Abstract
Objective
To investigate the association of body mass index (BMI) with asthma and analyze the risk factors of asthma persistence among overweight/obese adolescents and those with a high risk for obesity.
Methods
In this cross-sectional study, adolescents aged 11–17 years with complete general information and asthma diagnoses were selected from the National Health and Nutrition Examination Survey database. For adolescents without self-reported asthma, we performed matching according to age and sex at a case-to-control ratio of 1:3. Logistic regression analysis was employed to identify independent predictors of asthma occurrence followed by constructing a nomogram and comparing its efficacy to independent factors in predicting asthma occurrence. Besides, associations of BMI with asthma occurrence and persistence were evaluated. Finally, we obtained risk factors for asthma persistence in overweight/obese individuals and those at a high risk for obesity.
Results
Totally 753 adolescents with asthma and 2259 adolescents without asthma were included to analyze the occurrence of asthma. BMI and Hispanic Ethnicity were independent predictors of asthma occurrence and were included in nomogram construction. BMI had an efficiency comparable to that of the nomogram model in predicting asthma occurrence, which is superior to that of Hispanic Ethnicity. Of the 753 adolescents diagnosed with asthma, 464 were still diagnosed with asthma of at least a year's duration. Interestingly, BMI may have the ability to predict asthma persistence. Further, Hispanic Ethnicity and household income were significantly related to asthma occurrence among overweight/obese and high-risk obese individuals.
Conclusions
High BMI could independently predict increased asthma occurrence. Additionally, BMI may play an essential role in predicting asthma persistence. This study may help improve the diagnosis and reduce the occurrence of asthma.
Keywords: Asthma, Nomogram, BMI, Adolescents
Abbreviations
- NHANES
National Health and Nutrition Examination Survey
- BMI
body mass index
- ROC
receiver operating characteristic
- AUC
area under the curve
- DCA
decision curve analysis
- ROS
reactive oxygen species
- NF-κB
nuclear factor κ-light-chain-enhancer of activated B cells
- IL-1β
interleukin-1β
- TNF-α
tumor necrosis factor-α
- ER
endoplasmic reticulum
- TGF-β1
transforming growth factor β1
1. Introduction
Asthma is a common chronic and non-communicable disease characterized by airway hyper-responsiveness, inflammation, and periodic airflow obstruction, leading to respiratory symptoms, such as cough, wheezing, and breathlessness [1]. In 2019, current asthma and ever asthma occurred in 357.4 million and 645.2 million individuals, worldwide; more than 260 million people were affected by poorly controlled asthma with wheezing within the past 12 months according to Global Burden of Disease collaborators [2,3]. The number of asthmatics will increase by an additional 100 million by 2025 [4]. Asthma leads to a poorer quality of life for patients and increased healthcare costs due to frequent exacerbations, hospitalizations, and management of comorbidities [5]. In 2017, the total annual cost of treating asthma was $56 billion in the US, with an additional $5.9 billion in productivity losses [6]. In the UK, at least 6.3 million primary care consultations, 93,000 hospitalizations, and 1400 deaths from asthma each year cost the UK public sector more than £1.1 billion [7]. It is difficult to prevent asthma from an environmental or genetic perspective because of the diversity of allergens and the immutability of asthma [8]. Therefore, identifying modifiable risk factors for asthma to improve the patient's quality of life and reduce the disease burden is urgently needed.
Asthma often occurs in childhood and adolescence and is the leading cause of childhood hospitalization globally [9]. The prevalence of asthma in youngsters under 18 years of age has reached 13% and remained at 8.4% as of 2017 in the US [10]. It is estimated that 8%–9% of children and adolescents in high-income countries have asthma, while an increasing number of young people in low- and middle-income countries are affected by the disease [11]. A previous study has revealed that dietary intake is significantly associated with childhood asthma [8]. Dilini et al. adopted machine learning approaches to develop a childhood asthma prediction model [12]. Sauray et al. also used a machine learning approach to predict early childhood asthma persistence [12]. Studies have also revealed the effect of obesity on lung volumes and capacities, such as residual volume, expiratory reserve volume, and functional residual capacity with diverse results in adolescents with asthma [13,14]. On the other hand, due to the presence of asthma symptoms during exercise, physical activity would be decreased which might increase obesity risk [15]. Several factors have been associated with asthma in adolescents in recent years. Physiological, cognitive, emotional, and social changes specific to adolescents may increase exposure to various health risk factors such as a sedentary lifestyle and inadequate diet [16]. However, no systematic research has been conducted to evaluate the independent factors of asthma occurrence among adolescents.
Asthma persistence refers to the continuation of symptoms, and a large population of children who receive an incident asthma diagnosis ultimately does not experience persistent asthma symptoms [17]. In such cases, early diagnosis may lead to unnecessary treatment, potentially associated side effects, and altered quality of life for children and their families [18]. Thus, it is essential to explore a novel predictive tool to determine whether asthma persists as a chronic condition.
In this study, we downloaded high-quality data from the National Health and Nutrition Examination Survey (NHANES) database and adopted modern statistical techniques to investigate the potent risk factors for asthma occurrence and persistence in adolescents aged 11–17 years.
2. Materials and methods
2.1. Study design and population
All data used for analysis were obtained from the NHANES database (2011–2018) (https://www.cdc.gov/nchs/nhanes/index.htm), which was conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention. Detailed information on the NHANES procedures is available in the literature [19]. The NHANES is a program of studies designed to assess the health and nutritional status of adults and children in the US, which combines interviews and physical examinations. From 2011 to 2018, the NHANES included 39,156 participants, including 4852 adolescents aged 11–17 years. Among these adolescents, 4849 had asthma information. Finally, 3694 patients with complete general information were included in the analysis. In total, 753 adolescents diagnosed with asthma were enrolled in the final sample. For better statistical power, among the 2941 adolescents without diagnosed asthma, matching was performed according to age and sex, maintaining a ratio of 1:3 for cases, resulting in a final study sample of adolescents with diagnosed asthma (n = 753) and adolescents without diagnosed asthma (n = 2259). Of the 753 adolescents diagnosed with asthma, 464 and 282 were still diagnosed with asthma and without asthma, respectively, after one year.
All the participants provided written informed consent during the recruitment period. The NHANES is an open database; thus, Institutional Review Board approval was not required for this study.
2.2. Data collection
Data on asthma were recorded in the “Medical Conditions” section of the NHANES interview. The following questions were about the diagnosis of asthma: “Has a doctor or other health professional ever told that the survey participant has asthma?” “How old was when the survey participant first told had asthma?” followed by “Does the survey participant still have asthma?” Adolescents with asthma were included in the study to analyze the occurrence of asthma, and those who still had asthma after 1 year were enrolled to assess the persistence of asthma.
Age, sex, Hispanic Ethnicity, and household income of the participants were obtained from the “Demographic Variables & Sample Weights” data file.
The body mass index (BMI) of the participants was acquired from the “Body Measures” section of the “Examination Data”. BMI was classified according to 5th, 85th, and 95th percentiles for age and sex to identify children or teens with obesity: <5th percentile, underweight; 5th to <85th percentile, normal weight; 85th to <95th percentile, overweight; ≥95th percentile, obesity [20]. Given the obesity epidemic among American youths, we further split the 5th to <85th percentile into three groups: 5th to <35th percentile, 35th to <65th percentile, and 65th to <85th percentile to investigate whether the group from 65th to <85th is associated with asthma compared with lower BMI.
Information regarding household smokers was collected from the “Smoking-Household Smokers” section.
The energy, protein, dietary fiber, total fat, vitamin D, iron, calcium, and zinc information of the participants was generated from the “Total Nutrient Intakes” section of the NHANES dietary interview. All the data were extracted from the NHANES database (2011–2012, 2013–2014, 2015–2016, 2017–2018) based on questionnaires (See File S1).
2.3. Statistical analysis
All statistical analyses were performed using SPSS software (version 23.0) and R statistical software (version 4.2.1). Continuous variables are expressed as mean ± standard deviation or median (interquartile range). Continuous variables were compared using independent group t-tests and the Mann-Whitney test for normally and non-normally distributed data, respectively. Categorical variables are expressed as counts and percentages, and differences between groups were tested using the chi-square test or Fisher's test. Statistical significance was set at P < 0.05.
Univariate analysis was used to identify variables in logistic regression models. In addition, owing to the significant role of age and sex in asthma [21], we selected these factors for multivariate logistic regression analysis for asthma occurrence. The male was set as a reference level for sex, no for house smoker, Mexican American for Hispanic ethnicity since Mexican Americans have the lowest rates of asthma. A prediction model was established based on multivariate logistic regression analysis, and a nomogram was constructed and its efficacy was assessed by the calibration curve. The receiver operating characteristic (ROC) and area under the curve (AUC) were then adopted to examine the discriminative capacity of the model and independent parameters. The potential clinical usefulness of the model and the independent parameters were determined using decision curve analysis (DCA). Weighted multivariate logistic regression models were used to analyze the relationship between BMI and asthma occurrence. Three sequential models (model 1: non-adjusted; model 2: adjusted for age, sex, and Hispanic Ethnicity; model 3: adjusted for all covariates) were adopted to control for potential confounders. Finally, univariate analysis was also performed to evaluate the risk factors for asthma persistence among overweight/obese individuals and those with a high risk for obesity.
3. Results
3.1. Baseline characteristics of the participants
A total of 3012 eligible participants (1636 male and 1376 female) were enrolled in this study. The characteristics of the study population are shown in Table 1 and a flowchart of the study participants is shown in Fig. 1. There were significant differences in Hispanic Ethnicity, BMI, BMI category, household smoking status, vitamin D level, and dietary fiber content between adolescents with and without asthma (all P < 0.05). Notably, adolescents with asthma were more likely to be non-Hispanic black (30.94%) and non-Hispanic white (28.16%). They had higher BMIs but lower vitamin D and dietary fiber intake than those without asthma (P < 0.05). However, there were no significant differences in sex, age, household income, zinc, iron, calcium, total fat, protein, or energy between adolescents with and without asthma (all P > 0.05) (Table 1).
Table 1.
Characteristics of the study population by univariate analysis.
| Variables | Adolescents without asthma (n = 2259) | Adolescents with asthma (n = 753) | P-value |
|---|---|---|---|
| Sex | 1.000 | ||
| Male | 1227 (54.32) | 409 (54.32) | |
| Female | 1032 (45.68) | 344 (45.68) | |
| Age (years) | 14.00 [12.00, 16.00] | 14.00 [12.00, 16.00] | 0.837 |
| Hispanic ethnicity | 0.001 | ||
| Mexican American | 489 (21.65) | 124 (16.47) | |
| Other Hispanic | 217 (9.61) | 70 (9.30) | |
| Non-Hispanic White | 595 (26.34) | 212 (28.15) | |
| Non-Hispanic Black | 561 (24.83) | 233 (30.94) | |
| Other Race | 397 (17.57) | 114 (15.14) | |
| BMI (kg/m2) | 21.60 [18.90, 26.00] | 23.00 [20.10, 27.80] | <0.001 |
| BMI category | 0.002 | ||
| Underweight | 106 | 39 | |
| Healthy weight | 1840 | 568 | |
| Overweight | 202 | 98 | |
| Obese | 111 | 48 | |
| Household income ($) | 0.614 | ||
| <20000 | 363 (16.83) | 132 (18.08) | |
| 20000-74999 | 1133 (52.53) | 386 (52.88) | |
| ≥75000 | 661 (30.64) | 212 (29.04) | |
| Household smoker | 0.017 | ||
| No | 1744 (78.24) | 552 (74.00) | |
| Yes | 485 (21.76) | 194 (26.00) | |
| Zinc (mg) | 8.81 [5.90, 12.94] | 8.62 [5.56, 12.81] | 0.240 |
| Iron (mg) | 12.49 [8.23,18.47] | 11.87 [7.80,18.15] | 0.203 |
| Calcium (mg) | 857.00 [530.00,1285.00] | 829.00 [533.00,1235.00] | 0.339 |
| Vitamin D (μg) | 4.00 [1.50, 7.10] | 3.60 [1.10, 6.60] | 0.041 |
| Total fat (g) | 63.19 [42.21, 91.28] | 62.04 [42.67, 90.20] | 0.666 |
| Dietary fiber (g) | 12.90 [8.50, 18.80] | 12.30 [7.90, 17.80] | 0.019 |
| Protein (g) | 64.19 [45.37, 88.91] | 66.37 [43.87, 91.15] | 0.599 |
| Energy (kcal) | 1737.00 [1261.00, 2312.00] | 1732.00 [1225.00, 2334.00] | 0.590 |
Abbreviations: BMI, body mass index. Categorical variables are expressed as counts (percentages). Continuous variables are expressed as mean ± standard deviation or median (interquartile range). Statistical significance was set at P < 0.05.
Fig. 1.
The flowchart of the study population.
3.2. Selection of independent predictive factors
Univariate logistic regression analysis revealed that Hispanic Ethnicity, BMI, household smoking, and vitamin D in adolescents with asthma were significantly different from those in adolescents without asthma. In the multivariate logistic regression analysis, the two variables of BMI and Hispanic Ethnicity were statistically significant, indicating that BMI and Hispanic Ethnicity could independently predict asthma occurrence (P < 0.05) (Table 2).
Table 2.
Univariate and multivariate logistic regression analysis for risk factors for asthma occurrence.
| Parameters | OR | 95% CI | P-value | OR | 95% CI | Adjusted p-value |
|---|---|---|---|---|---|---|
| Univariate analysis | Multivariate analysis | |||||
| Age (years) | 1.015 | 0.974–1.059 | 0.474 | 0.992 | 0.950–1.037 | 0.727 |
| Sex | 1.000 | 0.847–1.180 | 1.000 | 0.961 | 0.810–1.140 | 0.648 |
| Household smoker | 1.261 | 1.041–1.528 | 0.018 | 1.167 | 0.959–1.419 | 0.124 |
| Body mass index (kg/m2) | 1.034 | 1.021–1.047 | <0.001 | 1.032 | 1.018–1.046 | <0.001 |
| Hispanic ethnicity | ||||||
| Mexican American | Referent | |||||
| Other Hispanic | 1.231 | 0.882–1.718 | 0.222 | 1.317 | 0.940–1.846 | 0.109 |
| Non-Hispanic White | 1.393 | 1.083–1.792 | 0.010 | 1.437 | 1.109–1.862 | 0.006 |
| Non-Hispanic Black | 1.654 | 1.288–2.123 | <0.001 | 1.614 | 1.250–2.086 | <0.001 |
| Other Race | 1.088 | 0.817–1.449 | 0.564 | 1.196 | 0.893–1.601 | 0.229 |
| Dietary fiber (g) | 0.991 | 0.981–1.001 | 0.069 | 0.997 | 0.987–1.008 | 0.584 |
| Vitamin D (μg) | 0.983 | 0.969–0.999 | 0.049 | 0.990 | 0.972–1.009 | 0.296 |
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval.
3.3. Nomogram model construction and evaluation
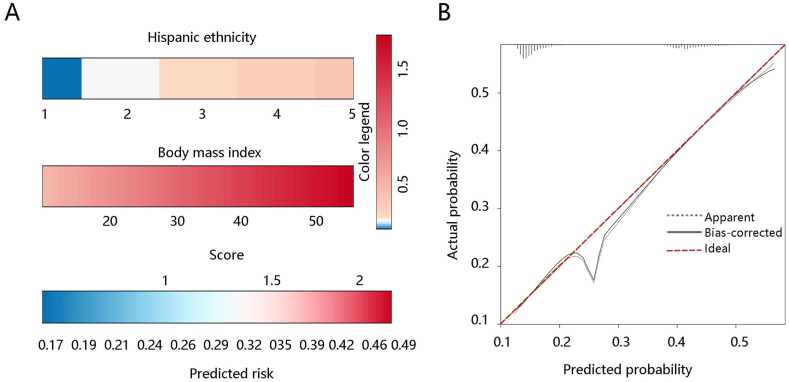
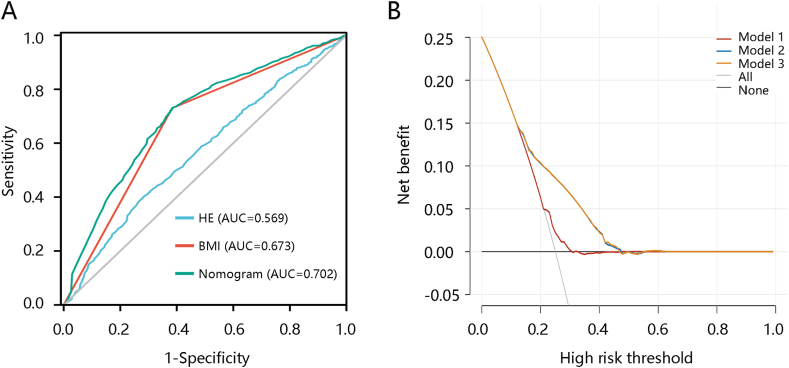
Based on the results of the multivariate logistic regression analysis, BMI and Hispanic Ethnicity were included in the asthma occurrence prediction model and presented as a nomogram. BMI contributed the most to asthma occurrence (Fig. 2A). The calibration curve illustrated good agreement between nomogram prediction and actual observations (Fig. 2B). ROC analysis was then conducted to evaluate how the model behaved in discriminating between adolescents with and without asthma. The results showed that the AUCs of the nomogram model, BMI, and Hispanic Ethnicity for predicting asthma occurrence were 0.702, 0.673, and 0.569, respectively (Fig. 3A). DCA results showed that the nomogram model had a prediction ability similar to that of BMI, which was superior to Hispanic Ethnicity (Fig. 3B). These results indicated that BMI had an efficiency comparable to that of the model in predicting asthma occurrence.
Fig. 2.
Nomogram construction and evaluation. (A) Nomogram construction to predict the risk of asthma. For HE, 1: Mexican American, 2: Other Hispanic, 3: Non-Hispanic White, 4: Non-Hispanic Black, 5: Other Race. (B) Nomogram evaluation by the calibration curve. Abbreviations: BMI, body mass index; HE, Hispanic Ethnicity.
Fig. 3.
Evaluation of the nomogram model and two independent predictors. The ability of the three models in predicting asthma occurrence using (A) receiver operating characteristic analysis and (B) decision curve analysis. Abbreviations: HE, Hispanic Ethnicity; BMI, body mass index; AUC, the area under the curve. Model 1, Hispanic Ethnicity; model 2, body mass index; model 3, nomogram.
3.4. Association of BMI with the asthma occurrence
To further explore the correlation between BMI and asthma occurrence, a weighted multivariate logistic regression analysis was conducted. In model 1, without adjustment, there were significant positive associations between the different BMI groups and asthma (P < 0.01). After adjusting for age, sex, and Hispanic Ethnicity in model 2 and after adjusting for all confounding factors in model 3, the results remained stable and statistically significant (Table 3). Moreover, the group from 65th to <85th is associated with an increased asthma risk compared with lower BMI (P < 0.001) (Table 4). These results confirm that BMI is positively associated with the occurrence of asthma, indicating that obese individuals have a higher risk of developing asthma.
Table 3.
Weighted ORs and 95% CIs for the relationship between BMI and asthma risk.
| BMI range | Model 1 |
Model 2 |
Model 3 |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| <5th percentile | Referent | Referent | Referent |
| 5th to < 85th percentile | 0.839 (0.574–1.226) | 0.854 (0.584–1.248) | 0.847 (0.578–1.240) |
| 85th to < 95th percentile | 1.319 (0.850–2.046) | 1.366 (0.879–2.122) | 1.319 (0.846–2.054) |
| ≥95th percentile | 1.175 (0.713–1.937) | 1.194 (0.724–1.969) | 1.106 (0.667–1.833) |
| P value trend | 0.008 | 0.006 | 0.021 |
Model 1: non-adjusted model.
Model 2: adjusted for age, gender, and Hispanic Ethnicity.
Model 3: adjusted for age, gender, Hispanic Ethnicity, household smoker, vitamin D, and dietary fiber.
Note: <5th percentile: Underweight; 5th to <85th percentile: Normal weight; 85th to <95th percentile: Overweight; ≥95th percentile: Obesity.
Abbreviations: BMI, body mass index; OR, odds ratio; 95% CI, 95% confidence interval.
Table 4.
Weighted ORs and 95% CIs for the relationship between BMI and asthma risk among individuals with normal weight.
| BMI range | Model 1 |
Model 2 |
Model 3 |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| 5th to < 35th percentile | Referent | Referent | Referent |
| 35th to < 65th percentile | 1.832 (1.460–2.300) | 1.842 (1.467–2.313) | 1.851 (1.472–2.329) |
| 65th to < 85th percentile | 1.897 (1.479–2.433) | 1.922 (1.497–2.467) | 1.889 (1.467–2.431) |
| P value trend | <0.001 | <0.001 | <0.001 |
Model 1: non-adjusted model.
Model 2: adjusted for age, gender, and Hispanic ethnicity.
Model 3: adjusted for age, gender, Hispanic ethnicity, household smoker, vitamin D, and dietary fiber.
Note: 5th to <35th percentile: low-risk group for overweight; 35th to <65th percentile: middle-risk group for overweight; 65th to <85th percentile: high-risk for overweight.
Abbreviations: BMI, body mass index; OR, odds ratio; 95% CI, 95% confidence interval.
3.5. Association of BMI with the asthma persistence
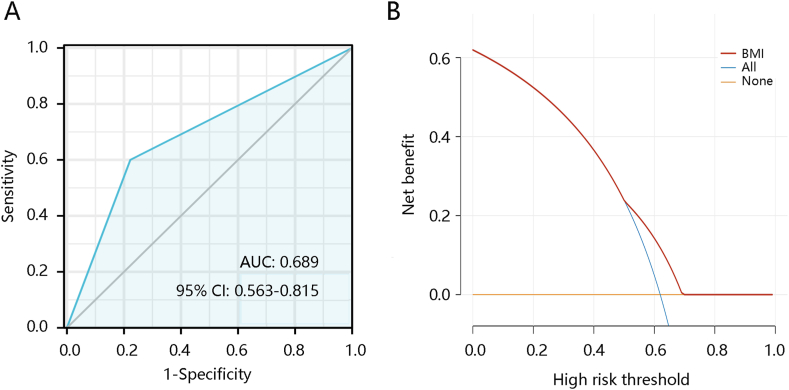
Owing to the significant ability of BMI in predicting asthma occurrence, we further analyzed its significance in predicting the persistence of asthma. Among the eligible participants with asthma (n = 753), 464 still had asthma and 282 did not have asthma after a year. The AUC of BMI for distinguishing participants who still had asthma from those without asthma was 0.689 (Fig. 4A). Fig. 4B shows the predictive ability of BMI. These findings indicate that BMI may play a vital role in predicting persistent asthma.
Fig. 4.
The ability of body mass index (BMI) in predicting asthma persistence. (A) Receiver operating characteristic analysis. (B) Decision curve analysis. Abbreviations: AUC, the area under the curve; 95% CI, 95% confidence interval; BMI, body mass index.
3.6. Risk factors for asthma persistence among overweight/obese and the high-risk group for obese individuals
Further, we focused on investigating potential risk factors related to asthma persistence among overweight/obese individuals and those with a high risk for obesity. Hispanic Ethnicity, and household income in adolescents without asthma were significantly different from those still with asthma. Adolescents still with asthma were more likely to be non-Hispanic black (40.89%). They had lower household income compared to those without asthma (P < 0.05) (Table 5).
Table 5.
Risk factors for asthma persistence among overweight/obese individuals.
| Variables | Adolescents without asthma (n = 111) | Adolescents still with asthma (n = 203) | P-value |
|---|---|---|---|
| Hispanic ethnicity | 0.001 | ||
| Mexican American | 33 (29.73) | 36 (17.73) | |
| Other Hispanic | 12 (10.81) | 11 (5.41) | |
| Non-Hispanic White | 27 (24.32) | 50 (24.63) | |
| Non-Hispanic Black | 22 (19.82) | 83 (40.89) | |
| Other Race | 17 (15.32) | 23 (11.33) | |
| Household income ($) | 0.008 | ||
| <20000 | 17 (15.9) | 49 (25.0) | |
| 20000-74999 | 55 (51.4) | 112 (57.1) | |
| ≥75000 | 35 (32.7) | 35 (17.9) | |
| Household smoker | 0.514 | ||
| No | 80 (73.39) | 139 (69.15) | |
| Yes | 29 (26.61) | 62 (30.85) | |
| Zinc (mg) | 7.66 [4.93, 12.11] | 8.16 [5.31, 11.73] | 0.667 |
| Iron (mg) | 11.80 [7.25, 16.99] | 11.04 [7.45, 17.12] | 0.996 |
| Calcium (mg) | 824.00 [533.00, 1206.00] | 804.00 [492.00, 1211.00] | 0.908 |
| Vitamin D (μg) | 4.00 [1.20, 6.60] | 3.20 [1.00, 6.50] | 0.232 |
| Total fat (g) | 55.24 [43.02, 89.91] | 58.81 [39.34, 88.49] | 0.920 |
| Dietary fiber (g) | 12.00 [7.70, 17.90] | 11.20 [7.10, 17.60] | 0.263 |
| Protein (g) | 62.99 [42.90, 82.41] | 62.40 [44.43, 90.95] | 0.390 |
| Energy (kcal) | 1638.00 [1229.00, 2273.00] | 1622.00 [1157.00, 2279.00] | 0.857 |
Note: Categorical variables are expressed as counts (percentages). Continuous variables are expressed as mean ± standard deviation or median (interquartile range). Statistical significance was set at P < 0.05.
4. Discussion
Asthma is one of the most common chronic diseases, involving airway remodeling and chronic inflammation [22]. It is a variable condition; hence, most patients with asthma experience either no symptoms for a long period or have relatively mild symptoms and then experience asthma attacks, which may be life-threatening [23]. Asthma prediction models provide a risk assessment for the future diagnosis of asthma in individuals without a prior diagnosis. The early models were rule-based systems based on the occurrence of early childhood wheezing episodes [24,25]. More recently, statistical models have been developed to identify preschoolers with asthma-like symptoms who are at high risk for future asthma diagnoses [26,27]. In this study, we constructed a nomogram model to predict asthma occurrence and found that BMI had comparable efficacy to the model in predicting asthma occurrence in adolescents aged 11–17 years. Moreover, BMI exhibited an acceptable performance in predicting asthma persistence, providing a potential guide for health strategies for its control and prevention in this population group.
We used data from the NHANES database (2011–2018) and conducted an initial univariate analysis to identify factors associated with asthma. Adolescents with asthma were more likely to be non-Hispanic black, followed by non-Hispanic white, consistent with Qu's study that non-Hispanic black children had a higher risk of asthma [8]. Similarly, Phuong et al. divided non-Hispanics into non-Hispanic white and non-Hispanic black and observed that non-Hispanic black was associated with an increased risk of childhood asthma, which may be attributed to economic status [28,29]. It has also been demonstrated that BMI is associated with adulthood asthma [30]. Fat-soluble vitamins A, D, and E exhibit various effects related to antioxidant and anti-inflammatory properties, lung development, and immune function [[31], [32], [33]]. Several studies have revealed the role of vitamins in asthma, and parental vitamin D supplementation may reduce susceptibility to asthma in the offspring [34,35]. In addition, low fiber intake is associated with increased systemic inflammation, contributing to asthma pathophysiology [36]. As expected, adolescents with asthma had higher BMIs but lower vitamin D and dietary fiber intake than those without asthma in this study. Drug costs are a major challenge in low and middle-income countries. Unavailable and unaffordable newer asthma treatments and approaches, and a lack of comprehensive health systems or research on the effectiveness and feasibility of implementation lead to a huge burden of asthma in these populations [37]. Although there was no significant difference in household income between the two groups among all the participants. Household income was significantly lower in obese and overweight individuals still with asthma compared to those without asthma. This might be due to participation bias.
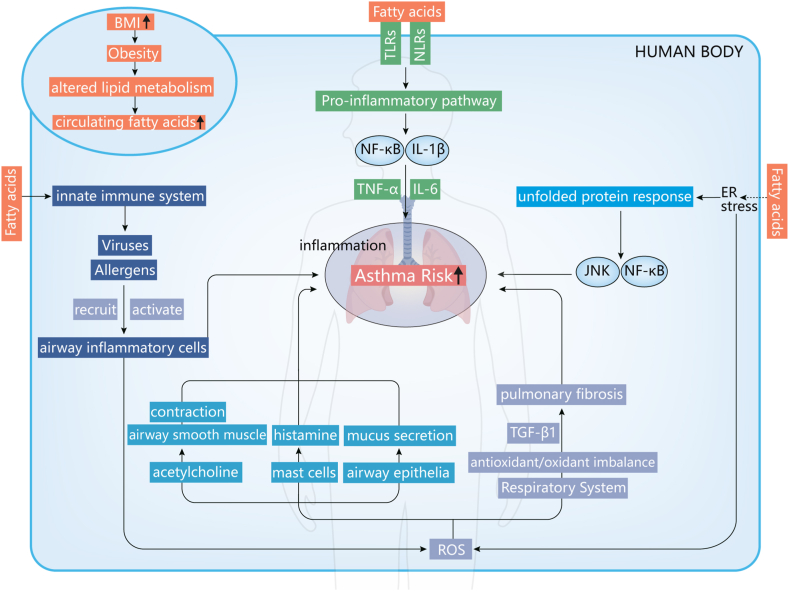
Subsequently, age, sex, household smoking status, Hispanic Ethnicity, BMI, vitamin D, and dietary fiber were integrated into multivariate logistic regression analysis, revealing that BMI and Hispanic Ethnicity were independent predictors of asthma occurrence. Another important finding of this study is the predictive role of BMI in adolescent asthma persistence. Obesity and excess fat accumulation may promote the occurrence and progression of asthma through metabolic, inflammatory, and mechanistic pathways [38]. Obese individuals have high circulating fatty acid levels because of altered lipid metabolism, inducing an inflammatory response and stimulating the innate immune system [39,40]. Fatty acids can stimulate the innate immune system by acting as ligands for pattern-recognition receptors. Patients with asthma have exaggerated immune responses to triggers such as allergens and viruses, which lead to the recruitment and activation of airway inflammatory cells and the release of reactive oxygen species (ROS). The binding of fatty acids to these receptors triggers proinflammatory pathways, including activation of nuclear factor κ-light-chain-enhancer of activated B cells (NF-κB) and release of interleukin-1β (IL-1β), promoting the release of cytokines such as IL-6 and tumor necrosis factor-α (TNF-α) [41]. Excess fatty acids can also be sensed within cells by organelles including the endoplasmic reticulum (ER). ER stress triggers the unfolded protein response, activating proinflammatory pathways including NF-Κb and c-Jun N-terminal kinase–activator protein 1 signaling pathway [42]. ER stress also accelerates ROS production, further amplifying the activity of NF-κB [43]. ROS induce airway smooth muscle contraction by increasing acetylcholine and promote histamine release by mast cells and mucus secretion by airway epithelial cells [44,45]. In addition, excessive ROS production may lead to a respiratory antioxidant/oxidant imbalance, leading to the emergence and persistence of transforming growth factor β1 (TGF-β1)-induced pulmonary fibrosis [46]. Therefore, adipose tissue might promote airway obstruction and affect lung function by secreting excessive proinflammatory factors such as IL-1β, TNF-α, IL-6, and TGF-β1 in the inflammatory and metabolic pathways, while the mechanical pathways affect lung function by increasing airway restriction, which promotes asthma [47]. The possible role of BMI in asthma is summarized in Fig. 5. The underlying mechanisms behind the association between BMI and asthma require validation in the future.
Fig. 5.
Possible mechanisms of body mass index involvement in asthma.
For strengths, we performed matching at a case-to-control ratio of 1:3 for better statistical power. Moreover, the novel argument focuses on just not the risk of asthma incidence but persistence. Understanding better about what characteristics are associated with long-term symptoms. However, the lack of association with household income between all enrolled participants with or without asthma might be related to participation bias, which should be validated in future research.
In conclusion, our study revealed that a high BMI is related to increased asthma occurrence and may play an essential role in predicting asthma persistence. Moreover, our study may help reduce asthma occurrence and improve disease control by changing lifestyle.
Consent for publication
Not applicable.
Data availability statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Funding
Not applicable.
Ethics approval
The Ethics Committee of Affiliated Hospital of Putian University agreed to submit the study for review and waived the need for ethical approval, as the data in this study are from public databases.
Author contributions
Ren-jie Li: Conceived and designed the experiments; Ren-jie Li, Ying-xu Wen: Performed the experiments; Ren-jie Li: Analyzed and interpreted the data; Ying-xu Wen: Contributed reagents, materials, analysis tools or data; Ren-jie Li, Ying-xu Wen: Wrote the paper
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Not applicable.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20092.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.National Asthma E., Prevention P. Expert panel report 3 (EPR-3): guidelines for the diagnosis and management of asthma-summary report 2007. J. Allergy Clin. Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. [DOI] [PubMed] [Google Scholar]
- 2.Song P., et al. Global, regional, and national prevalence of asthma in 2019: a systematic analysis and modelling study. J Glob Health. 2022;12 doi: 10.7189/jogh.12.04052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diseases G.B.D., Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L., et al. A dynamic nomogram for predicting the risk of asthma: development and validation in a database study. J. Clin. Lab. Anal. 2021;35(7) doi: 10.1002/jcla.23820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nurmagambetov T., Kuwahara R., Garbe P. The economic burden of asthma in the United States, 2008-2013. Ann Am Thorac Soc. 2018;15(3):348–356. doi: 10.1513/AnnalsATS.201703-259OC. [DOI] [PubMed] [Google Scholar]
- 6.Barnett S.B., Nurmagambetov T.A. Costs of asthma in the United States: 2002-2007. J. Allergy Clin. Immunol. 2011;127(1):145–152. doi: 10.1016/j.jaci.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Mukherjee M., et al. The epidemiology, healthcare and societal burden and costs of asthma in the UK and its member nations: analyses of standalone and linked national databases. BMC Med. 2016;14(1):113. doi: 10.1186/s12916-016-0657-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qu Y., et al. Dietary intake and asthma in preschoolers: a logistic lasso regression analysis. Front Pediatr. 2022;10 doi: 10.3389/fped.2022.870529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrante G., La Grutta S. The burden of pediatric asthma. Front Pediatr. 2018;6:186. doi: 10.3389/fped.2018.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCIH Statistics summary health statistics:national health interview survey. 2017. https://ftp.cdc.gov/pub/Health_Statistics/NCHS/NHIS/SHS/2017_SHS_Table_C-1.pdf Available from:
- 11.Cruz A.A., Stelmach R., Ponte E.V. Asthma prevalence and severity in low-resource communities. Curr. Opin. Allergy Clin. Immunol. 2017;17(3):188–193. doi: 10.1097/ACI.0000000000000360. [DOI] [PubMed] [Google Scholar]
- 12.Kothalawala D.M., et al. Development of childhood asthma prediction models using machine learning approaches. Clin. Transl. Allergy. 2021;11(9) doi: 10.1002/clt2.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Winck A.D., et al. Effects of obesity on lung volume and capacity in children and adolescents: a systematic review. Rev Paul Pediatr. 2016;34(4):510–517. doi: 10.1016/j.rppede.2016.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tosca M.A., et al. Obesity and asthma: an intriguing link in childhood and adolescence. Int. Arch. Allergy Immunol. 2021;182(12):1222–1225. doi: 10.1159/000517298. [DOI] [PubMed] [Google Scholar]
- 15.Lang D.M., et al. Physical activity in urban school-aged children with asthma. Pediatrics. 2004;113(4):e341–e346. doi: 10.1542/peds.113.4.e341. [DOI] [PubMed] [Google Scholar]
- 16.Elias B.C., et al. Factors associated with asthma in Brazilian adolescents: national adolescent school-based health survey (Pense-2012) Rev Paul Pediatr. 2019;37(4):406–413. doi: 10.1590/1984-0462/;2019;37;4;00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sears M.R. Predicting asthma outcomes. J. Allergy Clin. Immunol. 2015;136(4):829–836. doi: 10.1016/j.jaci.2015.04.048. ; quiz 837. [DOI] [PubMed] [Google Scholar]
- 18.Smit H.A., et al. Childhood asthma prediction models: a systematic review. Lancet Respir. Med. 2015;3(12):973–984. doi: 10.1016/S2213-2600(15)00428-2. [DOI] [PubMed] [Google Scholar]
- 19.Johnson C.L., et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat. 2013;2(161):1–24. [PubMed] [Google Scholar]
- 20.Kuczmarski R.J., et al. CDC growth charts: United States. Adv. Data. 2000;(314):1–27. [PubMed] [Google Scholar]
- 21.Shen T.C., et al. Association of interleukin-12a rs568408 with susceptibility to asthma in taiwan. Sci. Rep. 2017;7(1):3199. doi: 10.1038/s41598-017-03523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shang Y., et al. Exosomes from mmu_circ_0001359-modified ADSCs attenuate airway remodeling by enhancing FoxO 1 signaling-mediated M2-like macrophage activation. Mol. Ther. Nucleic Acids. 2020;19:951–960. doi: 10.1016/j.omtn.2019.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hussain Z., et al. Predicting the risk of asthma attacks in children, adolescents and adults: protocol for a machine learning algorithm derived from a primary care-based retrospective cohort. BMJ Open. 2020;10(7) doi: 10.1136/bmjopen-2019-036099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Castro-Rodriguez J.A. The Asthma Predictive Index: early diagnosis of asthma. Curr. Opin. Allergy Clin. Immunol. 2011;11(3):157–161. doi: 10.1097/ACI.0b013e3283464c4a. [DOI] [PubMed] [Google Scholar]
- 25.Amin P., et al. Optimum predictors of childhood asthma: persistent wheeze or the Asthma Predictive Index? J. Allergy Clin. Immunol. Pract. 2014;2(6):709–715. doi: 10.1016/j.jaip.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Martinez C.E., Sossa-Briceno M.P., Castro-Rodriguez J.A. Discriminative properties of two predictive indices for asthma diagnosis in a sample of preschoolers with recurrent wheezing. Pediatr. Pulmonol. 2011;46(12):1175–1181. doi: 10.1002/ppul.21493. [DOI] [PubMed] [Google Scholar]
- 27.van der Mark L.B., et al. Predicting asthma in preschool children at high risk presenting in primary care: development of a clinical asthma prediction score. Prim. Care Respir. J. 2014;23(1):52–59. doi: 10.4104/pcrj.2014.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vo P., et al. Individual factors, neighborhood social context and asthma at age 5 years. J. Asthma. 2017;54(3):265–272. doi: 10.1080/02770903.2016.1216563. [DOI] [PubMed] [Google Scholar]
- 29.Keet C.A., et al. Urban residence, neighborhood poverty, race/ethnicity, and asthma morbidity among children on Medicaid. J. Allergy Clin. Immunol. 2017;140(3):822–827. doi: 10.1016/j.jaci.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nwaru B.I., et al. Pubertal BMI change and adult-onset asthma in men: population-based cohort study in Sweden. Clin. Exp. Allergy. 2020;50(1):51–60. doi: 10.1111/cea.13534. [DOI] [PubMed] [Google Scholar]
- 31.Han Y.Y., et al. Diet and asthma: an update. Curr. Opin. Allergy Clin. Immunol. 2015;15(4):369–374. doi: 10.1097/ACI.0000000000000179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zosky G.R., et al. Vitamin D deficiency causes deficits in lung function and alters lung structure. Am. J. Respir. Crit. Care Med. 2011;183(10):1336–1343. doi: 10.1164/rccm.201010-1596OC. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Q. Natural forms of vitamin E: metabolism, antioxidant, and anti-inflammatory activities and their role in disease prevention and therapy. Free Radic. Biol. Med. 2014;72:76–90. doi: 10.1016/j.freeradbiomed.2014.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parr C.L., et al. Vitamin A and D intake in pregnancy, infant supplementation, and asthma development: the Norwegian Mother and Child Cohort. Am. J. Clin. Nutr. 2018;107(5):789–798. doi: 10.1093/ajcn/nqy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawes B.L., et al. Effect of vitamin D3 supplementation during pregnancy on risk of persistent wheeze in the offspring: a randomized clinical trial. JAMA. 2016;315(4):353–361. doi: 10.1001/jama.2015.18318. [DOI] [PubMed] [Google Scholar]
- 36.Berthon B.S., et al. Investigation of the association between dietary intake, disease severity and airway inflammation in asthma. Respirology. 2013;18(3):447–454. doi: 10.1111/resp.12015. [DOI] [PubMed] [Google Scholar]
- 37.Stolbrink M., et al. The availability, cost, and affordability of essential medicines for asthma and COPD in low-income and middle-income countries: a systematic review. Lancet Global Health. 2022;10(10):e1423–e1442. doi: 10.1016/S2214-109X(22)00330-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang W., et al. Association of weight change patterns across adulthood with incident asthma: a retrospective cohort study. Sci. Rep. 2022;12(1):9756. doi: 10.1038/s41598-022-13555-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hajer G.R., van Haeften T.W., Visseren F.L. Adipose tissue dysfunction in obesity, diabetes, and vascular diseases. Eur. Heart J. 2008;29(24):2959–2971. doi: 10.1093/eurheartj/ehn387. [DOI] [PubMed] [Google Scholar]
- 40.Hotamisligil G.S., Erbay E. Nutrient sensing and inflammation in metabolic diseases. Nat. Rev. Immunol. 2008;8(12):923–934. doi: 10.1038/nri2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vandanmagsar B., et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hotamisligil G.S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140(6):900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xue X., et al. Tumor necrosis factor alpha (TNFalpha) induces the unfolded protein response (UPR) in a reactive oxygen species (ROS)-dependent fashion, and the UPR counteracts ROS accumulation by TNFalpha. J. Biol. Chem. 2005;280(40):33917–33925. doi: 10.1074/jbc.M505818200. [DOI] [PubMed] [Google Scholar]
- 44.Cho Y.S., Moon H.B. The role of oxidative stress in the pathogenesis of asthma. Allergy Asthma Immunol Res. 2010;2(3):183–187. doi: 10.4168/aair.2010.2.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jesenak M., Zelieskova M., Babusikova E. Oxidative stress and bronchial asthma in children-causes or consequences? Front Pediatr. 2017;5:162. doi: 10.3389/fped.2017.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Liu Y.N., et al. Galangin attenuates airway remodelling by inhibiting TGF-beta1-mediated ROS generation and MAPK/Akt phosphorylation in asthma. Sci. Rep. 2015;5 doi: 10.1038/srep11758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bantula M., et al. Asthma and obesity: two diseases on the rise and bridged by inflammation. J. Clin. Med. 2021;10(2) doi: 10.3390/jcm10020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.