become endemic, the proportion of asymptomatic infections, the persons most affected by the disease and the disease presentation may change.
Furthermore, this literature review highlights the lack of research and knowledge on the epidemiology of dengue in Africa and on the clinical presentation of dengue in countries where the disease is emerging. Most of the published data on clinical presentation focused on children, while information regarding the clinical presentation in the adult population was scarce. These data are crucial for assisting public health authorities in adapting policies related to dengue surveillance and blood donors, as well as determining the need introducing dengue vaccines.
Ethical approval statement
Ethics approval was not required for this literature review.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Olga DE SANTIS reports financial support was provided by Swiss National Science Foundation. Olga DE SANTIS reports financial support was provided by the GlobalP3HS program for Global PhD Fellowship in Public Health Sciences funded by Marie Sklodowska-Curie Actions (Horizon 2020-COFUND).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20069.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
figs1.
References
- 1.« WHO | Dengue Guidelines for Diagnosis, Treatment, Prevention and Control. new edition ». WHO; 2017. http://www.who.int/rpc/guidelines/9789241547871/en/ consulté le 4 juillet. [PubMed] [Google Scholar]
- 2.Brady O.J., et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl. Trop. Dis. 2012;6(8) doi: 10.1371/journal.pntd.0001760. août. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanaway J.D., et al. The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016;16(6):712–723. doi: 10.1016/S1473-3099(16)00026-8. juin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhatt S., et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–507. doi: 10.1038/nature12060. avr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gubler D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002;10(2):100–103. doi: 10.1016/s0966-842x(01)02288-0. févr. [DOI] [PubMed] [Google Scholar]
- 6.Flahault A. [Emerging infectious diseases: the example of the Indian Ocean chikungunya outbreak (2005-2006)] Bull. Académie Natl. Médecine. 2007;191(1):113–124. ; discussion 125-128, janv. [PubMed] [Google Scholar]
- 7.Duong V., et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 2015;112(47):14688–14693. doi: 10.1073/pnas.1508114112. nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.« Factsheet about Dengue ». European Centre for Disease Prevention and Control; 2021. https://www.ecdc.europa.eu/en/dengue-fever/facts consulté le 25 juillet. [Google Scholar]
- 9.Clapham H.E., Cummings D.A.T., et, Johansson M.A. Immune status alters the probability of apparent illness due to dengue virus infection: evidence from a pooled analysis across multiple cohort and cluster studies. PLoS Negl. Trop. Dis. 2017;11(9) doi: 10.1371/journal.pntd.0005926. sept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ten Bosch Q.A., et al. Contributions from the silent majority dominate dengue virus transmission. PLoS Pathog. 2018;14(5) doi: 10.1371/journal.ppat.1006965. mai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Grange L., Simon-Loriere E., Sakuntabhai A., Gresh L., Paul R., et, Harris E. Epidemiological risk factors associated with high global frequency of inapparent dengue virus infections. Front. Immunol. 2014;5 doi: 10.3389/fimmu.2014.00280. juin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Santis O., et al. Investigation of dengue infection in asymptomatic individuals during a recent outbreak in La réunion. Viruses. 2023;15(3):742. doi: 10.3390/v15030742. mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ashshi A.M. The prevalence of dengue virus serotypes in asymptomatic blood donors reveals the emergence of serotype 4 in Saudi Arabia. Virol. J. 2017;14(1) doi: 10.1186/s12985-017-0768-7. Art. no 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stewart-Ibarra A.M., et al. The burden of dengue fever and chikungunya in southern coastal Ecuador: epidemiology, clinical presentation, and phylogenetics from the first two years of a prospective study. Am. J. Trop. Med. Hyg. 2018;98(5) doi: 10.4269/ajtmh.17-0762. Art. no 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alera M.T., et al. Incidence of dengue virus infection in adults and children in a prospective longitudinal cohort in the Philippines. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004337. Art. no 2, févr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson K.B., et al. A shorter time interval between first and second dengue infections is associated with protection from clinical illness in a school-based cohort in Thailand. J. Infect. Dis. 2014;209(3) doi: 10.1093/infdis/jit436. Art. no 3, févr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martínez-Vega R.A., et al. Peridomestic infection as a determining factor of dengue transmission. PLoS Negl. Trop. Dis. 2015;9(12) doi: 10.1371/journal.pntd.0004296. Art. no 12, déc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo S., et al. Seroprevalence of dengue IgG antibodies in symptomatic and asymptomatic individuals three years after an outbreak in Zhejiang Province, China. BMC Infect. Dis. 2018;18(1):23. doi: 10.1186/s12879-018-3000-5. Art. no 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.« WHO | Dengue and severe dengue ». http://www.who.int/mediacentre/factsheets/fs117/en/ WHO. consulté le 18 septembre 2017.
- 20.« dengue - chapter 4 - 2020 yellow book | travelers' health | CDC ». 2021. https://wwwnc.cdc.gov/travel/yellowbook/2020/travel-related-infectious-diseases/dengue consulté le 9 août.
- 21.Ratnam I., et al. Incidence and seroprevalence of dengue virus infections in Australian travellers to Asia. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31(6) doi: 10.1007/s10096-011-1429-1. Art. no 6, juin. [DOI] [PubMed] [Google Scholar]
- 22.Rai K.S. Genetics of Aedes albopictus. J. Am. Mosq. Control Assoc. 1986;2(4):429–436. déc. [PubMed] [Google Scholar]
- 23.Delatte H., Bagny L., Brengue C., Bouetard A., Paupy C., et, Fontenille D. The invaders: phylogeography of dengue and chikungunya viruses Aedes vectors, on the South West islands of the Indian Ocean. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. oct. 2011;11(7):1769–1781. doi: 10.1016/j.meegid.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 24.« GIDEON - Global Infectious Diseases and Epidemiology Network » . 2017. GIDEON - Global Infectious Diseases and Epidemiology Online Network.https://www.gideononline.com/ consulté le 19 août. [Google Scholar]
- 25.Jaenisch T., et al. Dengue expansion in africa—not recognized or not happening? Emerg. Infect. Dis. 2014;20(10) doi: 10.3201/eid2010.140487. oct. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Halstead S.B., Udomsakdi S., Scanlon J.E., et, Rohitayodhin S. Dengue and chikungunya virus infection in man in Thailand, 1962-1964. V. Epidemiologic observations outside Bangkok. Am. J. Trop. Med. Hyg. nov. 1969;18(6):1022–1033. doi: 10.4269/ajtmh.1969.18.1022. [DOI] [PubMed] [Google Scholar]
- 27.Guzmán M.G., et al. Epidemiologic Studies on Dengue in Santiago de Cuba, 1997. Am. J. Epidemiol. 2000;152(9):793–799. doi: 10.1093/aje/152.9.793. nov. [DOI] [PubMed] [Google Scholar]
- 28.Olivera-Botello G., et al. Tetravalent dengue vaccine reduces symptomatic and asymptomatic dengue virus infections in healthy children and adolescents aged 2-16 Years in Asia and Latin America. J. Infect. Dis. 2016;214(7):994–1000. doi: 10.1093/infdis/jiw297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matangkasombut P., et al. vol. 101. Department of Microbiology, Faculty of Science, Mahidol University; Bangkok, Thailand: 2020. (« Dengue Viremia Kinetics in Asymptomatic and Symptomatic Infection », Int. J. Infect. Dis.). (Matangkasombut P., ponpan.mat@mahidol.edu; Manopwisedjaroen K.; Thaloengsok S.) Department of Microbiology, Faculty of Science, Mahidol University, Bangkok, Thailand, Art. no (Matangkasombut P., ponpan.mat@mahidol.edu; Manopwisedjaroen K.; Thaloengsok S.) [DOI] [PubMed] [Google Scholar]
- 30.Ly S., et al. Asymptomatic dengue virus infections, Cambodia, 2012-2013. Emerg. Infect. Dis. 2019;25(7) doi: 10.3201/eid2507.181794. Art. no 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yoon I.-K., et al. Underrecognized mildly symptomatic viremic dengue virus infections in rural Thai schools and villages. J. Infect. Dis. 2012;206(3) doi: 10.1093/infdis/jis357. Art. no 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coelho I.C.B., et al. Dengue infection in children in fortaleza, Brazil: a 3-year school-based prospective cohort study. Am. J. Trop. Med. Hyg. 2020;103(1) doi: 10.4269/ajtmh.19-0521. Art. no 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jamjoom G.A., Azhar E.I., Kao M.A., et, Radadi R.M. Seroepidemiology of asymptomatic dengue virus infection in Jeddah, Saudi Arabia. Virol. Res. Treat. 2016;2016(7) doi: 10.4137/VRT.S34187. Art. no 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endy T.P. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in kamphaeng phet, Thailand. Am. J. Epidemiol. 2002;156(1):40–51. doi: 10.1093/aje/kwf005. juill. [DOI] [PubMed] [Google Scholar]
- 35.Chau T.N.B., et al. Dengue virus infections and maternal antibody decay in a prospective birth cohort study of Vietnamese infants. J. Infect. Dis. 2009;200(12) doi: 10.1086/648407. Art. no 12, déc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pengsaa K., Limkittikul K., Yoksan S., Wisetsing P., et, Sabchareon A. Dengue antibody in Thai children from maternally transferred antibody to acquired infection. Pediatr. Infect. Dis. J. oct. 2011;30(10):897–900. doi: 10.1097/INF.0b013e31821f07f6. [DOI] [PubMed] [Google Scholar]
- 37.Yap G., Li C., Mutalib A., Lai Y.-L., et, Ng L.-C. High rates of inapparent dengue in older adults in Singapore. Am. J. Trop. Med. Hyg. 2013;88(6):1065–1069. doi: 10.4269/ajtmh.12-0150. juin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lyerla R., et al. A dengue outbreak among camp participants in a caribbean island, 1995. J. Travel Med. 2000;7(2) doi: 10.2310/7060.2000.00022. Art. no 2, mars. [DOI] [PubMed] [Google Scholar]
- 39.Yoon I.-K., et al. Characteristics of mild dengue virus infection in Thai children. Am. J. Trop. Med. Hyg. 2013;89(6) doi: 10.4269/ajtmh.13-0424. Art. no 6, déc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mammen M.P., et al. Spatial and temporal clustering of dengue virus transmission in Thai villages. PLoS Med. 2008;5(11) doi: 10.1371/journal.pmed.0050205. Art. no 11, nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes M., et al. Index cluster study of dengue virus infection in Nicaragua. Am. J. Trop. Med. Hyg. 2010;83(3) doi: 10.4269/ajtmh.2010.10-0023. Art. no 3, sept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duong V., et al. Asymptomatic humans transmit dengue virus to mosquitoes. Proc. Natl. Acad. Sci. U. S. A. 2015;112(47) doi: 10.1073/pnas.1508114112. Art. no 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Anders K.L., et al. Households as foci for dengue transmission in highly urban vietnam. PLoS Negl. Trop. Dis. 2015;9(2) doi: 10.1371/journal.pntd.0003528. Art. no 2, févr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beckett C.G., et al. Early detection of dengue infections using cluster sampling around index cases. Am. J. Trop. Med. Hyg. 2005;72(6) Art. no 6, juin. [PubMed] [Google Scholar]
- 45.Dussart P., et al. Clinical and virological study of dengue cases and the members of their households: the multinational DENFRAME project. PLoS Negl. Trop. Dis. 2012;6(1) doi: 10.1371/journal.pntd.0001482. Art. no 1, janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vikram K., et al. vol. 153. National Institute of Malaria Research (ICMR); Delhi, India: 2016. « an epidemiological study of dengue in Delhi, India », Acta Trop. ((Vikram K.; Nagpal B.N., bnnagpal57@gmail.Com; Srivastava A.; Saxena R.; Anvikar A.; Das A.; Singh H.; Anushrita; Gupta S.K.; Valecha N.) National Institute of Malaria Research (ICMR), Delhi, India, Art. No (Vikram K.; Nagpal B.N., bnnagpal57@gmail.Com; Srivastava A.; Saxena R.; Anvikar A.; Das A.; Singh H.; Anushrita; Gupta S.K.; Valecha N.)). [DOI] [PubMed] [Google Scholar]
- 47.Wang T., et al. Evaluation of inapparent dengue infections during an outbreak in Southern China. PLoS Negl. Trop. Dis. 2015;9(3) doi: 10.1371/journal.pntd.0003677. Art. no 3, mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pengsaa K., Limkittikul K., Yoksan S., Wisetsing P., et, Sabchareon A. Dengue antibody in Thai children from maternally transferred antibody to acquired infection. Pediatr. Infect. Dis. J. 2011;30(10) doi: 10.1097/INF.0b013e31821f07f6. Art. no 10, oct. [DOI] [PubMed] [Google Scholar]
- 49.Tien N.T.K., et al. A prospective cohort study of dengue infection in schoolchildren in Long Xuyen, Viet Nam. Trans. R. Soc. Trop. Med. Hyg. 2010;104(9) doi: 10.1016/j.trstmh.2010.06.003. Art. no 9, sept. [DOI] [PubMed] [Google Scholar]
- 50.Olkowski S., et al. Reduced risk of disease during postsecondary dengue virus infections. J. Infect. Dis. 2013;208(6) doi: 10.1093/infdis/jit273. Art. no 6, sept. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Likosky W.H., Calisher C.H., Michelson A.L., Correa-Coronas R., Henderson B.E., et, Feldman R.A. AN epidemiologic study of dengue type 2 in Puerto Rico, 1969. Am. J. Epidemiol. 1973;97(4) doi: 10.1093/oxfordjournals.aje.a121508. Art. no 4, avr. [DOI] [PubMed] [Google Scholar]
- 52.Neff J.M., Morris L., Gonzalez-Alcover R., Coleman P.H., Lyss S.B., et, Negron H. Dengue fever in a PUERTO RICAN community. Am. J. Epidemiol. 1967;86(1) doi: 10.1093/oxfordjournals.aje.a120722. Art. no 1, juill. [DOI] [PubMed] [Google Scholar]
- 53.Rios I., Bailey R.E., Novak R.J., Sather G.E., Gubler D.J., et, Waterman S.H. Dengue transmission in two Puerto Rican communities in 1982. Am. J. Trop. Med. Hyg. 1985;34(3) doi: 10.4269/ajtmh.1985.34.625. Art. no 3, mai. [DOI] [PubMed] [Google Scholar]
- 54.Honório N.A., et al. Spatial evaluation and modeling of dengue seroprevalence and vector density in Rio de Janeiro, Brazil. PLoS Negl. Trop. Dis. 2009;3(11) doi: 10.1371/journal.pntd.0000545. Art. no 11, nov. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vasconcelos P.F., et al. [Dengue epidemic in Fortaleza, Ceará: randomized seroepidemiologic survey] Rev. Saude Publica. 1998;32(5) doi: 10.1590/s0034-89101998000500007. Art. no 5, oct. [DOI] [PubMed] [Google Scholar]
- 56.McBride W.J., Mullner H., LaBrooy J.T., et, Wronski I. The 1993 dengue 2 epidemic in Charters Towers, North Queensland: clinical features and public health impact. Epidemiol. Infect. 1998;121(1) doi: 10.1017/s0950268898001058. Art. no 1, août. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Morier L., Vazquez S., Soler M., Bravo J., Kouri G.P., et, Guzmán M.G. Dengue hemorrhagic fever in Cuba, 1981: a retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 1990;42(2) doi: 10.4269/ajtmh.1990.42.179. Art. no 2, févr. [DOI] [PubMed] [Google Scholar]
- 58.Yap G., Li C., Mutalib A., Lai Y.-L., et, Ng L.-C. High rates of inapparent dengue in older adults in Singapore. Am. J. Trop. Med. Hyg. 2013;88(6) doi: 10.4269/ajtmh.12-0150. Art. no 6, juin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yeh W.-T., Chen R.-F., Wang L., Liu J.-W., Shaio M.-F., et, Yang K.D. Implications of previous subclinical dengue infection but not virus load in dengue hemorrhagic fever. FEMS Immunol. Med. Microbiol. 2006;48(1) doi: 10.1111/j.1574-695X.2006.00127.x. Art. no 1. [DOI] [PubMed] [Google Scholar]
- 60.Méndez F., et al. Human and mosquito infections by dengue viruses during and after epidemics in a dengue-endemic region of Colombia. Am. J. Trop. Med. Hyg. 2006;74(4) Art. no 4, avr. [PubMed] [Google Scholar]
- 61.Rodrigues E.M.S., Dal-Fabbro A.L., Salomão R., Ferreira I.B., Rocco I.M., et, da Fonseca B.A.L. Epidemiologia da infecção pela dengue em Ribeirão Preto, SP, Brasil. Rev. Saude Publica. 2002;36(2) doi: 10.1590/S0034-89102002000200007. Art. no 2, avr. [DOI] [PubMed] [Google Scholar]
- 62.Centers for Disease Control and Prevention (Cdc) Dengue virus infections among travelers returning from Haiti--Georgia and Nebraska, October 2010. MMWR Morb. Mortal. Wkly. Rep. 2011;60(27) Art. no 27, juill. [PubMed] [Google Scholar]
- 63.Baaten G.G.G., Sonder G.J.B., Zaaijer H.L., van Gool T., Kint J.A.P.C.M., et, van den Hoek A. « travel-related dengue virus infection, The Netherlands. Emerg. Infect. Dis. 2006–2007;17(5) doi: 10.3201/eid1705.101125. Art. no 5, mai 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cobelens F.G.J., Groen J., Osterhaus A.D.M.E., Leentvaar‐Kuipers A., Dillen P.M.E.W., et, Kager P.A. Incidence and risk factors of probable dengue virus infection among Dutch travellers to Asia. Trop. Med. Int. Health. 2002;7(4) doi: 10.1046/j.1365-3156.2002.00864.x. Art. no 4. [DOI] [PubMed] [Google Scholar]
- 65.Potasman I., Srugo I., et, Schwartz E. Dengue seroconversion among Israeli travelers to tropical countries. Emerg. Infect. Dis. 1999;5(6) doi: 10.3201/eid0506.990615. Art. no 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seet R.C.S., Ooi E.E., Wong H.B., et, Paton N.I. An outbreak of primary dengue infection among migrant Chinese workers in Singapore characterized by prominent gastrointestinal symptoms and a high proportion of symptomatic cases. J. Clin. Virol. 2005;33(4) doi: 10.1016/j.jcv.2005.03.002. Art. no 4, août. [DOI] [PubMed] [Google Scholar]
- 67.Hayes C.G., et al. Dengue fever in U.S. Troops during operation restore hope, Somalia, 1992–1993. Am. J. Trop. Med. Hyg. 1995;53(1) doi: 10.4269/ajtmh.1995.53.89. Art. no 1, juill. [DOI] [PubMed] [Google Scholar]
- 68.Burke D.S., Nisalak A., Johnson D.E., et, Scott R.M. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 1988;38(1) doi: 10.4269/ajtmh.1988.38.172. Art. no 1, janv. [DOI] [PubMed] [Google Scholar]
- 69.Kosasih H., et al. The epidemiology, virology and clinical findings of dengue virus infections in a cohort of Indonesian adults in western java. PLoS Negl. Trop. Dis. 2016;10(2) doi: 10.1371/journal.pntd.0004390. Art. no 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Porter K.R., et al. Epidemiology of dengue and dengue hemorrhagic fever in a cohort of adults living in Bandung, West Java, Indonesia. Am. J. Trop. Med. Hyg. 2005;72(1) Art. no 1, janv. [PubMed] [Google Scholar]
- 71.Endy T.P., et al. Spatial and temporal circulation of dengue virus serotypes: a prospective study of primary school children in kamphaeng phet, Thailand. Am. J. Epidemiol. 2002;156(1) doi: 10.1093/aje/kwf006. Art. no 1, juill. [DOI] [PubMed] [Google Scholar]
- 72.Endy T.P., et al. Determinants of inapparent and symptomatic dengue infection in a prospective study of primary school children in kamphaeng phet, Thailand. PLoS Negl. Trop. Dis. 2011;5(3) doi: 10.1371/journal.pntd.0000975. Art. no 3, mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Balmaseda A., et al. High seroprevalence of antibodies against dengue virus in a prospective study of schoolchildren in Managua, Nicaragua. Trop. Med. Int. Health. 2006;11(6) doi: 10.1111/j.1365-3156.2006.01641.x. Art. no 6. [DOI] [PubMed] [Google Scholar]
- 74.Gordon A., et al. The Nicaraguan pediatric dengue cohort study: incidence of inapparent and symptomatic dengue virus infections, 2004-2010. PLoS Negl. Trop. Dis. 2013;7(9) doi: 10.1371/journal.pntd.0002462. Art. no 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Balmaseda A., et al. Trends in patterns of dengue transmission over four years of a pediatric cohort study in Nicaragua. J. Infect. Dis. 2010;201(1) doi: 10.1086/648592. Art. no 1, janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Capeding R.Z., et al. The incidence, characteristics, and presentation of dengue virus infections during infancy. Am. J. Trop. Med. Hyg. 2010;82(2) doi: 10.4269/ajtmh.2010.09-0542. Art. no 2, févr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Argüello D.F., et al. Incidence of dengue virus infection in school-aged children in Puerto Rico: a prospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 2015;92(3) doi: 10.4269/ajtmh.14-0231. Art. no 3, mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morrison A.C., et al. Epidemiology of dengue virus in iquitos, Peru 1999 to 2005: interepidemic and epidemic patterns of transmission. PLoS Negl. Trop. Dis. 2010;4(5) doi: 10.1371/journal.pntd.0000670. Art. no 5, mai. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tissera H., et al. Burden of dengue infection and disease in a pediatric cohort in urban Sri Lanka. Am. J. Trop. Med. Hyg. 2014;91(1) doi: 10.4269/ajtmh.13-0540. Art. no 1, juill. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.da Cunha R.V., Dias M., Nogueira R.M., Chagas N., Miagostovich M.P., et, Schatzmayr H.G. Secondary dengue infection in schoolchildren in a dengue endemic area in the state of Rio de Janeiro, Brazil. Rev. Inst. Med. Trop. Sao Paulo. 1995;37(6) doi: 10.1590/s0036-46651995000600008. Art. no 6, déc. [DOI] [PubMed] [Google Scholar]
- 81.Rigau-Perez J.G., Suarez E.L., Rodriguez-Figueroa L., et, Reiter P. Risk factors for dengue infection during an outbreak in yanes, Puerto Rico in 1991. Am. J. Trop. Med. Hyg. 1995;52(6) doi: 10.4269/ajtmh.1995.52.496. Art. no 6, juin. [DOI] [PubMed] [Google Scholar]
- 82.Guzmán M.G., et al. Epidemiologic Studies on Dengue in Santiago de Cuba, 1997. Am. J. Epidemiol. 2000;152(9) doi: 10.1093/aje/152.9.793. Art. no 9, nov. [DOI] [PubMed] [Google Scholar]
- 83.Vanwambeke S.O., et al. Multi-level analyses of spatial and temporal determinants for dengue infection. Int. J. Health Geogr. 2006;5:5. doi: 10.1186/1476-072X-5-5. janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yew Y.W., et al. Seroepidemiology of dengue virus infection among adults in Singapore. Ann. Acad. Med. Singapore. août 2009;38(8) Art. no 8. [PubMed] [Google Scholar]
- 85.Tsai J.-J., Lin P.-C., Tsai C.-Y., Wang Y.-H., et, Liu L.-T. Low frequency of asymptomatic dengue virus-infected donors in blood donor centers during the largest dengue outbreak in Taiwan. PLoS One. 2018;13(10) doi: 10.1371/journal.pone.0205248. Art. no 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mohammed H., et al. « Dengue virus in blood donations. Puerto Rico, 2005 », Transfusion (Paris) 2008;48(7) doi: 10.1111/j.1537-2995.2008.01771.x. Art. no 7. [DOI] [PubMed] [Google Scholar]
- 87.Dias L.L., Amarilla A.A., Poloni T.R., Covas D.T., Aquino V.H., et, Figueiredo L.T.M. Detection of dengue virus in sera of Brazilian blood donors. Transfusion (Paris) 2012;52(8) doi: 10.1111/j.1537-2995.2012.03729.x. Art. no 8, août. [DOI] [PubMed] [Google Scholar]
- 88.Slavov S.N., Hespanhol M.R., Ferreira A.R., Rodrigues E.S., Covas D.T., et, Kashima S. Silent dengue virus circulation among asymptomatic blood donors from a hyperendemic Brazilian region. Transfus. Med. Oxf. Engl. 2018;28(6) doi: 10.1111/tme.12521. Art. no 6. [DOI] [PubMed] [Google Scholar]
- 89.Sabino E.C., et al. Transfusion-transmitted dengue and associated clinical symptoms during the 2012 epidemic in Brazil. J. Infect. Dis. 2016;213(5) doi: 10.1093/infdis/jiv326. Art. no 5, mars. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Busch M.P., et al. Duration of dengue viremia in blood donors and relationships between donor viremia, infection incidence and clinical case reports during a large epidemic. J. Infect. Dis. 2016;214(1) doi: 10.1093/infdis/jiw122. Art. no 1, janv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Linnen J.M., et al. Dengue viremia in blood donors from Honduras, Brazil, and Australia. Transfusion (Paris) 2008;48(7) doi: 10.1111/j.1537-2995.2008.01772.x. Art. no 7. [DOI] [PubMed] [Google Scholar]
- 92.Rooks K., et al. Mitigating the risk of transfusion-transmitted dengue in Australia. J. Blood Transfus. 2016;2016 doi: 10.1155/2016/3059848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mangwana S. vol. 107. Sri Balaji Action Medical Institute; New Delhi, India: 2014. (« Dengue Viremia in Blood Donors in Northern India-Challenges of Emerging Dengue Outbreaks to Blood Transfusion Safety », Vox Sang). (Mangwana S.) Sri Balaji Action Medical Institute, New Delhi, India, Art. no (Mangwana S.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jain A., Jain et S., Chowdhury N. Seroprevalence of dengue in blood donors in an outbreak: experience of a blood bank in north India. Trop. Doct. 2019;49(3) doi: 10.1177/0049475519848947. Art. no 3, juill. [DOI] [PubMed] [Google Scholar]
- 95.Kulkarni R., Tiraki D., Wani D., Mishra A.C., et, Arankalle V.A. Risk of transfusion-associated dengue: screening of blood donors from Pune, western India. Transfusion (Paris) 2019;59(2) doi: 10.1111/trf.15007. Art. no 2. [DOI] [PubMed] [Google Scholar]
- 96.Liao Q., et al. An evaluation of asymptomatic Dengue infections among blood donors during the 2014 Dengue outbreak in Guangzhou, China. J. Med. Virol. 2017;89(11) doi: 10.1002/jmv.24883. Art. no 11. [DOI] [PubMed] [Google Scholar]
- 97.Zeng P., Liao Q., Gao Z., He M., et, Rong X. Sero-prevalence and viremia status of dengue virus among asymptomatic blood donors post epidemic outbreak in Chinese Guangzhou in 2015. Transfus. Med. Oxf. Engl. 2018;28(6) doi: 10.1111/tme.12551. Art. no 6. [DOI] [PubMed] [Google Scholar]
- 98.Harif N.F., Kader Z.S.A., Joshi S.R., et, M Yusoff N. Seropositive status of dengue virus infection among blood donors in North Malaysia. Asian J. Transfus. Sci. 2014;8(1) doi: 10.4103/0973-6247.126702. Art. no 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ribas-Silva et R.C., Eid A.A. Dengue antibodies in blood donors. Rev. Bras. Hematol. Hemoter. 2012;34(3) doi: 10.5581/1516-8484.20120048. Art. no 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Castro-Bonilla L., Coronel-Ruiz C., Parra-Alvarez S., Castellanos J.E., Porras-Ramírez A., et, Velandia-Romero M.L. Factors associated with dengue virus infection and reinfection in asymptomatic children in two Colombian municipalities. Am. J. Trop. Med. Hyg. 2018;99(6) doi: 10.4269/ajtmh.17-0617. Art. no 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Rafique I., et al. Asymptomatic dengue infection in adults of major cities of Pakistan. Asian Pac. J. Trop. Med. 2017;10(10) doi: 10.1016/j.apjtm.2017.09.013. Art. no 10, oct. [DOI] [PubMed] [Google Scholar]
- 102.Mohsin S.N., Ghafoor F., Saleem M., Ghous R., et, Aasim M. Seroprevalence of asymptomatic dengue infection in children in Lahore. Epidemiol. Infect. 2016;144(11) doi: 10.1017/S0950268816000522. Art. no 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iturrino-Monge R., et al. Seroprevalence of dengue virus antibodies in asymptomatic Costa Rican children, 2002-2003: a pilot study. Rev. Panam. Salud Publica Pan Am. J. Public Health. 2006;20(1) doi: 10.1590/s1020-49892006000700005. Art. no 1, juill. [DOI] [PubMed] [Google Scholar]
- 104.Ferri C.A., et al. Asymptomatic dengue virus cases in misiones, Argentina: a seroprevalence study in the university population. Microbes Infect. 2019;21(3–4) doi: 10.1016/j.micinf.2018.12.003. Art. no 3-4, mai. [DOI] [PubMed] [Google Scholar]
- 105.C T A., Pereira da C., et al. High prevalence of dengue antibodies and the arginine variant of the FcγRIIa polymorphism in asymptomatic individuals in a population of Minas Gerais State, Southeast Brazil. Immunogenetics. 2018;70(6) doi: 10.1007/s00251-017-1046-y. Art. no 6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supplementary material/referenced in article.