Abstract
Orchidaceae are diverse plants whose bioactive compounds have various biological activities. New hybrids of Dendrobium have been generated to gain characteristics shared with their ancestors. Dendrobium Pearl Vera (designated as DH) is derived from parents used for dermatological treatments and cosmetics. However, the phytoconstituents and biological properties of DH have not been reported. The current study investigated extracts from DH plants using four solvents (water, methanol, ethanol, or 2-propanol). The propanolic extract (DH-P) contained the highest phenolic and flavonoid contents, along with a high scavenging performance for free radicals. In total, 25 tentative constituents in the DH-P matrix were identified, consisting of amino acids, nucleotides, and three types of secondary metabolites: furan, phenolics, and alkaloids. The DH-P inhibited human tyrosinase in vitro in a concentration-dependent manner of the phenolic content. Furthermore, there was no significant difference between DH-P with 10 μg/ml phenolic content and 0.75 mM kojic acid (a commercial whitening agent) on the inhibition of human tyrosinase. Incubation with DH-P containing at least 15 μg/ml phenolic content greatly inhibited the proliferation of human melanoma; however, the cell viability was not affected by the phenolic content at 5 μg/ml or less. The half-maximal inhibitory concentration (IC50) of the phenolic content in DH-P on melanoma viability was 12.90 ± 1.04 μg/ml. Melanin production in vivo by human melanoma incubated with 5 μg/ml phenolic content in DH-P was reduced significantly, compared to 2.5 μg/ml phenolic content in DH-P, 100 μg/ml arbutin, and in control. The identified components, including 5-hydroxymethyl-2-furaldehyde, salicylic acid, nicotinamide, acetophenone, cytidine, adenosine, proline, or valine, have been reported to be associated with depigmentation, antioxidant, and anticancer. This research revealed, for the first time, the tentative phytoconstituents of Dendrobium Pearl Vera and their biological activities, thus demonstrating the potential use of DH-P in dermal applications.
Keywords: Antioxidant, Anticancer, Mass spectrometry, Orchid, Phytochemicals, Whitening
1. Introduction
The Orchidaceae is a diverse plant family with an estimated 30,000 species, most of which are distributed in tropical areas and on high mountains. Dendrobium spp. are one of the largest groups (more than 1000 species) in this family [1,2]. Dendrobium spp. are the first-ranked of floriculture exports from Thailand to global markets and are of interest for use in industrial material applications [3,4].
Natural products are increasingly demanded in food and cosmetic industries; therefore, many scientists are investigating new resources with biological activities for use as new drugs and bioactive components. The Dendrobium genus is one of the most important genera for medicinal applications. It has long been used for improving human health and curing various skin diseases and age-related disorders [1,4]. At least 20 species of Dendrobium, including D. canaliculatum, have been used to treat dermatological disorders [4]. With more patients with hyperpigmentation, this skin color disorder could progressively develop into melanogenesis [5]. The extract from D. tosaense can exhibit antitumor activity by inhibiting the melanogenesis of B16/F10 cells [6]. Some Dendrobium species inhibited the activity of tyrosinase, an enzyme involved in the production of melanin pigment causing dark spots on the skin [5,7]. Some phytoconstituents in plants have been utilized in herbal cosmetics as anti-hyperpigmentry agents, such as arbutin, ascorbic acid, genistein, ellagic acids, and quercetin [5]. Dendrobium Sonia Earsakul was reported to have high antioxidant activity, similar to vitamin C, which can prevent cell damage and destruction [8]. D. bigibbum is one of the six Dendrobium spp. listed in the New Inventory of Existing Cosmetic Ingredients in China Launched [4]. Recently, phytochemicals from Dendrobium spp. have gained attention for their benefits for skin improvement; nevertheless, the composition and accumulation of beneficial phytochemicals could vary from species to species and are as yet unknown.
Different types of bioactive compounds with medicinal and pharmaceutical functions, such as polyphenols, flavonoids, alkaloids, and polysaccharides, are endogenously produced and accumulated in different parts of Dendrobium spp., depending on the constituents [1,2,4]. Alkaloids and polysaccharides have several medicinal properties related to age-pathogenic and cancer prevention [1,2]. Phenolics and flavonoids are well-known for their bioactive agents as free radical scavengers or metal ion chelators [9,10]. Common phytoconstituents, such as quercetin, kaempferol, coumarin, and gigantol, can be found in many plants, including orchids, reported to show antioxidant, antiaging, antimicrobial, anti-inflammatory, and anticancer activities [2,9,11]. Compounds in medicinal orchids are also cytotoxic toward several cancer cells [2]. For example, dendrobine, an alkaloid found in D. nobile, induced apoptotic cell death in A549 lung cancer cells [12]; furthermore, gigantol in D. draconis suppressed the metastasis of lung cancer cells [2].
New hybrids of Dendrobium have been created frequently to generate new characteristics for ornamental purposes. They are one of the popular cultivars in Thailand's floriculture due to its beauty and long-lasting flowers. Although many new orchid species have been created and taxonomically identified, their phytochemicals and biological properties remain unknown [2], including for Dendrobium Pearl Vera (designated as DH in this study). DH plant is made from a cross between D. bigibbum and Dendrobium Topaz Dream and is the third generation of hybridization (with D. canaliculatum and D. bigibbum as ancestors) [4,13]. Wang (2021) reported that D. canaliculatum has been used to treat sores, while D. bigibbum is listed as cosmetic ingredients in China [4]. The genetic material of DH plant contains 93.75% of D. bigibbum and 6.25% of D. canaliculatum [13], suggesting that the DH characteristics should be similar to D. bigibbum. We hypothesized that DH potentially contains bioactive compounds with pharmacological properties that could be utilized, especially for skin improvement applications. Therefore, its bioactive substances and biological functions were the aims of our investigation.
We used solvents not previously investigated with regard to DH to optimize the phenolic and flavonoid yields. Then, we examined the tentative bioactive compounds using high-resolution mass spectrometry (HR-MS) and investigated three biological properties (antioxidative, anticarcinogenic, and depigmentation). This is the first report on the inhibitory effects of DH extract on free radicals, skin cancer cells, and tyrosinase activity from in vitro and human cell-based assays, as well as the phytoconstituents of the DH extract.
2. Materials and methods
2.1. Chemicals, reagents, and cell lines
Standards used for HPLC and HR-MS were quercetin (Sigma® Q4951 and Fluka® 83370), kaempferol (Biopurify® BP0820), dendrobine (Cayman® 26067), gigantol (Sigma® Sml2036), and coumarin (Sigma® C4261). Human skin cell line melanoma SK-MEL-28 (ATCC® HTB-72) was used in the cell viability study. Other chemicals, reagents, and media used were analytical and molecular biology grades.
2.2. Sample preparation and extraction
Whole plants of tissue-cultured orchid hybrid, Dendrobium Pearl Vera hybrid (DH), were collected, ground, and then frozen using liquid nitrogen. The ground sample was extracted using four different types of solvent (deionized water, methanol, ethanol, and 2-propanol) with a 1:50 (g/ml) ratio. Samples were macerated at room temperature for 24 h. Then, the filtrate was collected after passing through filter paper (Whatman No.1). Each solvent was used to extract thrice and pooled. The filtrates were concentrated in a rotary evaporator (Buchi R-100; Switzerland) and dried using nitrogen gas. The viscous extracts were redissolved in 95% ethanol to a concentration of 1 mg/ml before use in further experiments.
2.3. Determination of total phenolic content
The total phenolic content was determined using the Folin-Ciocalteu colorimetric method with a minor modification [14]. Briefly, 20 μl of the extracts and standard were placed in a 96-well microplate, and 100 μl of 10% (v/v) Folin-Ciocalteu reagent was added. The mixture was incubated at room temperature for 3 min. Then, 80 μl of 1 M sodium carbonate (Na2CO3) was added and further incubated in the dark at room temperature for 20 min. Subsequently, the absorbance of the mixture was read at 765 nm using a microplate reader (Tecan M200; Switzerland). Each sample was performed in triplicate. A standard curve (Y = 0.0037×, R2 = 0.9993) was generated using gallic acid at concentrations of 0, 12.5, 25, 50, 100, and 200 μg/ml. The result was expressed as micrograms of gallic acid equivalent per gram of fresh tissue (μg GAE/g of tissue) [10,14].
2.4. Determination of total flavonoid content
The total flavonoid content was determined using an aluminum chloride colorimetric assay in the microplate platform with a minor modification [15]. Briefly, 104 μl of distilled water and 60 μl of methanol were placed in each well. Then, 20 μl of standard and each extract were added, followed by 8 μl of 0.5 M potassium acetate and 5% (w/v) aluminum chloride. The mixture was shaken for 5 min and then incubated in the dark at room temperature for 30 min. The absorbance of the mixture was read at 415 nm using a microplate reader (Tecan M200; Switzerland). Each sample was performed in triplicate. A standard curve (Y = 0.0076×, R2 = 0.9962) was generated using quercetin at concentrations of 0, 12.5, 25, 50, 100, and 200 μg/ml. The result was expressed as micrograms of quercetin equivalent per gram of fresh tissue (μg QE/g of tissue) [10,15].
2.5. Quantification of quercetin and kaempferol using HPLC
Samples (1 mg/ml) were passed through a 0.20 μm nylon filter and analyzed using HPLC (Waters e2695; Waters Corp.; USA) for quercetin and kaempferol. A methanol-to-acetonitrile-to-water ratio containing 1% acetic acid at 40:15:45, as an isocratic eluent, was run at a flow rate of 1.0 ml/min through a Promosil column (C18; 250 × 4.6 mm). The column chamber was set at 35 °C. The sample was injected for 10 μl and analyzed in triplicate. The active compounds were simultaneously quantified at 360 nm. A standard curve was generated using the Empower 3 software for quercetin (Y = 3.10e+004× - 3.72e+004) and kaempferol (Y = 4.95e+004x + 5.93e+004). The standards were prepared at a 2-fold serial concentration of 0–200 μg/ml.
2.6. Screening of chemical compositions using HR-MS analysis
Propanolic extract was used to identify the metabolites in the DH plant. Dendrobine, quercetin, coumarin, and gigantol were made in mixtures at a concentration of 10 pg/μl and used as standards to evaluate their presence in the samples. The DH extract was prepared at a concentration of 40 ng/μl and 40 pg/μl. The samples were separated on a Vanquish UHPLC system (Thermo Fisher Scientific; USA) and analyzed using an untargeted approach on a quadrupole orbitrap mass spectrometer (Q Exactive HF; Thermo Fisher Scientific; USA). A chromatographic separation used a reverse phase C18 column (Kinetex XB; 100 × 2.1 mm, 2.6 μm). Then, the fragmented ions were matched to the mass spectral library. The mobile phase consisted of A (0.2% formic acid-water) and B (0.2% formic acid-methanol). The gradient program was applied as: 1–5% B for 0.3 min, 5–40% B from 0.3 to 4.5 min, 80% B for 1.4 min as a column washing phase, and 1% B for 1.6 min as a re-equilibration phase, resulting in a total runtime of 7.5 min. The flow rate was set at 500 μl/min, and the injection volume was 10 μl. Electrospray ionization (ESI) was performed in positive mode. The MS scan range was m/z 100–1,000, and the resolution was set to 60,000 (at m/z 200) for MS1 and 15,000 (at m/z 200) for MS2. The collision energy was 30 eV. Instrument control was performed using the Xcalibur software (Thermo Fisher Scientific; USA).
2.7. DPPH radical scavenging capacity
DPPH assay was performed in the microplate platform with minor modifications [16]. The standard and the extracts (each 20 μl) were placed in wells. Then, 160 μl of 150 μM DPPH radical solution was added and incubated in the dark at room temperature for 30 min. The scavenging activity of the samples against DPPH radicals was detected at 517 nm. Each sample was run in triplicate. Ethanol was used as the control. A standard curve (Y = 0.1689×, R2 = 0.998) was generated using ascorbic acid (AA) at concentrations in the range 0–500 μM. Scavenging activity was calculated using the formula:
| % Inhibition = (1 – Acontrol / Asample) × 100 |
where A is the absorbance value.
2.8. ABTS radical scavenging capacity
ABTS assay was performed in the microplate platform with minor modifications [17]. Active ABTS+ radicals were prepared at a 1:60 (v/v) ratio in methanol to obtain the absorbance at 1.1. The reaction was done by mixing 10 μl of the extracts or standard and 190 μl of ABTS+ working solution. The mixture was incubated in the dark at room temperature for 2 h. The scavenging activity was detected at 734 nm. Each sample was run in triplicate. Ethanol was used as the control. A standard curve (Y = 0.1736×, R2 = 0.995) was generated using AA at 0–500 μM serial concentrations. Scavenging activity was calculated using the same formula as for DPPH and reported as % inhibition.
2.9. Ferric reducing antioxidant power assay
The FRAP assay was conducted using a microplate platform [18] by mixing 10 μl of the extracts or standard and 140 μl of FRAP working reagent. The reaction was incubated in the dark at 37 °C for 30 min. The absorbance of the mixture was read at 593 nm. Each sample was run in triplicate. Ethanol was used as the control. A standard curve (Y = 0.0027×, R2 = 0.999) was generated using AA at serial concentrations in the range 0–1000 μM. Antioxidant power was reported as the FRAP value per milligram of extract.
2.10. Cell-free human tyrosinase inhibition
The human tyrosinase activity in a cell-free model was investigated with 10 μg/ml of phenolic content in DH-E or DH-P using a Tyrosinase Inhibitor Screening Assay Kit (Abcam; UK). The samples were mixed with tyrosinase enzyme and buffer and then incubated at 25 °C for 10 min before the reaction was started by adding tyrosinase substrate solution. The activity was carried out at 37 °C in the microplate reader in kinetic mode to measure the absorption at 510 nm every 2 min for 45 min. The percentage of inhibition was calculated by comparison with the control and 0.75 mM kojic acid.
| % Inhibition = (Acontrol – Asample) / Acontrol × 100% |
where A is the absorbance value.
Various concentrations of phenolic content in DH-P were performed using the same method to investigate any concentration-dependent effect.
2.11. Cell culture and viability test
Human melanoma SK-MEL-28 was cultured with Dulbecco's modified eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin at 37 °C under 5% CO2. Before the experiments were carried out, cell synchronization was performed by growing cells overnight with DMEM supplemented with only 0.4% FBS and 1% antibiotics.
Various concentrations of phenolic content from DH-P were prepared in the complete medium for the viability assay using PrestoBlue (ThermoFisher; USA). The cells at passage 15, 17, and 20 were seeded in 96-well plates at a density of 10,000 cells/well and cultured with DH-P extracts for 48 h. The cells grown in the 10% FBS complete medium containing the same amount of 95% ethanol were used as the control. Those cells cultured in 0.4% FBS complete medium for the same amount of time and conditions were referred to as the number of cells seeded on day 0. Five replications were performed for each treatment. Cell viability was measured at the absorbance of 570 nm and was calculated using the formula:
| % Cell viability = Asample / Acontrol × 100% |
where A is the absorbance value.
2.12. Melanin production by human melanoma
Serum-starved melanoma was seeded at 100,000 cells per well in a 24-well plate. Every sample was cultured with 10% FBS complete medium containing 100 nM of α-MSH for 48 h. The effects of DH-P extracts containing 2.5 or 5 μg/ml of phenolic content on melanin content were compared to those cultured with 100 μg/ml arbutin and the control. After the incubation, the culture medium was removed and washed with cold phosphate buffer saline (PBS) 3 times before the dried cells were kept at −80 °C until the melanin measurement was performed. All cell samples were added with 1 N NaOH, scraped from the plate, and heated at 60 °C for 1 h. The melanin content was calculated by comparing the absorption at 405 nm with the melanin standard curve.
2.13. Statistical and data analyses
One-way analysis of variance (ANOVA) was used in the analysis. Means were compared using Tukey's comparison at the p < 0.05 significance level. For MS analysis, raw files generated by Q Exactive HF were searched in the Compound Discoverer Software 3.1 (Thermo Fisher Scientific; USA). All identified compounds with a match factor >75 were considered and manually reviewed using the Xcalibur 4.0 Qual browser (Thermo Fisher Scientific; USA). The obtained MS1 and MS2 spectra were compared to reference spectra taken from mzcloud (Copyright© 2013–2020; HighChem LLC; Slovakia). The mass tolerance was restricted to 5 ppm.
3. Results and discussion
3.1. Phenolic and flavonoid content in DH extracts
Extractions of DH were performed using four solvents—water (DH-W), methanol (DH-M), ethanol (DH-E), and 2-propanol (DH-P), arranged in order from high to low polarity index, respectively. The bioactive substances in the extracts at a concentration of 1 mg/ml were quantified for total phenolic and flavonoid contents using colorimetric assays, as well as for quercetin and kaempferol using high performance liquid chromatography (HPLC), shown in Table 1. The total phenolic and flavonoid contents among the extracts were significantly different (p < 0.05). The DH-P extract had the significantly highest amount of phenolic and flavonoid substances, while DH-W had the lowest. Wakeel et al. (2019) reported that the chemical polarity of the solvents affected the quantity and extraction efficiency, depending on what type of chemical structures had accumulated in the woad (Isatis tinctoria) [19]. They observed that phenolic and flavonoid contents in the extract increased when the solvent polarity index increased but suddenly reduced if the solvent polarity was very high. Dendrobium spp. contain terpenes, stilbenoids, alkaloids, bibenzyls, phenanthrenes, flavonoids, and polysaccharides [2], ranging from low to high polarity. Propanol has moderate polarity and has the lowest polarity index compared to the other solvents used in the current study; nevertheless, it is the most efficient solvent for the extraction of bioactive compounds in DH, based on the current results. Quercetin decreased as the solvent polarity increased and was not detected in DH-W, corresponding with the decreased content of total phenolics and flavonoids. Thus, phytochemicals, especially phenolics and flavonoids, in DH were moderate-to-low in polarity. Kaempferol was not detected in any of the samples. Both quercetin and kaempferol are flavonoids with several pharmaceutical effects and are commonly present in plants, including orchids [8,11]. This fact could imply that DH might accumulate kaempferol at a concentration below the limit of detection and quantification; thus, clearly kaempferol (if at all) accumulates less than quercetin in DH.
Table 1.
Amounts of bioactive compounds in extracts.
| Sample | Solvent | Total phenolics (mg of GAE per g extract)1/ |
Total flavonoids (mg of QE per g extract)1/ |
Quercetin (mg per g extract)1/ |
Kaempferol (mg per g extract) |
|---|---|---|---|---|---|
| DH-W | Deionized water | 1.8 ± 0.6c | 1.1 ± 0.4d | ND | ND |
| DH-M | Methanol | 10.9 ± 0.6b | 5.1 ± 0.4c | 0.63 ± 0.00a | ND |
| DH-E | Ethanol | 13.3 ± 1.6b | 28.5 ± 1.6b | 0.64 ± 0.00a | ND |
| DH-P | 2-Propanol | 20.0 ± 1.6a | 32.5 ± 1.6a | 0.65 ± 0.01a | ND |
1/ = Mean (±SD) in same column followed by different lowercase superscripts are significantly different at p < 0.05 according to Tukey's test. GAE = Gallic acid equivalent; QE = Quercetin equivalent; ND = Not detectable.
Plant growth stages and parts must be considered for quantifying metabolic accumulation [1,20]. Wang et al. (2022) reported that D. officinale accumulated high alkaloids and flavonoids in leaves, gigantol in stems and roots, and polysaccharides in stems [20]. D. nobile contained higher alkaloid contents in the stems of immature plants rather than in mature ones [21]. In the current study, DH plants of approximately 180-day age (Fig. 1) were harvested from tissue culture. The extraction used a whole plant (including roots, stems, and leaves) to cover the full range of available metabolites in the plant. Importantly, plant extraction on an industrial scale has bottlenecks in cultivating plants to reach maturity (more than 12 months for Dendrobium) and the low accumulation of bioactive agents, especially alkaloids [2,12,21]. In addition, cultivating plants in a natural environment has a high risk of exposure to pathogens and microorganisms. Thus, the high market demand for natural products has promoted the investigation of biotechnological approaches to induce the production of secondary metabolites in plant tissue-cultured or fermentation systems by feeding them with elicitors or precursors, such as UV lights, plant hormones, or abiotic stresses.
Fig. 1.
Plant characteristics of Dendrobium Pearl Vera hybrid (DH) at 90 days (left) and 180 days (right). Plants were grown from protocorm-like bodies in a tissue-culture system. Scale bar: 2 cm.
3.2. Metabolites and biosynthesis relationships in DH plant
The current study used an untargeted metabolomic approach based on HR-MS (Orbitrap system) to investigate and identify the chemical composition in the DH-P extract (Fig. 2A–C). The MS1 isotope patterns (Fig. 2B) and MS2 fragments (Fig. 2C) in the MS spectra were manually evaluated to avoid false-positive identifications, such as in the analysis of 4-guanidinobutyric acid. We identified 25 tentative compounds from the DH-P matrix (Table 2). In addition, four compounds (dendrobine, coumarin, quercetin, and gigantol) were included in the study as references because they have been reported to accumulate in Dendrobium [2,12]. The tentative substances were categorized into nutritional compounds, such as amino acids and nucleotides, that are essential for plant growth and development, as well as secondary metabolites associated with the plant growth itself and their usefulness for further applications. The 12 secondary metabolites were 1 furan, 3 phenolic, and 8 alkaloid compounds (Table 2). Analytical quantification and validation of the compounds associated with biological activities (Table 2), such as 5-HMF, salicylic acid, and nicotinamide, will be investigated in future work.
Fig. 2.
Chromatograms and mass spectral patterns of 4-guanidinobutyric acid, an anticarcinogenic agent. (A) Extracted ion chromatogram, (B) Precursor ion (MS1), and (C) Fragment ions (MS2).
Table 2.
Tentative compounds in DH-P and standard compounds identified through Orbitrap and Compound Discoverer analysis.
| Tentative Compound | Formula [H+ adduct] |
RT (min) | Precursor ion (m/z) adduct | Type of metabolite | Biological activity and function | References |
|---|---|---|---|---|---|---|
| I. Secondary metabolites | ||||||
| Choline | C5H14NO | 0.37 | 104.1070 | Alkaloid | Vitamin-like, neurotransmitter, regulation of water resorption and photosynthesis for plants | [31] |
| 5-Hydroxymethyl-2-furaldehyde (5′HMF) | C6H7O3 | 0.41 | 127.0389 | Furan | Suppression of cancer cell migration and invasion | [30] |
| Trigonelline | C7H8NO2 | 0.43 | 138.0550 | Alkaloid | Hypoglycemic, hypolipidemic, and neuroprotective agent | [28] |
| Tropine | C8H16NO | 0.49 | 142.1227 | Alkaloid | Substance in the biosynthesis of alkaloid compounds | [22] |
| 4-Guanidinobutyric acid (4-GBA) |
C5H12N3O2 | 0.50 | 146.0924 | Alkaloid | Anticancer and reduction of gastric lesions | [32] |
| Salicylic acid | C7H7O3 | 0.53 | 139.0390 | Phenolic | Plant hormone and antimicrobial | [23] |
| Pipecolic acid | C6H12NO2 | 0.55 | 130.0863 | Alkaloid | Immune regulator and a precursor for the biosynthesis of biological metabolites | [25] |
| Nicotinamide | C6H7N2O | 0.63 | 123.0553 | Alkaloid | A precursor of nicotinic acid | [28] |
| Acetophenone | C8H9O | 0.77 | 121.0648 | Phenolic | Antimicrobial and inhibition of superoxide anion in neutrophil | [33] |
| 5-Aminolevulinic acid | C5H10NO3 | 0.95 | 132.1019 | Alkaloid | Signaling molecule and enhancement of plant growth under abiotic stress | [26] |
| 4-Acetamidobutanoic acid | C6H12NO3 | 1.19 | 146.0811 | Alkaloid | A precursor of the neurotransmitter GABA | [27] |
| 3,5-di-tert-Butyl-4-hydroxy benzaldehyde (BHT-CHO) | C15H23O2 | 5.88 | 235.1693 | Phenolic | Antioxidant and antimicrobial | [34] |
| II. Nucleotide and derivatives | ||||||
| Cytidine | C9H14N3O5 | 0.51 | 244.0927 | |||
| Dihydrothymine | C5H9N2O2 | 0.56 | 129.0658 | Intermediate in thymine metabolism | ||
| Adenosine | C10H14N5O4 | 1.09 | 268.1039 | |||
| III. Amino acid and derivatives | ||||||
| dl-Arginine | C6H15N4O2 | 0.36 | 175.1189 | |||
| Asparagine | C4H9N2O3 | 0.38 | 133.0607 | |||
| l-Aspartic acid | C4H8NO4 | 0.39 | 134.0447 | |||
| l-Glutamic acid | C5H10NO4 | 0.40 | 148.0604 | |||
| D-(+)-Proline | C5H10NO2 | 0.44 | 116.0706 | |||
| l-Valine | C5H12NO2 | 0.52 | 118.0862 | |||
| l-Pyroglutamic acid | C5H8NO3 | 0.83 | 130.0500 | |||
| l-Norleucine | C6H14NO2 | 0.88 | 132.1020 | Suppression of metastasis of gastric and breast cancer cells | [24] | |
| Leucine | C6H14NO2 | 0.93 | 132.1020 | |||
| l-Phenylalanine | C9H12NO2 | 1.48 | 166.0863 | |||
| IV. Standard compound | ||||||
| Dendrobine | C16H26NO2 | 3.14 | 264.1955 | Alkaloid | Induction of cell apoptosis in lung cancer cells | [12] |
| Coumarin | C9H7O2 | 4.56 | 147.0440 | Phenolic | Antioxidant, UV protective, and anticancer activities | [9] |
| Quercetin | C15H11O7 | 5.37 | 303.0498 | Flavonol | Antioxidant, UV protective, and anticancer activities | [9] |
| Gigantol | C16H19O4 | 5.42 | 275.1273 | Bibenzyl | Suppression of metastasis of lung cancer cells | [2] |
A plant's metabolism is known to be highly interconnected with plant growth and development to defend itself from stresses due to environmental changes and pathogen attacks. In other words, metabolites can be very dynamic in their functions, being nutritive in some compounds (intermediates or final products), while in others, they may have biological activities [22,23]. Plant extracts often present precursors, such as amino acids, phytohormones, and sugars, that provide a broad range of metabolic changes during plant growth and stress responses [23]. Of these nutritive substances, amino acids are abundant and most involved in the biosynthesis of medicinal metabolites [12,23]. Ten amino acids and derivatives were observed in the DH-P matrix (Table 2). Interestingly, the amino acid l-norleucine was identified, which has been reported to exhibit anticancer activity [24].
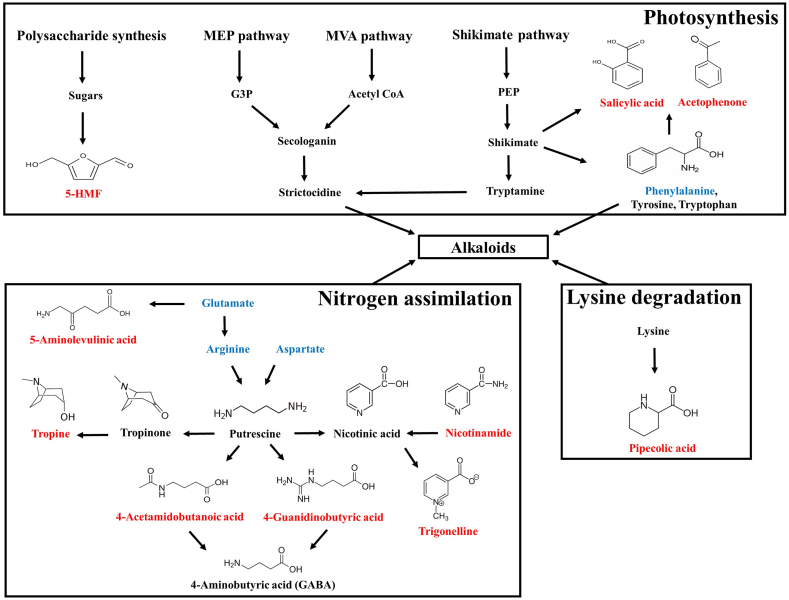
Active substances found in Dendrobium species are alkaloids, phenanthrenes, bibenzyls, flavonoids, and polysaccharides [1,2,22]. Notably, alkaloids are the most active constituents in Dendrobium spp. with remarkable medicinal properties, for example, anticancer, neuroprotective, anti-hypersensitive, antipyretic, and immunomodulatory activities [12,22]. The biosynthesis and relationship among some of the tentative substances were proposed in our study (Fig. 3). Several articles provide good reviews and illustrations on the biosynthetic pathways of alkaloids in Dendrobium spp. based on Omic studies [12,20,22]. Wang et al. (2020) suggested that alkaloids in Dendrobium spp. are usually derived from shikimate, mevalonate (MVA) and methylerythritol phosphate (MEP) pathways [22]. Furthermore, l-amino acids, consisting of lysine, arginine, phenylalanine, tyrosine, and tryptophan, are the main substances involved in the production of most alkaloids [12]. Strictosidine, derived from the MVA and MEP pathway, is an essential precursor for alkaloid biosynthesis [22]. Pipecolate is claimed to be a product of l-lysine degradation [25]. Glutamate is an amino acid from nitrogen assimilation and a precursor of arginine and ornithine, and it is a source for 5-aminolevulinic acid biosynthesis in higher plants through the Beal pathway [26]. Putrescine, the important intermediate in alkaloid biosynthesis produced from arginine or ornithine, can convert to tropine and a neurotransmitter ɣ-aminobutyric acid (GABA) through 4-aminobutanal [27]. Arginine and aspartic acid are thought to be involved with the nicotine biosynthesis pathway, as is trigonelline [28].
Fig. 3.
Plausible interconnections and relationships among tentative metabolites in DH plant. The scheme is based on the literature cited in the text. Red and blue letters represent metabolites and amino acids identified in the current study, respectively. Black letters represent substances involved, but not identified from the study, in the biosynthetic pathways.
The Shikimate pathway is upstream of phenylpropanoid and alkaloid biosynthesis in plants and is essential to produce many secondary metabolites [22]. Aromatic amino acids, including phenylalanine, tyrosine, and tryptophan, are generated from the shikimate pathway and serve as precursors, especially phenylalanine, for phenolic and flavonoid compounds, such as salicylic acid and acetophenone [23,29]. 5-Hydroxymethyl-2-furaldehyde (5′HMF), a suppressor of cancer cell migration and invasion, is a furan sugar product from sugar degradation generated by non-enzymatic reactions [30]. Based on our investigation, an untargeted approach revealed some essential elements and bioactive metabolites in the DH plant that are somehow interconnected.
3.3. Biological activities of DH extract
3.3.1. Scavenging of free radicals
The extracts were investigated for scavenging activity for free radicals, such as DPPH, ABTS, and ferric ions (Fig. 4). DH extracts at a concentration of 1 mg/ml were used to compare the scavenging efficiency and capacity levels with the standard ascorbic acid (AA). The DH extracts were able to inhibit free radicals; however, their capacity was lower than ascorbic acid (Fig. 4). Among the samples, the DH-P extract had the greatest scavenging activity on free radicals tested, with inhibition levels of approximately 29% and 42% for DPPH and ABTS free radicals, respectively (Fig. 4A). Its inhibitory activity was comparable with ascorbic acid at a concentration of 170 μM for DPPH and 241 μM for ABTS, calculated from a standard curve. The scavenging capacity for free radicals was gradually reduced in DH-E and DH-M, respectively, and was lowest in DH-W. A similar trend of scavenging performance was observed in the ferric reducing antioxidant power (FRAP) assay, except there was no significant difference between DH-E and DH-P (Fig. 4B). The scavenging activity was directly correlated with the total phenolic and flavonoid contents in the extracts (Table 1). The most active constituents in the extracts from Dendrobium are terpenes, stilbenoids, alkaloids, bibenzyls, phenanthrenes, flavonoids, and polysaccharides [1,2]. Phenolic and flavonoid substances are both well-known as bioactive agents for the inhibition of free radicals causing cell damage and destruction and as chelators for metal ions (such as iron and copper), with which they could generate highly reactive hydroxyl radicals via Fenton reactions [9,10,35]. The antioxidant activity of phenolics and flavonoids is mainly due to the variation of positions and numbers of hydroxyl groups on substances that can quench free radicals or chelate metal ions through different chemical mechanisms [35]. Notably, phenolics and flavonoids are the largest groups of secondary metabolites accumulated in plants and beneficially display antioxidant activity to rejuvenate skin age. For example, the phenolic compounds in Dendrobium Sonia Earsakul exhibited high antioxidant activity comparable to vitamin C, and it has been developed for usage in cosmetics [3,8]. Furthermore, D. bigibbum, an ancestor of the DH plant, has been listed in the New Inventory of Existing Cosmetic Ingredients in China Launched [4]. Our results could support the utilization of DH extract for skin improvement.
Fig. 4.
Antioxidant activity of DH extracts on free radicals. (A) Inhibitory effects on DPPH and ABTS free radicals and (B) Reducing power on ferric ions (Fe3+). Different lowercase letters on bars of particular free radicals indicate significant differences at p < 0.05, according to Tukey's test. AA, ascorbic acid at 500 μM (A) and 125 μM (B), were compared scavenging efficiency and capacity with extracts.
3.3.2. Cell-free human tyrosinase inhibition
The DH-E and DH-P extracts were investigated for their inhibitory effects on human tyrosinase due to their high contents of phenolics and flavonoids and favorable antioxidant activity. In summary, 10 μg/ml of the phenolics in DH-E and DH-P (Fig. 5A) had a percentage of inhibition activity over the control of 67.19% ± 1.56% and 82.81% ± 1.56%, respectively. The statistical analysis revealed that the inhibition activity of DH-P was not statistically different from 0.75 mM kojic acid, which had 94.43% ± 3.19% inhibition. Although the extracts were used at equal amounts of phenolic content, DH-E was prepared from a higher amount of crude extract than DH-P but had lower inhibition activity. For this reason, various concentrations of phenolics only in the DH-P were further used in the analysis, and 2-fold dilutions of the DH-P extract were prepared. The results showed the inhibition of human tyrosinase by DH-P in a concentration-dependent manner (Fig. 5B). Phenolics at 5 μg/ml showed similar inhibition activity (81.13% ± 0.13%) to 10 μg/ml phenolics, indicating saturation of the dose-response curve. It has been well-proven that synthetic and natural phenolic compounds have inhibitory effects on animal and human tyrosinase, the key enzyme involved in the production of melanin pigment [5,36]. Some Dendrobium spp. also can inhibit the activity of tyrosinase [7]. This result demonstrated that DH-P extract could inhibit human tyrosinase in vitro and might contain promising substances that could be useful in reducing the skin dark pigment, melanin. Therefore, the biological activity of DH-P extract on human skin cells was further investigated.
Fig. 5.
Inhibitory effect on cell-free human tyrosinase. (A) Inhibitory activity of 10 μg/ml phenolics in DH-E and DH-P extracts, compared to 0.75 mM kojic acid; (B) Inhibitory activity of DH-P extract at various concentrations of phenolic content. Data represent percentages of values in a reaction containing only solvent, without any inhibitors. Values are mean ± SEM of three independent experiments with statistical analysis based on one-way ANOVA, where ** indicates a significant difference at p < 0.01.
3.3.3. Effect of DH-P on the proliferation of human melanoma cells
It was preliminarily observed that SK-MEL-28, an aggressive type of skin cancer, responded to 100 μg/ml of DH extract obtained from four different solvents in different ways on day 2 but notably reduced the survival rate at day 4 (Supplementary Fig. 1). Regardless of the exposure time, DH-P strongly inhibited the growth of melanoma (more than 85% inhibition). Since DH-P contains high amounts of phenolics and flavonoids that can inhibit melanoma cells [37,38], its effect on the viability of melanoma was investigated in this study. Various amounts of phenolic content in DH-P were prepared and used to treat melanoma cells for 48 h. The viability was calculated as the percentage of those cells growing in a complete medium. Fig. 6 demonstrates that DH-P affected the viability of SK-MEL-28 in a concentration-dependent manner. Furthermore, 15 μg/ml of phenolics or higher in DH-P could inhibit the proliferation of the melanoma, as shown by the percentage of viability being lower than 45.27% of the growth-starved cells (dashed line in Fig. 6). In contrast, a phenolic content at 5 μg/ml or lower did not change the viability rate of cells from the control. The IC50 of the phenolics in DH-P was 12.90 ± 1.04 μg/ml, as enumerated from a transformed viability curve. This result was consistent with other studies observing that extracts from Dendrobium spp. could reduce cell growth and metastasis toward cancer cell lines [2,12]. The inhibitory effect of DH-P on cancer cell growth was relevant not only to the amounts of phenolics and flavonoids but also to other chemical constituents, including 5-HMF, 4-GBA, and l-norleucine (Table 2). The crude methanolic extract of Glochidion velutinum, an evergreen plant, had cytotoxic activity against prostate cancer and breast cancer cell lines. It was proposed that epigallocatechin gallate, ellagic acid, isovitexin, and rutin, identified by LC-MS/MS, might be responsible for the anticancer activity [39]. D. nobile and D. draconis inhibited lung cancer cells [2,12]. Therefore, investigating the cytotoxicity on other cancer cell lines with crude DH-P extract would also be worth studying.
Fig. 6.
Effect of phenolic content in DH-P on human melanoma cell viability. Experiments were individually conducted in triplicate using cells at passage 15, 17, and 20 and incubated for 48 h. The bar graph presents the mean ± SEM of percentage values over those of cells grown in a complete medium. The dashed line represents the percentage of growth-starved cells cultured with low serum media.
3.3.4. Depigmentation activity of human melanoma cells by DH-P
DH-P containing phenolic contents of 2.5 or 5 μg/ml was used to examine the effect of reducing melanin content produced by human melanoma, compared to 100 μg/ml of arbutin, a widely used whitening agent. Neither concentration of phenolics affected cell viability (Fig. 6); however, they showed different inhibition effects on human tyrosinase in vitro (Fig. 5B). Fig. 7 shows that the phenolic content at 5 μg/ml significantly reduced the melanin produced by human melanoma compared to the lower amount of phenolics in DH-P, arbutin, and the control. The melanin contents measured in the treatment with 5 μg/ml phenolic DH-P, 2.5 μg/ml phenolic DH-P, arbutin, and control media were 17.44 ± 3.89, 31.27 ± 1.14, 34.33 ± 0.69 and 37.82 ± 0.65 mg/ml, respectively. Quercetin and kaempferol have been reported to increase the melanin content and tyrosinase activity of human malignant melanoma HMV II cells [40]; however, in the current study, those compounds were found in DH extracts in trace amounts (Table 1) and might not be expected to contribute to increasing the melanin content under these circumstances.
Fig. 7.
Effect of phenolic content in DH-P on α-MSH-induced melanin content. Melanin content at 100 nM of α-MSH-activated melanoma was determined by measuring absorption at 405 nm. Treatment with 2.5 or 5 μg/ml phenolic DH-P was compared to 100 μg/ml arbutin and the media control after 48 h of incubation. Values of melanin content are mean ± SEM of three independent experiments using cells at passage 15, 17, and 20. Significant differences were determined using one-way ANOVA, where ** and *** indicate p < 0.01 and p < 0.001, respectively.
Among the tentative compounds identified (Table 2), several bioactive ingredients might play a role in reducing melanin content. 5′HMF reduced the melanin content and transcription and expression of tyrosinase enzyme in B16 mouse melanoma cells [41]. Salicylic acid, a phenolic compound, reduced the percentages of melanin content and tyrosinase activity in murine melanoma cells and human epidermal melanocytes compared to the control [42]. Although the effects of nicotinamide on melanin synthesis by mono-layered melanocytes and melanocyte-keratinocyte co-culture are controversial [43]. Recently, nicotinamide mononucleotide showed an inhibitory effect on melanin production by melanocytes of aged (but not for young) persons through the cAMP/Wnt signaling pathway [44]. Twelve acetophenone (phenolic) derivatives at micromolar levels could inhibit mushroom tyrosinase activity and melanin production, including a reduced proliferation of B16F10 murine melanoma cells [45]. In addition, they could predict the moiety interacting with the enzyme's active site. Cytidine, a common nucleoside, could inhibit the process of melanosome transfer from melanocytes to keratinocytes and reduce the melanin content in a co-culture model [46]. Moreover, various concentrations of adenosine showed different effects on melanin production. At 4 μM adenosine increased the melanin content and tyrosinase activity of B16 murine melanoma cells, but it reduced those effects at high doses of 40 and 400 μM [47]. Amino acids are the monomer component of peptides and proteins; Boo (2020) collectively reported both inhibitory and increased effects of peptides on melanin production and related enzymes [48]. Interestingly, proline and valine were found in DH-P; they are the constituents of dipeptides (proline-serine and valine-serine) that had an inhibitory effect on the melanin content in Mel-Ab murine melanocyte cells [49].
In summary, this current study demonstrated that DH-P extract contained high phenolics and flavonoids with antioxidant activity. The natural products in the DH extract could inhibit human tyrosinase in vitro and decrease melanin production by human melanoma cells. At the right concentration, these extracts also revealed anticancer proliferation activity in melanoma. Tentative bioactive components were proposed to contribute to such biological functions and their biosynthesis in the plant. Therefore, DH-P extract has the potential to be formulated as an ingredient for skin care and cosmetic products.
Author contribution statement
Napachanok Mongkoldhumrongkul Swainson, Attawan Aramrak: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Thanyawan Pengoan, Rungpailin Khonsap, Pilairath Meksangsee, Gerhard Hagn: Performed the experiments.
Christopher Gerner: Contributed reagents, materials, analysis tools or data.
Funding statement
This work was supported by the Office of the Ministry of Higher Education, Science, Research and Innovation; and the Thailand Science Research and Innovation through the Kasetsart University Reinventing University Program 2021, Bangkok, Thailand. CordyBiotech Co., Ltd. provided partial research funding.
Data availability statement
Data included in article/supplementary material/referenced in article.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Attawan Aramrak reports financial support and equipment, drugs, or supplies were provided by CordyBiotech Co., Ltd. Attawan Aramrak reports a relationship with CordyBiotech Co., Ltd. that includes: equity or stocks and funding grants. Attawan Aramrak has patent pending to Attawan Aramrak and Chan Maketon. Corresponding author was received the plant material from CordyBiotech Co., Ltd.
Acknowledgments
Language editing was serviced by Dr. Andrew Warner, Academic English Editor at Kasetsart University Research & Development Institute (KURDI), Bangkok, Thailand.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2023.e20197.
Appendix A. Supplementary data
The following is the supplementary data to this article:
figs1.
References
- 1.Cakova V., Bonte F., Lobstein A. Dendrobium: sources of active ingredients to treat age-related pathologies. Aging Dis. 2017 Dec 1;8(6):827–849. doi: 10.14336/AD.2017.0214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Śliwiński T., Kowalczyk T., Sitarek P., Kolanowska M. Orchidaceae-derived anticancer agents: a review. Cancers. 2022 Jan 31;14(3):754. doi: 10.3390/cancers14030754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanlayavattanakul M., Louritha N., Chaikul P. Biological activity and phytochemical profiles of Dendrobium: a new source for specialty cosmetic materials. Ind. Crops Prod. 2018 May 7;120(15):61–70. doi: 10.1016/j.indcrop.2018.04.059. [DOI] [Google Scholar]
- 4.Wang Y.H. Traditional uses and pharmacologically active constituents of Dendrobium plants for dermatological disorders: a review. Nat Prod Bioprospect. 2021 Oct;11(5):465–487. doi: 10.1007/s13659-021-00305-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rathee P., Kumar S., Kumar D., Kumari B., Yadav S.S. Skin hyperpigmentation and its treatment with herbs: an alternative method. Futur J Pharm Sci. 2021 Jul 7:132. doi: 10.1186/s43094-021-00284-6. [DOI] [Google Scholar]
- 6.Chan C.F., Wu C.T., Huang W.Y., Lin W.S., Wu H.W., Huang T.K., Chang M.Y., Lin Y.S. Antioxidation and melanogenesis inhibition of various Dendrobium tosaense extracts. Molecules. 2018 Jul 21;23(7):1810. doi: 10.3390/molecules23071810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Athipornchai A., Jullapo N. Tyrosinase inhibitory and antioxidant activities of orchid (Dendrobium spp.) South Afr. J. Bot. 2018 Sep 25;119:188–192. doi: 10.1016/j.sajb.2018.09.003. [DOI] [Google Scholar]
- 8.Shafazila T.S., Lee P.M., Hung L.K. International Conference on Science and Social Research (CSSR 2010) Kuala Lumpur; Malaysia: 2010 Dec. Radical scavenging activities of extract and solvent-solvent partition fractions from Dendrobium Sonia ‘red bom’ flower; pp. 762–765. [DOI] [Google Scholar]
- 9.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: an overview. Medicines (Basel) 2018 Aug 25;5(3):93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gülçin I., Bursal E., Sehitoğlu M.H., Bilsel M., Gören A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from erzurum, Turkey. Food Chem. Toxicol. 2010 Aug-Sep;48(8–9):2227–2238. doi: 10.1016/j.fct.2010.05.053. [DOI] [PubMed] [Google Scholar]
- 11.Chinembiri T.N., du Plessis L.H., Gerber M., Hamman J.H., du Plessis J. Review of natural compounds for potential skin cancer treatment. Molecules. 2014 Aug 6;19(8):11679–11721. doi: 10.3390/molecules190811679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song C., Ma J., Li G., Pan H., Zhu Y., Jin Q., Cai Y., Han B. Natural composition and biosynthetic pathways of alkaloids in medicinal Dendrobium species. Front. Plant Sci. 2022 May 6;13 doi: 10.3389/fpls.2022.850949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.OrchidRoots [Internet] 2022. https://orchidroots.org/ September 14. Available from:
- 14.Attard E.A. Rapid microtitre plate folin-ciocalteu method for the assessment of polyphenols. Cent. Eur. J. Biol. 2013 Jan;8:48–53. doi: 10.2478/s11535-012-0107-3. [DOI] [Google Scholar]
- 15.Bhattacharyya P., Kumaria S., Diengdoh R., Tandon P. Genetic stability and phytochemical analysis of the in vitro regenerated plants of Dendrobium nobile lindl., an endangered medicinal orchid. Meta Gene. 2014;2:489–504. doi: 10.1016/j.mgene.2014.06.003. Jul 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herald T.J., Gadgil P., Tilley M. High-throughput micro plate assays for screening flavonoid content and DPPH-scavenging activity in sorghum bran and flour. J. Sci. Food Agric. 2012 Aug 30;92(11):2326–2331. doi: 10.1002/jsfa.5633. [DOI] [PubMed] [Google Scholar]
- 17.Thaipong K., Boonprakob U., Crosby K., Cisneros-Zevallos L., Byrne D.H. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006 Jun;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 18.Bolanos de la Torre A.A., Henderson T., Nigam P.S., Owusu-Apenten R.K. A universally calibrated microplate ferric reducing antioxidant power (FRAP) assay for foods and applications to manuka honey. Food Chem. 2015 May 1;174:119–123. doi: 10.1016/j.foodchem.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Wakeel A., Jan S.A., Ullah I., Shinwari Z.K., Xu M. Solvent polarity mediates phytochemical yield and antioxidant capacity of isatis tinctoria. PeerJ. 2019 Oct 9;7 doi: 10.7717/peerj.7857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang Y., Tong Y., Adejobi O.I., Wang Y., Liu A. Research advances in multi-omics on the traditional Chinese herb Dendrobium officinale. Front. Plant Sci. 2022 Jan 11;12 doi: 10.3389/fpls.2021.808228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y.W., Shi Y.C., Zhang S.B. Metabolic and transcriptomic analyses elucidate a novel insight into the network for biosynthesis of carbohydrate and secondary metabolites in the stems of a medicinal orchid Dendrobium nobile. Plant Divers. 2022 Oct 27 doi: 10.1016/j.pld.2022.10.004. In pres. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Z., Zhao M., Cui H., Li J., Wang M. Transcriptomic landscape of medicinal Dendrobium reveals genes associated with the biosynthesis of bioactive components. Front. Plant Sci. 2020 Apr 28;11:391. doi: 10.3389/fpls.2020.00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schäfer M., Brütting C., Baldwin I.T., Kallenbach M. High-throughput quantification of more than 100 primary- and secondary-metabolites, and phytohormones by a single solid-phase extraction based sample preparation with analysis by UHPLC-HESI-MS/MS. Plant Methods. 2016 May 26;12:30. doi: 10.1186/s13007-016-0130-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He T., Jin M., Xu C., Ma Z., Wu F., Zhang X. The homeostasis-maintaining metabolites from bacterial stress response to bacteriophage infection suppress tumor metastasis. Oncogene. 2018 Oct;37(43):5766–5779. doi: 10.1038/s41388-018-0376-z. [DOI] [PubMed] [Google Scholar]
- 25.Wang C., Liu R., Lim G.H., de Lorenzo L., Yu K., Zhang K., Hunt A.G., Kachroo A., Kachroo P. Pipecolic acid confers systemic immunity by regulating free radicals. Sci. Adv. 2018;4(5):eaar4509. doi: 10.1126/sciadv.aar4509. May 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y., Liao W., Dawuda M.M., Hu L., Yu J. 5-Aminolevulinic acid (ala) biosynthetic and metabolic pathways and its role in higher plants: a review. Plant Growth Regul. 2019 Mar 2;87:357–374. doi: 10.1007/s10725-018-0463-8. [DOI] [Google Scholar]
- 27.Chen D., Shao Q., Yin L., Younis A., Zheng B. Polyamine function in plants: metabolism, regulation on development, and roles in abiotic stress responses. Front. Plant Sci. 2019 Jan 10;9:1945. doi: 10.3389/fpls.2018.01945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashihara H., Ludwig I.A., Katahira R., Yokota T., Fujimura T., Crozier A. Trigonelline and related nicotinic acid metabolites: occurrence, biosynthesis, taxonomic considerations, and their roles in planta and in human health. Phytochem Rev. 2015 Oct;14:765–798. doi: 10.1007/s11101-014-9375-z. [DOI] [Google Scholar]
- 29.Dong F., Yang Z., Baldermann S., Kajitani Y., Ota S., Kasuga H., Imazeki Y., Ohnishi T., Watanabe N. Characterization of L-phenylalanine metabolism to acetophenone and 1-phenylethanol in the flowers of camellia sinensis using stable isotope labeling. J. Plant Physiol. 2012;169(3):217–225. doi: 10.1016/j.jplph.2011.12.003. Feb 15. [DOI] [PubMed] [Google Scholar]
- 30.Chow P.H., Kourghi M., Pei J.V., Nourmohammadi S., Yool A.J. 5-Hydroxymethyl-Furfural and structurally related compounds block the ion conductance in human aquaporin-1 channels and slow cancer cell migration and invasion. Mol. Pharmacol. 2020 Jul;98(1):38–48. doi: 10.1124/mol.119.119172. [DOI] [PubMed] [Google Scholar]
- 31.Wessler I., Kilbinger H., Bittinger F., Kirkpatrick C.J. The biological role of non-neuronal acetylcholine in plants and humans. Jpn. J. Pharmacol. 2001 Jan;85(1):2–10. doi: 10.1254/jjp.85.2. [DOI] [PubMed] [Google Scholar]
- 32.Hwang I.Y., Jeong C.S. Inhibitory effects of 4-guanidinobutyric acid against gastric lesions. Biomol Ther (Seoul). 2012 Mar;20(2):239–244. doi: 10.4062/biomolther.2012.20.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stuppner H., Reinisch O., Wiedermann C.J., Wagner H. Acetophenones - compounds from plant origin with inhibitory effects on neutrophil in vitro respiration burst activity. Phytomedicine. 1995 Apr;1(4):283–286. doi: 10.1016/S0944-7113(11)80003-6. [DOI] [PubMed] [Google Scholar]
- 34.Angelini P., Girometta C., Tirillini B., Moretti S., Covino S., Cipriani M., D'Ellena E., Angeles G., Federici E., Savino E., Cruciani G., Venanzoni R. A comparative study of the antimicrobial and antioxidant activities of inonotus hispidus fruit and their mycelia extracts. Int. J. Food Prop. 2019 Apr 25;22(1):768–783. doi: 10.1080/10942912.2019.1609497. [DOI] [Google Scholar]
- 35.Desmet S., Morreel K., Dauwe R. Origin and function of structural diversity in the plant specialized metabolome. Plants. 2021;10(11):2393. doi: 10.3390/plants10112393. Nov 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Panzella L., Napolitano A. Natural and bioinspired phenolic compounds as tyrosinase inhibitors for the treatment of skin hyperpigmentation: recent advances. Cosmetics. 2019;6(4):57. doi: 10.3390/cosmetics6040057. Oct 1. [DOI] [Google Scholar]
- 37.Gado F., Digiacomo M., Salsano J.E., Macchia M., Manera C. Phenolic compounds in prevention and treatment of skin cancers: a review. Curr. Med. Chem. 2021;28(33):6730–6752. doi: 10.2174/0929867328666210324160324. Oct 25. [DOI] [PubMed] [Google Scholar]
- 38.Liu-Smith F., Meyskens F.L. Molecular mechanisms of flavonoids in melanin synthesis and the potential for the prevention and treatment of melanoma. Mol. Nutr. Food Res. 2016 Jun;60(6):1264–1274. doi: 10.1002/mnfr.201500822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shah S.L., Bashir K., Rasheed H.M., Rahman J.U., Ikram M., Shah A.J., Majrashi K.A., Alnasser S.M., Menaa F., Khan T. LC-MS/MS-Based metabolomic profiling of constituents from Glochidion velutinum and its activity against cancer cell lines. Molecules. 2022;27(24):9012. doi: 10.3390/molecules27249012. Dec 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Takekoshi S., Nagata H., Kitatani K. Flavonoids enhance melanogenesis in human melanoma cells. Tokai J. Exp. Clin. Med. 2014;39(3):116–121. Sep. 20. [PubMed] [Google Scholar]
- 41.Bito T., Koseki K., Asano R., Ueda N., Yamada T., Yabuta Y., Ichiyanagi T., Ishihara A., Watanabe K., Watanabe F. 5-Hydroxymethyl-2-Furaldehyde purified from Japanese pear (pyrus pyrifolia nakai cv. Nijisseiki) juice concentrate inhibits melanogenesis in B16 mouse melanoma cells. Biosci. Biotechnol. Biochem. 2020 Nov;84(11):2374–2384. doi: 10.1080/09168451.2020.1792762. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Jiang R., Zhou J., Xu X., Sun Z., Li J., Chen X., Li Z., Yan X., Zhao D., Zheng Z., Sun L. Salicylic acid in ginseng root alleviates skin hyperpigmentation disorders by inhibiting melanogenesis and melanosome transport. Eur. J. Pharmacol. 2021;910 doi: 10.1016/j.ejphar.2021.174458. Nov 5. [DOI] [PubMed] [Google Scholar]
- 43.Boo Y.C. Mechanistic basis and clinical evidence for the applications of nicotinamide (niacinamide) to control skin aging and pigmentation. Antioxidants. 2021;10(8):1315. doi: 10.3390/antiox10081315. Aug 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brito S., Baek J.M., Cha B., Heo H., Lee S.H., Lei L., Jung S.Y., Lee S.M., Lee S.H., Kwak B.M., Chae S., Lee M.G., Bin B.H. Nicotinamide mononucleotide reduces melanin production in aged melanocytes by inhibiting cAMP/wnt signaling. J. Dermatol. Sci. 2022 Jun;106(3):159–169. doi: 10.1016/j.jdermsci.2022.05.002. [DOI] [PubMed] [Google Scholar]
- 45.Hałdys K., Goldeman W., Anger-Góra N., Rossowska J., Latajka R. Monosubstituted acetophenone thiosemicarbazones as potent inhibitors of tyrosinase: synthesis, inhibitory studies, and molecular docking. Pharmaceuticals. 2021;14(1):74. doi: 10.3390/ph14010074. Jan 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Diwakar G., Klump V., Lazova R., Pawelek J. Evidence for glycosylation as a regulator of the pigmentary system: key roles of sialyl(α2-6)gal/GalNAc-terminated glycans in melanin synthesis and transfer. Glycoconj. J. 2015 Aug;32(6):413–420. doi: 10.1007/s10719-015-9605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim M.Y., Lee H.E., Im M., Lee Y., Kim C.D., Lee J.H., Seo Y.J. Effect of adenosine on melanogenesis in b16 cells and zebrafish. Ann. Dermatol. 2014 Apr;26(2):209–213. doi: 10.5021/ad.2014.26.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boo Y.C. Up- or downregulation of melanin synthesis using amino acids, peptides, and their analogs. Biomedicines. 2020;8(9):322. doi: 10.3390/biomedicines8090322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee H.E., Kim E.H., Choi H.R., Sohn U.D., Yun H.Y., Baek K.J., Kwon N.S., Park K.C., Kim D.S. Dipeptides inhibit melanin synthesis in mel-ab cells through down-regulation of tyrosinase. KOREAN J. PHYSIOL. PHARMACOL. 2012;16(4):287–291. doi: 10.4196/kjpp.2012.16.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.