Purpose
The study aim to construct an effective model for predicting the survival period of COVID-19 patients. Methods: Clinical data of 386 COVID-19 patients were collected from December 2022 to January 2023. The patients were randomly divided into training and validation cohorts in a 7:3 ratio. LASSO regression and multivariate Cox regression analyses were used to identify prognostic factors, and a nomogram was constructed. Nomogram was evaluated using decision curve analysis, receiver operating characteristic curve, consistency index (c-index), and calibration curve. Results: 86 patients (22.3%) died. A new nomogram for predicting the survival was established based on age, resting oxygen saturation, Blood urea nitrogen (BUN), c-reactive protein-to-albumin ratio (CAR), and pneumonia visual score. The decision curve indicated high clinical applicability. The nomogram c-indexes in the training and validation cohorts were 0.846 and 0.81, respectively. The area under the curves (AUCs) for the 15-day and 30-day survival probabilities were 0.906 and 0.869 in the training cohort, and 0.851 and 0.843 in the validation cohort. The calibration curves demonstrated consistency between predicted and actual survival probabilities. Conclusions: Our nomogram has the capacity to assist clinical practitioners in estimating the survival rate of COVID-19 patients, thereby facilitating more optimal management strategies and therapeutic interventions with substantial clinical applicability.
Keywords: Coronavirus, COVID-19, Nomogram, Prediction, Survival
1. Introduction
The World Health Organization dubbed the SARS-CoV-2 infection “coronavirus disease 2019 (COVID-19)" after the discovery of a brand-new coronavirus (SARS-CoV-2) in December 2019 [1,2]. Since its development, COVID-19 has spread throughout China and the world, causing significant morbidity and mortality [3]. It poses a severe risk to public health, and healthcare systems worldwide are already under much strain. SARS-CoV-2 had been documented in 227 nations as of 30 March 2022, with more than 485 million confirmed cases of COVID-19 and more than 6 million deaths globally attributed to COVID-19 [4]. According to a Chinese Center for Disease Control and Prevention estimate, there are around 44,500 confirmed SARS-CoV-2 infections, with up to 15.8% of those patients in serious or critical condition [5]. COVID-19 pneumonia cases soared dramatically across the country after changes to China's national epidemic prevention strategy in December 2022 lifted the category A infectious diseases ban on COVID-19. The majority of COVID-19 individuals experience a minor sickness. However, some patients, particularly the elderly and those with concomitant conditions like diabetes, who are more likely to be impacted and develop severe respiratory disease, which increases mortality, have rapid deterioration [6,7]. Precise prognostic evaluation by clinical physicians facilitates the formulation of optimal treatment strategies. Moreover, it aids in resource allocation within healthcare institutions and fosters effective doctor-patient and doctor-family communication. Thus, it is imperative for clinicians to predict patients' outcomes upon admission, identifying those at heightened risk of unfavorable outcomes. Furthermore, provide active, supportive treatment to these patients to improve their prognosis.
A plethora of models or variables have been proposed in recent studies to predict the progression and outcome of COVID-19 patients. For instance, one study found that C-reactive protein (CRP) significantly increased in the early stages of developing severe disease, which could predict the severity and prognosis of patients in the early stage [8]. Another study found that combining the two parameters to create the neutrophil-to-lymphocyte ratio (NLR) can more accurately reflect systemic inflammation in the body and confirmed that it was an independent risk factor for death in critically ill patients [9,10]. Skeletal muscle function was previously used to identify COVID-19 patients with a bad prognosis [11,12]. Despite some studies exploring the predictive power of various variables and models in relation to COVID-19, a significant proportion of these investigations were conducted either in patient populations outside of China or prior to 2022. Therefore, it remains imperative to conduct additional research to determine the validity and clinical utility of these predictive models in the current context of the COVID-19 pandemic in China.
To address this, our research aims to apply a novel approach to develop and validate a straightforward and efficacious early prediction model for COVID-19 patients. This model will take into consideration a range of critical clinical factors, including age,sex, body mass index (BMI), Smoking history, chronic medical conditions, vital signs, pneumonia visual score, treatment regimen, and laboratory biochemical markers. This approach can aid doctors in identifying high-risk populations and enhancing intervention management, which will boost prognosis and lower mortality. Following the lifted of category A limitations on COVID-19 in China, this study is the first to propose a straightforward and useful early prognosis model for COVID-19 patients, which is in line with the clinical traits of the current new coronavirus strain in that country.
2. Methods
2.1. Patient selection
The present retrospective study was conducted at Zhongshan Hospital Xiamen University, and patient enrollment took place between December 2022 and January 2023. Here are the COVID-19 diagnostic standards: 1. the presence of COVID-19 infection-related clinical symptoms; 2. one or more of the pathogenic or serological findings listed below: (1) positive nucleic acid test for novel coronavirus; (2) positive antigen test for novel coronavirus; (3) positive novel coronavirus isolation and culture. (4) the level of novel coronavirus-specific IgG antibodies in recovery is at least four times higher than in the acute period. Patients with clinical classifications of medium size and higher were included in this investigation, and severe/critical high-risk groups were also included. A minimum follow-up period of 30 days was maintained for all recruited patients, and the final outcome was recorded as either survival or death. The Ethics Committee of Zhongshan Hospital Xiamen University approved this study (Ethics approval number:2023-086).
2.2. Data collection
For each patient, the following data were gathered: (1) general clinical characteristics of age, gender, body mass index (BMI), and smoking history; (2)vital signs, including heart rate, resting oxygen saturation, systolic blood pressure, temperature, and consciousness; (3) clinical symptoms, such as fever, cough, expectoration, fatigue, myalgia, and gastrointestinal symptoms; (4)A simple CT visual scoring method described by Giulia Besutti et al. [19]. was used to determine the severity of pneumonia, and the patients were divided into two groups based on the severity: <40% and ≥40%. (5) co-occurring diseases, such as hypertension, diabetes, chronic obstructive pulmonary disease (COPD),cardiovascular, chronic renal insufficiency, abnormal liver function, senile dementia, stroke, malignant tumor; (6) Laboratory tests included blood assays (e.g., leukocyte count, neutrophils count, lymphocytes count, monocyte count), inflammatory markers (procalcitonin (PCT), c-reactive protein (CRP), lactate dehydrogenase (LDH)), coagulation profile, liver and renal function (e.g., albumin (ALB), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatinine (Crea), blood urea nitrogen (BUN)), and cardiac enzymes. PLR (platelet-to-lymphocyte ratio), LMR (lymphocyte-to-monocyte ratio), NLR (neutrophil-to-lymphocyte ratio), and CAR (c-reactive protein-to-albumin ratio) are also included. (7)treatment and outcomes information. All cohort patients were randomly divided into two groups with a 7:3 ratio: the training cohort was used to build the prediction model, and the validation cohort was used to evaluate its performance.
2.3. Statistical analysis
Data analysis was performed using SPSS 22.0 software and R version 4.2.2. Normally distributed variables were described in terms of absolute number and percentage, mean, and standard deviation, while nonparametric variables were reported as median and interquartile range. Categorical variables were analyzed using chi-square or Fisher's exact test, while continuous variables were compared using t-test or Mann-Whitney U test. The study defined the period from admission to death or end of follow-up as survival time. In the training cohort, the least absolute shrinkage and selection operator (LASSO) logistic regression model was used to identify potential prognostic factors from all available indicators. These factors were then included in a multivariate Cox regression analysis to determine significant prognostic factors for COVID-19 survival. Using these significant factors, a short-term survival prediction model were developed and visualized using nomograms. The Harrell's concordance index (C-index), calibration curve, receiver operating characteristic (ROC) curve, and decision curve analysis (DCA) were used to evaluate the discriminatory ability of the survival prediction models. A significance level of P < 0.05 was used.
3. Results
3.1. Demographic and clinical characteristics of patients with COVID-19
The study enrolled 386 patients with COVID-19 who were admitted to Zhongshan Hospital at Xiamen University. Demographic and clinical data were collected and all patients were followed for at least 30 days after admission. The study cohort consisted of 251 male patients (65.0%) and 135 female patients (35.0%), with a mean age of 76 years (interquartile range (IQR): 65–84 years). The most common symptoms among patients were cough (85.8%), expectoration (72.3%), fever >37.3 °C (67.1%), and shortness of breath (36.3%). Less frequent symptoms included fatigue (16.6%), chest tightness (14.0%), gastrointestinal symptoms (13.5%), and myalgia (12.2%). Hypertension was the most common coexisting disease among patients (52.1%), followed by diabetes (34.7%). Other coexisting conditions such as cardiac disease, chronic renal insufficiency, malignant tumors, COPD, stroke, and abnormal liver function were present in 30.3%, 22.7%, 12.2%, 11.1%, 9.6%, and 9.3% of patients, respectively. Antiviral therapy was the most common treatment for hospitalized patients (59.6%), followed by anticoagulation (50.8%) and glucocorticoids (50.5%). Abnormal laboratory results included decreased lymphocyte count and increased levels of infection-related biomarkers (e.g. CRP, PCT, LDH, neutrophil, leukocyte); other abnormal laboratory findings included d-dimer, ALB, Troponin T, and NT-proBNP. Of the 386 patients enrolled, 86 died and 300 were discharged after treatment. The study cohort was randomly divided into a training group (70%) and a validation group (30%) with no significant differences in their demographic and clinical characteristics (P > 0.05). Table 1 summarizes the demographic and clinical data for both groups.
Table 1.
Demographic and Clinical Features, Treatment, Laboratory findings, and Outcomes of Patients in the Training and Validation Cohorts.
| Characteristics | Sum (n = 386) | Training Cohort (n = 270) | Validation Cohort (n = 116) | P | |
|---|---|---|---|---|---|
| Sex,n(%) | Female | 135(34.97) | 94(34.82) | 41(35.35) | 0.88 |
| Male | 251(65.03) | 176(65.18) | 75(64.65) | ||
| Age,median[IQR] | 76.00[65.00,84.00] | 75.00[65.00,84.00] | 76.00[66.00,83.00] | 0.74 | |
| Heart rate on Admission, median[IQR] | 89.00[78.00,103.00] | 91.00[79.00,104.00] | 88.00[78.00,99.00] | 0.15 | |
| Systolic blood pressure, median[IQR] | 130.00[115.00,144.00] | 130.00[114.00,145.00] | 130.00[116.00,141.00] | 0.76 | |
| Resting oxygen Saturation, median[IQR] | 96.00[94.00,98.00] | 96.00[94.00,98.00] | 96.00[94.00,98.00] | 0.77 | |
| Temperature, median[IQR] | 36.50[36.50,36.90] | 36.60[36.50,36.90] | 36.50[36.50,36.90] | 0.45 | |
| BMI,median[IQR] | 22.77[21.11,24.99] | 22.77[21.30,25.28] | 22.77[20.70,24.41] | 0.25 | |
| Consciousness,n(%) | Awake | 361(93.52) | 254(94.07) | 107(92.24) | 0.69 |
| Lethargic | 15(3.89) | 9(3.33) | 6(5.17) | ||
| Unconscious | 10(2.59) | 7(2.59) | 3(2.5) | ||
| Smoking history,n(%) | No | 334(86.53) | 234(86.67) | 100(86.21) | 0.90 |
| Yes | 52(13.47) | 36(13.33) | 16(13.79) | ||
| Pneumonia visual score,n(%) | <40% | 298(77.20) | 90(77.59) | 208(77.04) | 0.91 |
| ≥40% | 88(22.80) | 26(22.41) | 62(22.96) | ||
| Cough,n(%) | Yes | 331(85.75) | 233(86.30) | 98(84.48) | 0.64 |
| Expectoration,n(%) | Yes | 279(72.28) | 188(69.63) | 91(78.45) | 0.08 |
| Fever,n(%) | Yes | 259(67.10) | 186(68.89) | 73(62.93) | 0.25 |
| Shortness of breath,n (%) | Yes | 140(36.27) | 104(38.52) | 36(31.03) | 0.16 |
| Fatigue,n(%) | Yes | 64(16.58) | 41(15.19) | 23(19.83) | 0.26 |
| Chest tightness,n(%) | Yes | 54(13.99) | 38(14.07) | 16(13.79) | 0.94 |
| Gastrointestinal Symptoms,n(%) | Yes | 52(13.47) | 32(11.85) | 20(17.24) | 0.16 |
| Myalgia,n(%) | Yes | 47(12.18) | 32(11.85) | 15(12.93) | 0.77 |
| History of hypertension,n(%) | No | 185(47.93) | 131(48.52) | 54(46.55) | 0.72 |
| Yes | 201(52.07) | 139(51.48) | 62(53.45) | ||
| Diabetes history,n(%) | No | 252(65.28) | 182(67.41) | 70(60.34) | 0.18 |
| Yes | 134(34.72) | 88(32.59) | 46(39.66) | ||
| Cardiovascular disease,n(%) | No | 269(69.69) | 185(68.52) | 84(72.41) | 0.45 |
| Yes | 117(30.31) | 85(31.48) | 32(27.59) | ||
| COPD,n(%) | No | 343(88.86) | 235(87.04) | 108(93.10) | 0.08 |
| Yes | 43(11.14) | 35(12.96) | 8(6.90) | ||
| Chronic renal insufficiency,n(%) | No | 298(77.20) | 208(77.04) | 90(77.59) | 0.91 |
| Yes | 88(22.80) | 62(22.96) | 26(22.41) | ||
| Abnormal liver function,n(%) | No | 350(90.67) | 247(91.48) | 103(88.79) | 0.41 |
| Yes | 36(9.33) | 23(8.52) | 13(11.21) | ||
| Senile dementia,n(%) | No | 374(96.89) | 260(96.30) | 114(98.28) | 0.30 |
| Yes | 12(3.11) | 10(3.70) | 2(1.72) | ||
| Stroke,n(%) | No | 349(90.42) | 245(90.74) | 104(89.66) | 0.74 |
| Yes | 37(9.58) | 25(9.26) | 12(10.34) | ||
| Malignant tumor,n(%) | No | 339(87.82) | 239(88.52) | 100(86.21) | 0.52 |
| Yes | 47(12.17) | 31(11.48) | 16(13.79) | ||
| Antiviral therapy,n(%) | No | 156(40.42) | 107(39.63) | 49(42.24) | 0.63 |
| Yes | 230(59.59) | 163(60.37) | 67(57.76) | ||
| Glucocorticoids,n(%) | No | 191(49.48) | 135(50.00) | 56(48.28) | 0.76 |
| Yes | 195(50.52) | 135(50.00) | 60(51.72) | ||
| Anticoagulation,n(%) | No | 190(49.22) | 137(50.74) | 53(45.69) | 0.36 |
| Yes | 196(50.78) | 133(49.26) | 63(54.31) | ||
| Prone positioning,n(%) | No | 281(72.80) | 201(74.44) | 80(68.97) | 0.27 |
| Yes | 105(27.20) | 69(25.56) | 36(31.03) | ||
| Thymalfasin,n(%) | No | 280(72.54) | 193(71.48) | 87(75.00) | 0.48 |
| Yes | 106(27.46) | 77(28.52) | 29(25.00) | ||
| Leukocyte, 109/L median[IQR] | 6.75[4.98,9.70] | 6.75[4.98,9.54] | 6.79[4.99,9.73] | 0.88 | |
| Neutrophil, 109/L median[IQR] | 5.10[3.36,7.81] | 4.96[3.28,7.81] | 5.23[3.65,7.71] | 0.58 | |
| Lymphocyte, 109/L median[IQR] | 0.93[0.62,1.41] | 0.94[0.62,1.38] | 0.90[0.62,1.49] | 0.60 | |
| Monocyte, 109/L median[IQR] | 0.50[0.33,0.75] | 0.50[0.34,0.76] | 0.49[0.33,0.70] | 0.48 | |
| Hemoglobing g/L,median[IQR] | 123.00[108.00,137.00] | 125.00[109.00,138.00] | 121.00[108.00,136.00] | 0.25 | |
| Platelets, 109/L median[IQR] | 178.00[128.00,233.00] | 180.00[128.00,234.00] | 178.00[130.00,225.00] | 0.52 | |
| PLR,median[IQR] | 189.04[120.00,290.51] | 190.27[119.85,291.49] | 187.91[124.68,277.78] | 0.79 | |
| NLR,median[IQR] | 5.42[2.99,10.46] | 5.42[2.69,10.12] | 5.72[3.46,10.58] | 0.45 | |
| CAR,median[IQR] | 2.06[0.49,3.92] | 2.01[0.46,4.00] | 2.09[0.67,3.73] | 0.64 | |
| LMR,median[IQR] | 2.00[1.13,3.30] | 1.96[1.13,3.33] | 2.12[1.14,3.23] | 0.90 | |
| D-dimer,mg/L median[IQR] | 0.94[0.48,2.07] | 0.94[0.47,2.05] | 0.94[0.60,2.14] | 0.60 | |
| NT-proBNP,ng/L median[IQR] | 565.52[151.67,2168.00] | 588.00[164.00,2285.00] | 556.00[147.00,1859.00] | 0.70 | |
| Troponin T,ng/L median[IQR] | 21.70[10.70,54.90] | 20.66[10.70,57.60] | 24.50[12.50,49.00] | 0.39 | |
| CRP,mg/L median[IQR] | 70.98[18.68,131.28] | 70.83[16.05,134.14] | 71.27[23.23,128.55] | 0.61 | |
| PCT,ng/mL median[IQR] | 0.16[0.07,0.63] | 0.16[0.07,0.60] | 0.16[0.08,0.83] | 0.22 | |
| ALB,g/L median[IQR] | 35.20[31.70,38.90] | 35.40[31.90,38.90] | 34.90[31.40,37.90] | 0.24 | |
| AST,U/L median[IQR] | 35.10[25.30,51.60] | 34.80[25.30,49.80] | 36.30[24.80,55.60] | 0.65 | |
| ALT,U/L median[IQR] | 21.90[14.20,35.60] | 22.00[13.90,35.40] | 21.30[14.90,39.50] | 0.61 | |
| ALP,U/L median[IQR] | 74.00[57.60,92.60] | 74.70[58.50,93.00] | 70.80[56.50,90.30] | 0.44 | |
| Crea, umol/L median[IQR] | 82.00[64.00,119.20] | 81.70[63.10,116.10] | 82.90[64.60,122.00] | 0.52 | |
| BUN,mmol/L median[IQR] | 6.50[4.70,10.70] | 6.50[4.70,10.50] | 6.50[4.80,11.30] | 0.95 | |
| CK,U/L median[IQR] | 135.60[75.00,253.50] | 139.20[72.90,250.30] | 133.70[77.80,273.00] | 0.65 | |
| CKMB,U/L median[IQR] | 13.50[10.00,19.50] | 13.20[10.00,18.10] | 14.50[10.00,23.00] | 0.25 | |
| LDH,U/L median[IQR] | 268.09[208.10,347.90] | 263.20[205.60,338.38] | 281.90[209.40,361.70] | 0.37 | |
| Outcome,n(%) | Discharged | 300(77.72) | 210(77.78) | 90(77.59) | 0.97 |
| Died | 86(22.28) | 60(22.22) | 26(22.41) | ||
| Hospital stay,d mean(±SD) | 10.37 ± 6.45 | 10.02 ± 6.44 | 11.17 ± 6.40 | 0.12 | |
| Hospital costs, yuan median[IQR] | 11773.39[6338.00,19778.00] | 11040.00[6075.00,19080.55] | 13262.00[7016.00,20650.00] | 0.31 |
Abbreviations: BMI, body mass index; COPD, chronic obstructive pulmonary disease; PLR, platelet-to-lymphocyte ratio; NLR,neutrophil-to-lymphocyte ratio; CAR,c-reactive protein-to-albumin ratio; LMR,lymphocyte-to-monocyte ratio; CRP,C-reactive protein; PCT,procalcitonin; ALB,albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; ALP, Alkaline phosphatase; Crea, creatinine; BUN, blood urea nitrogen; CK.creatine kinase; CKMB, Creatine Kinase Isoenzyme; LDH, lactate dehydrogenase; IQR, interquartile range.
3.2. Assessing prognostic factors for COVID-19 patient survival
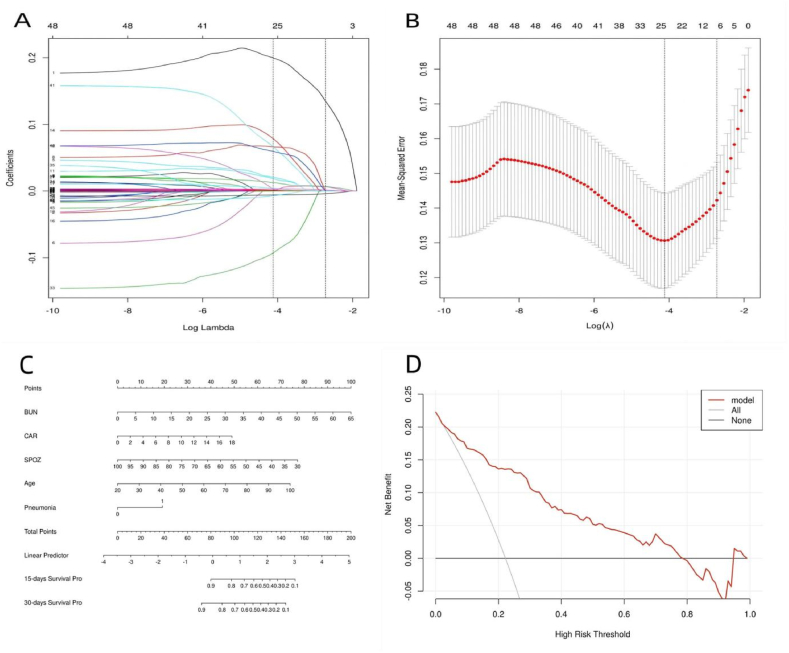
In this study, we aimed to identify the prognostic factors associated with the survival of COVID-19 patients. To this end, we analyzed 270 patient records that included demographic information, vital signs, medical history, treatment information, and laboratory findings. Our analysis using LASSO regression showed that factors such as age, resting oxygen saturation, blood urea nitrogen (BUN), lactate dehydrogenase (LDH), c-reactive protein-to-albumin ratio (CAR), and pneumonia visual score were significant predictors of survival (Fig. 1A and B). These factors were subsequently incorporated into multivariate Cox regression analyses (Table 2), which revealed that age, resting oxygen saturation, BUN levels, CAR levels, and pneumonia visual scores were key determinants of the survival of patients with COVID-19.
Fig. 1.
(A) LASSO coefficient profiles of the all indicators. (B) Ten-fold cross-validation for tuning parameter selection in the LASSO model.(C) Nomogram for Predicting Short-Term Survival of COVID-19 Patients. (D)Decision curve analysis compares the net clinical benefits of three scenarios in predicting patient survival: a perfect prediction model (gray line), not screening patients at all (horizontal solid black line), and screening based on the nomogram (red thick dash line).
Table 2.
Multivariate cox analysis of potential prognostic factors identified by LASSO regression in the training cohort.
|
Factors |
Coefficients | Hazard Ratio (95%CI) | P | ||
|---|---|---|---|---|---|
| Resting oxygen saturation | −0.038 | 0.963(0.936,0.990) | 0.008 | ||
| Age | 0.040 | 1.041(1.014,1.069) | 0.002 | ||
| BUN | 0.064 | 1.066(1.038,1.095) | 0.000 | ||
| LDH | 0.000 | 1(1.000,1.001) | 0.240 | ||
| CAR | 0.101 | 1.107(1.014,1.208) | 0.023 | ||
| Pneumonia visual score | |||||
| <40% | Reference | ||||
| ≥40% | 0.79 | 2.203 | 1.247 | 3.891 | 0.007 |
Abbreviations:BUN, blood urea nitrogen; CAR,c-reactive protein-to-albumin ratio.
LHD, lactate dehydrogenase; CI, confidence interval.
3.3. Construction of survival nomogram
A final Cox regression analysis was conducted to evaluate the impact of five independent predictors (age, resting oxygen saturation, BUN, CAR, and pneumonia visual score) on the likelihood of survival in COVID-19 patients. The results of the analysis are presented in Table 3 and demonstrate the fitted coefficients and hazard ratios of each predictor in the model. Based on these results, a predictive nomogram was developed to estimate the 15-day and 30-day probabilities of survival for patients with COVID-19 (Fig. 1C). To use the nomogram in clinical practice, an individual patient's values for each predictor are plotted on the corresponding variable axis. The number of points received for each variable value is calculated by plotting a line upward, and the sum of these scores is located on the total points axis. This total score can then be used to determine the 15-day and 30-day survival probabilities by drawing a straight line. To evaluate the clinical applicability of our risk prediction nomogram, decision curve analysis (DCA) was performed. The results suggest that the model was clinical applicability in predicting the of survival of COVID-19 patients (Fig. 1D).
Table 3.
Coefficients, Hazard Ratios, and 95% Confidence Intervals of the five Predictors in the Final Model.
|
Factors |
Coefficients | Hazard Ratio (95%CI) | P |
|---|---|---|---|
| Resting oxygen saturation | −0.044 | 0.957(0.933,0.982) | 0.001 |
| Age | 0.040 | 1.041(1.014,1.069) | 0.002 |
| BUN | 0.064 | 1.067(1.039,1.095) | 0.000 |
| CAR | 0.112 | 1.119(1.029,1.215) | 0.008 |
| Pneumonia visual score | |||
| <40% | Reference | ||
| ≥40% | 0.827 | 2.287(1.014,1.069) | 0.004 |
Abbreviations:BUN, blood urea nitrogen; CAR,c-reactive protein-to-albumin ratio; CI, confidence interval.
3.4. Evaluation of the survival nomogram
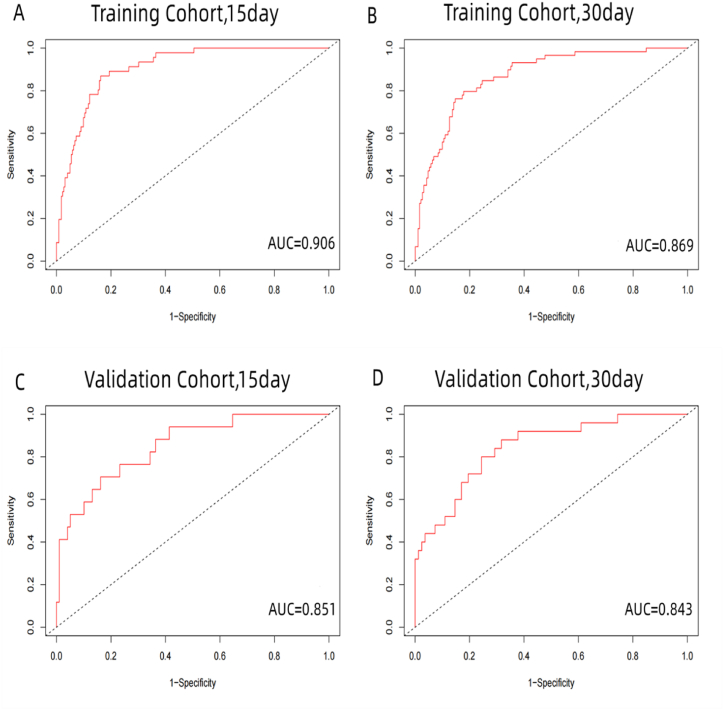
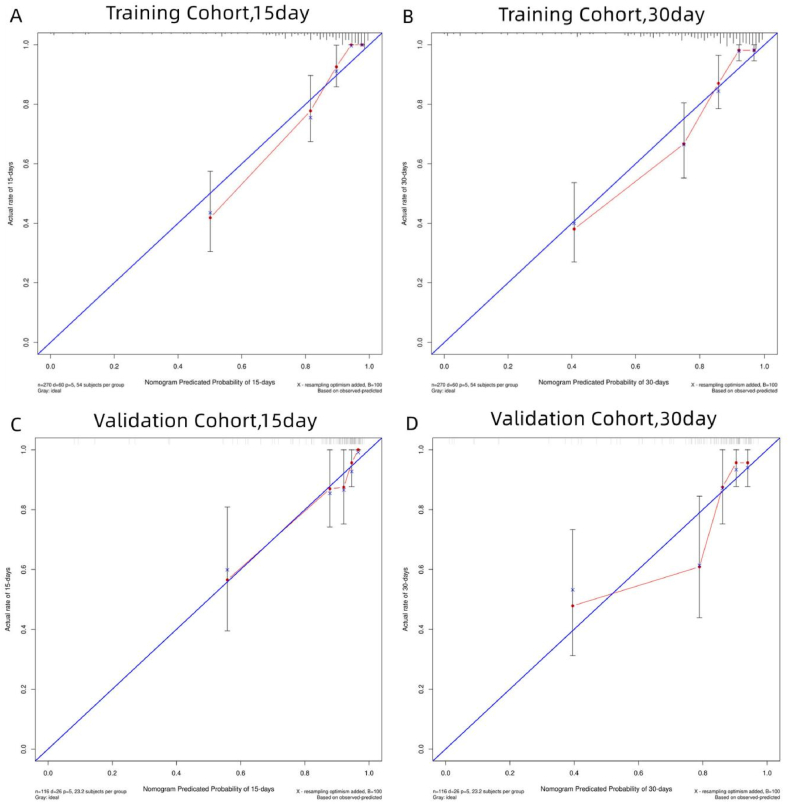
The results of our predictive model demonstrate its efficacy in predicting patient survival. In the training cohort, the c-index was found to be 0.846, and in the validation cohort, it was 0.810 (Table 4). To further assess the performance of the model, Receiver Operating Characteristic (ROC) curves were plotted for 15- and 30-day survival, yielding an area under the curve (AUC) of 0.906 and 0.869 in the training cohort, and 0.851 and 0.843 in the validation cohort (Fig. 2A–D). These results indicate that the predictive model has a high level of discrimination. Calibration plots, comparing the predicted survival probabilities to actual observations, showed good agreement in both the training and validation cohorts, indicating a well-calibrated model (Fig. 3A–D).
Table 4.
Performance of nomogram for prediction of 15-day and 30-day survival.
|
Cohort |
AUC |
AUC |
|
|---|---|---|---|
| C-Index | 15- Day | 30- Day | |
| Training cohort (n = 270) | 0.846 | 90.61 | 86.90 |
| Validation cohort (n = 116) | 0.81 | 85.09 | 84.34 |
Abbreviations: AUC, area under the receiver operating characteristic curve; C-index, concordance index.
Fig. 2.
The ROC curve and AUC of the nomogram in the training and validation cohort. A and B indicate the ROC curve and AUC of the nomogram in predicting 15- and30-day survival in the training cohort, while C and D illustrate 15-and 30-day survival prediction in the validation cohort. Abbreviations: AUC, area under the ROC curve; ROC, receiver operating characteristic.
Fig. 3.
Calibration plot of the nomogram in the training and validation cohorts. The calibration plot for predicting 15-day (A) and 30-day (B) survival in the training cohort and for predicting 15-day (C) and 30-day (D) survival in the validation cohort.
4. Discussion
The novel coronavirus (SARS-CoV-2) is the causative agent of COVID-19, an acute infectious disease characterized by its rapid transmission and the substantial impact it has on human health and healthcare systems [13]. Certain demographic groups, including those with pre-existing medical conditions, possess a heightened vulnerability to acquiring infections and tend to exhibit more severe outcomes, including mortalities [14]. In an effort to better understand the impact of COVID-19 on human health, this study endeavors to establish a predictive model for the survival outcomes of patients diagnosed with the virus. The model is a well-performing nomogram that is easy to use and has good discrimination ability, accuracy, and clinical utility. This model can aid in the early detection of high-risk patients, prompt early intervention and treatment, and optimize the allocation and utilization of hospital beds and medical resources rationally and efficiently, thus mitigating resource shortages and improving patient outcomes.
Numerous studies have reported the risk factors associated with severe COVID-19 cases [15,16]. However, most of these studies were based on patient populations outside China or before 2022 and may not accurately reflect the current pathogenicity of COVID-19. This study aimed to identify the significant factors related to the survival rate of COVID-19 by analyzing the clinical data of 386 COVID-19 patients collected from Zhongshan Hospital Xiamen University between December 2022 and January 2023 using LASSO regression and multivariate Cox regression analysis. The results indicated that age, oxygen saturation, BUN, CAR, and pneumonia visual score were significantly associated with the survival rate and were utilized to develop a prognostic nomogram. To evaluate the nomogram's clinical applicability, decision curve analysis (DCA) was done. Evaluation using the c-index, AUC values, and calibration plots demonstrated good discrimination ability and calibration performance in predicting the 15-day and 30-day survival probabilities of COVID-19 patients, indicating its satisfactory performance.
Furthermore, our nomogram only includes five easily accessible features, including age, oxygen saturation, BUN, CAR, and pneumonia visual score. Clinical research has demonstrated that aged people are more susceptible to bad results. Elderly people are more likely to contract COVID-19 [17], in addition, elderly individuals are also more prone to developing complications such as respiratory difficulties, low blood pressure, and cardiac arrhythmias, which could result in severe consequences. Therefore, these patients should be highly prioritized to improve their health and survival outcomes. The Diagnosis and Treatment Guidelines for Novel Coronavirus Infection suggest that patients with a resting oxygen saturation ≤93% should be defined as severe cases [18], indicating that patients with low oxygen saturation have a more serious condition and poorer prognosis. Pulmonary CT images provide a direct representation of the severity of pneumonia. Numerous articles have reported on visual quantification scores for COVID-19 patients, concluding that patients with higher scores for pneumonia have poorer clinical outcomes [19,20]. Visual, quantitative analysis based on CT images demonstrates high consistency, diagnostic solid capability, and the ability to reflect clinical classification. When combined with clinical information, it can accurately assess the clinical severity of COVID-19 and guide clinical treatment.
We have observed a significant correlation between elevated levels of BUN and poor prognosis among COVID-19 patients. Previous literature reports evidence of renal disease in over 40% of patients, with over 13% had elevated serum creatinine and urea nitrogen values [21]. Notably, the presence of renal disease is associated with higher hospital mortality rates [22]. These patients exhibit a pro-inflammatory state, defects in innate and adaptive immune cell populations [23], and a higher risk of upper respiratory tract infection and pneumonia [24]. In fact, COVID-19 and renal disease are interrelated; firstly, the novel coronavirus may directly cause cellular injury to renal tissue [25]; secondly, viral antigen-immune complexes deposition or viral-induced specific immune effect mechanisms may impair the kidney; and thirdly, viral-induced cytokines or mediators may indirectly impact renal tissue such as hypoxia, shock, and rhabdomyolysis [26]. Therefore, clinicians should pay attention to elevated renal disease markers such as BUN among hospitalized COVID-19 patients. Early detection and effective intervention of renal involvement may help reduce COVID-19 patient mortality.
Previous research has found that patients with higher levels of inflammation at the time of admission are more likely to develop severe COVID-19 [27]. High cytokine and chemokine production in these patients causes deregulation of the innate immune system and attracts inflammatory cells that enter lung tissue and cause immunological damage [28]. As a result, inflammation is a predictor of severity and prognosis in these patients. CAR (c-reactive protein-to-albumin ratio) is a marker of inflammation; C-reactive protein (CRP) is a signature inflammatory factor produced in the liver that rises fast in response to cellular injury and infection. In contrast, albumin is a non-inflammatory substance produced in the liver that does not increase in response to inflammation. As a result, the CRP/albumin ratio (CAR) is thought to be a more reliable measure of inflammatory conditions. It can assess the severity of disorders such as coronary artery disease, infection, and malignancy [15]. CRP, PCT, LDH, PLR, LMR, NLR, and CAR were all included in this investigation. CAR was found to be a risk factor for poor prognosis in patients with COVID-19, and patients with lower CAR had higher survival.
This study has several limitations. Firstly, this is a single-center retrospective study, and there may be some unavoidable biases. Clinical outcomes for patients under different medical conditions may differ. Secondly, the patients in this study were recruited during the peak of the COVID-19 outbreak when the epidemic was fully lifted, medical resources were scarce and clinical physicians had limited treatment experience in China. Thirdly, the length of hospital stay and end-of-life status of the patients are influenced by other factors such as economics, family, and social factors. Fourthly, our study followed up with patients for 30 days. Some patients have yet to be discharged, and their condition may change anytime. Long-term survival outcomes require further study.
Despite these limitations, the results of this study indicate that our nomogram is capable of predicting the prognosis of COVID-19 patients and has good discrimination and calibration abilities. The model can optimally utilize medical resources and provide appropriate care and interventions to patients, thereby improving prognosis and reducing mortality rates. Nevertheless, additional investigations are warranted to evaluate the practical feasibility of the nomogram in real-world settings.
Ethics statement
This study was approved by the Ethics Committee of Zhongshan Hospital Xiamen University (Ethics approval number:2023-086). Patients were consented by an informed consent process that was reviewed by the Ethics Committee of Zhongshan Hospital Xiamen University and certify that the study was performed in accordance with the ethical standards as laid down in the Declaration of Helsinki.
Funding source declaration
This work was supported by the projects of Xiamen Science and Technology Commission (3502Z20209028).
Author contribution statement
Jinxin Xu: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Wenshan Zhang: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yingjie Cai: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Jingping Lin: Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Chun Yan: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Meirong Bai: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data.
Yunpeng Cao: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Sunkui Ke: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Yali Liu: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20137.
Contributor Information
Sunkui Ke, Email: xmzsksk2021@163.com.
Yali Liu, Email: Elliy@163.com.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu Y., Gayle A.A., Wilder-Smith A., et al. The reproductive number of COVID-19 is higher compared to SARS coronavirus. J. Trav. Med. 2020;27 doi: 10.1093/jtm/taaa021. taaa021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 coronavirus pandemic. Available online. at: https://wwwworldometersinfo/coronavirus/(accessed March 30, 2022).
- 5.Zheng Yi, Chang Xiong, Liu Yuquan, et al. Epidemiological and clinical characteristics analysis of COVID-19 in the surrounding areas of Wuhan, Hubei Province in 2020. Pharmacol. Res. 2020 Jul;157 doi: 10.1016/j.phrs.2020.104821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen N., Zhou M., Dong X., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Escadafal C., Incardona S., Fernandez-Carballo B.L., et al. The goodand the bad: using C reactive protein to distinguish bacterial fromnon-bacterial infection among febrile patients in low-resourcesettings. BMJ Glob. Health. 2020;5(5) doi: 10.1136/bmjgh-2020-002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Adamstein N.H., Macfadyen J.G., Rose L.M., et al. The neutrophil-lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur. Heart J. 2021;42(9):896–903. doi: 10.1093/eurheartj/ehaa1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kerboua K.N. A cost-effective nomogram to guide therapeutic interventions in COVID-19. Immunol. Invest. 2021;50(1):92–100. doi: 10.1080/08820139.2020.1773850. [DOI] [PubMed] [Google Scholar]
- 11.Ufuk Furkan, Demirci Mahmut, Sagtaset al Ergin. The prognostic value of pneumonia severity score and pectoralis muscle Area on chest CT in adult COVID-19 patients. Eur. J. Radiol. 2020 Oct;131 doi: 10.1016/j.ejrad.2020.109271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Amy Attaway, Welch Nicole, Dasarathy Dhweeja, et al. Acute skeletal muscle loss in SARS-CoV-2 infection contributes to poor clinical outcomes in COVID-19 patients. J Cachexia Sarcopenia Muscle. 2022 Oct;13(5):2436–2446. doi: 10.1002/jcsm.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhu F., Cao Y., Xu S., et al. Reply to comments on”Co-infection of SARS-CoV-2 and HIV in a patient in Wuhan city, China. J. Med. Virol. 2020 Apr:8. doi: 10.1002/jmv.25838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu X.-W., Wu X.-X., Jiang X.-G., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zavalaga-Zegarra Hernán J., Palomino-Gutierrez Juan J., Ulloque-Badaracco Juan R., et al. C-reactive protein-to-albumin ratio and clinical outcomes in COVID-19 patients: a Systematic review and meta- AnalysisTrop Med Infect Dis. 2022 Aug 16;7(8):186. doi: 10.3390/tropicalmed7080186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu Bin, Song Ke-Han, Yao Yi, et al. Individualized model for predicting COVID-19 deterioration in patients with cancer: a multicenter retrospective study. Cancer Sci. 2021 Jun;112(6):2522–2532. doi: 10.1111/cas.14882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roberta Eduarda Grolli, Maiqueli Eduarda Dama Mingoti, Amanda Gollo Bertollo, et al. Impact of COVID-19 in the mental health in elderly: psychological and biological updates. Mol. Neurobiol. 2021 May;58(5):1905–1916. doi: 10.1007/s12035-020-02249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.General Office of National Health Commission of the People's Republic of China . Trial tenth ed. vol. 18. February 2023. General comprehensive affairs department of national administration of traditional Chinese medicine of the people's Republic of China.new coronavirus infection diagnosis and treatment plan. (China Medicine). 2. [Google Scholar]
- 19.Besutti Giulia, Rossi Paolo Giorgi, Iotti Valentina, et al. Accuracy of CT in a cohort of symptomatic patients with suspected COVID-19 pneumonia during the outbreak peak in Italy. Eur. Radiol. 2020 Dec;30(12):6818–6827. doi: 10.1007/s00330-020-07050-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chung M., Bernheim A., Mei X., et al. CT imaging features of 2019 novel coronavirus (2019-nCoV) Radiology. 2020 Apr;295(1):202–207. doi: 10.1148/radiol.2020200230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng Yichun, Luo Ran, Wang Kun, et al. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020 May;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Congjie, Sun Huiyuan, Li Xinna, et al. Development and validation of a nomogram for the early prediction of acute kidney injury in hospitalized COVID-19 patients. Front. Public Health. 2022 Nov 24;10 doi: 10.3389/fpubh.2022.1047073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Betjes M.G. Immune cell dysfunction and inflammation in end-stage renal disease. Nat. Rev. Nephrol. 2013;9:255–265. doi: 10.1038/nrneph.2013.44. [DOI] [PubMed] [Google Scholar]
- 24.Sibbel S., Sato R., Hunt A. The clinical and economic burden of pneumonia in patients enrolled in Medicare receiving dialysis: a retrospective, observational cohort study. BMC Nephrol. 2016;17:199. doi: 10.1186/s12882-016-0412-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peiris J.S.M., Chu C.M., Cheng V.C.C. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A., Zarychanski R., Pinto R. Critically ill patients with 2009 influenza A(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 27.Gong Jiao, Ou Jingyi, Qiu Xueping, et al. A tool for early prediction of severe coronavirus disease 2019 (COVID-19): a multicenter study using the risk nomogram in Wuhan and Guangdong, China. Clin. Infect. Dis. 2020 Jul 28;71(15):833–840. doi: 10.1093/cid/ciaa443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhary Shalki, Sharma Kajal, Silakari Om. The interplay between inflammatory pathways and COVID-19: a critical review on pathogenesis and therapeutic options. Microb. Pathog. 2021 Jan;150 doi: 10.1016/j.micpath.2020.104673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.