Abstract
This study aims to elucidate the evolution of catfish research publications over recent decades, identify emerging research clusters, examine keyword patterns, determine major contributors (including authors, organizations, and funding agencies), and analyze their collaborative networks and citation bursts on a global scale. The USA, Brazil, China, and India collectively contribute approximately 67% of the total catfish research publications, with a marked increase in prevalence since 2016. The most frequently occurring and dominant keywords are “channel catfish” and “responses,” respectively. Intriguingly, our findings reveal 28 distinct article clusters, with prominent clusters including “yellow catfish,” “channel catfish”, “pectoral girdle,” “African catfish”, “Rio Sao Francisco basin,” “Edwardsiella ictaluri,” and “temperature mediated”. Concurrently, keyword clustering generates seven main clusters: “new species”, “growth performance”, “heavy metal”, “gonadotropin-releasing”, “essential oil”, and “olfactory receptor”. This study further anticipates future research directions, offering fresh perspectives on the catfish literature landscape. To the best of our knowledge, this is the first article to conduct a comprehensive mapping review of catfish research publications worldwide.
Keywords: Freshwater fishes, African catfish, Aeromonas infection, Gonadotropin receptor, Ictalurus punctatus (Rafinesque), Edwardsiella ictaluri, Lipid metabolism, Flavobacterium columnar
Catfishes live in marine and freshwater environments and contain both highly endangered and successful invasive species. Due to their worldwide diversity and distribution, they are considered interesting models for scientific research and publication, in various fields of study. In order to fully understand global academic trends and corresponding trajectories about catfish research, the mapping review is proposed based on the 20,139 articles from the metabase of Web of Science Core Collection from its inception until December 2022 and visualized by the scientometric software of CiteSpace.
1. Introduction
Catfishes are among one of the most surprising fish groups in the world. Catfish are characterized based on their cylindrical body with a flattened ventral and prominent barbell(s), mostly scale-less compared to other teleost fishes. Catfishes are energetic bottom-dwelling movement fish and opportunistic feeders who actively search for food, especially at night. They are distributed worldwide, except in the Antarctic. They are also one of the most diverse groups of ray-finned fish globally, containing both highly endangered and the world's worst invasive alien species [1,2]. In the study of catfish taxonomy, approximately 40 families have been identified. Historically, the number of catfish species was reported as 3093 in 2005 [3]. By 2011, this count increased to 3407 species [4], indicating a notable growth in the understanding and discovery of catfish biodiversity. With an increasing number of catfish species found in the world [5,6], it is expected that it can reach up to 5000 species by the end of 2030. This is also because catfishes can be found in marine and freshwater environments and are also exceptionally important as an aquatic product.
Due to their diversity, distribution, and ecological importance, catfish have become one of the potential model organisms for scientific research and publication, in various fields of study such as toxicology, evolutionary biology, fisheries, parasitology, neurology, immunology, and economics. For example, catfish are hardy species, and they are a suitable candidate for toxicology-related experiments since they are high tolerance to low dissolved oxygen which was potentially induced by the heavy metals’ contaminants [[7], [8], [9]]. Historically, fossil catfish have been reported from North America that lived in the Green River Formation, Wyoming around 50 million years ago [10]. Additionally, identification of the African drainage basin is also beneficial through the widespread presence of African sharp-tooth catfish, Clarias gariepinus in the wild [11]. This indicates the importance of this group of fish in evolutionary biology and biogeographical related studies.
Based on the recent report by the FAO's Cultured Aquatic Species Information Programme [12], catfishes have been cultured in almost 90 countries globally, such as (i) North African catfish, Clarias gariepinus which is popularly farmed in Brazil, South Africa, Kenya, Mali, Nigeria, (ii) Channel catfish, Ictalurus punctatus mostly cultured in Russia, China, USA, Mexico, and Cuba, and (iii) Striped catfish, Pangasianodon hypophthalmus which is mainly cultivated in Vietnam and Thailand. Catfish aquaculture is a multifaceted process that involves factors such as nutrition, biotechnology, and reproduction. The demand for catfish which provides a significant portion of the protein supply, fats, fatty acids, vitamin D, selenium, phosphorus and calcium, which are essential for human growth and development [13] is not only limited to the whole fish and fillets but for its various value-added products such as surimi, pickles, sausages, and noodles [14]. Similarly, the fish is also exploited for fish protein hydrolysate (FPH), hydroxyapatite (HA), and lipid fraction [15,16]. Consistently, Gelatine produced from fish skin during fillet processing is used in the production of edible films because of its low melting point, low oxygen permeability, and better film-forming ability [17].
The steady increase in the rates of per capita consumption of catfish [18] is also contributed through the expansion of the successful breeding technology and seed production of this group in term of economic benefits to communities worldwide [19]. The escalating demand for catfish has significant economic implications, particularly for countries classified as low- and middle-income, such as Bangladesh, Vietnam, and Kenya. In these nations, commercial catfish production has not only created job opportunities and boosted labor income but has also been a substantial source of annual tax revenue at both the state and federal levels [18,20,21]. They are also being used for agriculture-economics related experiments [22,23]. Furthermore, parasitic infection can contribute to significant economic losses in catfish aquaculture. Previous studies also show that catfish has become one of the potential model fish for parasitology related study [24,25]. The growth, disease resistance, and survival of catfish populations are at risk due to inadequate nutrition. To ensure that the fish are healthy and optimally developing a balanced and complete diet is essential [26,27].
The catfish family is also a vital player in the aquarium industry, commercial, and sport fishing worldwide. Breeding is critical in aquaculture, and catfish-induced breeding uses various techniques. One commonly used method involves synthetic hormones to stimulate ovulation and augment egg fertilization rates [28,29]. Biotechnology, including gene banking and transgenic fish, has the potential to transform aquaculture practices. These innovative techniques improve catfish growth and disease resistance and ultimately increase aquaculture yield [30,31]. Additionally, catfish diets vary based on their age, size, and species. Omnivorous by nature, catfish can consume a vast array of natural foods and artificial feeds. Proper nutrition, consisting of protein, lipids, carbohydrates, vitamins, and minerals, is essential for catfish aquaculture. For example, in sub-Saharan Africa, pond rearing is a popular catfish farming technique, with the C. gariepinus being the most cultivated species. Successful catfish aquaculture in the region depends on the artificial reproduction and pond rearing of these fish, with the Kaiser's handbook providing in-depth information on these techniques [32].
However, due to the vast literature on catfish research in scientific databases, and which direction catfish research in the future, broader views on this topic are truly needed. Additionally, there is a lack of studies about the current situation and trends of catfish research in the world. The scientometric analysis is recently used to identify trends in any selected knowledge, which is one of the interdisciplinary fields of computer science, information science, and statistics. It was a useful tool to detect emerging topics and reveal any collaboration pattern among contributors. Most of the recent development of scientometrics is around eliciting research questions and informing researchers about state-of-the-art analysis, according to the objective of each scientometric study.
The primary objective of this comprehensive study is to employ a robust combination of qualitative and scientometric analyses to critically evaluate the global body of literature on catfish research, with the ultimate aim of discerning emerging trends and shaping the future trajectory of this vital field. To the best of our knowledge, this is the first investigation to provide an in-depth, scientometric-based assessment of catfish-related research spanning 48 years, from 1975 to 2022.
Specifically, our objectives are to systematically analyze the existing literature in order to:
-
I.
Quantify the total number of publications within the designated timeframe, thereby elucidating the historical development and growth of catfish research;
-
II.
Identify the key contributors, both individuals, and institutions, who have driven advancements in this domain;
-
III.
Examine the collaborative networks that have emerged between researchers, organizations, and countries or continents, highlighting the extent of global cooperation in catfish studies;
-
IV.
Conduct a thorough cluster network analysis to discern distinct research foci and themes that characterize this field;
-
V.
Investigate the distribution of keywords to uncover prevalent and evolving research topics; and
-
VI.
Synthesize these findings to forecast future trends and directions within the knowledge domain of catfish research.
By presenting the results of our scientometric analysis, we aim to provide valuable insights that can inform the allocation of funding for future research endeavors and assist early career researchers in understanding the current state of the field. Ultimately, our findings will help to guide and shape the trajectory of catfish research, promoting progress and innovation in this vital area of scientific inquiry.
2. Materials and methods
To accomplish the objectives of this study, we have carefully chosen and adapted the most relevant scientometric analysis methods and techniques, as delineated by Chen [33], Chen and Song [34], and Chen [35]. The Web of Science Core Collection (WoSCC) database, specifically the Science Citation Index Expanded (SCIE) version, was selected as the sole source of data for this investigation. This decision was based on the high quality of indexed content within the WoSCC, its well-structured search capabilities, comprehensive coverage, and compatibility with the chosen analytical software, CiteSpace.
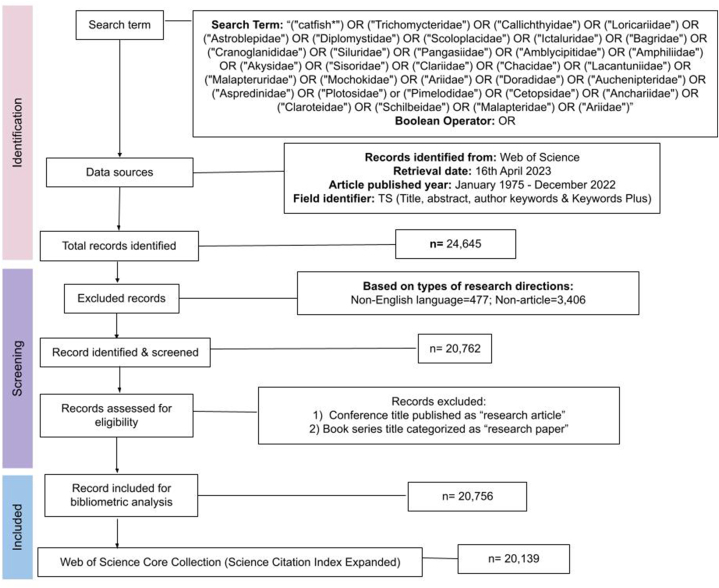
Given that there are approximately one thousand described catfish species globally, we opted to utilize the term “catfish” in our search string to capture all relevant information and associated articles within the WoSCC database. To further ensure the inclusion of literature pertaining specifically to taxonomy and systematics, we also incorporated keywords related to catfish families into our search strategy (Fig. 1). The search process was conducted on April 16th, 2023, and encompassed keywords that referenced both catfish and their respective family names. Our approach entailed three stages: identification, screening, and inclusion. We systematically filtered the search results at every phase using defined criteria. This included publication year, article type, and a specific focus on articles published in English, ensuring a robust dataset for subsequent analysis. In the initial stage, we identified a total of 20,756 records pertinent to the scope of our study. As we transitioned to the screening phase, we refined our dataset by applying the criteria of the Science Citation Index Expanded (SCIE) (n = 20,139). SCIE, being a subset of the Web of Science Core Collection, prioritizes highly cited and reliable scientific resources, thereby enhancing the validity and integrity of our dataset.
Fig. 1.
The frameworks of article structure diagram for the study on catfish research.
To undertake the scientometric analysis and depict the associated data, we utilized the CiteSpace software (Advanced version 6.2.R2, 64-bit). For reproducibility, the following parameters were set in the software: Time slicing was configured from 1975 to 2022, considering slices of 1 years each. We set a cited threshold and opted to visualize the top N references per slice. Visualization scaling was set, with the pruned network visualization option turned On. For node representation, we focused on cited references, authors, and institutions. Linking was facilitated using the Pathfinder and pruning approach, while terms were sourced from titles, abstracts, author keywords, and keyword plus. Using these specific settings, the software enabled us to produce co-citation analyses of influential references and co-occurrence of keywords, thus mapping out relationships between terms. Through the meticulous application of these techniques, our aim was to furnish an exhaustive and pivotal snapshot of the catfish research paradigm, providing insights to propel further studies and advancements in this crucial area.
3. Results
3.1. Publication years
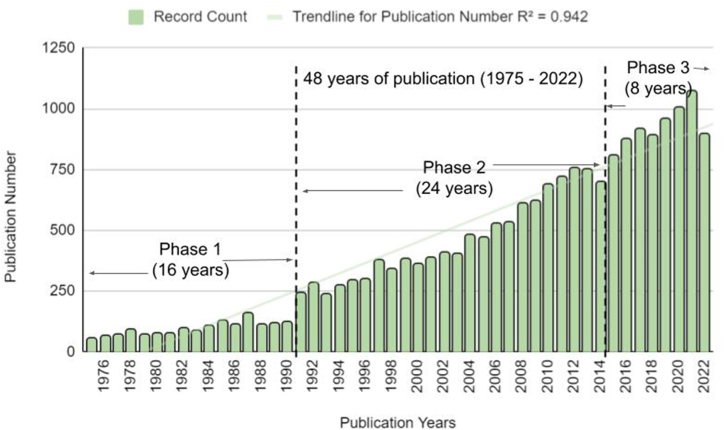
Our analysis reveals that the progression of catfish research can be delineated into three distinct phases of scientific publication, characterized by the increasing growth of articles: (i) Phase 1, referred to as the “initial development phase,” spans from 1975 to 1990; (ii) Phase 2, or the “steady development phase,” encompasses the years 1991–2014; and (iii) Phase 3, the “rapid development phase,” covers the period from 2015 to 2022 (Fig. 2). Intriguingly, the trendline for the number of publications, generated using Microsoft Excel, indicates that catfish research began garnering significant attention starting in 2016, with a rapid increase in publications up until 2021. The observed decrease in publications for 2022 is likely attributable to delays in the release of the final Web of Science database, which is dependent upon data processing and quality control checks by publishers. Furthermore, the sudden increase in total publications from 1990 to 1991 can be ascribed to “artifact” effects referring to inconsistencies in data resulting from search string restrictions.
Fig. 2.
Publication trends of catfish research based on the metadata from Web of Science Core Collection, Science Citation Index Expanded, from 1975 until 2022, with three main phases of development.
3.2. Major contributors
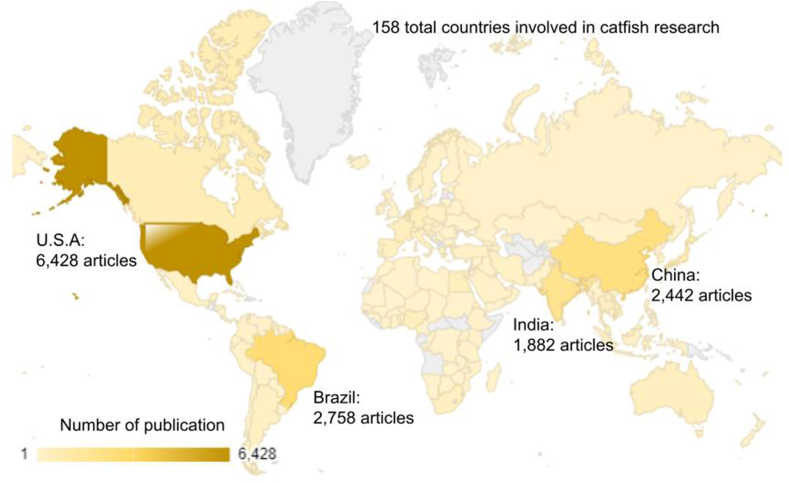
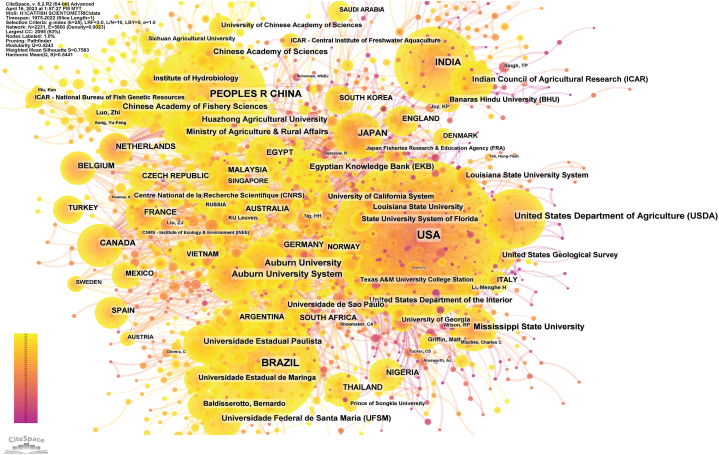
Our metadata analysis identified a total of 37,572 authors, 7080 organizational entries, 8686 distinct funding agencies, 1729 source titles (i.e., publication sources or journals), and 661 publishers. The study indicates that a significant proportion of countries globally (n = 158) are actively involved in catfish-related research. As depicted in Fig. 3, geographical representation demonstrates a higher prevalence of literature originating from the USA, Brazil, China, and India, collectively accounting for approximately 67% of total catfish research publications. This is expected, as these countries rank among the world's leading catfish producers. In line with this finding, the top 12 funding agencies supporting catfish research, which contribute nearly one-third of the total funding agencies worldwide, are predominantly based in the USA, Brazil, China, and India.
Fig. 3.
Publication distribution of catfish research in the world, between 1975 until 2022, with top four countries was indicated in the maps, with darker colors representing the highest total number of publications, whereas lighter shades representing fewer publications.
Additionally, prior research indicates that over half of all catfish species are endemic to the Americas [36,37]. This regional biodiversity may directly or indirectly contribute to the elevated volume of catfish research publications originating from the Americas.
3.3. Collaborative networks
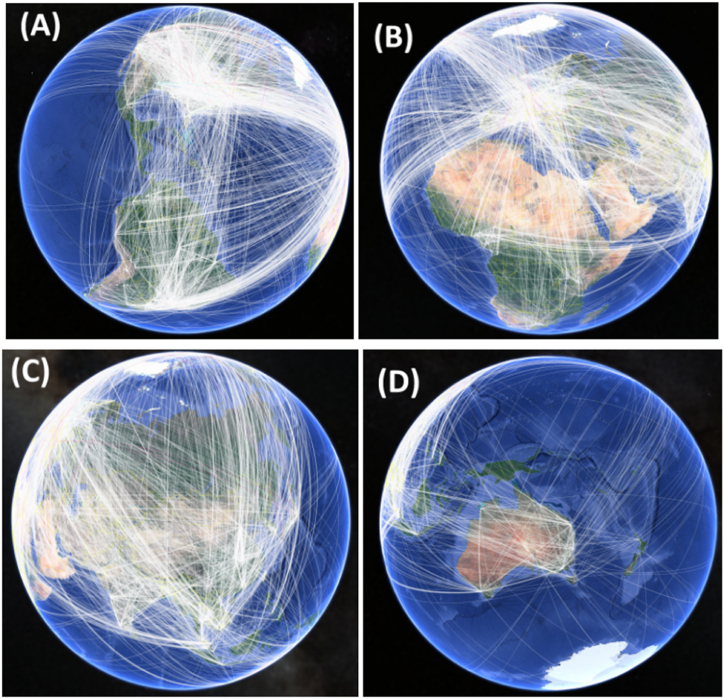
The collaborative networks among major contributors to catfish research from 1975 to 2022 are depicted in Fig. 4. Results demonstrate that American organizations (Fig. 4A) and those from the European continent (Fig. 4B) are closely connected in the diagram, indicating a high degree of interconnectivity and numerous instances of authors publishing across institutions. Both regions act as sources and destinations within the network. Asian countries are also actively involved in catfish research and collaboration (Fig. 4C) with other continents. However, fewer collaborative networks were observed between the African (Fig. 4B) and Oceanian continents (Fig. 4D). Investigating the nature of collaborative research or networks between countries or institutions could generate interest among early-career researchers and funding agencies. To further explore the data in three dimensions, the same metadata can be visualized using Google Earth 3D.
Fig. 4.
Collaboration networks between major contributors of catfish research from 1975 until 2022 based on Web of Science Core Collection, Science Citation Index Expanded through the Google Earth 3D software with (A) America Continent, (B) Europe and African Continent, (C) Asia Continent and (D) Oceania Continent. The white line showing the connection and collaboration research between countries and institutions, where thickness level indicates higher number of collaborative networks between continent.
Fig. 5 presents a cooperation network clustering generated by CiteSpace, illustrating the connections among researchers and institutions. Major contributing institutions include the United States Department of Agriculture (USDA), Auburn University, and Mississippi State University from the USA; the Indian Council of Agricultural Research from India; the Ministry of Agriculture and Rural Affairs from China; São Paulo State University from Brazil; Egyptian Knowledge Bank from Egypt; and the Chinese Academy of Sciences and Chinese Academy of Fishery Sciences from China. Collectively, these institutions have contributed to more than 5837 publications, accounting for over 28% of the total number of publications in the field. This finding underscores the significance of their collective impact on catfish research and emphasizes the importance of fostering international collaboration in driving the advancement of knowledge and innovation in this domain.
Fig. 5.
Collaborative network of countries and organizations in catfish related research from 1975 to 2022: Node size reflects the volume of publications in each country or region, while connection thickness indicates the degree of cooperation between them.
3.4. Thematic cluster analysis of research
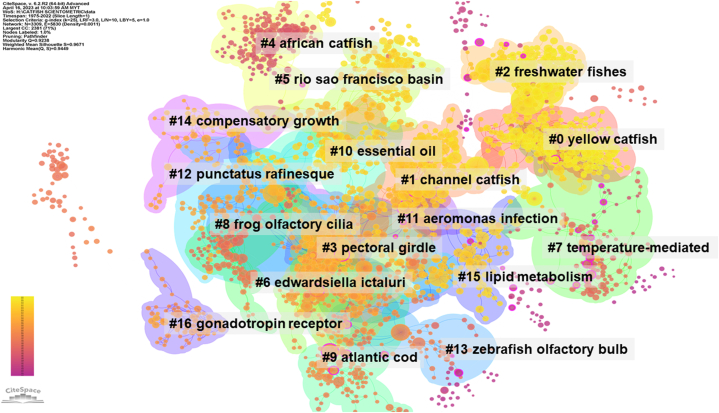
Thematic cluster analysis of the catfish research has been shared in Fig. 6. The key takeaways from the 10 major clusters are elucidated below:
-
a.
Cluster 0: Yellow Catfish Research - Predominantly centered around the “yellow catfish Pelteobagrus fulvidraco”. The cluster brings to light research focused on the emergence of new species related to the yellow catfish, alongside potential applications or implications in the area of sedative efficacy. The papers within this cluster are relatively recent, with an average publication year of 2017, indicating contemporary investigations [[38], [39], [40], [41]].
-
b.
Cluster 1: Channel Catfish Insights - This cluster pivots on the “channel catfish” and predominantly delves into in-depth analyses of this species. Moreover, sedative efficacy emerges as a recurrent theme, echoing the research interest in improving fish welfare in aquaculture practices. The average publication year for this cluster is 2012 [[42], [43], [44], [45]].
-
c.
Cluster 2: Intersecting Themes - While “new species” features as the core topic, a notable overlap with “channel catfish” is observed. An enhanced focus on sedative efficacy further underscores its significance in modern catfish research. This cluster aligns temporally with Cluster 0, representing studies from 2017 [[46], [47], [48], [49]].
-
d.
Cluster 3: Morphological Focus - Here, emphasis shifts from species-centric studies to anatomical areas, particularly the “pectoral girdle”. Nonetheless, the recurrent theme of sedative efficacy persists, with the cluster showcasing publications majorly from the early 2000s (2001) [[50], [51], [52], [53]].
-
e.
Cluster 4: African Catfish Dynamics - This cluster, with its focus on the “african catfish Clarias gariepinus”, delves into the hormonal aspect of catfish research, particularly the pituitary gonadotropin-releasing hormone. Given the average year of 1985, it points towards foundational or pioneering works within this domain [[54], [55], [56], [57], [58]].
-
f.
Cluster 5: Geographical Explorations - Moving away from species-centric studies, this cluster, with its spotlight on the “rio sao francisco basin”, emphasizes geographically oriented catfish research. The “southernmost record” theme further underscores regional significance, with most studies from around 2011 [[59], [60], [61], [62]].
-
g.
Cluster 6: Pathogenic Interactions - Rooted in “channel catfish”, this cluster introduces “Edwardsiella ictaluri”, a known pathogen, hinting at studies concerning disease dynamics in aquaculture. Published primarily around 2003, it might reflect responses to disease outbreaks or advances in aquaculture health [[63], [64], [65], [66], [67]].
-
h.
Cluster 7: Temperature Concerns - While still pivoting around “channel catfish”, the spotlight here shifts to “temperature-mediated processes”. This theme, combined with the timeline (1987), may signify early insights into how temperature variations impact catfish behavior or physiology [[68], [69], [70], [71]].
-
i.
Cluster 8: Cross-species Investigations - This cluster intriguingly ties “channel catfish” research to “frog olfactory cilia”, suggesting possible interdisciplinary explorations. Given its 1991 average publication year, it might reflect a phase of innovative methodological borrowing or comparative studies [72].
-
j.
Cluster 9: Broader Fish Studies - While rooted in “channel catfish”, the cluster introduces “atlantic cod” as a theme, pointing towards broader ichthyological interests. The theme “delta linkage”, combined with the 1996 average year, could hint at genetic or evolutionary studies within this period.
Fig. 6.
Reference co-citation clustering network of catfish-related publications: Node sizes represent citation frequency, and node colors, progressing from magenta (1975) to yellow (2022), depict the evolution of research over time. Colored connections indicate co-citation relationships, with the network further subdivided into major 17 clusters through network clustering analysis.
The co-citation clustering analysis of catfish research publications reveals the multidisciplinary nature of the field and emphasizes the increasing significance of understanding catfish biology, ecology, and aquaculture in the context of environmental change, sustainability, and food security. Common themes across several clusters include investigations into the impacts of environmental factors, such as temperature, water quality, and disease, on catfish physiology, behavior, and population dynamics. Several clusters underscore the importance of species-specific knowledge and geographic studies in catfish research. For example, clusters centered on yellow catfish (Cluster #0), channel catfish (Cluster #1), and African catfish (Cluster #4) highlight the diverse physiological and ecological adaptations of different catfish species, as well as their responses to various stressors. These clusters also emphasize the need to understand the broader implications of catfish biology, behavior, and ecology for aquaculture practices and sustainable fisheries management.
Some clusters exhibit overlapping research themes, such as the exploration of sedative efficacy in both yellow catfish (Cluster #0) and channel catfish (Cluster #1). These clusters demonstrate the field's focus on optimizing aquaculture practices, ensuring fish welfare, and improving the efficiency of catfish production. Additionally, clusters focusing on new species discoveries (Cluster #2 and Cluster #5) highlight the importance of taxonomic and biogeographic research in catfish ecology, conservation, and management. The average years for each cluster provide insights into the temporal development of catfish research. Clusters with more recent average years, such as yellow catfish (Cluster #0) and new species discoveries (Cluster #2), suggest emerging research areas that have gained momentum in recent years. In contrast, clusters with earlier average years, such as African catfish (Cluster #4) and temperature-mediated processes (Cluster #7), represent more established research domains that have provided foundational knowledge for subsequent investigations.
The global review of catfish research, mapped through these co-citation clusters, unveils a multidisciplinary landscape. From taxonomical pursuits and immune dynamics to environmental interactions and sensory biology, these insights capture the complexity and richness of catfish research.
3.5. Keywords distribution and research focus
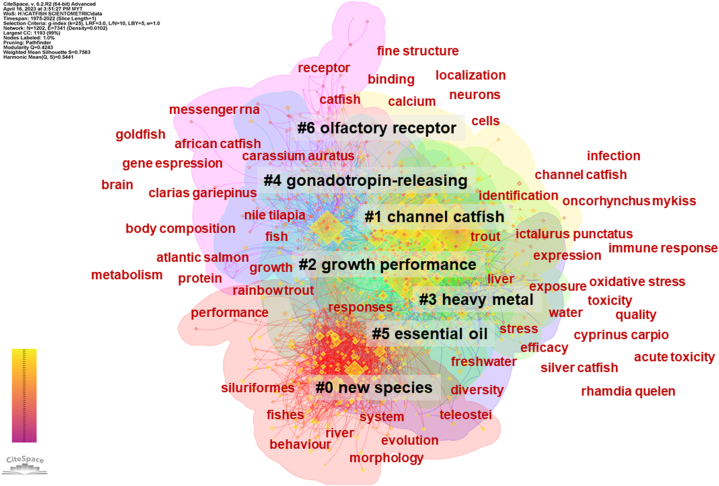
The keyword clustering analysis of catfish research publications provides insights into the temporal progression and thematic relationships among the different research areas (Fig. 7). By examining the average years for each cluster, we can discern emerging trends, and establish research domains, and shifts in research focus over time.
-
a.
Cluster #0: Taxonomy and Diversity (Major descriptors: “teleostei,” “fishes,” “evolution,” “siluriformes,” “river,” “diversity,” “system,” “behaviour morphology”) - This cluster places a pronounced emphasis on the discovery of “new species” of catfish, underscored by descriptors like “evolution” and “siluriformes.” Insights from this cluster, particularly from the year 2007, underline the breadth and depth of catfish biodiversity in aquatic ecosystems such as rivers. Additionally, the focus on “behaviour morphology” suggests investigations into behavioral and structural adaptations of these species [[73], [74], [75], [76]].
-
b.
Cluster #1: Immune Dynamics in Channel Catfish (Major descriptors: “channel catfish,” “Ictalurus punctatus,” “trout oncorhynchus mykiss,” “identification,” “expression,” “immune response,” “infection”) - Circling around “channel catfish,” this cluster from 2003 delves deep into the immunological responses of catfish, especially against infections. The co-citation with “trout Oncorhynchus mykiss” hints at comparative immunological studies between diverse fish species [[77], [78], [79], [80]].
-
c.
Cluster #2: Nutritional Aspects and Metabolic Insights (Major descriptors: “fish,” “rainbow trout,” “growth,” “atlantic salmon,” “body composition,” “protein,” “nile tilapia,” “performance,” “metabolism”) - With “growth performance” at its core, this 2002 cluster pivots on the physiological and metabolic aspects of catfish and other fish species. The descriptors suggest a keen interest in nutritional inputs, body composition, and metabolic rates, vital for aquaculture efficiency [[81], [82], [83], [84]].
-
d.
Cluster #3: Environmental Stressors and Their Effects (Major descriptors: “water,” “toxicity,” “oxidative stress,” “exposure,” “liver,” “Cyprinus carpio,” “quality”) - Under the overarching theme of “channel catfish” and “heavy metal,” the research from 2003 in this cluster seems to explore the effects of environmental contaminants, especially heavy metals, on catfish health. The focus on “oxidative stress” and “liver” hints at cellular and organ-level investigations [8,[85], [86], [87]].
-
e.
Cluster #4: Neuroendocrinology in African Catfish (Major descriptors: “african catfish,” “Clarias gariepinus,” “gene expression,” “Carassium auratus,” “goldfish,” “brain,” “messenger rna”) - Centered around “african catfish” and the “gonadotropin-releasing hormone,” this 2000 cluster delves into the neuroendocrine mechanisms in catfish. The exploration of gene expression and its implications on catfish behavior and physiology is evident [54,[88], [89], [90]].
-
f.
Cluster #5: Essential Oils and Catfish Stress Management (Major descriptors: “responses,” “freshwater,” “stress,” “silver catfish,” “efficacy,” “Rhamdia quelen,” “acute toxicity”) - Anchored in 2008, this cluster studies the application and efficacy of “essential oil” in mitigating stress and potential toxicities in catfish, offering potential alternative treatments in aquaculture health [[91], [92], [93], [94]].
-
g.
Cluster #6: Sensory Biology in Rainbow Trout (Major descriptors: “catfish,” “cells,” “calcium,” “binding,” “neurons,” “fine structure,” “receptor,” “localization”) - This cluster from 1994, with its focus on “rainbow trout” and “olfactory receptor neuron,” delves into the sensory biology of catfish, exploring the intricate mechanisms of olfaction, potentially critical for behaviors such as foraging and mating [95,96].
Fig. 7.
Keyword clustering network of catfish-related publications: Node sizes represent citation frequency, and node colors, progressing from magenta (1975) to yellow (2022), depict the evolution of research over time. Colored connections indicate co-citation relationships, with the network further subdivided into 7 clusters through network clustering analysis.
In summary, the overall analysis of these clusters underscores the multifaceted nature of catfish research, encompassing diverse themes, such as evolutionary biology, neuroendocrine regulation, health and immunity, environmental stressors, and aquaculture practices. By examining the similarities, differences, and temporal trends among clusters, researchers can identify knowledge gaps, prioritize future research efforts, and develop targeted strategies to address pressing challenges facing catfish populations and the industries that rely on them. There will be four (4) different groups for this type of analysis which are:
-
•
Emerging research areas: Clusters with more recent average years, such as Cluster #5 (Essential Oil Applications and Stress Mitigation in Catfish; 2008) and Cluster #0 (New Species and Evolutionary Insights; 2007), suggest that these research themes have gained momentum in recent years. These clusters represent innovative approaches to understanding catfish biology and ecology and explore potential applications of natural products and new discoveries in catfish evolution and biodiversity.
-
•
Established research domains: Clusters with earlier average years, such as Cluster #4 (African Catfish and Neuroendocrine Regulation; 2000) and Cluster #2 (Growth Performance and Nutritional Requirements; 2002), represent more established research areas that have provided foundational knowledge for subsequent investigations. These clusters have contributed significantly to our understanding of catfish biology, including the role of neuroendocrine regulation in reproduction and growth, and the optimization of nutritional requirements and feeding strategies in aquaculture.
-
•
Similar clusters: Some clusters exhibit overlapping research themes, such as Cluster #1 (Channel Catfish Health and Immune Response) and Cluster #3 (Heavy Metal Toxicity and Channel Catfish Health). Both clusters focus on the health and well-being of catfish, investigating aspects like immune response, disease management, and the effects of environmental stressors, such as heavy metal exposure. The similarity between these clusters reflects the field's interest in catfish welfare and the development of strategies to ensure the sustainable management of catfish populations.
-
•
Distinct clusters: Clusters that are more distinct from each other include Cluster #6 (Olfactory System and Neuronal Signalling in Catfish) and Cluster #2 (Growth Performance and Nutritional Requirements). Cluster #6 primarily focuses on the molecular and cellular aspects of olfaction in catfish, while Cluster #2 investigates the physiological and metabolic processes related to growth and nutrition in various fish species. The disparity between these clusters demonstrates the broad scope of catfish research and the diverse array of topics that researchers are exploring.
4. Discussion
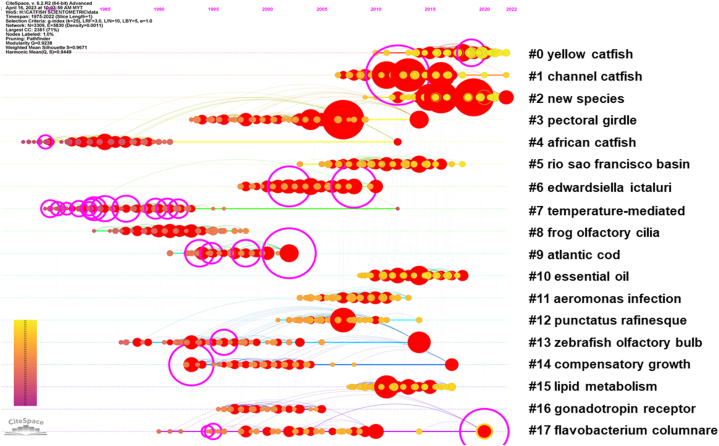
The employment of timeline co-citation analysis (Fig. 8) in the present study constitutes a powerful scientometric technique for the identification and elucidation of emergent research clusters within the domain of catfish ecology and associated fields. By delineating the chronological development of the extant literature, this analysis has facilitated the discernment of four research clusters experiencing citation bursts, specifically, cluster #0 ″yellow catfish in relation to ecology and sedative,” cluster #1 ″channel catfish in relation to ecology and sedative,” cluster #2 ″new species discoveries,” and cluster #17 ″Flavobacterium columnare."
Fig. 8.
A timeline co-citation analysis for catfish study, references with strong citation bursts are shown as red circle, whereas references with high centrality are shown in yellow circle, and purple circle indicated the burstness
These clusters serve to underscore the multifaceted nature of catfish research, accentuating the imperative to comprehend the ecological, environmental, and economic ramifications of these foci. For example, the exploration of yellow and channel catfish in the context of ecology and sedatives reflects the burgeoning interest in refining aquaculture practices to ensure optimal fish welfare and minimize stress-induced mortality. Concurrently, the cluster devoted to new species discoveries emphasizes the critical role of taxonomic research in advancing knowledge of biodiversity, biogeography, and conservation requirements. Lastly, the Flavobacterium columnare cluster directs attention to the mounting concern surrounding bacterial pathogens and the pressing need for the development of enhanced disease prevention and management strategies in aquaculture environments. The present study makes a significant impact on the scientific realm by elucidating the research trends and emerging focal points within catfish research. This scientometric analysis provides researchers with an overview of the current state of knowledge, enabling them to recognize the gaps and formulate new research questions accordingly.
However, despite these advancements, several research gaps still exist. Firstly, more comprehensive studies are needed to elucidate the complex interactions between catfish ecology and the various factors influencing their population dynamics and welfare. Understanding these interactions will enable better predictions and management strategies for catfish conservation and aquaculture. Secondly, the potential socio-economic impacts of the catfish population shift remain largely underexplored. As communities often rely on catfish for their livelihoods, it is crucial to investigate the implications of changes in catfish populations for human well-being. Lastly, there is a need for more interdisciplinary research, integrating perspectives from ecology, aquaculture, economics, and social sciences. Such an approach will facilitate a more holistic understanding of the challenges and opportunities associated with catfish research.
As we continue the general discussion, we will delve deeper into the four research clusters identified by the timeline co-citation analysis. By examining the contributions and implications of each cluster, we aim to provide a more comprehensive understanding of the current state of knowledge in the field of catfish research. This in-depth analysis will elucidate the specific aspects of each cluster, the interconnections among them, and their collective significance to the broader scientific community. The insights gained from this investigation will not only enrich our understanding of the complex interactions within catfish research but also contribute to shaping future research directions and informing policy decisions.
4.1. Optimizing aquaculture practices: the interplay between Catfish ecology, welfare, and sedative use
The sustainable development of the catfish aquaculture industry hinges on optimizing the intricate interplay between catfish ecology, welfare, and sedative use. This requires a multidisciplinary approach, integrating knowledge from fish ecology, animal welfare science, and aquaculture management practices. Recent scientometric analyses have mapped the evolution of research in this area, providing valuable insights into the factors influencing catfish welfare and productivity.
A crucial aspect of catfish welfare is understanding the impact of ecological factors on fish health and performance. Dunham and Elaswad [97] emphasized the significance of water quality, temperature, and dissolved oxygen levels in their review, suggesting that proper management of these factors can substantially improve overall welfare and productivity. Similarly, Majhi et al. [98] demonstrated that the implementation of biofloc-based water treatment systems improved water quality, reduced stress, and enhanced growth performance, supporting the notion that ecological factors play a significant role in catfish welfare. Handling and transport are key areas where catfish welfare is paramount, as these processes can induce stress, compromising immune function and increasing disease susceptibility [99,100]. The use of appropriate sedatives and maintaining optimal water quality are essential strategies to minimize stress during these procedures.
The use of sedatives is integral to catfish aquaculture, as they alleviate stress during handling, transport, and medical interventions. By reducing stress, sedatives enhance fish welfare, promote growth, and decrease disease susceptibility, thereby optimizing productivity. Bowker et al. [101] conducted a comprehensive review of sedative options for fish research, revealing that the fish response to chemical sedatives is primarily influenced by sedative dose, water temperature, and to a lesser extent, fish size, and dissolved oxygen levels. Their study demonstrated that higher doses and warmer temperatures accelerated induction, while warmer temperatures also promoted faster recovery. Öğretmen and Gökçek [102] provided a comparative analysis of three anesthetic agentsclove oil, 2-phenoxyethanol, and eugenol assessing their efficacy on captive-bred African catfish, Clarias gariepinus. They concluded that clove oil was the most suitable anesthetic among the evaluated agents.
Akinrotimi et al. [103] evaluated the efficacy of aqueous extracts of clove seed as an anesthetic, specifically examining its effects on hematological parameters in Clarias gariepinus. The study demonstrated size-related induction times and significant alterations in hematological variables at higher concentrations while maintaining a 100% survival rate. In contrast, Cupp et al. [104] investigated the effectiveness of alternative sedatives, such as AQUI-S®20E (iso-eugenol), in reducing stress during fish transport in high loading densities. They found AQUI-S®20E to be an effective alternative with fewer side effects on fish welfare. The potential risks associated with sedative use in fish farming warrant careful consideration. Zeng et al. [105] assessed the effects of eugenol exposure on catfish behavior and physiology, finding alterations in swimming behavior, blood biochemistry, and gill morphology. These results emphasize the need for careful monitoring of sedative use to avoid negative impacts on fish welfare. De Lima Silva et al. [91] investigated the anesthetic properties of Ocimum gratissimum L. essential oil (EO) in silver catfish (Rhamdia quelen), revealing effective anesthetization at concentrations exceeding 30 mg L-1 without adverse effects or mortality. The authors deduced that O. gratissimum EO serves as a secure and efficient anesthetic for silver catfish, with its mechanism likely involving interaction with the GABAA -benzodiazepine receptor.
Conte [106] conducted a comprehensive analysis of the literature on catfish welfare and sedative use, highlighting the need for further research on alternative sedatives and their potential impacts on fish behavior, physiology, and immune function. The author also emphasized the importance of developing standardized protocols for sedative use to ensure consistency across the industry and minimize potential risks to fish welfare. Bowker et al. [107] investigated the effects of different sedatives on the stress response and recovery of African catfish during transport, concluding that eugenol and benzocaine were more effective at reducing stress than MS-222 and recommending further research to optimize their use in catfish aquaculture. Several researchers have proposed a holistic approach to optimize catfish welfare in aquaculture, encompassing ecological factors, handling practices, and sedative use [108,109]. This underscores the need for a multidisciplinary approach to developing best practices for catfish farming. Additionally, studies have demonstrated the potential benefits of integrating environmental enrichment strategies, such as the addition of natural substrates and refuges, in catfish farming to improve welfare and reduce stress. These strategies have been shown to increase growth rates, reduce aggression, and improve overall welfare, suggesting that incorporating environmental enhancements into aquaculture practices can optimize catfish welfare and productivity [[110], [111], [112]].
In summary, the complex interplay between catfish ecology, welfare, and sedative use is crucial for the sustainable development of the catfish industry. A multidisciplinary approach is required to optimize catfish farming practices, integrating knowledge from fish ecology, animal welfare science, and aquaculture management. Future research should focus on evaluating alternative sedatives, developing standardized protocols for sedative use, and investigating the potential benefits of environmental enrichment strategies to ensure the continued growth and sustainability of the catfish industry.
4.2. Taxonomic discoveries and conservation implications: unravelling the hidden diversity of Catfish species
Catfish comprise a diverse and ecologically significant group of freshwater and marine fish, with over 3000 species distributed across the globe [3]. Recent advances in molecular and morphological techniques have facilitated a more comprehensive understanding of catfish species' hidden diversity, leading to the discovery of novel taxa and refining our knowledge of their evolutionary relationships.
Recent studies employing molecular and morphological techniques have unveiled new species and revised taxonomic classifications, underscoring the importance of an integrative taxonomic approach for informing conservation and management strategies. Sullivan et al. [113] and Lujan et al. [114] both discovered new species and revaluated phylogenetic relationships within the catfish genus Noturus and the armored catfish family Loricariidae, respectively. Sacranto et al. [115] and de Borba et al. [116] both utilized DNA barcoding with the mitochondrial cytochrome oxidase subunit I (COI) gene to investigate the genetic diversity of morphologically similar freshwater fish species in South Brazil and to differentiate distinct Ancistrus lineages from the Amazon and Paraguay river basins in Brazil, respectively. These findings emphasize the crucial role of molecular techniques, such as DNA barcoding, in identifying cryptic species and preventing species mislabelling, commercial fraud, and protecting aquatic habitats and endangered species. Carvalho et al. [117] applied DNA barcoding to investigate species authentication in commercial fish products sold under the common name surubim (Pseudoplatystoma spp.) in Brazilian markets, discovering that approximately 80% of analyzed samples were mislabelled.
Focusing on different regions, Li et al. [118] and Shibatta et al. [119] both described new genera of catfish, Creteuchiloglanis and Rhyacoglanis, by examining unique morphological traits and employing phylogenetic analyses. Rodiles-Hernández et al. [120] discovered a new family of catfish, the Lacantuniidae, based on a single specimen from a remote river in Mexico, exemplifying the potential for new taxonomic discoveries even in relatively well-studied regions. These discoveries highlight the importance of continued exploration in diverse ecosystems to uncover new species and better understand the biogeographical history of catfish. In a comprehensive taxonomic revision, Dias and Zawadzki [121] delved into the Neotropical catfish genus Hypostomus, shedding light on the hidden diversity of these fishes and providing a framework for future research on their ecology, evolution, and conservation. This study underscores the significance of re-evaluating existing taxonomic classifications and integrating novel techniques to uncover previously unrecognized diversity.
In 2022, a significant number of new catfish species were discovered worldwide, highlighting the vast diversity and ongoing exploration of aquatic life. Reis and Lehmann [122] identified a new genus of Hypopopomatinae armored catfish in the northern regions of South America, accompanied by the reclassification of 18 species. Additionally, Esmaeili et al. [123] discovered Mystus cyrusi from the Kol River drainage in southern Iran, and Pinna and Dagosta [124] revised the taxonomy of the genus Paracanthopoma, unveiling nine new species. New species discoveries spanned various geographical locations, such as Parotocinclus hardmani from the Potaro River tributaries in Guyana [125], Liobagrus brevispina from the Nan-Jiang River in China, and Clarias monsembulai from the Salonga National Park in the Democratic Republic of the Congo [126]. Furthermore, discoveries included Mystus irulu from the Western Ghats of India [127], Glyptothorax yuensis from the Yu River in Myanmar [128], and Amblyceps hmolaii from the Kaladan River drainage in India [129].
These recent taxonomic discoveries illustrate the hidden diversity of catfish species and the critical role of integrative taxonomy in informing conservation and management efforts. The combination of molecular and morphological techniques has proven invaluable for uncovering cryptic species, refining our understanding of their evolutionary relationships, and providing insights into their biogeographical history. Embracing an integrative, multidisciplinary approach to taxonomy and conservation is essential to ensure the adequate protection and management of these ecologically significant catfish species for future generations. By continuing to explore and document the remarkable diversity of aquatic organisms, researchers can contribute to a more comprehensive understanding of the world's freshwater and marine ecosystems, ultimately supporting more effective conservation strategies and management practices.
4.3. Disease dynamics and management strategies in Catfish aquaculture
The sustainable development of the aquaculture industry relies on understanding and optimizing the interplay between ecological factors, animal welfare, and management practices. Catfish are susceptible to various diseases, including bacterial, viral, and parasitic infections, which can significantly impact their welfare and productivity. Studies have identified major diseases affecting catfish such as Columnaris disease, caused by Flavobacterium columnare; Edwardsiella ictaluri, causing enteric septicemia; Aeromonas hydrophila [130], Streptococcus iniae [131]; channel catfish virus disease [132] and proliferative gill disease [133].
Enteric septicemia of catfish (ESC) is a severe disease instigated by the pathogenic bacterium Edwardsiella ictaluri, significantly impacting catfish populations. Initially documented in 1976, ESC afflicts catfish of various sizes, including market-sized fish, often resulting in high mortality rates. The disease is commonly observed when water temperatures range between 20 and 30 °C. ESC presents with a variety of symptoms, such as erythematous and white ulcers, erythematous patches in the head or ventral regions, and erythematous papules at the cranial foramen between the eyes, potentially leading to a “hole-in-head” manifestation [134]. Ever since its discovery, there have been numerous outbreaks of ESC reported worldwide. For instance, the inaugural incidence of E. ictaluri infection in Chinese-farmed yellow catfish (Pelteobagrus fulvidraco) transpired in May 2006 at a farm in Liaoning Province, China. This outbreak culminated in cumulative mortality of up to 50%. Management and treatment strategies for ESC typically necessitate the use of medicated feed containing antibiotics such as sulfadimethoxine-ormetoprim and florfenicol [130].
Aeromonas hydrophila, a pervasive Gram-negative bacterium, is implicated in the development of motile aeromonad septicemia (MAS), a disease responsible for notable morbidity and mortality within catfish aquaculture. Infections by this opportunistic microorganism typically arise due to stress factors such as inadequate water quality, elevated stocking densities, and handling practices, which collectively weaken the fish's immune response [135,136]. Clinical presentations of A. hydrophila infections in catfish encompass erratic swimming patterns, lethargy, anorexia, exophthalmia, and external hemorrhagic manifestations. Internally, afflicted fish may exhibit hemorrhagic and necrotic lesions in organs such as the liver, spleen, and kidney, ultimately progressing to septicemia [137,138].
Flavobacterium columnare, the causative agent of Columnaris disease, has emerged as a significant concern in catfish aquaculture due to its high morbidity and mortality rates. The increasing trend of publications on this bacterium can be attributed to its economic impact and the growing awareness of its implications for catfish welfare [139]. A comprehensive study had been done on the pathogenicity, detection, and control of F. columnare, highlighting the importance of understanding its biology, virulence factors, and host-pathogen interactions to develop effective management strategies [140,141].
Recent studies have investigated various approaches to prevent and control F. columnare infections in catfish. For instance, Laanto et al. [142] demonstrated the potential of phage therapy as an alternative to antibiotics, while Mousa et al. [143] explored the use of essential oils as a natural, eco-friendly solution. The study revealed that trans-cinnamaldehyde (TC) effectively inhibited the growth of E. ictaluri, F. columnare, and A. hydrophila, causing cell wall lysis and leakage of cytoplasmic contents. Catfish receiving TC-supplemented feed demonstrated a higher survival rate and reduced bacterial concentrations during an E. ictaluri challenge trial. These findings suggest that TC could serve as a safe and efficient alternative to antimicrobials, mitigating the development and spread of antimicrobial-resistant strains.
Vaccination has also been explored as a strategy to mitigate the impact of F. columnare in catfish aquaculture. Studies have shown the use of modified live F. columnare vaccine [144] and the use of recombinant F. columnare vaccine [145] provided significant protection against the bacterium, suggesting that vaccination could be a viable approach for disease management. Environmental factors have been identified as critical determinants of F. columnare prevalence in catfish aquaculture. Studies have highlighted the role of water hardness, water temperature, and dissolved oxygen levels in disease outbreaks, emphasizing the need for proper water management to prevent infections [146]. Progress in diagnostic methodologies has played a crucial role in expanding the research corpus on F. columnare. Serological examinations, including enzyme-linked immunosorbent assays (ELISA), latex agglutination tests, fluorescent antibody tests, and lateral-flow assays, have proven valuable for field implementation [147,148]. Moreover, Panangala et al. [149] devised a rapid, sensitive, and specific loop-mediated isothermal amplification (LAMP) assay to detect F. columnare, enabling prompt intervention and disease management in catfish farming operations. In conclusion, the recent surge in publications on F. columnare can be attributed to the significant economic and welfare implications of the bacterium for the catfish aquaculture industry.
5. Conclusions
In conclusion, the present study offers a comprehensive, data-driven synthesis of the existing body of research on catfish, elucidating key trends, hotspots, and frontiers within the field. Our in-depth analysis of 28 distinct clusters of catfish references not only highlights the richness and diversity of this research domain but also enables us to propose three promising avenues for future inquiry.
Moreover, our examination of seven keyword clusters, derived from co-occurrence analysis, reveals the interconnectedness of various elements within catfish research and grants a deeper understanding of the overarching themes propelling advancements in this field. As a result, we strongly advocate for strategic cooperation and collaboration among researchers, particularly between those based in the Americas, Africa, and Oceania. By fostering a global network of expertise, we can collectively leverage resources and cutting-edge techniques to elevate catfish research on an international scale. Looking forward, the discovery of new species within the catfish group necessitates rigorous scientific approaches that incorporate a variety of methodologies, such as morphological, DNA, geographical, and behavioral analyses. By integrating these diverse techniques, researchers will be better equipped to accurately identify and classify novel catfish species, ultimately contributing to a more precise and detailed understanding of their biology and ecology.
Additionally, the extensive fossil record of catfish, spanning millions of years, offers a distinctive avenue for enhancing our understanding of historical aquatic ecosystem dynamics. The examination of these ancient specimens can yield invaluable insights into the enduring patterns and effects on finfish populations and broader aquatic habitats. By building upon the findings presented herein, future research efforts can not only expand our comprehension of catfish biology, ecology, and evolution but also generate actionable insights that inform policy and conservation decisions on a global scale. Ultimately, this will enable us to better safeguard the ecological integrity of our planet's aquatic ecosystems, in the face of mounting environmental challenges.
Author contributions
Conceptualization, CST, MNA and NMN; methodology, MNA, FL and RMP; software, CST, MNA and ZAK; validation, DT, GTI and HG; formal analysis, CST, MNA; investigation, CST, MNA and NMN; resources, FL, RMP and DT; data curation, GTI, ZAK an HG; writing original draft preparation, CST and MNA; writing review and editing, CST, NMN and ZAK; visualization, FL, RMP, DT, GTI, ZAK, and HG; supervision, MNA; funding acquisition, GTI and ZAK. All authors have read and agreed to the published version of the manuscript.
Funding
Research funding and Article Processing Charge (APC) for this mapping review was supported in part by funds provided by USDA-NIFA Sustainable Agriculture Systems, Grant No. 2019-69012-29905 to GTI and ZAK. Title of the project: Empowering US Broiler Production for Transformation and Sustainability USDA-NIFA (Sustainable Agriculture Systems): No. 2019-69012-29905.
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Data availability statement
The authors do not have permission to share data.
Additional information
No additional information is available for this paper.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The present review was also supported by the Ministry of Higher Education (MOHE), under the Malaysia's Long-Term Research Grant Scheme (LRGS; project code: LRGS/1/2019/UPM/1) at Universiti Malaysia Terengganu, as part of the project entitled Stock Enhancement, Sustainable Production, and Culture of Patin Buah (Pangasius nasutus) for Improvement of Fishermen's Livelihood and Aquaculture Industry in Malaysia (53340) to RMP, NMD and MNA.
Contributor Information
Thirukanthan Chandra Segaran, Email: thiru@umt.edu.my.
Mohamad Nor Azra, Email: azramn@umt.edu.my.
Rumeaida Mat Piah, Email: rumeaida@umt.edu.my.
Fathurrahman Lananan, Email: fathurrahman@unisza.edu.my.
Guillermo Téllez-Isaías, Email: gtellez@uark.edu.
Huan Gao, Email: huanmr@163.com, gaoh@jou.edu.cn.
Donald Torsabo, Email: donald.torsabo@uam.edu.ng.
Zulhisyam Abdul Kari, Email: zulhisyam.a@umk.edu.my.
Noordiyana Mat Noordin, Email: diyananoordin@umt.edu.my.
References
- 1.IUCN Red List. 2023. https://www.iucnredlist.org/search?query=catfish&searchType=species [Google Scholar]
- 2.Lowe S., Browne M., Boudjelas S., De-Poorter M. 2000. 100 of the world's worst invasive alien species A selection from the global invasive species database; p. 12.http://www.iucngisd.org/gisd/pdf/100English.pdf Published by The Invasive Species Specialist Group (ISSG) a specialist group of the Species Survival Commission (SSC) of the World Conservation Union (IUCN), vol.12, First published as special lift-out in Aliens 12, December 2000. Updated and reprinted version:November 2004. [Google Scholar]
- 3.Ferraris C.J. Checklist of catfishes, recent and fossil (Osteichthyes: siluriformes), and catalogue of siluriform primary types. Zootaxa. 2007;1418:8. doi: 10.11646/zootaxa.1418.1.1. [DOI] [Google Scholar]
- 4.Armbruster J.W. In: Michaletz P.H., Travnichek V.H., editors. vol. 77. American Fisheries Society; USA: 2011. Global catfish biodiversity; pp. 15–37. (Conservation, Ecology, and Management of Catfish: the Second International Symposium). [DOI] [Google Scholar]
- 5.Jokar M., Kamangar B.B., Ghaderi E., Freyhof J. Glyptothorax sardashtensis, a new species of torrent catfish from the upper Lesser Zab drainage in Iran (Teleostei: sisoridae) Zootaxa. 2023;5254:476–492. doi: 10.11646/zootaxa.5254.4.2. [DOI] [PubMed] [Google Scholar]
- 6.Mejia E., Ferraro G.A., Buckup P.A. A new species of Rineloricaria (Siluriformes: Loricariidae) from coastal drainages of Rio de Janeiro, southeastern Brazil. Neotrop. Ichthyol. 2023;21:1. doi: 10.1590/1982-0224-2022-0083. [DOI] [Google Scholar]
- 7.Rejab M.R.M., Manam N.K.A., Fauzi N.S., Mohamed S., Ngah N. Ecotoxicity of Furcraea gigantae leaves on nontarget organism. Biosci. Res. 2020;17:64–70. https://www.isisn.org/BR17(SI-1)2020/64-70-17(SI-1)2020BR20-07.pdf [Google Scholar]
- 8.Turan F., Eken M., Ozyilmaz G., Karan S., Uluca H. Heavy metal bioaccumulation, oxidative stress and genotoxicity in African catfish Clarias gariepinus from Orontes River. Ecotoxicology. 2020;29:1522–1537. doi: 10.1007/s10646-020-02253-w. [DOI] [PubMed] [Google Scholar]
- 9.Tripathi S., Purchase D., Chandra R., Nadda A.K., Chaturvedi P. Emerging pollutants characterization, mitigation and toxicity assessment of sewage wastewater treatment plant- India: a case study. J. Contam. Hydrol. 2023;254 doi: 10.1016/j.jconhyd.2023.104139. [DOI] [PubMed] [Google Scholar]
- 10.Grande L. Paleontology of the Green River Formation, with a review of the fish fauna. Bull. Geol. Surv. Wyoming. 1984;63:1–333. http://npshistory.com/publications/fobu/gsw-bul-63.pdf [Google Scholar]
- 11.Van-Steenberge M.W., Vanhove M.P.M., Manda A.C., Larmuseau M.H.D., Swart B.L., Khang'Mate F., Hellemans B., Houdt J.V., Micha J.C., Koblmuller S., Roodt-Wilding R., Volckaert F.A.M. Unravelling the evolution of Africa's drainage basins through a widespread freshwater fish, the African sharptooth catfish Clarias gariepinus. J. Biogeogr. 2020;47:1739–1754. doi: 10.1111/jbi.13858. [DOI] [Google Scholar]
- 12.FAO . Food and Agriculture Organization (FAO) of the United Nations; 2023. Cultured Aquatic Species Fact Sheets: Cultured Aquatic Species Information Programme.https://www.fao.org/fishery/en/culturedspecies/search April 17th, 2023. [Google Scholar]
- 13.Khalili-Tilami S., Sampels S. Nutritional value of fish: lipids, proteins, vitamins, and minerals. Reviews in Fisheries Science & Aquaculture. 2018;26(2):243–253. doi: 10.1080/23308249.2017.1399104. [DOI] [Google Scholar]
- 14.Rathod N.B., Pagarkar A.U., Pujari K.H., Shingare P.E., Satam S.B., Phadke G.G., Gaikwad B.V. Status of valuable components from pangasius: a review. International Journal of Current Microbiology and Applied Sciences. 2018;7(4):2106–2120. doi: 10.20546/ijcmas.2018.704.241. [DOI] [Google Scholar]
- 15.Halim N.R.A., Yusof H.M., Sarbon N.M. Functional and bioactive properties of fish protein hydolysates and peptides: a comprehensive review. Trends Food Sci. Technol. 2016;51:24–33. doi: 10.1016/J.TIFS.2016.02.007. [DOI] [Google Scholar]
- 16.Nam P.V., Van-Hoa N., Anh T.T.L., Trung T.S. Towards zero-waste recovery of bioactive compounds from catfish (Pangasius hypophthalmus) by-products using an enzymatic method. Waste and Biomass Valorization. 2020;11(8) doi: 10.1007/s12649-019-00758-y. [DOI] [Google Scholar]
- 17.Nurdiani R., Prihanto A.A., Firdaus M., Aini F.N., Nabilah F.A., Talib R.A., Huda N. Physicochemical characteristics of Pangasius sp. skin-gelatin-based-edible film enriched with silver nanoparticles. F1000Research. 2023;12 doi: 10.12688/f1000research.129024.1-. [DOI] [Google Scholar]
- 18.FAO . Food and Agriculture Organization (FAO) of the United Nations; Rome, Italy: 2020. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. [DOI] [Google Scholar]
- 19.Munguti J., Odeke-Iteba J. In: Catfish, Atamanalp M., editors. United Kingdom; London: 2022. Advances in african catfish (Clarias gariepinus) seed-production techniques in Kenya. 1-11. [DOI] [Google Scholar]
- 20.De Silva S.S., Phuong N.T. Striped catfish farming in the Mekong Delta, Vietnam: a tumultuous path to a global success. Rev. Aquacult. 2011;3:45–73. doi: 10.1111/j.1753-5131.2011.01046.x. [DOI] [Google Scholar]
- 21.Marttin F., de Graaf G. 2023. Poverty Alleviation through Fish Culture: Homestead Cafish Culture in Bangladesh.https://www.fao.org/3/Y2419e/y2419e06.htm 17th April 2023. [Google Scholar]
- 22.Endut A., Lananan F., Hamid S.H.A., Jusoh A., Nik W.N.W. Balancing of nutrient uptake by water spinach (Ipomoea aquatica) and mustard green (Brassica juncea) with nutrient production by African catfish (Clarias gariepinus) in scaling aquaponic recirculation system. Desalination Water Treat. 2016;57(60):29531–29540. doi: 10.1080/19443994.2016.1184593. [DOI] [Google Scholar]
- 23.Khatib M.A.M., Mat Jais A.M. A brief overview of the integrated fish farming of three commercially popular fish species (snakehead, Tilapia and catfish) in Malaysia. Malaysian Journal of Applied Sciences. 2021;6(2):105–112. doi: 10.37231/myjas.2021.6.2.301. [DOI] [Google Scholar]
- 24.Ibrahim A., Adetola J.O., Kolawole A.E., Yusuf A.A. Natural occurrence of Diplostomum spp. in farm-raised African catfish (Clarias gariepinus) from Oyo state, Nigeria. International Journal of Veterinary Science and Medicine. 2016;4:41–44. doi: 10.1016/j.ijvsm.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Afolabi O.J., Olususi F.C., Odeyemi O.O. Comparative study of African catfish parasites from cultured and natural habitats. Bull. Natl. Res. Cent. 2020;44:163. doi: 10.1186/s42269-020-00419-4. [DOI] [Google Scholar]
- 26.Rapatsa M., Moyo N. Aquaculture Nutrition; 2022. A Review and Meta-Analysis of the Effects of Replacing Fishmeal with Insect Meals on Growth of Tilapias and Sharptooth Catfish. 2022. [DOI] [Google Scholar]
- 27.Xiong Y., Yu G., Wang J., Wu J., Wang D., He Y., Mei J. Effects of feeding rate and dietary protein levels on the breeding performance of female yellow catfish (Pelteobagrus fulvidraco) Aquacult. 2022;53(1) doi: 10.1111/are.15570. [DOI] [Google Scholar]
- 28.Araujo G.S., Silva J.W.A., da-Cotas J., Pereira L. Fish farming techniques: current situation and trends. J. Mar. Sci. Eng. 2022;10(11) doi: 10.3390/jmse10111598. [DOI] [Google Scholar]
- 29.Xu L., Zhao M., Ryu J.H., Hayman E.S., Fairgrieve W.T., Zohar Y., Luckenbach J.A., Wong T.T. Reproductive sterility in aquaculture: a review of induction methods and an emerging approach with application to Pacific Northwest finfish species. Rev. Aquacult. 2023;15(1) doi: 10.1111/raq.12712. [DOI] [Google Scholar]
- 30.Coogan M., Alston V., Su B., Khalil K., Elaswad A., Khan M., Johnson A., Xing D., Li S., Wang J., Simora R.M. Improved growth and high inheritance of melanocortin-4 receptor (mc4r) mutation in CRISPR/Cas-9 gene-edited Channel Catfish, Ictalurus punctatus. Mar. Biotechnol. 2022;24(5) doi: 10.1007/s10126-022-10146-8. [DOI] [PubMed] [Google Scholar]
- 31.Yang L., Sun N., Zeng H., Wang Y., Chen W., Ding Z., Liu Y., Wang J., Meng M., Shen Y., Kang J., Ma X., Lv W., Chen J., Meyer A., Guo B., He S. Science China Life Sciences; 2023. Enlarged Fins of Tibetan Catfish Provide New Evidence of Adaptation to High Plateau. [DOI] [PubMed] [Google Scholar]
- 32.Kaiser F., Schlachter M., Schulz C., Figueiredo-Silva C. Dietary supplementation with chromium DL-methionine enhances growth performance of african catfish (Clarias gariepinus) Aquacult. Nutr. 2023 doi: 10.1155/2023/7092657. 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen C. Science mapping: a systematic review of the literature. Journal of Data and Information Science. 2017;2:1–40. doi: 10.1515/jdis-2017-0006. [DOI] [Google Scholar]
- 34.Chen C., Song M. Visualizing a field of research: a methodology of systematic scientometric reviews. PLoS One. 2019;14:10. doi: 10.1371/journal.pone.0223994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C. Lean Publishing; Victoria, Canada: 2021. How to Use CiteSpace (Advance) p. 137.http://leanpub.com/howtousecitespace [Google Scholar]
- 36.Miller S.M., Mitchell M.A. In: Manual of Exotic Pet Practice. Mitchell M.A., Tully T.N., editors. Amsterdam; Netherlands: 2009. Ornamental fish; pp. 39–72. [DOI] [Google Scholar]
- 37.Jin Y., Liu S., Yuan Z., Yang Y., Tan S., Liu Z. In: Genomics in Aquaculture. MacKenzie S., Jentoft S., editors. Academic Press; Massachusetts, USA: 2016. Catfish genomic studies: progress and perspectives. [DOI] [Google Scholar]
- 38.Topic-Popovic N., Strunjak‐Perovic I., Coz‐Rakovac R., Barisic J., Jadan M., Persin Berakovic A., Sauerborn-Klobucar R. Tricaine methane‐sulfonate (MS‐222) application in fish anaesthesia. J. Appl. Ichthyol. 2012;28(4):553–564. doi: 10.1111/j.1439-0426.2012.01950.x. [DOI] [Google Scholar]
- 39.Gökçek K., Öğretmen F., Kanyilmaz M. Efficacy of clove oil, 2-phenoxyethanol and benzocaine on European catfish, Silurus glanis linnaeus, 1758. Turk. J. Fish. Aquat. Sci. 2017;17(1):129–133. doi: 10.4194/1303-2712-v17_1_15. [DOI] [Google Scholar]
- 40.Brijs J., Sundell E., Hjelmstedt P., Berg C., Senčić I., Sandblom E., Axelsson M., Lines J., Bouwsema J., Ellis M., Saxer A. Humane slaughter of African sharptooth catfish (Clarias gariepinus): effects of various stunning methods on brain function. Aquaculture. 2021;531 doi: 10.1016/j.aquaculture.2020.735887. [DOI] [Google Scholar]
- 41.Neiffer D.L., Stamper M.A. Fish sedation, anesthesia, analgesia, and euthanasia: considerations, methods, and types of drugs. ILAR J. 2009;50(4):343–360. doi: 10.1093/ilar.50.4.343. [DOI] [PubMed] [Google Scholar]
- 42.Stehly G.R., Gingerich W.H. Evaluation of AQUI‐STM (efficacy and minimum toxic concentration) as a fish anaesthetic/sedative for public aquaculture in the United States. Aquacult. Res. 1999;30(5):365–372. doi: 10.1046/j.1365-2109.1999.00339.x. [DOI] [Google Scholar]
- 43.Small B.C., Chatakondi N. Routine measures of stress are reduced in mature channel catfish during and after AQUI-S anesthesia and recovery. N. Am. J. Aquacult. 2005;67(1):72–78. doi: 10.1577/FA04-028.1. [DOI] [Google Scholar]
- 44.Wang J., Xiong G., Bai C., Liao T. Anesthetic efficacy of two plant phenolics and the physiological response of juvenile Ictalurus punctatus to simulated transport. Aquaculture. 2021;538 doi: 10.1016/j.aquaculture.2021.736566. [DOI] [Google Scholar]
- 45.Small B.C. Anesthetic efficacy of metomidate and comparison of plasma cortisol responses to tricaine methanesulfonate, quinaldine and clove oil anesthetized channel catfish Ictalurus punctatus. Aquaculture. 2003;218(1–4):177–185. doi: 10.1016/S0044-8486(02)00302-2. [DOI] [Google Scholar]
- 46.Trajano E. Population ecology of Trichomycterus itacarambiensis, a cave catfish from eastern Brazil (Siluriformes, Trichomycteridae) Environ. Biol. Fish. 1997;50(4):357–369. doi: 10.1023/A:1007366119261. [DOI] [PubMed] [Google Scholar]
- 47.Thoni R.J., Gurung D.B. Parachiloglanis bhutanensis, a new species of torrent catfish (Siluriformes: sisoridae) from Bhutan. Zootaxa. 2014;3869(3):306–312. doi: 10.11646/zootaxa.3869.3.5. [DOI] [PubMed] [Google Scholar]
- 48.Mazungula D.N., Chakona A. An integrative taxonomic review of the Natal Mountain catfish, Amphilius natalensis Boulenger 1917 (Siluriformes, Amphiliidae), with description of four new species. J. Fish. Biol. 2021;99(1):219–239. doi: 10.1111/jfb.14714. [DOI] [PubMed] [Google Scholar]
- 49.Reis R.E., Pereira E.H., Armbruster J.W. Delturinae, a new loricariid catfish subfamily (Teleostei, Siluriformes), with revisions of Delturus and Hemipsilichthys. Zool. J. Linn. Soc. 2006;147(2):277–299. doi: 10.1111/j.1096-3642.2006.00229.x. [DOI] [Google Scholar]
- 50.Fine M.L., Friel J.P., McElroy D., King C.B., Loesser K.E., Newton S. Pectoral spine locking and sound production in the channel catfish Ictalurus punctatus. Copeia. 1997:777–790. doi: 10.2307/1447295. [DOI] [Google Scholar]
- 51.Diogo R., Chardon M., Vandewalle P. Osteology and myology of the cephalic region and pectoral girdle of the Chinese catfish Cranoglanis bouderius, with a discussion on the autapomorphies and phylogenetic relationships of the Cranoglanididae (Teleostei: siluriformes) J. Morphol. 2002;253(3):229–242. doi: 10.1002/jmor.10000. [DOI] [PubMed] [Google Scholar]
- 52.Bosher B.T., Newton S.H., Fine M.L. The spines of the channel catfish, Ictalurus punctatus, as an anti‐predator adaptation: an experimental study. Ethology. 2006;112(2):188–195. doi: 10.1111/j.1439-0310.2006.01146.x. [DOI] [Google Scholar]
- 53.Shkil F., Kapitanova D., Borisov V., Veretennikov N., Roux N., Laudet V. Direct development of the catfish pectoral fin: an alternative pectoral fin pattern of teleosts. Dev. Dynam. 2022;251(11):1816–1833. doi: 10.1002/dvdy.509. [DOI] [PubMed] [Google Scholar]
- 54.Bosma P.T., Rebers F.E., Dijk W.V., Willems P.H., Goos H.J.T., Schulz R.W. Inhibitory and stimulatory interactions between endogenous gonadotropin-releasing hormones in the African catfish (Clarias gariepinus) Biol. Reprod. 2000;62(3):731–738. doi: 10.1095/biolreprod62.3.731. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar S., Subhedar N. β-endorphin and gonadotropin-releasing hormone in the forebrain and pituitary of the female catfish, Clarias batrachus: double-immunolabeling study. Gen. Comp. Endocrinol. 2000;118(1):39–47. doi: 10.1006/gcen.1999.7437. [DOI] [PubMed] [Google Scholar]
- 56.Dubois E.A., Slob S., Zandbergen M.A., Peute J., Goos H.T. Gonadal steroids and the maturation of the species-specific gonadotropin-releasing hormone system in brain and pituitary of the male African catfish (Clarias gariepinus) Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2001;129(2–3):381–387. doi: 10.1016/S1096-4959(01)00328-1. [DOI] [PubMed] [Google Scholar]
- 57.Schulz R.W., Bosma P.T., Zandbergen M.A., Van der Sanden M.C., Van Dijk W., Peute J., Bogerd J., Goos H.J. Two gonadotropin-releasing hormones in the African catfish, Clarias gariepinus: localization, pituitary receptor binding, and gonadotropin release activity. Endocrinology. 1993;133(4):1569–1577. doi: 10.1210/en.133.4.1569. [DOI] [PubMed] [Google Scholar]
- 58.Bogerd J., Diepenbroek W.B., Hund E., van Oosterhout F., Teves A.C., Leurs R., Blomenrohr M. Two gonadotropin-releasing hormone receptors in the African catfish: no differences in ligand selectivity, but differences in tissue distribution. Endocrinology. 2002;143(12):4673–4682. doi: 10.1210/en.2002-220578. [DOI] [PubMed] [Google Scholar]
- 59.Wosiacki W.B. New species of the catfish genus Trichomycterus (Siluriformes, Trichomycteridae) from the headwaters of the rio São Francisco basin, Brazil. Zootaxa. 2004;592(1):1–12. doi: 10.11646/ZOOTAXA.592.1.1. [DOI] [Google Scholar]
- 60.Carvalho T.P., Cardoso A.R., Friel J.P., Reis R.E. Two new species of the banjo catfish Bunocephalus Knerii (Siluriformes: aspredinidae) from the upper and middle rio São Francisco basins, Brazil. Neotrop. Ichthyol. 2015;13:499–512. doi: 10.1590/1982-0224-20140152. [DOI] [Google Scholar]
- 61.Penido I.S., Pessali T.C., Zawadzki C.H. When destruction comes first: two new species of Hypostomus Lacépède, 1803 (Siluriformes: Loricariidae) from a deeply‐impacted river in the Rio São Francisco basin in Brazil. J. Fish. Biol. 2021;98(5):1371–1384. doi: 10.1111/jfb.14674. [DOI] [PubMed] [Google Scholar]
- 62.Quevedo R., Reis R.E. Pogonopoma obscurum: a new species of loricariid catfish (Siluriformes: Loricariidae) from southern Brazil, with comments on the genus Pogonopoma. Copeia. 2002;2002(2):402–410. doi: 10.1643/0045-8511(2002)002[0402:POANSO]2.0.CO. 2. [DOI] [Google Scholar]
- 63.Klesius P.H., Shoemaker C.A. Development and use of modified live Edwardsiella ictaluri vaccine against enteric septicemia of catfish. Advances in veterinary medicine. 1999;41:523–537. doi: 10.1016/s0065-3519(99)80039-1. [DOI] [PubMed] [Google Scholar]
- 64.Lim C., Klesius P.H., Li M.H., Robinson E.H. Interaction between dietary levels of iron and vitamin C on growth, hematology, immune response and resistance of channel catfish (Ictalurus punctatus) to Edwardsiella ictaluri challenge. Aquaculture. 2000;185(3–4):313–327. doi: 10.1016/S0044-8486(99)00352-X. [DOI] [Google Scholar]
- 65.Welker T.L., Lim C., Yildirim‐Aksoy M., Shelby R., Klesius P.H. Immune response and resistance to stress and Edwardsiella ictaluri challenge in channel catfish, Ictalurus punctatus, fed diets containing commercial whole‐cell yeast or yeast subcomponents. Journal of the world Aquaculture Society. 2007;38(1):24–35. doi: 10.1111/j.1749-7345.2006.00070.x. [DOI] [Google Scholar]
- 66.Russo R., Shoemaker C.A., Panangala V.S., Klesius P.H. In vitro and in vivo interaction of macrophages from vaccinated and non-vaccinated channel catfish (Ictalurus punctatus) to Edwardsiella ictaluri. Fish Shellfish Immunol. 2009;26(3):543–552. doi: 10.1016/j.fsi.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 67.Shoemaker C.A., Klesius P.H., Evans J.J. In ovo methods for utilizing the modified live Edwardsiella ictaluri vaccine against enteric septicemia in channel catfish. Aquaculture. 2002;203(3–4):221–227. doi: 10.1016/S0044-8486(01)00631-7. [DOI] [Google Scholar]
- 68.Martins M.L., Xu D.H., Shoemaker C.A., Klesius P.H. Temperature effects on immune response and hematological parameters of channel catfish Ictalurus punctatus vaccinated with live theronts of Ichthyophthirius multifiliis. Fish Shellfish Immunol. 2011;31(6):774–780. doi: 10.1016/j.fsi.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 69.Lange M.D., Webster C.D. The effect of temperature on the mucosal IgM antibody response to DNP-KLH in channel catfish (Ictalurus punctatus) Fish Shellfish Immunol. 2017;70:493–497. doi: 10.1016/j.fsi.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 70.Bly J.E., Clem L.W. Temperature-mediated processes in teleost immunity: in vitro immunosuppression induced by in vivo low temperature in channel catfish. Vet. Immunol. Immunopathol. 1991;28(3–4):365–377. doi: 10.1016/0165-2427(91)90127-X. [DOI] [PubMed] [Google Scholar]
- 71.Singh S.P., Sharma J.G., Ahmad T., Chakrabarti R. Effect of water temperature on the physiological responses of Asian catfish Clarias batrachus (Linnaeus 1758) Asian Fish Sci. 2013;26(1):26–38. [Google Scholar]
- 72.Miyamoto T., Restrepo D., Cragoe E.J., Teeter J.H. IP 3-and cAMP-induced responses in isolated olfactory receptor neurons from the channel catfish. J. Membr. Biol. 1992;127:173–183. doi: 10.1007/BF00231505. [DOI] [PubMed] [Google Scholar]
- 73.Dagosta F.C., De Pinna M. The fishes of the Amazon: distribution and biogeographical patterns, with a comprehensive list of species. Bull. Am. Mus. Nat. Hist. 2019;2019(431):1–163. doi: 10.1206/0003-0090.431.1.1. [DOI] [Google Scholar]
- 74.Dos Reis R.B., Frota A., Depra G.D.C., Ota R.R., Da Graca W.J. Freshwater fishes from Paraná State, Brazil: an annotated list, with comments on biogeographic patterns, threats, and future perspectives. Zootaxa. 2020;4868(4):451–494. doi: 10.11646/ZOOTAXA.4868.4.1. [DOI] [PubMed] [Google Scholar]
- 75.Hegde S., Kumar G., Engle C., Hanson T., Roy L.A., Cheatham M., Avery J., Aarattuthodiyil S., van Senten J., Johnson J., Wise D. Technological progress in the US catfish industry. J. World Aquacult. Soc. 2022;53(2):367–383. doi: 10.1111/jwas.12877. [DOI] [Google Scholar]
- 76.Day J.J., Steell E.M., Vigliotta T.R., Withey L.A., Bills R., Friel J.P., Genner M.J., Stiassny M.L. Exceptional levels of species discovery ameliorate inferences of the biogeography and diversification of an Afrotropical catfish family. Mol. Phylogenet. Evol. 2023;182 doi: 10.1016/j.ympev.2023.107754. [DOI] [PubMed] [Google Scholar]
- 77.Elibol-Flemming B., Waldbieser G.C., Wolters W.R., Boyle C.R., Hanson L.A. Expression analysis of selected immune-relevant genes in channel catfish during Edwardsiella ictaluri infection. J. Aquat. Anim. Health. 2009;21(1):23–35. doi: 10.1577/H08-009.1. [DOI] [PubMed] [Google Scholar]
- 78.Liu H., Peatman E., Wang W., Abernathy J., Liu S., Kucuktas H., Terhune J., Xu D.H., Klesius P., Liu Z. Molecular responses of ceruloplasmin to Edwardsiella ictaluri infection and iron overload in channel catfish (Ictalurus punctatus) Fish Shellfish Immunol. 2011;30(3):992–997. doi: 10.1016/j.fsi.2010.12.033. [DOI] [PubMed] [Google Scholar]
- 79.Li C., Zhang Y., Wang R., Lu J., Nandi S., Mohanty S., Terhune J., Liu Z., Peatman E. RNA-seq analysis of mucosal immune responses reveals signatures of intestinal barrier disruption and pathogen entry following Edwardsiella ictaluri infection in channel catfish, Ictalurus punctatus. Fish Shellfish Immunol. 2012;32(5):816–827. doi: 10.1016/j.fsi.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 80.Yang Q., Pan Y.L., Wang K.Y., Wang J., He Y., Wang E.L., Liu T., Geng Y., Chen D.F., Huang X.L. OmpN, outer membrane proteins of Edwardsiella ictaluri are potential vaccine candidates for channel catfish (Ictalurus punctatus) Mol. Immunol. 2016;78:1–8. doi: 10.1016/j.molimm.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 81.Li M.H., Robinson E.H., Oberle D.F., Lucas P.M. Effects of various corn distillers by‐products on growth, feed efficiency, and body composition of channel catfish, Ictalurus punctatus. Aquacult. Nutr. 2010;16(2):188–193. doi: 10.1111/j.1365-2095.2009.00650.x. [DOI] [Google Scholar]
- 82.Tang Q., Wang C., Xie C., Jin J., Huang Y. Dietary available phosphorus affected growth performance, body composition, and hepatic antioxidant property of juvenile yellow catfish Pelteobagrus fulvidraco. Sci. World J. 2012 doi: 10.1100/2012/987570. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hung L.T., Binh V.T.T., Thanh Truc N.T., Tham L.H., Ngoc Tran T. Effects of dietary protein and lipid levels on growth, feed utilization and body composition in red‐tailed catfish juveniles (Hemibagrus wyckioides, Chaux & Fang 1949) Aquacult. Nutr. 2017;23(2):367–374. doi: 10.1111/anu.12401. [DOI] [Google Scholar]
- 84.Dong G.F., Yang Y.O., Yao F., Chen L., Yue D.D., Yu D.H., Huang F., Liu J., Liu L.H. Growth performance and whole‐body composition of yellow catfish (Pelteobagrus fulvidraco Richardson) under feeding restriction. Aquacult. Nutr. 2017;23(1):101–110. doi: 10.1111/anu.12366. [DOI] [Google Scholar]
- 85.Asagba S.O., Eriyamremu G.E., Igberaese M.E. Bioaccumulation of cadmium and its biochemical effect on selected tissues of the catfish (Clarias gariepinus) Fish Physiol. Biochem. 2008;34:61–69. doi: 10.1007/s10695-007-9147-4. [DOI] [PubMed] [Google Scholar]
- 86.Opasola O.A., Adeolu A.T., Iyanda A.Y., Adewoye S.O., Olawale S.A. Bioaccumulation of heavy metals by Clarias gariepinus (african catfish) in asa river, ilorin, kwara state. Journal of Health and Pollution. 2019;9(21) doi: 10.5696/2156-9614-9.21.190303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Alvarado C., Cortez-Valladolid D.M., Herrera-López E.J., Godínez X., Ramírez J.M. Metal bioaccumulation by carp and catfish cultured in lake chapala, and weekly intake assessment. Appl. Sci. 2021;11(13):6087. doi: 10.3390/app11136087. [DOI] [Google Scholar]
- 88.Taufek N., Harmin S.A., Christianus A. Effect of chicken gonadotropin-releasing hormone (cGnRH-II) on plasma steroid hormone, maturation and ovulation in African catfish, Clarias gariepinus (Burchell) Afr. J. Biotechnol. 2009;8(23) doi: 10.5897/AJB09.1195. [DOI] [Google Scholar]
- 89.Am Shokr E. Effect of gonadotropin releasing hormone injection on physiological changes and reproductive hormones in Clarias gariepinus. Egyptian Journal of Aquatic Biology and Fisheries. 2020;24(1):119–129. doi: 10.21608/ejabf.2020.69364. [DOI] [Google Scholar]
- 90.Abdel-Latif H.M., Shukry M., Saad M.F., Mohamed N.A., Nowosad J., Kucharczyk D. Effects of GnRHa and hCG with or without dopamine receptor antagonists on the spawning efficiency of African catfish (Clarias gariepinus) reared in hatchery conditions. Anim. Reprod. Sci. 2021;231 doi: 10.31018/jans.v13i2.2695. [DOI] [PubMed] [Google Scholar]
- 91.de Lima Silva L., Parodi T.V., Reckziegel P., de Oliveira Garcia V., Bürger M.E., Baldisserotto B., Malmann C.A., Pereira A.M., Heinzmann B.M. Essential oil of Ocimum gratissimum L.: anesthetic effects, mechanism of action and tolerance in silver catfish, Rhamdia quelen. Aquaculture. 2012;350:91–97. doi: 10.1590/1414-431X20133013. [DOI] [Google Scholar]
- 92.Silva L.L., Garlet Q.I., Benovit S.C., Dolci G., Mallmann C.A., Bürger M.E., Baldisserotto B., Longhi S.J., Heinzmann B.M. Sedative and anesthetic activities of the essential oils of Hyptis mutabilis (Rich.) Briq. and their isolated components in silver catfish (Rhamdia quelen) Braz. J. Med. Biol. Res. 2013;46:771–779. doi: 10.1016/j.aquaculture.2012.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dos Santos A.C., Junior G.B., Zago D.C., Zeppenfeld C.C., da Silva D.T., Heinzmann B.M., Baldisserotto B., da Cunha, Ma Anesthesia and anesthetic action mechanism of essential oils of Aloysia triphylla and Cymbopogon flexuosus in silver catfish (Rhamdia quelen) Vet. Anaesth. Analg. 2017;44(1):106–113. doi: 10.1111/vaa.12386. [DOI] [PubMed] [Google Scholar]
- 94.Aydın B., Barbas L.A.L. Sedative and anesthetic properties of essential oils and their active compounds in fish: a review. Aquaculture. 2020;520 doi: 10.1016/j.aquaculture.2020.734999. [DOI] [Google Scholar]
- 95.Hansen A., Rolen S.H., Anderson K., Morita Y., Caprio J., Finger T.E. Correlation between olfactory receptor cell type and function in the channel catfish. J. Neurosci. 2003;23(28):9328–9339. doi: 10.1523/JNEUROSCI.23-28-09328.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Derby C.D., Sorensen P.W. Neural processing, perception, and behavioral responses to natural chemical stimuli by fish and crustaceans. J. Chem. Ecol. 2008;34:898–914. doi: 10.1007/s10886-008-9489-0. [DOI] [PubMed] [Google Scholar]
- 97.Dunham R.A., Elaswad A. Catfish biology and farming. Annual review of animal biosciences. 2018;6:305–325. doi: 10.1146/annurev-animal-030117-014646. [DOI] [PubMed] [Google Scholar]
- 98.Majhi S.S., Singh S.K., Biswas P., Debbarma R., Parhi J., Ngasotter S., Waikhom G., Meena D.K., Devi A.G., Mahanand S.S., Xavier K.M. Effect of stocking density on growth, water quality changes and cost efficiency of butter catfish (ompokbimaculatus) during seed rearing in a biofloc system. Fishes. 2023;8(2):61. doi: 10.3390/fishes8020061. [DOI] [Google Scholar]
- 99.Refaey M.M., Tian X., Tang R., Li D. Changes in physiological responses, muscular composition and flesh quality of channel catfish Ictalurus punctatus suffering from transport stress. Aquaculture. 2017;478:9–15. doi: 10.1016/j.aquaculture.2017.01.026. [DOI] [Google Scholar]
- 100.Zheng T., Song Z., Tao Y., Qiang J., Ma J., Lu S., Xu P. Transport stress induces innate immunity responses through TLR and NLR signaling pathways and increases mucus cell number in gills of hybrid yellow catfish (Tachysurus fulvidraco♀× Pseudobagrus vachellii♂) Fish Shellfish Immunol. 2022;127:166–175. doi: 10.1016/j.fsi.2022.06.015. [DOI] [PubMed] [Google Scholar]
- 101.Bowker J.D., Trushenski J.T., Glover D.C., Carty D.G., Wandelear N. Sedative options for fish research: a brief review with new data on sedation of warm-, cool-, and coldwater fishes and recommendations for the drug approval process. Rev. Fish Biol. Fish. 2015;25:147–163. doi: 10.1007/s11160-014-9374-6. [DOI] [Google Scholar]
- 102.Öğretmen F., Gökçek K. Comparative efficacy of three anesthetic agents on juvenile African catfish, Clarias gariepinus (Burchell, 1822) Turk. J. Fish. Aquat. Sci. 2013;13(1) doi: 10.4194/1303-2712-v13_1_07. [DOI] [Google Scholar]
- 103.Akinrotimi O.A., Gabriel U.U., Edun O.M. The efficacy of clove seed extracts as an anaesthetic agent and its effect on haematological parameters of African catfish (Clarias gariepinus) International Journal Aquaculture Fishery Science. 2015;1(2):42–47. doi: 10.17352/2455-8400.000008. [DOI] [Google Scholar]
- 104.Cupp A.R., Hartleb C.F., Fredricks K.T., Gaikowski M.P. Effectiveness of eugenol sedation to reduce the metabolic rates of cool and warm water fish at high loading densities. Aquacult. Res. 2016;47(1):234–242. doi: 10.1111/are.1248. [DOI] [Google Scholar]
- 105.Zeng X., Dong H., Wu J., Wang W., Duan Y., Chen J., Zhang J. Essential oil of Magnolia denudata is an effective anesthetic for spotted seabass (Lateolabrax maculatus): a test of its effect on blood biochemistry, physiology, and gill morphology. Fish Physiol. Biochem. 2022;48(5):1349–1363. doi: 10.1007/s10695-022-01124-x. [DOI] [PubMed] [Google Scholar]
- 106.Conte F.S. Stress and the welfare of cultured fish. Appl. Anim. Behav. Sci. 2004;86(3–4):205–223. doi: 10.1016/j.applanim.2004.02.003. [DOI] [Google Scholar]
- 107.Bowker J.D., Trushenski J.T., Glover D.C., Carty D.G., Wandelear N. Sedative options for fish research: a brief review with new data on sedation of warm-, cool-, and coldwater fishes and recommendations for the drug approval process. Rev. Fish Biol. Fish. 2015;25:147–163. doi: 10.1007/s11160014-9374-6. [DOI] [Google Scholar]
- 108.Ojelade O.C., Iyasere O., Durosaro S., Ikililu, ABULRAHEEM. Akinde A., Ibukun, Ishola . 2022. Behavioural and Physiological Assessment of Juveniles of African Catfish (Clarias gariepinus) Experimentally Challenged with Paraffin Oil. [DOI] [Google Scholar]
- 109.dos Santos Teixeira N., Marques L.S., Rodrigues R.B., Gusso D., Pinheiro G.T., Machado T.L.F., Streit Jr, Dp Effects of anesthetic MS-222 on stress and reproduction of South American silver catfish (Rhamdia quelen) males. Anim. Reprod. Sci. 2021;225 doi: 10.1016/j.anireprosci.2020.106669. [DOI] [PubMed] [Google Scholar]
- 110.Williams T.D., Readman G.D., Owen S.F. Key issues concerning environmental enrichment for laboratory-held fish species. Lab. Anim. 2009;43(2):107–120. doi: 10.1258/la.2007.007023. [DOI] [PubMed] [Google Scholar]
- 111.Näslund J., Johnsson J.I. Environmental enrichment for fish in captive environments: effects of physical structures and substrates. Fish Fish. 2016;17(1):1–30. doi: 10.1111/faf.12088. [DOI] [Google Scholar]
- 112.Arechavala‐Lopez P., Cabrera‐Álvarez M.J., Maia C.M., Saraiva J.L. Environmental enrichment in fish aquaculture: a review of fundamental and practical aspects. Rev. Aquacult. 2022;14(2):704–728. doi: 10.1111/raq.12620. [DOI] [Google Scholar]
- 113.Sullivan J.P., Lundberg J.G., Hardman M. A phylogenetic analysis of the major groups of catfishes (Teleostei: siluriformes) using rag1 and rag2 nuclear gene sequences. Mol. Phylogenet. Evol. 2006;41(3):636–662. doi: 10.1016/j.ympev.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 114.Lujan N.K., Armbruster J.W., Lovejoy N.R., López-Fernández H. Multilocus molecular phylogeny of the suckermouth armored catfishes (Siluriformes: Loricariidae) with a focus on subfamily Hypostominae. Mol. Phylogenet. Evol. 2015;82:269–288. doi: 10.1016/j.ympev.2014.08.020. [DOI] [PubMed] [Google Scholar]
- 115.Scaranto B.M.S., Ribolli J., Zaniboni‐Filho E. DNA barcoding reveals blend of silver catfish Rhamdia species from fish farms in Southern Brazil. Aquacult. Res. 2018;49(5):1907–1913. doi: 10.1111/are.13646. [DOI] [Google Scholar]
- 116.de Borba R.S., Mariotto S., Centofante L., Henrique Zawadzki C., Pasquali Parise-Maltempi P. Molecular discrimination of Ancistrus lineages (Siluriformes: Loricariidae) using barcode DNA tool. Mitochondrial DNA Part A. 2019;30(4):602–608. doi: 10.1080/24701394.2019.1597071. [DOI] [PubMed] [Google Scholar]
- 117.Carvalho D.C., Neto D.A., Brasil B.S., Oliveira D.A. DNA barcoding unveils a high rate of mislabeling in a commercial freshwater catfish from Brazil. Mitochondrial DNA. 2011;22(sup1):97–105. doi: 10.3109/19401736.2011.588219. [DOI] [PubMed] [Google Scholar]
- 118.Li X., Dao W., Zhou W. Redefinition of the Glyptonsternine genus Pareuchiloglanis (Teleostei: sisoridae), with descriptions of three new genera. J. Fish. Biol. 2023;102(1):53–74. doi: 10.1007/s00343-021-1319-z. [DOI] [PubMed] [Google Scholar]
- 119.Shibatta O.A., Vari R.P. A new genus of Neotropical rheophilic catfishes, with four new species (Teleostei: siluriformes: Pseudopimelodidae) Neotrop. Ichthyol. 2017;15 doi: 10.1590/1982-0224-20160132. [DOI] [Google Scholar]
- 120.Rodiles-Hernández R., Hendrickson D.A., Lundberg J.G., Humphries J.M. Lacantunia enigmatica (Teleostei: siluriformes) a new and phylogenetically puzzling freshwater fish from Mesoamerica. Zootaxa. 2005;1000(1):1–24. doi: 10.11646/ZOOTAXA.1000.1.1. [DOI] [Google Scholar]
- 121.Dias A.C., Zawadzki C.H. Hypostomus hermanni redescription and a new species of Hypostomus (siluriformes: Loricariidae) from upper paraná river basin, Brazil. Neotrop. Ichthyol. 2021;19 doi: 10.1590/1982-0224-20160132. [DOI] [Google Scholar]
- 122.Reis Roberto E., Pablo Lehmann A. A new genus of armored catfish (Siluriformes: Loricariidae) from the Greater Amazon, with a review of the species and description of five new species. Neotrop. Ichthyol. 2022;20 doi: 10.1590/1982-0224-2022-0002. [DOI] [Google Scholar]
- 123.Esmaeili H.R., Sayyadzadeh, GOLNAZ. Zarei F.A.T.A.H., Eagderi S., Mousavi-Sabet H. Mystus cyrusi, a new species of bagrid catfish (Teleostei: bagridae) from Middle East. Zootaxa. 2022;5099(3):325–343. doi: 10.11646/zootaxa.5099.3.2. [DOI] [PubMed] [Google Scholar]
- 124.Pinna M.D., Dagosta F.C.P. A taxonomic review of the vampire catfish genus Paracanthopoma Giltay, 1935 (Siluriformes, Trichomycteridae), with descriptions of nine new species and a revised diagnosis of the genus. Papéis Avulsos Zool. (São Paulo) 2022;62 doi: 10.11606/1807-0205/2022.62.072. [DOI] [Google Scholar]
- 125.Lehmann A.P., Lujan N.K., Reis R.E. A new species of armored catfish (Loricariidae: hypoptopomatinae) syntopic and superficially similar to Parotocinclus collinsae, from the Potaro River basin, Guyana. Ichthyology & Herpetology. 2022;110(1):69–76. doi: 10.1643/i2021065. [DOI] [Google Scholar]
- 126.Bernt M.J., Stiassny M.L. A new species of air-breathing catfish (clariidae: Clarias) from Salonga national Park, democratic republic of the Congo. Am. Mus. Novit. 2022;2022(3990):1–20. doi: 10.1206/3990.1. [DOI] [Google Scholar]
- 127.Vijayakrishnan B., Praveenraj J. Mystus irulu, a new species of bagrid catfish from the Western Ghats of Karnataka, India (Teleostei: bagridae) Zootaxa. 2022;5120(3):443–448. doi: 10.11646/ZOOTAXA.5120.3.10. [DOI] [PubMed] [Google Scholar]
- 128.Shangningam B., Kosygin L. Glyptothorax yuensis, a new species of sisorid catfish (Teleostei: sisoridae) from Myanmar. Zootaxa. 2022;5129(1):118–128. doi: 10.11646/zootaxa.5129.1.7. [DOI] [PubMed] [Google Scholar]
- 129.Singh M., Lalronunga S., Ramliana L. Integrative taxonomy peeps a new torrent catfish species of genus Amblyceps (Siluriformes: amblycipitidae) in Kaladan River of Mizoram, India. Mol. Biol. Rep. 2022;49(6):4565–4572. doi: 10.1007/s11033-022-07302-7. [DOI] [PubMed] [Google Scholar]
- 130.Wise A.L., LaFrentz B.R., Kelly A.M., Khoo L.H., Xu T., Liles M.R., Bruce T.J. A review of bacterial co-infections in farmed catfish: components, diagnostics, and treatment directions. Animals. 2021;11(11):3240. doi: 10.3390/ani11113240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Chen D.F., Wang K.Y., Geng Y., Wang J., Huang X.L., He M. Pathological changes in cultured channel catfish Ictalurus punctatus spontaneously infected with Streptococcus iniae. Diseases of aquatic organisms. 2011;95(3):203–208. doi: 10.3354/dao02354. [DOI] [PubMed] [Google Scholar]
- 132.Davis K.B., Griffin B.R., Gray W.L. Effect of dietary cortisol on resistance of channel catfish to infection by Ichthyopthirius multifiliis and channel catfish virus disease. Aquaculture. 2003;218(1–4):121–130. doi: 10.1016/S0044-8486(02)00630-0. [DOI] [Google Scholar]
- 133.Wise D.J., Griffin M.J., Terhune J.S., Pote L.M., Khoo L.H. Induction and evaluation of proliferative gill disease in channel catfish fingerlings. J. Aquat. Anim. Health. 2008;20(4):236–244. doi: 10.1577/H08-023.1. [DOI] [PubMed] [Google Scholar]
- 134.Zhou T., Yuan Z., Tan S., Jin Y., Yang Y., Shi H., Wang W., Niu D., Gao L., Jiang W., Gao D. A review of molecular responses of catfish to bacterial diseases and abiotic stresses. Front. Physiol. 2018;9:1113. doi: 10.3389/fphys.2018.01113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Hossain M.J., Sun D., McGarey D.J., Wrenn S., Alexander L.M., Martino M.E., Xing Y., Terhune J.S., Liles M.R. An Asian origin of virulent Aeromonas hydrophila responsible for disease epidemics in United States-farmed catfish. mBio. 2014;5(3) doi: 10.1128/mBio.00848-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bebak J., Wagner B., Burnes B., Hanson T. Farm size, seining practices, and salt use: risk factors for Aeromonas hydrophila outbreaks in farm-raised catfish, Alabama, USA. Prev. Vet. Med. 2015;118(1):161–168. doi: 10.1016/j.prevetmed.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 137.Grizzle J.M., Kiryu Y. Histopathology of gill, liver, and pancreas, and serum enzyme levels of channel catfish infected with Aeromonas hydrophila complex. J. Aquat. Anim. Health. 1993;5(1):36–50. doi: 10.1577/1548-8667(1993)005<0036:HOGLAP>2.3.CO. 2. [DOI] [Google Scholar]
- 138.Baumgartner W.A., Ford L., Hanson L. Lesions caused by virulent Aeromonas hydrophila in farmed catfish (Ictalurus punctatus and I. punctatus× I. furcatus) in Mississippi. J. Vet. Diagn. Invest. 2017;29(5):747–751. doi: 10.1177/1040638717708584. [DOI] [PubMed] [Google Scholar]
- 139.Shoemaker C.A., Olivares-Fuster O.S.C.A.R., Arias C.R., Klesius P.H. Flavobacterium columnare genomovar influences mortality in channel catfish (Ictalurus punctatus) Vet. Microbiol. 2008;127(3–4):353–359. doi: 10.1016/j.vetmic.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 140.Wahli T., Madsen L. Flavobacteria, a never-ending threat for fish: a review. Curr. Clin. Microbiol. Rep. 2018;5:26–37. doi: 10.1007/s40588-018-0086-x. [DOI] [Google Scholar]
- 141.Beck B.H., Peatman E. US-Japan Aquaculture Panel Symposium. 2014. Examining host-pathogen interactions at mucosal surfaces reveals novel molecular targets for columnaris disease intervention; p. 32. [Google Scholar]
- 142.Laanto E., Bamford J.K., Ravantti J.J., Sundberg L.R. The use of phage FCL-2 as an alternative to chemotherapy against columnaris disease in aquaculture. Front. Microbiol. 2015;6:829. doi: 10.3389/fmicb.2015.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mousa A.A., Ramachandran R., Ozdemir O., Karsi A., Abdelhamed H. Dietary trans-cinnamaldehyde improves oxidative stress response of channel catfish (Ictalurus punctatus) following Edwardsiella ictaluri infection. Aquaculture. 2021;532 doi: 10.1016/j.aquaculture.2020.735985. [DOI] [Google Scholar]
- 144.Shoemaker C.A., Klesius P.H., Drennan J.D., Evans J.J. Efficacy of a modified live Flavobacterium columnare vaccine in fish. Fish Shellfish Immunol. 2011;30(1):304–308. doi: 10.1016/j.fsi.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Lange M.D., Abernathy J., Farmer B.D. Evaluation of a recombinant Flavobacterium columnare DnaK protein vaccine as a means of protection against columnaris disease in channel catfish (Ictalurus punctatus) Front. Immunol. 2019;10:1175. doi: 10.3389/fimmu.2019.01175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Straus D.L., Farmer B.D., Beck B.H., Bosworth B.G., Torrans E.L., Tucker C.S. Water hardness influences Flavobacterium columnare pathogenesis in channel catfish. Aquaculture. 2015;435:252–256. doi: 10.1016/j.aquaculture.2014.10.003. [DOI] [Google Scholar]
- 147.Eissa I., Derwa H., Ismaiel M., El-Lamie M., Elsayed T. Recent advances in diagnosis of flavobacteriosis among Some freshwater fishes. Suez canal veterinary medical journal. SCVMJ. 2015;20(2):31–42. doi: 10.21608/SCVMJ.2015.64567. [DOI] [Google Scholar]
- 148.Mishra S., Seshagiri B., Rathod R., Sahoo S.N., Choudhary P., Patel S., Behera D.K., Ojha D.K., Jena A., Namburu P.K., Swain P. Recent advances in fish disease diagnosis, therapeutics, and vaccine development. Frontiers in Aquaculture Biotechnology. 2023:115–145. doi: 10.1016/B978-0-323-91240-2.00011-7. [DOI] [Google Scholar]
- 149.Panangala V.S., Shoemaker C.A., Van Santen V.L., Dybvig K., Klesius P.H. Multiplex-PCR for simultaneous detection of 3 bacterial fish pathogens, Flavobacterium columnare, Edwardsiella ictaluri, and Aeromonas hydrophila. Dis Aquat Organ. 2007;74(3):199–208. doi: 10.3354/dao074199.PMID:17465305. 2007 Mar 13. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors do not have permission to share data.