Abstract
Until now, many methods have been proposed to treat cancer, such as radiation therapy and drug therapy, but none of them have caused a complete cure for cancer. Heavy metal complexes such as cisplatin are among the compounds used as drugs in chemotherapy against cancer cells. These compounds cause cell death and have anti-cancer properties, but they have side effects. The biochemical mechanism of cisplatin is related to its interaction with DNA through covalent binding. To reduce the toxicity of metallodrugs, new complexes can be designed containing S, S- bidentate ligands such as diethyldithiocarbamate. Moreover, anti-cancer compounds probably interact with proteins, such as HSA, before passing the cancerous cell membrane and DNA as a target. So, the function of proteins and their stabilities are expected to change. In this research, the mode of binding of [Pt (bpy) (amyl.dtc)]NO3 complex with BSA was evaluated by various thermodynamic methods. Negative binding enthalpy and entropy changes amounts show that the connection between the Platinum compound and BSA occurs via the van Der Waals type of hydrogen bond. The negative Gibbs free energy change was obtained through isothermal titration, which showed interaction proceeds spontaneously. Moreover, the emission titration data showed that protein fluorescence quenching by platinum agent titration is static. Binding, quenching constants, and binding site number were obtained by the Stern-Volmer equation, and only one binding site was determined for this interaction. A Scatchard plot with a positive slope shows the Pt agent-BSA formation is proceeding positively cooperative. The kinetic study displayed that the absorption monitoring followed the second-order model. Finally, molecular docking simulation showed that the position of the Pt agent on protein is placed I under region II.
Keywords: Bovine serum albumin, Docking simulation, Anticancer Pt compound, Dithiocarbamate
1. Introduction
Cancer, which occurs with the uncontrolled growth and proliferation of cells in a part of the body, results from environmental factors and genetic disorders; today, it is considered the main cause of death in the world [1,2]. Cisplatin is one of the inorganic compounds still sold as an anti-cancer drug; However, in general, this class of drugs is associated with side effects such as gastrointestinal complications and has high treatment costs [3]. Although cisplatin has a good effect on some cancers (such as ovarian and stomach cancer), it has an irreversible destructive effect. It affects the kidneys, and its side effects include toxic effects on kidneys, hair loss, hemolytic anemia, nerve damage, and electrolyte imbalance, and its use is limited due to these toxic effects. Therefore, its performance can be improved by reducing the side effects and increasing the drug level [4,5]. Cisplatin can bond with biomolecules containing S atoms, which inactivates it and reduces toxicity [[6], [7], [8]]. So, many ligand and metal compounds have been synthesized and reported as anti-oxidants, anti-esterases, and anti-cancer agents [[9], [10], [11], [12]]. Therefore, using various metals in anti-cancer agents, like Pd, can lead to fewer side effects and toxicity than platinum compounds. Also, using compounds with sulfur-donating ligands, such as thiosulfate or diethyldithiocarbamate [6,13,14]. One of the most abundant proteins in plasma is albumin, whose role is to regulate blood osmotic pressure, and its interaction with small molecules provides useful information about the properties of the complex. The structure of this protein is spherical, which is responsible for binding and transporting various small ligands in the blood [15]. Bovine serum albumin is one of the proteins that has been studied extensively. BSA has been widely used as a typical protein to peruse the aggregation process of proteins [[16], [17], [18]]. Amyloid nanofibers obtained from bovine serum albumin have been used as one of the most important carriers of medicinal substances in chemotherapy, including cancer, and due to their high binding capacity, they are of great interest for both hydrophilic and hydrophobic drugs. BSA, as a carrier protein, has three homologous (II, I, and III) domains, (L9-L1) Loops, and two related to subdomains A and B [[19], [20], [21]].
Molecular docking studies are used in drug design and discovery and are important for understanding biological regulatory mechanisms. Since these interactions take place through a specific site on the surface of the macromolecule, to understand their function, we need information about ligand- or complex-protein formation, which can include information such as the number of sets of binding sites and how many binding sites there are in each set. In this study, [Pt(bpy)(amyl.dtc)]NO3 (Scheme 1) was selected as an anti-cancer agent, and its interaction with BSA was evaluated by using experimental spectroscopic methods (UV–Vis and fluorescence spectroscopy) based on the report [22], and theoretical docking simulation. The synthesis method and structural data have been presented in Ref. [23]. Cooperativity, the number of binding sites, the Stern-Volmer constant, and thermodynamic and kinetic parameters were evaluated by spectral methods. In addition, the molecule was optimized and analyzed to determine the binding ability and the mode of binding on BSA by a simulation study.
Scheme 1.

Structure of [Pt(bpy)(amyl.dtc)]NO3 complex.
2. Experimental section
2.1. Material and methods
Recently, [Pt(bpy)(amyl.dtc)]NO3 complex has been synthesized and reported as an anti-cancer drug [23]. All materials used in this study were purchased from Sigma-Aldrich (USA). Bovine serum albumin manufactured by Sigma A3782-100 MG was used to prepare BSA solution (1 mg/1 mL Tris buffer). The absorption monitoring was done between 200 and 700 nm by a PerkinElmer Accurately Lambda 25 spectrophotometer (USA). Also, all emissions were detected by a Scinco FS-2 fluorescence spectrophotometer (Korea).
2.2. Experimental bovine serum albumin binding
2.2.1. Isothermal titration study
To investigate bovine serum albumin-Pt agent formation, UV absorption was measured in the range of 200–400 nm [22,24]. The protein absorptions were monitored in the absence and presence of various dosages of the Pt complex at 27 and 37 °C.
2.2.2. Thermal stability study
In this step, Tm, the temperature at which half of BSA unfolded, was evaluated [25,26]. The absorption of both Solutions of BSA (2 mg/mL) and BSA-[Pt(bpy)(amyl.dtc)]NO3 were detected at 278 nm by the temperature increasing from 25 to 60 °C, separately, where the complex concentration is 1 mM.
2.2.3. Fluorescence spectroscopy
To record the fluorescence emission spectrum of BSA, the range of the fluorescence spectrum was adjusted between 200 and 700 nm (λex = 280 nm), and the width of the excitation and emission gap was set to 5 nm [[27], [28], [29]]. 1500 μl of 0.2 mg/mL BSA solution was placed in the cell, and its emission spectrum was followed by titration of 50 μL Pt agent (10−6 M) injection, and the emission spectrum was recorded.
2.3. BSA-Pt complex computer simulation
One of the promising technologies in the drug development process is computer-aided drug discovery [[30], [31], [32]]. The advanced AutoDock program is one of the most important molecular docking software at the international level, which is very useful in the discovery and rational development of pharmaceutical molecules. This software can calculate the number and type of chemical interactions between the ligand and the macromolecular receptor (proteins or nucleic acids) based on the binding free energy using force field functions. Before any simulation, the structure of the complex must be optimized. To get the most stable conformations of the Pt complex the structure-optimizing calculations were carried out by applying DFT/B3LYP method with 6–31G and LANL2DZ basis sets [33]. Pt (II) complex was used as a ligand, and BSA as a receptor in the molecular docking. BSA protein with PDB ID: 4F5S was downloaded as a PDB file from the protein database. Interaction of the BSA with Pt (II) complex was performed by the molecular docking program AutoDock 4.2.3 package. To perform molecular docking, the first box building was done with the maximum box size to perform blind docking, and by analyzing the results, the box size and the binding site of the complex to the protein were determined. The main docking and the final analysis were then performed.
3. Result and discussion
3.1. Binding isotherm of BSA to Pt-complex
Serum albumins are the carriers of numerous drugs. Because of sharp absorption at 280 nm [34], UV/Vis methods make it easy to study to obtain more information on drug-protein interactions. Fig. 1 shows the UV–vis monitoring of BSA in the attendance and absence of various concentrations of the [Pt(bpy)(amyl.dtc)]NO3 complex. The decreasing trend in the diagram (Fig. 1a and b) shows that a new complex protein is formed. By using the Amax diagram in terms of ʋ،(ʋ = [complex]b/[BSA]t), the values of L1/2 (the amount of the complex which can make half of BSA abnormal) were obtained. The less dosage leads to fewer side effects if this complex is used as a medicine in cancer treatment [35]. Different thermodynamic parameters such as the binding constant, Kb, and the standard binding Gibbs free energy changes, , can be obtained according to equations (1), (2)) [34]:
| (1) |
| (2) |
In equation (1), A0 is, the initial absorbance of free BSA, A∞ is the final absorbance of bound BSA at 280 nm, and A is the measured absorbance at particular complex concentrations (Fig. 2a and b). By drawing the plot of 1/A-A0 vs 1/[complex], Fig. 2a′ and 2b′, the binding constant was obtained. According to these graphs and the binding constant reported in Table 1, it can be concluded rising temperature leads to decreasing binding constant, and the larger binding constant shows a strong interaction between the Pt complex and BSA protein [31]. To compare this data with a clinical Pt drug, cisplatin, this investigation was done with Human Serum Albumin, HSA, at room temperature [35], reported in Fig. 3a and b, respectively. According to Fig. 2, Fig. 3, Kb can be obtained. With the help of ΔG°b and Kb values, other thermodynamic parameters were determined through the van't Hoff condition [36] (equations (3), (4))):
| (3) |
| (4) |
Fig. 1.
UV–Vis monitoring of BSA (15 M) interactions (-- -) by [Pt(bpy)(amyl.dtc)]NO3 titrating (4–40 μM) at (a) 27 and (b) 37 °C and in Tris buffer (pH = 7.4). The arrow illustrates the direction of change in intensity upon increasing the concentration of the complex.
Fig. 2.
BSA absorbance vs. ʋ and the linear plot of 1/ΔA vs. 1/[complex] at (a, a′) 27 and (b, b′) 37 °C.
Table 1.
Experimental half-saturation concentration L1/2 and thermodynamic parameters values of BSA-[Pt (bpy) (amyl.dtc)]NO3 and HSA-cisplatin formation.
| Complex | T (K) | [L]1/2 | Kb M−1)) |
(kJmol−1) | (kJmol−1) | (Jmol−1K−1) |
|---|---|---|---|---|---|---|
| [Pt (bpy) (amyl.dtc)]NO3 | 300 | 0.1 | 6778 | −22 | -62.1 | -133.6 |
| 310 | 0.11 | 3038 | −22.7 | −126.9 | ||
| Cisplatin | 298 | 0.18 | 1183 | −17.6 | – | – |
Fig. 3.
(a) HSA absorbance vs. ʋ at 25 °C and (b) the linear plot of 1/ΔA vs. 1/[cisplatin].
Thermodynamic parameters are presented in Table 1. According to these values, the type of connection can be determined [36]. The results display that the main forces driving the interaction are van der Waals and hydrogen binding forces [37].
3.2. Binding isotherm
The binding analysis of experimental data is done by binding isotherms and Scatchard diagrams, which are always associated with problems. Still, one fact that is very popular today is the concept of binding capacity [38]. The binding capacity can be gotten using equation (5) [38]:
| (5) |
Where ʋ is defined by equation (6) [22] as follows:
| (6) |
Therefore, considering the slope of the isotherm plot (Fig. 4), the binding capacity value can be obtained.
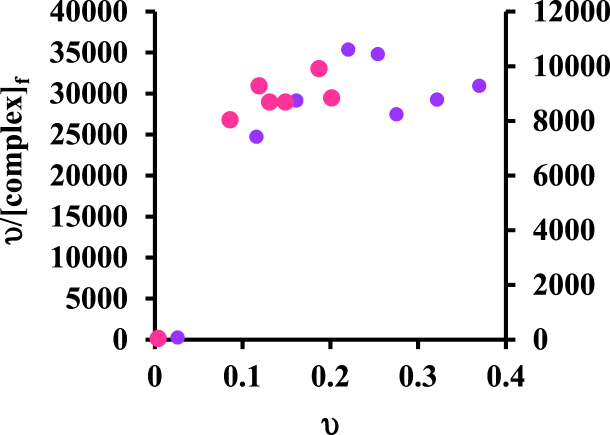
Fig. 4.
Binding isotherm of BSA with Pt-complex at 37 °C ( ) and 27 °C (
) and 27 °C ( ).
).
Since the binding isotherms drawn at two temperatures are single steps, this system has one binding set [38]. Equation (5) can be rewritten to the linear form (equation (7)) as follow:
| (7) |
In this expression, nH is called the Hill coefficient. According to this equation, if a system is identical and dependent, the diagram of RTθ/ʋ vs ʋ should be linear (Fig. 5a and b).
Fig. 5.
Changes of RTθ/ν in terms of ν for the interaction of BSA with [Pt(bpy)(amyl.dtc)]NO3 complex at (a) 27 and (b) 37 °C.
Due to the linearity of the binding capacity diagram, RTθ/ʋ in terms of ʋ, only one proper set of binding sites was observed on BSA for this complex [39].
3.3. Binding analysis with scatchard diagram
By drawing the Scatchard plot (υ/[L] vs υ), we can obtain main data on protein binding with drugs, such as the number of binding sites and the binding constant. So, if this graph is linear, it is non-cooperative; if the slope of the graph is positive and the curve of the graph is downward, it shows positive cooperation, and the opposite case shows negative cooperation. The Scatchard diagram is drawn for the interaction of this complex with bovine serum albumin at two temperatures of 27 and 37 °C (Fig. 6). We use Hill's equation (equation (8)) to obtain Hill binding constant, KH, and Hill coefficient, nH, as follows [6]:
| (8) |
Fig. 6.
Schematic Scatchard plot of protein binding with of [Pt(bpy)(amyl.dtc)]NO3 complex at temperatures 37 °C ( ) and 27 °C (
) and 27 °C ( ).
).
Considering the positive slope of the graph and the low depression of the graphs, it can be concluded that this interaction proceeds via a positive, cooperative mechanism [6,40].
By drawing the graph of Ln (ʋ/g-ʋ) in terms of Ln [complex]f (Fig. 7), Hill's constant, KH, and Hill's coefficient, nH, were obtained. These values are reported in Table 2. In these graphs, the Hill coefficient is more than one at 27 and 37 °C, with positive, cooperative behavior in this interaction [41].
Fig. 7.
Hill plot of protein binding with [Pt (bpy) (amyl.dtc)]NO3 complex at 37 °C ( ) and 27 °C (
) and 27 °C ( ).
).
Table 2.
Hill parameter and binding constant of BSA interaction with complex.
| complex | T (°C) | g | Kb (M−1) | KH (M−1) | nH |
|---|---|---|---|---|---|
| [Pt(bpy)(amyl.dtc)]NO3 | 27 37 |
3.8 1.9 |
6.7 × 102 3.04 × 102 |
19.1 22.92 |
1.8 1.8 |
The values related to molar and binding Gibbs free energy are obtained through equation (9) as follow [38,42]:
| (9) |
Fig. 8 shows the graph of molar binding Gibbs free energy changes versus Ln [complex]f at both mentioned temperatures. The negative slope indicates positive cooperativity in related interactions [43].
Fig. 8.
ΔG°b,υ graph versus Ln [complex]f for BSA interaction with [Pt (bpy) (amyl.dtc)]NO3 at two temperatures of 27 (●) and 37 (●) °C.
3.4. BSA thermal stability
The thermal stability of protein can be studied based on [L]1/2 value and the ratio of [L]/[BSA] [42] in the absence and presence of the drug. Tm was determined by UV scanning of 2 mg/mL solution of BSA at 278 nm in the presence and absence of 10−4 M Platinum agent. In this test, the temperature increased from the ambient temperature by 1 °C per minute until it reached 60 °C. The value of Tm for the BSA in the presence of the platinum agent is about 47 °C, while this value is only about 37 °C for the protein (Fig. 9a). This study was repeated using cisplatin as a controller, and HSA thermal changing was continued to 95 °C (Fig. 9b) [35]. The results show Pt complex-BSA formation leads to a stable system [42].
Fig. 9.
Absorption changes (at 280 nm) vs temperature for (a) BSA in the absence and the presence of [Pt (bpy) (amyl.dtc)]NO3 from 25 to 65 °C and (b) HSA-cisplatin from 25 to 95 °C.
3.5. UV–visible kinetic study
Kinetic studies consider arranged information about the binding mode of BSA with Pt agent by the time checking recorded 1 min at 278 nm wavelength until 1 h (Fig. 10a and b) [39,43]. Based on equations (10), (11)) and plotting the charts vs time (seconds), the rate constant of the BSA-Pt agent can be determined.
| (10) |
| (11) |
In these two formulas, A0 is initial absorption, A∞ is final absorption, A is absorption in successive times, k is a rate constant, and t is time. To determine the order type of the mechanism in the interaction of the above complex with bovine serum albumin, the following graphs were drawn at two temperatures of 27 and 37 °C (Fig. 11a and b). According to equations (10), (11)), since the square linear regression coefficient, R2, in these graphs is more linear for the second-order reaction than the first-order reaction, it is concluded that the kinetics is a second-order type for both 27 and 37 °C during occupying of all binding sites. In biological systems, protein interaction should depend on drug and protein concentrations in binding. So, it will lead to limit protein binding and interaction with drugs. It means probably side effects decrease since this matter is important in research in the drug design field [44].
Fig. 10.
Absorption changes over time in the interaction of [Pt (bpy) (amyl.dtc)]NO3 with BSA at (a) 27 and (b) 37 °C.
Fig. 11.
Investigating the first-order (a) and second-order (b) kinetics model in the interaction of [Pt (bpy) (amyl.dtc)]NO3 complex with BSA at two temperatures of 27 ( ) and 37 °C (
) and 37 °C ( ).
).
3.6. BSA emission quenching
Bovine serum albumin has amino acids that have fluorescent properties, so fluorescence spectroscopy is widely used to evaluate its interaction with metal compounds [43]. There are two types of tryptophan in BSA: tryptophan 212, which is in the hydrophobic part, and tryptophan 134, which is on the molecule's surface [44,45]. So, fluorescence emission monitoring was done for BSA-free and BSA-complex systems, and decreasing intensity was observed, along with increasing complex concentration, in the BSA-Pt agent. In this test, we set the excitation wavelength to 280 nm (Fig. 12), and the quenching indicates drug binding to the protein [22].
Fig. 12.
Fluorescence quenching of BSA (λex = 280 nm) in the presence of various concentrations of Pt compound (0–210 μΜ); insert: Stern-Volmer diagrammatic of protein quenching.
The Stern-Volmer equation (equation (12)) was utilized to evaluate the experimentally obtained emission data, and the fluorescence quenching proceed [6,40,41]:
| (12) |
In this relationship, Fo, F, τ0, Q, kq, Ksv, and n are fluorescence emissions in the absence and presence of the Pt complex, the lifetime (10−8 s) of the fluorophore within the nonappearance of Pt agent, as ligand, which is equal to, the concentration of the ligand, the rate constant; and quenching constant and binding site numbers on protein, respectively [46]. To obtain Ksv, low concentration regions are considered at the ratio of 1:1 (ligand/protein), and linear Fo/F plot vs [Q] in various by reducing the fluorescence emission. Based on Ksv = kq.τ equation, kq is also calculated (more than 2 × 1010 M−1S−1), and static interaction is confirmed [47,48].
Using different methods, Kb and the number of binding sites per protein molecule can be determined by equation (13), which has been applied as follows:
| (13) |
In this respect, Fo and F, respectively, show the intensity of the fluorescence in the absence and presence of the Ligand, and the [Q] parameters represent the quencher ligand concentration.
Based on equation (13), the values of n and Kb are evaluated [49] by plotting experimental emission data as the Log vs the log [Q] as shown in Fig. 13 (Kb = 5 × 106 M−1 and n = 1.3). Also, Kb values exhibit the strength of the interaction between BSA and Pt agent [50,51].
Fig. 13.

Linear diagram of vs Log [complex].
3.7. Molecular docking study
The advanced AutoDock program is one of the most important docking simulation software at the international level, which is very useful in the discovery and rational development of pharmaceutical molecules. Using force field functions, this software can calculate the chemical interactions between the ligand and the macromolecular receptor (proteins or nucleic acids) based on the binding free energy [52,53]. The BSA code, which was downloaded, is 4F5S from the PDB. During the data analysis, the appropriate grid size was set with a distance of 0.375 Å along the three axes. Concurring with the Gibbs energy, the restraint consistency was calculated, and the esteem of Ki for the Pt agent was 41.29 nM (nanomolar). As shown in Fig. 14, there was no hydrogen bound. In this interaction, one hydrogen bond with the amino acid glutamine and eight hydrophobic bonds with the amino acids alanine, asparagine, glycine, lysine, phenylalanine, and valine were observed. The comes about of molecular docking indicates the binding position of the Pt compound on BSA site I within the subdomain IIA.
Fig. 14.
Docking pose of Pt complex interaction with HSA through hydrogen and hydrophobic binding.
4. Conclusions
Cisplatin treats human cancers such as bladder and ovarian cancer, head and neck cancer, and testicular cancer. But its clinical utilization is constrained due to a few genuine side impacts. Hence, it is conceivable to utilize S, S giver ligands such as dithiocarbamate to get ready anti-cancer drugs and decrease a few side impacts. In this research, the interaction ability of [Pt (bpy) (amyl.dtc)]NO3 with BSA was investigated by using fluorescence emission titration, UV–Vis. absorption monitoring, and molecular docking simulation. Thermodynamic parameters were obtained. The enthalpy and entropy values were −62.1, −133.58 kJ/mol, and −126.91 kJ/mol.K at 27 and 37 °C, respectively, which display that the driving forces during the interaction are van der Waals and hydrogen binding. The negative Gibbs free energy obtained showed a spontaneous interaction. Using the spectroscopic kinetic model, the linear regression coefficient in the graph for the second-order reaction is higher than the first-order reaction, so it can be concluded that the kinetic prefers second order, and binding of the complex on BSA depends on drug and protein concentrations. Moreover, the emission titration results showed that protein fluorescence quenching by the Pt complex is a static quenching mechanism by the Stern-Volmer equation. The results showed that there is only one binding site in the interaction of the complex with BSA. Molecular docking showed that the location of this complex on BSA is position I under region IIA.
Funding statement
The work was supported by Payame Noor University and the Chemistry and Chemical Engineering Research Center of Iran.
Authors contributions
Zahra Arabpour Shiraz: Performed the experiments, Analyzed the data, and wrote the Experimental sections.
Nasrin Sohrabi: Designed the experiment , analyzed all data and interpreted the data and wrote and edited the paper.
Mahboube Eslami Moghadam: Prepared materials, analyzed the theoretical data and wrote and edited all the paper.
Mohsen Oftadeh: Analyzed tools or data and wrote the titration section.
Data availability statement
Data will be made available on request.
Ethics approval
Not applicable. This article does not contain any studies with human participants or animals performed by any of the authors.
Declaration of competing interest
There is not any conflict in reviewing our manuscripts, such as former advisors, students, or recent collaborators.
Acknowledgments
We are thankful to the Research Council of Payame Noor University for the fiscal support.
Contributor Information
Nasrin Sohrabi, Email: nsohrabi99@pnu.ac.ir, sohrabnas99@gmail.com.
Mahboube Eslami Moghadam, Email: eslami_moghadam@ccerci.ac.ir, eslami_moghadam@yahoo.com.
Abbreviations
- amyl.dtc
n-pentyl dithiocarbamate
- BSA
Bovine Serum Albumin
- bpy
bipyridine
- dtc
Dithiocarbamate
- LGA
Lamarckian genetic algorithm
- PDB
Protein Data Bank
- Panc1
Human pancreatic cell line
- Tm
melting temperature
- UV–vis
ultraviolet–visible
References
- 1.Pecorino L. Oxford university press; 2021. Molecular Biology of Cancer: Mechanisms, Targets, and Therapeutics. [Google Scholar]
- 2.Pitot H.C. 2002. The Molecular Basis of Human Cancer. [Google Scholar]
- 3.Rahiminezhad A., Moghadam M.E., Divsalar A., Mesbah A.W. How can the cisplatin analogs with different amine act on DNA during cancer treatment theoretically? J. Mol. Model. 2022;28:1–12. doi: 10.1007/s00894-021-04984-x. https://doi:10.1007/s00894-021-04984-x [DOI] [PubMed] [Google Scholar]
- 4.Reedijk J., Lohman P.H.M. Cisplatin: synthesis, antitumour activity and mechanism of action. Pharm. Weekbl. Sci. 1985;7:173–180. doi: 10.1007/BF02307573. https://doi:10.1007/BF02307573 [DOI] [PubMed] [Google Scholar]
- 5.Arunkumar P.A., Viswanatha G.L., Radheshyam N., Mukund H., Belliyappa M.S. Science behind cisplatin-induced nephrotoxicity in humans: a clinical study. Asian Pac. J. Trop. Biomed. 2012;2(8):640–644. doi: 10.1016/S2221-1691(12)60112-9. https://doi:10.1016/S2221-1691(12)60112-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eslami-Moghadam M., Rezaeisadat M., Mansouri-Torshizi H., Hosseinzadeh S., Daneshyar H. New anticancer potential Pt complex with tertamyl dithiocarbamate ligand: synthesis, DNA targeting behavior, molecular dynamic and biological activity. J. Mol. Liq. 2023;379 https://doi:10.1016/j.molliq.2023.121651 [Google Scholar]
- 7.Hashemi Z.H., Eslami-Moghadam M., Mirzaei M., Notash B. Biological activity of two anticancer Pt complexes with a cyclohexylglycine ligand against a colon cancer cell line: theoretical and experimental study. ACS Omega. 2022;7(44):39794–39811. doi: 10.1021/acsomega.2c03776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bazsefidpar P., Eftekhar E., Jahromi M.Z., Nikpoor A.R., Eslami Moghadam M., Zolghadri S. In-vitro cytotoxicity and in-vivo antitumor activity of two platinum complexes with 1, 3-dimethyl pentyl glycine ligand against breast cancer. J. Inorg. Biochem. 2023;241 doi: 10.1016/j.jinorgbio.2023.112144. https://doi:10.1016/j.jinorgbio [DOI] [PubMed] [Google Scholar]
- 9.Raza M.A., Farwa U., Danish M., Ozturk S., Aagar A.A., Dege N., Al-Sehemi Computational modeling of imines based anti-oxidant and anti-esterases compounds: synthesis, single crystal, and In-vitro assessment. Comput. Biol. Chem. 2023;104 doi: 10.1016/j.compbiolchem.2023.107880. https://doi:10.1016/j.compbiolchem.2023.107880 [DOI] [PubMed] [Google Scholar]
- 10.Kundu B.K., Pragti, Carlton Ranjith W.A., Shankar U., Kannan R.R., Mobin S.M., Mukhopadhyay S. Cancer-targeted chitosan–biotin-conjugated mesoporous silica nanoparticles as carriers of zinc complexes to achieve enhanced chemotherapy in vitro and in vivo. ACS Appl. Bio Mater. 2021;5(1):190–204. doi: 10.1021/acsabm.1c01041. https://doi:10.1021/acsabm.1c01041 [DOI] [PubMed] [Google Scholar]
- 11.Simsek O., Ashfaq M., Tahir M.N., Ozturk S., Agar E. Synthesis and charaterizations of the schiff base derived from 2-hydroxy-5-nitrobenzaldehyde along with hirshfeld surface analysis and computational study. J. Struct. Chem. 2023;64(5):942–953. https://doi:10.1134/s0022476623050128 [Google Scholar]
- 12.Ghasemzadeh-Mohammadi V., Zamani B., Afsharpour M., Mohammadi A. Extraction of caffeine and catechins using microwave-assisted and ultrasonic extraction from green tea leaves: an optimization study by the IV-optimal design. Food Sci. Biotechnol. 2017;26:1281–1290. doi: 10.1007/s10068-017-0182-3. https://doi:10.1007/s10068-017-0182-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseini S.A., Eslami Moghadam M., Saeidifar M., Saboury A.A. Biological effect and molecular docking of anticancer palladium and platinum complexes with morpholine dithiocarbamate on human serum albumin as a blood carrier protein. Can. J. Physiol. Pharmacol. 2018;96(12):1276–1285. doi: 10.1139/cjpp-2017-0693. https://doi:10.1139/cjpp-2017-0693 [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh S., Eslami-Moghadam M., Sheshmani S., Shahvelayati A.S. Some new anticancer platinum complexes of dithiocarbamate derivatives against human colorectal and pancreatic cell lines. J. Biomol. Struct. Dyn. 2020;38(8):2215–2228. doi: 10.1080/07391102.2019.1627909. https://doi:10.1080/07391102.2019.1627909 [DOI] [PubMed] [Google Scholar]
- 15.Kundu B.K., Mukhopadhyay S. Target based chemotherapeutic advancement of ruthenium complexes. Coord. Chem. Rev. 2021;448 doi: 10.1016/j.ccr.2021.214169. [DOI] [Google Scholar]
- 16.Babcock J.J., Brancaleon L. Bovine serum albumin oligomers in the E-and B-forms at low protein concentration and ionic strength. Int. J. Biol. Macromol. 2013;53:42–53. doi: 10.1016/j.ijbiomac.2012.10.030. https://doi:10.1016/j.ijbiomac.2012.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hornok V. Serum albumin nanoparticles: problems and prospects. Polymers. 2021;13(21):3759. doi: 10.3390/polym13213759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hong S., Choi D.W., Kim H.N., Park C.G., Lee W., Park H.H. Protein-based nanoparticles as drug delivery systems. Pharmaceutics. 2020;12(7):604. doi: 10.3390/pharmaceutics12070604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jafari A., Eslami Moghadam M., Mansouri-Torshizi H. Green synthesis and bioactivity of aliphatic N-substituted Glycine derivatives. ACS Omega. 2023;8(33):30158–30176. doi: 10.1021/acsomega.3c02828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taboada P., Barbosa S., Castro E., Mosquera V. Amyloid fibril formation and other aggregate species formed by human serum albumin association. J. Phys. Chem. B. 2006;110(42):20733–20736. doi: 10.1021/jp064861r. https://doi:10.1021/jp064861r [DOI] [PubMed] [Google Scholar]
- 21.Scattolin T., Voloshkin V.A., Visentin F., Nolan S.P. A critical review of palladium organometallic anticancer agents. Cell Reports Physical Science. 2021;2(6) https://doi:10.1016/j.xcrp.2021.100446 [Google Scholar]
- 22.Arabpour Shiraz Z., Sohrabi N., Eslami Moghadam M., Oftadeh M. Dynamic and thermodynamic investigation on the interaction of bovine serum albumin with an anticancer Pt complex containing dithiocarbamate using molecular docking and spectroscopic methods. Polycyclic Aromat. Compd. 2023;43(3):2823–2843. https://doi:10.1080/10406638.2022.2058027 [Google Scholar]
- 23.Hosseinzadeh S., Eslami Moghadam M., Sheshmani S., Shahvelayati A.S. New anticancer Pd and Pt complexes of tertamyl dithiocarbamate and DACH ligands against HT29 and Panc1 cell lines. J. Biomol. Struct. Dyn. 2022;40(15):6910–6920. doi: 10.1080/07391102.2021.1891972. https://doi:10.1080/07391102.2021.1891972 [DOI] [PubMed] [Google Scholar]
- 24.Dirix C., Duvetter T., Loey A.V., Hendrickx M., Heremans K. The in-situ observation of the temperature and pressure stability of recombinant Aspergillus aculeatus pectin methylesterase with Fourier transform IR spectroscopy reveals an unusual pressure stability of β-helices. Biochem. J. 2005;392(3):565–571. doi: 10.1042/BJ20050721. https://doi:10.1042/BJ20050721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaker B., Ahmad S., Lee J., Jung C., Na D. vol. 137. 2021. Silico methods and tools for drug discovery.https://doi:10.1016/j.compbiomed.2021.104851 (Comput. Biol. Med). [DOI] [PubMed] [Google Scholar]
- 26.Laurie A.T.R., Jackson R.M. Methods for the prediction of protein-ligand binding sites for structure-based drug design and virtual ligand screening. Curr. Protein Pept. Sci. 2006;7(5):395–406. doi: 10.2174/138920306778559386. https://doi:10.2174/138920306778559386 [DOI] [PubMed] [Google Scholar]
- 27.Heydari M., Eslami-Moghadam M., Tarlani A., Farhangian H. DNA as a target for anticancer phen-imidazole Pd (II) complexes. Appl. Biochem. Biotechnol. 2017;182:110–127. doi: 10.1007/s12010-016-2314-2. https://doi:10.1007/s12010-016-2314-2 [DOI] [PubMed] [Google Scholar]
- 28.Buddanavar A.T., Nandibewoor S.T. Multi-spectroscopic characterization of bovine serum albumin upon interaction with atomoxetine. J. pharm. Analysis. 2017;7(3):148–155. doi: 10.1016/j.jpha.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ilmi R., Kansız S., Al-Rasbi N.K., Dege N., Raithby P.R., Khan M.S. Towards white light emission from a hybrid thin film of a self-assembled ternary samarium (III) complex. New J. Chem. 2020;44(15):5673–5683. doi: 10.1039/C9NJ06287D. [DOI] [Google Scholar]
- 30.Jia P., Pei J., Wang G. Pan, Zhu X., Y Wu Y., Ouyang L. The roles of computer-aided drug synthesis in drug development. Green Synthesis and Catalysis. 2022;3(1):11–24. doi: 10.1016/j.gresc.2021.11.007. [DOI] [Google Scholar]
- 31.Shiekhzadeh A., Sohrabi N., Eslami-Moghadam M., Oftadeh M. Kinetic and thermodynamic investigation of human serum albumin interaction with anticancer glycine derivative of platinum complex by using spectroscopic methods and molecular docking. Appl. Biochem. Biotechnol. 2020;190(2):506–528. doi: 10.1007/s12010-019-03078-y. https://doi:10.1007/s12010-019-03078-y [DOI] [PubMed] [Google Scholar]
- 32.Kansız S., Tolan A., Azam M., Dege N., Alam M., Sert Y., İçbudak H. vol. 218. 2022. Polyhedron.https://doi:10.1016/j.poly.2022.115762 (Acesulfame Based Co (II) Complex: Synthesis, Structural Investigations, Solvatochromism, Hirshfeld Surface Analysis and Molecular Docking Studies). [Google Scholar]
- 33.Arbuznikov A.V. Hybrid exchange correlation functionals and potentials: concept elaboration. J. Struct. Chem. 2007;48:S1–S31. https://doi:10.1007/s10947-007-0147-0 [Google Scholar]
- 34.Eslami-Moghadam M., Saidifar M., Rostami-Charati F.D., Ajloo M., Ghadamgahi Molecular dynamic simulation and spectroscopic investigation of some cytotoxic palladium (II) complexes interaction with human serum albumin. Comb. Chem. High Throughput Screening. 2014;17(9):781–789. doi: 10.2174/1386207317666140926124647. https://doi:10.2174/1386207317666140926124647 [DOI] [PubMed] [Google Scholar]
- 35.Eslami-Moghadam M., Sadeghi M., Mansouri-Torshizi H., Saidifar M. High cancer selectivity and improving drug release from mesoporous silica nanoparticles in the presence of human serum albumin in cisplatin, carboplatin, oxaliplatin, and oxalipalladium treatment. Eur. J. Pharm. Sci. 2023;187 doi: 10.1016/j.ejps.2023.106477. https://doi:10.1016/j.ejps.2023.106477 [DOI] [PubMed] [Google Scholar]
- 36.He C., Liu X., Jiang Z., Geng S., Ma H., Liu B. Interaction mechanism of flavonoids and α-glucosidase: experimental and molecular modelling studies. Foods. 2019;8(9):355. doi: 10.3390/foods8090355. https://doi:10.3390/foods8090355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eslami-Moghadam M., Divsalar A., Abolhosseini Shahrnoy A., Saboury A.A. Synthesis, cytotoxicity assessment, and interaction and docking of novel palladium (II) complexes of imidazole derivatives with human serum albumin. J. Biomol. Struct. Dyn. 2016;34(8):1751–1762. doi: 10.1080/07391102.2015.1090345. https://doi:10.1080/07391102.2015.1090345 [DOI] [PubMed] [Google Scholar]
- 38.Bordbar A.K., Saadati Z., Sohrabi N. Analysis of ligand binding process using binding capacity concept. Acta Biochim. Pol. 2004;51(4):963–970. [PubMed] [Google Scholar]
- 39.Shiekhzadeh A., Sohrabi N., Eslami-Moghadam M., Divsalar A., Soltani N., Oftadeh M., Fateminasab F. Spectroscopic and docking molecular study of new anticancer Pt complex binding with human serum albumin. Nucleosides, Nucleotides Nucleic Acids. 2021;40(4):369–392. doi: 10.1080/15257770.2021.1880010. https://doi:10.1080/15257770.2021.1880010 [DOI] [PubMed] [Google Scholar]
- 40.Moghadam M.E., Rezaeisadat M., Shahryari E., Mansouri-Torshizi H., Heydari M. Biological interaction of Pt complex with imidazole derivative as an anticancer compound with DNA: experimental and theoretical studies. Int. J. Biol. Macromol. 2023;249 doi: 10.1016/j.ijbiomac.2023.126097. https://doi:10.1016/j.ijbiomac.2023.126097 [DOI] [PubMed] [Google Scholar]
- 41.Sandua N., Chiloma C.G., Davidb M., Florescub M. Biophysical interaction of levothyroxine with bovine serum albumin: a spectroscopic assay. J. Biomol. Struct. Dyn. 2020;40(3):1139–1151. doi: 10.1101/2020.04.29.068510. [DOI] [PubMed] [Google Scholar]
- 42.Bonin J.P., Sapienza P.J., Wilkerson E., Goldfarb D., Wang L., Herring L., Lee A.L. Positive cooperativity in substrate binding by human thymidylate synthase. Biophys. J. 2019;117(6):1074–1084. doi: 10.1016/j.bpj.2019.08.015. https://doi:10.1016/j.bpj.2019.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shiekhzadeh A., Sohrabi N., Eslami-Moghadam M., Oftadeh M., Divsalar A. Effect of presence of aliphatic Glycine in the anti-cancer platinum complex structure on human serum albumin binding. J. Pharmac. Innova. 2022;17:353–365. https://doi:10.1007/s12247-020-09508-3 [Google Scholar]
- 44.Hadian Rasanani S., Eslami-Moghadam M., Soleimani E., Divsalar A., Tarlani A. Improving activity of anticancer oxalipalladium analog by the modification of oxalate group with isopentylglycine. J. Coord. Chem. 2017;70(22):3769–3789. https://doi:10.1080/00958972.2017.1395417 [Google Scholar]
- 45.Topală T., Bodoki A., Oprean L., Oprean R. Bovine serum albumin interactions with metal complexes. Clujul Med. 2014;87(4):215–219. doi: 10.15386/cjmed-357. https://doi:10.15386/cjmed-357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vignesh G., Arunachalam S., Vignesh S., James R.A. BSA binding and antimicrobial studies of branched polyethyleneimine–copper (II) bipyridine/phenanthroline complexes. Spectrochim, Acta, Part A. 2012;96:108–116. doi: 10.1016/j.saa.2012.05.009. https://doi:10.1016/j.saa.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 47.Ehteshami M., Rasoulzadeh F., Mahboob S., Rashidi M.R. Characterization of 6-mercaptopurine binding to bovine serum albumin and its displacement from the binding sites by quercetin and rutin. J. Lumin. 2013;135:164–169. https://doi:10.1016/j.jlumin.2012.10.044 [Google Scholar]
- 48.Bowers A.N., Santra K., Trujillo-Rodríguez M.J., Song A., Emaus M.N., Petrich J.W., Anderson J.L. Fluorescence quenching of the SYBR Green I-dsDNA complex by in situ generated magnetic ionic liquids. Anal. Bioanal. Chem. 2020;412:2743–2754. doi: 10.1007/s00216-020-02538-3. https://doi:10.1007/s00216-020-02538-3 [DOI] [PubMed] [Google Scholar]
- 49.Kundu B.K., Upadhyay S.N., Sinha N., Ganguly R., Grabchev I., Pakhira S., Mukhopadhyay S. Pyrene-based fluorescent Ru (II)-arene complexes for significant biological applications: catalytic potential, DNA/protein binding, two photon cell imaging and in vitro cytotoxicity. Dalton Trans. 2022;51(10) doi: 10.1039/D1DT04093F. 3937 – 3935. [DOI] [PubMed] [Google Scholar]
- 50.Hafshajani K.T., Sohrabi N., Eslami-Moghadam M., Oftadeh M. Spectroscopy and molecular dynamic study of the interaction of calf thymus DNA by anticancer Pt complex with butyl glycine ligand. Spectrochim. Acta, Part A. 2023;299 doi: 10.1016/j.saa.2023.122826. https://doi:10.1016/j.saa.2023.122826 [DOI] [PubMed] [Google Scholar]
- 51.Kragh-Hansen U. Molecular aspects of ligand binding to serum albumin. Pharmacol. Rev. 1981;33(1):17–53. [PubMed] [Google Scholar]
- 52.R Laurie A.T., Jackson R.M. Methods for the prediction of protein-ligand binding sites for structure-based drug design and virtual ligand screening. Curr. Protein Pept. Sci. 2006;303(5665):1813–1818. doi: 10.2174/138920306778559386. https://doi:10.2174/138920306778559386 [DOI] [PubMed] [Google Scholar]
- 53.Jorgensen W.L. The many roles of computation in drug discovery. Science. 2004;303(5665):1813–1818. doi: 10.1126/science.1096361. https://doi:10.1126/science.1096361 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.