Abstract
Acinic cell carcinoma (ACCA), a type of malignant epithelial neoplasm, tends to occur in the parotid gland, and is occasionally found within the breast. Published literature regarding primary ACCA of the breast is scarce, and the number of reports may be fewer than 100. At present, full clinical details have not been published. As an extremely rare disorder, ACCA cannot be definitively diagnosed depending on microscopic structure alone and often requires the assistance of immunohistochemistry. Currently, universal therapies are not available. Here, we present a 47-year-old patient with a history of a palpable mass in the outer upper quadrant of the left breast for more than 2 years, which had obviously increased in size in the last half year. This patient was definitively diagnosed with primary ACCA of the breast. Neoadjuvant chemotherapy was performed preoperatively, and drug sensitivity tests based on primary tumor cells were conducted after surgery and successfully screened chemotherapy schemes for the patient's greater benefit. The whole treatment course followed the guidelines for invasive breast cancer. The patient was free of symptoms for 14 months after surgery. Long-term follow-up is in progress. Altogether, to further broaden the understanding of primary ACCA of the breast, we detail the diagnosis and treatment of one patient and review the relevant literature.
Keywords: Acinic cell carcinoma, Breast cancer, Primary acinic cell carcinoma of the breast, ACCA
1. Introduction
Acinic cell carcinoma (ACCA), a type of malignant epithelial neoplasm exhibiting acinic cell differentiation, exhibits a predilection to occur in the parotid gland and is found within the breast. Published English literature regarding cases of primary ACCA of the breast is scare, and the number of reports may even be fewer than 100; this remains a significant barrier for the treatment of such patients [1]. Primary ACCA of the breast was classified as a special type of breast cancer by WHO 2003, and the 2012 version began to classify it as a subtype of triple-negative breast cancer (TNBC) [2,3]. This classification was also used in the latest fifth edition of the WHO classification of breast tumors [4].
Clinically, the presentation of breast ACCA is indistinguishable from other types of breast cancer. Its ultrastructural and immunohistochemical features share unique similarity to ACCA of the parotid gland [5]. Histologically, ACCA of the breast is characterized by small acinar structures and diffuse infiltrative growth. Those clear or granular epithelial cells, when viewed under the microscope, are arranged in a microglandule and solid manner, and zymogen granules can be seen in the cytoplasm of partial cells. Immunohistochemically, the majority of tumor cells express lysozyme, cytokeratin (CK), epithelial membrane antigen (EMA), and S-100. In the present case, gross cystic disease fluid protein 15 (GCDFP-15) was positive locally. Periodic acid Schiff (PAS) staining was also positive, while estrogen receptor (ER), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2) and myoepithelial markers, such as calponin and p63, were negative [6].
2. Case presentation
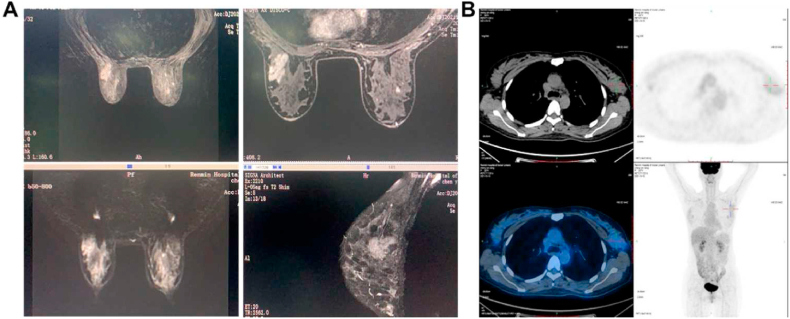
A 47-year-old woman with no previous medical history self-reported a palpable mass in the outer upper quadrant of the left breast that had been present for more than 2 years, and the mass had obviously increased in size over the last half year. The patient had no relevant family history or history of previous surgery. Regular color Doppler ultrasound revealed an irregular low echo area in the left breast, and the axillary lymph node showed multiple echo structures (Fig. 1A). The molybdenum target showed a distorted structure in the upper outer quadrant of the left mammary gland with spotty calcium salt deposition (Fig. 1B). MRI also confirmed the presence of a mass (Fig. 2A). Positron emission tomography with integrated computed tomography (PET-CT) showed that the cross sectional size of the mass was approximately 4.1 cm x 2.7 cm x 2.9 cm. Whole-body PET imaging demonstrated no bone, lung, liver, or brain metastasis (Fig. 2B).
Fig. 1.
Preoperative subsidiary exams (first part). (A) Regular color Doppler ultrasound revealed irregular hypoechoic areas in the left breast and clear echo boundaries of multiple lymph nodes in the left axilla, and there was no obvious abnormal blood flow signal in the bilateral breasts. (B) The molybdenum target showed a patchy tortuous structure in the upper outer quadrant of the left mammary gland accompanied by punctate calcification and calcium salt deposition, but no definite mass shadow was found.
Fig. 2.
Preoperative subsidiary exams (second part). (A) MRI showed that the shape, size and location of the bilateral breasts were symmetrical, with heterogeneous fibro glandular and multiple nodules. There was an irregular mass in the upper outer quadrant of the left breast with an increased number of left axillary lymph nodes. (B) Positron emission tomography with integrated computed tomography (PET-CT) showed an isodense soft tissue mass in the upper outer quadrant of the left breast, with ill-defined boundaries and uniform density. The cross-section size of the mass was approximately 4.1 cm x 2.7 cm x 2.9 cm with abnormal radioactive distribution, and presented hypermetabolism in the early stage (SUVmax: 2.3) and hypometabolism in the delayed phase (SUVmax: 1.2). There were several small lymph nodes in the left axillary region, some with mildly concentrated radioactivity. Whole-body PET imaging demonstrated no bone, lung, liver, or brain metastasis.
3. Clinical course
To confirm the diagnosis, the left mammary mass and axillary lymph nodes (ALNs) were cytologically examined by fine needle aspiration biopsy cytology (FNAC). The pathologic tissue obtained from the first puncture was small, and the pathology department of the Renmin Hospital of Wuhan University suggested that it was an alveolar carcinoma, while the other two hospitals suggested that it was a carcinoma involving micro-glandular adenosis (CMGA) and mammary analogue secretory carcinoma (MASC), respectively.
A second puncture was conducted due to the inconsistent results obtained from the three hospitals. Microscopically, the tumor cells presented a variety of arrangement patterns, such as vesicular, tubular, and striated, and no vascular or nerve invasion was seen. Immunohistochemistry results revealed: androgen receptor (AR, moderate strength positive), E-Cadherin (+), Ki-67 (+, positive index about 40% in the area of hotspots), SOX10 (+), S100 (+), gross cystic disease fluid protein of 15 (GCDFP-15, +), PAS-diastase (PASD, +), and the other specific results are summarized in Table 1. PD-L1 immunohistochemical assay result: PD-L1 (combined positive score = 3) (Detection platform: Dako Link48; Antibody clone number: 22C3). This case was diagnosed as primary acinic cell carcinoma of the breast by the Department of Pathology, Renmin Hospital of Wuhan University (Wuhan, China). These results were also confirmed after joint consultation of the pathology departments of two other hospitals.
Table 1.
The summarized results of immunohistochemistry (preoperative and postoperative).
| Antibody | Preoperative | Postoperative |
|---|---|---|
| ER | N | N |
| PR | N | N |
| HER2 | N | N |
| AR | moderate strength positive in 20% cells | strongly positive in 70% cells |
| SOX10 | positive in most of the area | P |
| GCDFP 15 | positive in most of the area | – |
| CK5/6 | N | N |
| CK7 | – | P |
| P63 | N | N |
| E-Cadherin | P | P |
| P120 (on membrane) | P | P |
| S-100 | P | P |
| EMA | P | P |
| Lysozyme | P | – |
| CEA | N | – |
| CD68 | – | N |
| Ki-67 | positive index approximately 40% in the area of hotspots | positive in 2% cells |
| PASD | P | P |
| P53 | Mt | – |
| TRPS1 | P | – |
| FOXC1 | weakly positive | – |
| PD-L1 | CPS = 3 | – |
After discussion in a multidisciplinary meeting, the patient received neoadjuvant chemotherapy (carboplatin in combination with weekly nab-paclitaxel) before the operation. Additionally, Keytruda, known as pembrolizumab, was applied for immunotherapy. The chemotherapy regimens are detailed in Table S1. The patient presented grade 2 myelosuppression in a neoadjuvant chemotherapy course with the lowest white blood cell count of 2.50 x 109 cells/L and the lowest platelet count of 55.00 x 109 cells/L. After two cycles, there was no dramatic reduction in mass size by MRI reexamination. Herein, left axillary lymph node dissection, modified radical left mastectomy, and right prophylactic simple mastectomy were performed under general anesthesia after the patient's platelet levels recovered to normal. The dissected left sentinel node and ALNs are displayed in Fig. S1A and B, respectively. On gross examination, the maximum residual infiltration foci in the primary lesions of the left breast were 3.5 cm × 2.5 cm grayish white masses with ill demarcated and slightly hard texture (Fig. S1C). Microscopically, the tumor cells presented diffuse infiltrative growth and a variety of morphological arrangements, including nested sheets and alveolar and tubular structures (Fig. 3A). The tumor cells contained abundant granular eosinophilic cytoplasm with clear cytoplasm and eccentric nuclei, and light-stained secretions were retained in the intercellular microcapsule space (Fig. 3B). No intravascular tumor, thrombus, or nerve invasion was detected. Histochemical assays showed PAS-diastase (PASD) staining was positive (Fig. 3C), and immunohistochemical, epithelial membrane antigen (EMA), S-100 and Gross cystic disease fluid protein 15 (GCDFP-15) were also positive in tumor cells (Fig. 3D–F). In contrast to the results of FNAC performed before neoadjuvant chemotherapy, a small amount of eosinophilic degeneration of the cytoplasm and nuclear pyknosis were observed in the parenchymal tumors. Of 46 dissected lymph nodes, 26 contained metastatic deposits (Fig. S1D). Of these, 9 lymph nodes had a therapeutic response, while 17 lymph nodes were nonresponders. The right breast mass was considered a benign condition, consistent with preoperative judgment. The immunohistochemistry results of the surgically removed left breast mass showed that androgen receptor (AR) was strongly positive (70%), while Ki-67 was weakly positive (2%). All immunohistochemical results are summarized in Table 1.
Fig. 3.
Pathological features and immunophenotype of this case. (A) The tumor cells presented diffuse infiltrative growth and a variety of morphological arrangements, including nested sheets and alveolar and tubular structures (HE, × 40). Blue arrows point to tumor cells in various morphological arrangements. (B) Microscopically, the tumor cells contained abundant granular eosinophilic cytoplasm. There were vacuolated cells with clear cytoplasm and eccentric nuclei, and lightly stained secretions were retained in the intercellular microcapsule space (HE, × 100). The orange arrow points to the abundant granular eosinophilic cytoplasm contained in the tumor cells. The green arrow points to the eccentric nuclei of the vacuolar cell, while the red arrow is the light stained secretions in the microcystic interstitial space. (C) Secretory granules in the cytoplasm of acinus cells were PAS-diastase stain (PASD)-positive ( × 40). (D) Tumor cells were positive for epithelial membrane antigen (EMA) ( × 40). (E) Tumor cells were positive for S-100 ( × 40). (F) Tumor cells focally expressed gross cystic disease fluid protein 15 (GCDFP-15) ( × 40).
After the drug sensitivity test, the ECT scheme (epirubicin plus cyclophosphamide and paclitaxel sequential regimen) was chosen to start postoperative chemotherapy one week after surgery. Keytruda was also used for immunotherapy. See Table S2 for details. Oral capecitabine was maintained (3 g in the morning and at night) for one year.
4. Discussion
Currently, published English literature regarding cases of primary ACCA of the breast, a special type of triple-negative breast cancer (TNBC), is scarce. In this case, both SOX10 and GCDFP15, markers suggestive of mammary origin, were positive, indicating that the patient's mass was indeed of primary mammary origin.
It is difficult to distinguish ACCA from carcinoma involving microglandular adenosis (CMGA) and mammary analog secretory carcinoma (MASC) at the histological level. Classic CMGA is usually a mass with no definite abnormality or unclear boundaries, and is often accidently discovered at the time of section examination due to calcification or adenoma of the breast [7]. Microscopically, the glands in CMGA are surrounded by a basal layer, while there is no basal layer in ACCA. S-100 was diffusely positive and EMA was negatively expressed in most CMGA cases, while lysozyme, S-100, and EMA were diffusely strongly positive in ACCA, which can be used to distinguish them from each other [8]. MASCs, which have abundant cytoplasm, vary in shape from vacuolar to granular and are eosinophilic under microscopy. ETV6-NTRK3 fusion, the most specific diagnostic marker for MASC [9]. The accuracy of ETV gene rearrangement detection carried out using the FISH technique for diagnostic MASC was 95%. FISH analysis and microscopic findings can distinguish MASC from ACCA [8]. Here, the patient was definitively diagnosed with ACCA based on the combination of cytology, ultrastructure, and immunohistochemistry after excluding the differential diagnosis.
Regarding treatment strategies, there is a lack of adequate follow-up data due to the small number of cases reported thus far. A summary of the reported treatment modalities and details for the treatment of acinic cell carcinoma of the breast is provided in Table 2. Surgery (total mastectomy or partial mastectomy) is still the accepted primary treatment with chemotherapy (according to guidelines for the treatment of invasive breast cancer) followed by adjuvant radiotherapy and endocrine therapy [6]. If the mass is too large or has invaded the skin, neoadjuvant chemotherapy should be given first, and radical surgery should be performed after the mass is reduced. The difference between this case and other cases is that the postoperative chemotherapy regimen does not rely solely on medication guidance and the clinical experience of the physician. The patient in the current case also sought the help of modern sophisticated medical techniques, including a drug sensitivity test, culminating in the ECT protocol. This customized individualized treatment plan in the era of precision medicine is undoubtedly beneficial to patients.
Table 2.
Summary of the reported treatment modalities and details for the treatment of acinic cell carcinoma of the breast.
| References | Sex/age | Surgery | therapeutic regimen | Outcome |
|---|---|---|---|---|
| Roncaroli et al. (1996) [10] | F/42 | MRM + ALND | CT | AW (1 year after surgery) |
| Schmitt et al. (2000) [11] | F/79 | MRM + ALND | RT | NED (21 months after surgery) |
| Damiani et al. (2000) [5] | F/42 | MRM | Neo-CT + CT (cyclophosphamide) | AW (5 year after surgery) |
| F/35 | MRM + ALND | Neo-CT + CT (cyclophosphamide) | AW (1 year after surgery) | |
| F/80 | BCS | HT | AW (1 year after surgery) | |
| Coyne et al. (2002) [12] | F/49 | MRM + ALND | Neo-CT + CT (Doxorubicin, cyclophosphamide, methotrexate, and 5-fluorouracil, and after 12 months received an additional course of chemotherapy including mitomycin, mitomycin C and methotrexate and epirubicin due to liver metastases) | Liver metastasis (1 year after surgery) and finally died 3 years after initial diagnosis |
| Peintinger et al. (2004) [13] | F/36 | BCS + ALND | CT + RT | Lung metastases occurred 8 years after surgery, and the patient is currently healthy (2 years after resection of the pulmonary mass) |
| Huo et al. (2011) [14] | F/40 | MRM + ALND | Neo-CT (Paclitaxel, fluorouracil, doxorubicin, and cyclophosphamide) + RT + HT | NED (1 year after surgery) |
| F/30 | BCS + ALND | CT + RT | Bone metastases and died (34 months after the initial diagnosis) | |
| Choh et al. (2012) [15] | F/79 | BCS + SLND | RT | NED (after nine months of treatment) |
| Ripamonti et al. (2013) [16] | F/44 | MRM + SLND | HT | AW |
| Winkler et al. (2013) [10] | F/56 | MRM + SLND | Neo-CT (Four cycles of intensive doses of doxorubicin and paclitaxel) + HT | NED (2 years after surgery) |
| Shingu et al. (2013) [17] | F/41 | BCS + SLND | RT (50 Gy-/25 fractions) + CT (docetaxel 75 mg/m2 and cyclophosphamide 600 mg/m2 intravenously every 3 weeks for 4 cycles) | NED (3 years after surgery) |
| Zhao et al. (2014) [18] | F/38 | MRM + ALND | CT (6 cycles) | NED (10 months after surgery) |
| Zhong et al. (2014) [19] | F/50 F/40 F/59 F/42 F/56 F/42 F/50 F/61 F/35 F/34 F/46 |
BCS BCS Mastectomy BCS Mastectomy Mastectomy Mastectomy Mastectomy BCS Mastectomy Mastectomy |
CT + RT CT + RT CT CT + RT CT CT CT CT CT + RT CT CT |
NED NED NED NED Lung metastasis NED NED NED NED NED NED |
| Conlon et al. (2016) [20] | F/47 | Bilateral mastectomy + ALND | CT + RT + HT | right internal mammary lymph nodes metastasis and alive with disease 6 years after the initial diagnosis |
| F/49 | BCS | Neo-CT | NED (18 months after surgery) | |
| Kawai et al. (2016) [21] | F/49 | MRM + SLND | CT | DR |
| Kim et al. (2017) [22] | F/40 | BCS | CT + RT | DR |
| F/38 | BCS | CT + RT | NED | |
| F/47 | BCS | CT + RT | NED | |
| F/46 | BCS | CT + RT | NED | |
| Waever et al. (2021) [23] | F/42 | Mastectomy + SLND | Neo-CT (received antenatal doxorubicin and cyclophosphamide, and postpartum paclitaxel) | NED |
| Sarsiat et al. (2022) [24] | F/59 | BCS + SLND | Neo-CT (3 cycles of epirubicin 100 mg/m2, 5-fluorouracil 500 mg/m2, and cyclophosphamide 500 mg/m2 were administered every 21 days, followed by 3 cycles of docetaxel 100 mg/m2) + CT | Peritoneal metastases and finally died |
| Present case | F/47 | Bilateral mastectomy + ALND | Neo-CT (6 cycles of Nab-Paclitaxel 80 mg/m2 were administered every 7 days, 2 cycles of Carboplatin 600 mg and Keytruda 200 mg were administered every 21 days) + CT (4 cycles of Epirubicin 150 mg and Cyclophosphamide 1 g were administered every 21 days, followed by 2 cycles of Nab-Paclitaxel 350 mg and Carboplatin 520 mg were administered every 21 days, meanwhile, 11 cycles of Keytruda 200 mg) + oral capecitabine was maintained (3 g in the morning and at night) for one year | NED (14 months after surgery) |
Note: F, female; M, male; MRM, modified radical mastectomy; ALND, Axillary lymph node dissection; CT, chemotherapy; AW, Alive and well; RT, Radiation therapy; NED, no evidence of disease; Neo-CT, Neoadjuvant chemotherapy; BCS, Breast-conserving surgery; HT, Hormone therapy; SLND, Sentinel lymph node dissection; DR, disease recurrence.
In terms of immunotherapy, Keytruda, a specific antibody against PD-1 on the surface of T cells, can block the inhibitory signal transduction of immune cells and promote the differentiation and maturation of T cells, resulting in an enhanced tumor killing effect. Keytruda was approved for the treatment of high-risk early triple-negative breast cancer patients based on a phase III clinical trial [25]. This study confirmed that Keytruda reduced the risk of disease progression by 37% (HR, 0.63; 95% CI, 0.48–0.82; P = 0.00031), and its use significantly improved patient survival. Keytruda can be used either as a neoadjuvant therapy or as a single agent as an adjuvant therapy after surgery. For such patients, immunotherapy as the first-line regimen is still superior to traditional chemotherapy. The benefit-risk profile of a multicenter, randomized, controlled clinical trial in locally advanced or metastatic non-small cell lung cancer demonstrated that Keytruda significantly prolonged progression-free survival in a significant proportion of patients, regardless of their PD-L1 levels [26]. Compared with neoadjuvant chemotherapy alone, Keytruda combined with chemotherapy as neoadjuvant chemotherapy plus postoperative Keytruda monotherapy provided a statistically and clinically significant improvement in event-free survival. In addition, the 2021 NCCN Breast cancer guidelines add Keytruda combined with chemotherapy as a first-line treatment for advanced triple-negative breast cancer [27]. Taking all these considerations into account, the current patient was treated with Keytruda both pre- and postoperatively, despite the fact that the CPS score for PD-L1 in our patient was not very high, at only 3 points (Detection platform: Dako Link48; Antibody clone number: 22C3).
Regarding the prognosis of this disease, different experts hold different opinions. A review of an extensive list of cases demonstrated that most cases had no recurrence, distant metastases could occur in bone, liver, or lung in very few cases, and occasionally death was reported [28,29]. In contrast, Damiani et al. followed 6 patients with breast ACCA and suggested that patients may be critically associated with poor prognosis when the tumor grade is high and there are other adverse characteristics [5]. Sarsiat et al. argued that ACCA is not always associated with a good prognosis by reporting a case of primary ACCA of the breast with poor outcome [24]. The mainstream opinion is that ACCA is a low-grade malignant tumor with a good prognosis despite its triple-negative phenotype [13]. In the case of this patient, we are optimistic regarding prognosis based on the following arguments. First, after two cycles of preoperative neoadjuvant chemotherapy combined with immunotherapy, we examined the patient's intraoperatively removed mass thoroughly and found that AR positivity increased from 20% preoperatively to 70% postoperatively (Table 1). According to a 2012 prognostic analysis of 287 TNBC patients, the expression rate of AR in TNBC was approximately 25.8%, which was significantly correlated with longer disease-free survival (DFS) and overall survival (OS); this indicates that ER-/PR-/AR + patients had a better prognosis, and multivariate analysis revealed that AR was an independent prognostic factor for TNBC [30]. Second, the postoperative value of Ki-67, a marker of cell proliferation, was decreased, and the pathologist also found preoperative Ki-67 hotspot areas that were not seen after neoadjuvant chemotherapy. Although the patient's MRI results showed no significant reduction in the mass, we believe that the immunohistochemical pathology level results of AR and Ki-67 have a higher level of evidence than MRI. These results to be evidence that the patient benefited from neoadjuvant chemotherapy.
The patient was free of symptoms for 14 months after surgery. No obvious lesions were found in multiple postoperative imaging examinations, suggesting that our treatment strategy was effective, but the long-term prognosis still requires follow-up monitoring.
5. Conclusions
As breast ACCA imaging findings are usually occult, immunohistochemical examination is an important modality for accurate diagnosis. Since the limited understanding of this kind is extremely rare, medication and surgery regimens mostly follow the invasive BC treatment guidelines. To date, however, full clinical details have not been published in any of the cases. We presented a detailed and complete diagnosis and treatment process for a patient diagnosed with primary ACCA of the breast. We believe that carboplatin in combination with weekly nab-paclitaxel neoadjuvant chemotherapy is beneficial to patients with high expression of Ki-67 in fine needle aspiration biopsy cytology to a certain extent. Moreover, precision medicine is also of great help in the formulation of postoperative chemotherapy regimens.
In summary, the aim of this study was to provide a reference for other patients and promote the understanding of this kind of disease in the future. Additional studies are urgently needed to clarify the biological characterization and effective treatment of breast ACCA.
Ethics approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Clinical Research Ethics Committee of Renmin Hospital of Wuhan University (WDRY2020-K218).
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images.
Funding
This work was supported by the Cross-innovation talent project in Renmin Hospital of Wuhan University (grant number JCRCZN-2022-016), the Undergraduate education quality construction comprehensive reform project (grant number 2022ZG282) and the National Natural Science Foundation of China (grant number 82071655).
Author contribution statement
All authors listed have significantly contributed to the investigation, development and writing of this article. </p>
Data availability statement
Data will be made available on request.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests.Yan-Xiang Cheng reports administrative support was provided by Cross-innovation talent project in Renmin Hospital of Wuhan University. Yan-Xiang Cheng reports statistical analysis was provided by Undergraduate education quality construction comprehensive reform project. Yan-Xiang Cheng reports administrative support was provided by National Natural Science Foundation of China.
Acknowledgments
The authors acknowledge the valuable contributions of specialists from the surgery, internal medicine, radiotherapy, pathology, and imaging departments and thank the patient for his support and cooperation.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.heliyon.2023.e20160.
Note: ER, PR, HER2 and AR are hormone receptors in breast cancer. SOX10 and GCDFP15 are markers of the initial origin of the tumor in the mammary gland. CK5/6 and CK7 are cytokeratin's that mark epithelial cells of origin, while P63 is a sensitive and specific marker of myoepithelial cells. Double positive expression of E-cadherin and P120 suggests breast ductal invasive carcinoma. S-100, EMA and Lysozyme were used to differentiate ACCA from carcinoma involving microglandular adenosis. CEA is a tumor marker of epithelial origin. CD68 is a lymphoid-derived marker. Ki-67 is a marker of cell proliferation. PAS-diastase stains were used to evaluate the intracellular glycogen. P53, a tumor suppressor gene, is the most frequently mutated gene in invasive breast cancer. TRPS1 is a new target of TNBC, while FOXC1 is a key transcription factor involved in TNBC invasion and metastasis. Abbreviations: N, negative; P, positive; -, no report; ER, estrogen receptor; PR, progesterone receptor; HER2, Human epidermal growth factor receptor 2; CK5/6, cytokeratin 5/6; CK7, cytokeratin 7; CEA, carcinoembryonic antigen; PASD, PAS-diastase; AR, androgen receptor; GCDFP 15, gross cystic disease fluid protein of 15; EMA, epithelial membrane antigen; Mt, wild type; TRPS1, trichorhinophalangeal syndrome type 1; FOXC1, forkhead box C1; CPS, combined positive score.
Contributor Information
Jing-Ping Yuan, Email: yuanjingping@whu.edu.cn.
Yan-Xiang Cheng, Email: rm001050@whu.edu.cn.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Ajkunic A., Skenderi F., Shaker N., Akhtar S., Lamovec J., Gatalica Z., Vranic S. Acinic cell carcinoma of the breast: a comprehensive review. Breast. 2022;66:208–216. doi: 10.1016/j.breast.2022.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Frank G.A., Danilova N.V., Andreeva I., Nefedova N.A. [WHO classification of tumors of the breast, 2012] Arkh. Patol. 2013;75(2):53–63. [PubMed] [Google Scholar]
- 3.Sinn H.P., Kreipe H. Focusing on Issues and Updates from the 3rd Edition. Breast Care (Basel) Vol. 8. 2013. A brief overview of the WHO classification of breast tumors; pp. 149–154. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan P.H., Ellis I., Allison K., Brogi E., Fox S.B., Lakhani S., Lazar A.J., Morris E.A., Sahin A., Salgado R., et al. The 2019 World Health Organization classification of tumours of the breast. Histopathology. 2020;77(2):181–185. doi: 10.1111/his.14091. [DOI] [PubMed] [Google Scholar]
- 5.Damiani S., Pasquinelli G., Lamovec J., Peterse J.L., Eusebi V. Acinic cell carcinoma of the breast: an immunohistochemical and ultrastructural study. Virchows Arch. 2000;437(1):74–81. doi: 10.1007/s004280000206. [DOI] [PubMed] [Google Scholar]
- 6.Limite G., Di Micco R., Esposito E., Sollazzo V., Cervotti M., Pettinato G., Varone V., Benassai G., Amato B., Pilone V., et al. Acinic cell carcinoma of the breast: review of the literature. Int. J. Surg. 2014;12(Suppl 1):S35–S39. doi: 10.1016/j.ijsu.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Kravtsov O., Jorns J.M. Microglandular adenosis and associated invasive carcinoma. Arch. Pathol. Lab Med. 2020;144(1):42–46. doi: 10.5858/arpa.2019-0049-RA. [DOI] [PubMed] [Google Scholar]
- 8.Chang E.D., Lee E.J., Lee A.W., Kim J.S., Kang C.S. Primary acinic cell carcinoma of the breast: a case report with an immunohistochemical and ultrastructural studies. J Breast Cancer. 2011;14(2):160–164. doi: 10.4048/jbc.2011.14.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lannon C.L., Sorensen P.H. ETV6-NTRK3: a chimeric protein tyrosine kinase with transformation activity in multiple cell lineages. Semin. Cancer Biol. 2005;15(3):215–223. doi: 10.1016/j.semcancer.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 10.Winkler N., Morrell G. Factor RE: invasive carcinoma with acinic cell-like features of the breast. Breast J. 2013;19(3):334–335. doi: 10.1111/tbj.12119. [DOI] [PubMed] [Google Scholar]
- 11.Schmitt F.C., Ribeiro C.A., Alvarenga S., Lopes J.M. Primary acinic cell-like carcinoma of the breast--a variant with good prognosis? Histopathology. 2000;36(3):286–289. doi: 10.1046/j.1365-2559.2000.0872f.x. [DOI] [PubMed] [Google Scholar]
- 12.Coyne J.D., Dervan P.A. Primary acinic cell carcinoma of the breast. J. Clin. Pathol. 2002;55(7):545–547. doi: 10.1136/jcp.55.7.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peintinger F., Leibl S., Reitsamer R., Moinfar F. Primary acinic cell carcinoma of the breast: a case report with long-term follow-up and review of the literature. Histopathology. 2004;45(6):645–648. doi: 10.1111/j.1365-2559.2004.01957.x. [DOI] [PubMed] [Google Scholar]
- 14.Huo L., Bell D., Qiu H., Sahin A., Wu Y., Sneige N. Paneth cell-like eosinophilic cytoplasmic granules in breast carcinoma. Ann. Diagn. Pathol. 2011;15(2):84–92. doi: 10.1016/j.anndiagpath.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Choh C.T., Komar V., Courtney S.P. Primary acinic cell carcinoma of the breast: a rare lesion with good prognosis. Breast J. 2012;18(6):610–611. doi: 10.1111/tbj.12013. [DOI] [PubMed] [Google Scholar]
- 16.Ripamonti C.B., Colombo M., Mondini P., Siranoush M., Peissel B., Bernard L., Radice P., Carcangiu M.L. First description of an acinic cell carcinoma of the breast in a BRCA1 mutation carrier: a case report. BMC Cancer. 2013;13:46. doi: 10.1186/1471-2407-13-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shingu K., Ito T., Kaneko G., Itoh N. Primary acinic cell carcinoma of the breast: a clinicopathological and immunohistochemical study. Case Rep Oncol Med. 2013;2013 doi: 10.1155/2013/372947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao Y., Li W., Lang R., Yang Y., Gao X., Zheng Y., Zhang C., Fu X., Fu L. Primary acinic cell carcinoma of the breast: a case report and review of the literature. Int. J. Surg. Pathol. 2014;22(2):177–181. doi: 10.1177/1066896913483898. [DOI] [PubMed] [Google Scholar]
- 19.Zhong F., Bi R., Yu B., Cheng Y., Xu X., Shui R., Yang W. Carcinoma arising in microglandular adenosis of the breast: triple negative phenotype with variable morphology. Int. J. Clin. Exp. Pathol. 2014;7(9):6149–6156. [PMC free article] [PubMed] [Google Scholar]
- 20.Conlon N., Sadri N., Corben A.D., Tan L.K. Acinic cell carcinoma of breast: morphologic and immunohistochemical review of a rare breast cancer subtype. Hum. Pathol. 2016;51:16–24. doi: 10.1016/j.humpath.2015.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawai H., Sugimoto R., Iga N., Ikeda H., Yoshida R., Waki N., Ishizaki M., Nishi H., Yamashita K. [A case of primary acinic cell carcinoma(ACC)of the breast] Gan To Kagaku Ryoho. 2016;43(12):2019–2021. [PubMed] [Google Scholar]
- 22.Kim M., Kim M., Chung Y.R., Park S.Y. Mammary carcinoma arising in microglandular adenosis: a report of five cases. J Pathol Transl Med. 2017;51(4):422–427. doi: 10.4132/jptm.2016.11.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weaver K.D., Isom J., Esnakula A., Daily K., Asirvatham J.R. Acinic cell carcinoma of the breast: report of a case with immunohistochemical and next-generation sequencing studies. Int. J. Surg. Pathol. 2021;29(8):882–886. doi: 10.1177/10668969211008508. [DOI] [PubMed] [Google Scholar]
- 24.Sarsiat L., Watkinson G., Turnbull A., Diana A., Oikonomidou O. Primary acinic cell carcinoma of the breast is associated with a poor outcome: a case report and literature review. Mol Clin Oncol. 2022;16(2):43. doi: 10.3892/mco.2021.2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmid P., Cortes J., Pusztai L., McArthur H., Kummel S., Bergh J., Denkert C., Park Y.H., Hui R., Harbeck N., et al. Pembrolizumab for early triple-negative breast cancer. N. Engl. J. Med. 2020;382(9):810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 26.Mok T.S.K., Wu Y.L., Kudaba I., Kowalski D.M., Cho B.C., Turna H.Z., Castro G., Jr., Srimuninnimit V., Laktionov K.K., Bondarenko I., et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819–1830. doi: 10.1016/S0140-6736(18)32409-7. [DOI] [PubMed] [Google Scholar]
- 27.Gradishar W.J., Moran M.S., Abraham J., Aft R., Agnese D., Allison K.H., Blair S.L., Burstein H.J., Dang C., Elias A.D., et al. NCCN guidelines(R) insights: breast cancer, version 4.2021. J. Natl. Compr. Cancer Netw. 2021;19(5):484–493. doi: 10.6004/jnccn.2021.0023. [DOI] [PubMed] [Google Scholar]
- 28.Hirokawa M., Sugihara K., Sai T., Monobe Y., Kudo H., Sano N., Sano T. Secretory carcinoma of the breast: a tumour analogous to salivary gland acinic cell carcinoma? Histopathology. 2002;40(3):223–229. doi: 10.1046/j.1365-2559.2002.01346.x. [DOI] [PubMed] [Google Scholar]
- 29.Kinkor Z., Skalova A. [Acinic cell-like differentiation in invasive ductal carcinoma and in ductal hyperplasia of the breast--report of two cases] Cesk. Patol. 2005;41(1):29–33. [PubMed] [Google Scholar]
- 30.He J., Peng R., Yuan Z., Wang S., Peng J., Lin G., Jiang X., Qin T. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: a retrospective analysis based on a tissue microarray. Med. Oncol. 2012;29(2):406–410. doi: 10.1007/s12032-011-9832-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.