Abstract
Four ferredoxin isoproteins were identified in the C4 plant Zea mays L. by analysis of extracts from leaves, mesocotyls, and roots of the young seedlings. The relative amounts of the isoproteins isolated from the photosynthetic and nonphotosynthetic organs were different. All the isoproteins were present in the leaves of green and etiolated plants, whereas two out of the four isoproteins were not detected in the roots or in the mesocotyls. During the greening of etiolated seedlings, the level of the two isoproteins unique to the leaf increased markedly. Analysis of the cellular and subcellular distribution of the two major leaf isoproteins showed that one isoprotein was present in the chloroplasts of both mesophyll and bundle sheath cells, whereas the other was only found in the chloroplasts of bundle sheath cells. This is the first report of the cell-specific expression of ferredoxin isoproteins in the leaves of a C4 plant.
Full text
PDF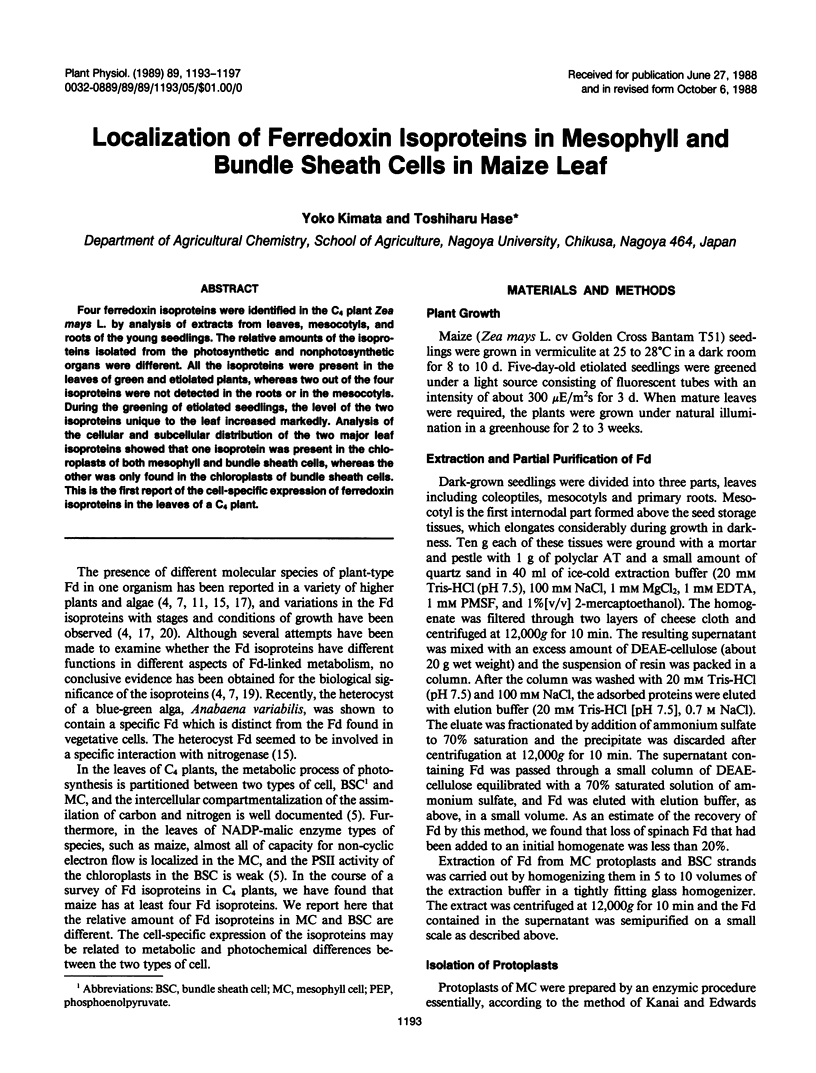



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brill W. J., Westphal J., Stieghorst M., Davis L. C., Shah V. K. Detection of nitrogenase components and other nonheme iron proteins in polyacrylamide gels. Anal Biochem. 1974 Jul;60(1):237–241. doi: 10.1016/0003-2697(74)90149-3. [DOI] [PubMed] [Google Scholar]
- Hutson K. G., Rogers L. J., Haslett B. G., Boulter D., Cammack R. Comparative studies on two ferredoxins from the cyanobacterium Nostoc strain MAC. Biochem J. 1978 Jun 15;172(3):465–477. doi: 10.1042/bj1720465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins C. L., Boag S. Isolation of Bundle Sheath Cell Chloroplasts from the NADP-ME Type C(4) Plant Zea mays: Capacities for CO(2) Assimilation and Malate Decarboxylation. Plant Physiol. 1985 Sep;79(1):84–89. doi: 10.1104/pp.79.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai R., Edwards G. E. Separation of mesophyll protoplasts and bundle sheath cells from maize leaves for photosynthetic studies. Plant Physiol. 1973 Jun;51(6):1133–1137. doi: 10.1104/pp.51.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathnam C. K., Edwards G. E. Distribution of Nitrate-assimilating Enzymes between Mesophyll Protoplasts and Bundle Sheath Cells in Leaves of Three Groups of C(4) Plants. Plant Physiol. 1976 Jun;57(6):881–885. doi: 10.1104/pp.57.6.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Oaks A., Jacquot J. P., Vidal J., Gadal P. An electron transport system in maize roots for reactions of glutamate synthase and nitrite reductase : physiological and immunochemical properties of the electron carrier and pyridine nucleotide reductase. Plant Physiol. 1985 Jun;78(2):374–378. doi: 10.1104/pp.78.2.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uedan K., Sugiyama T. Purification and characterization of phosphoenolpyruvate carboxylase from maize leaves. Plant Physiol. 1976 Jun;57(6):906–910. doi: 10.1104/pp.57.6.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS D. E., REISFELD R. A. DISC ELECTROPHORESIS IN POLYACRYLAMIDE GELS: EXTENSION TO NEW CONDITIONS OF PH AND BUFFER. Ann N Y Acad Sci. 1964 Dec 28;121:373–381. doi: 10.1111/j.1749-6632.1964.tb14210.x. [DOI] [PubMed] [Google Scholar]