Abstract
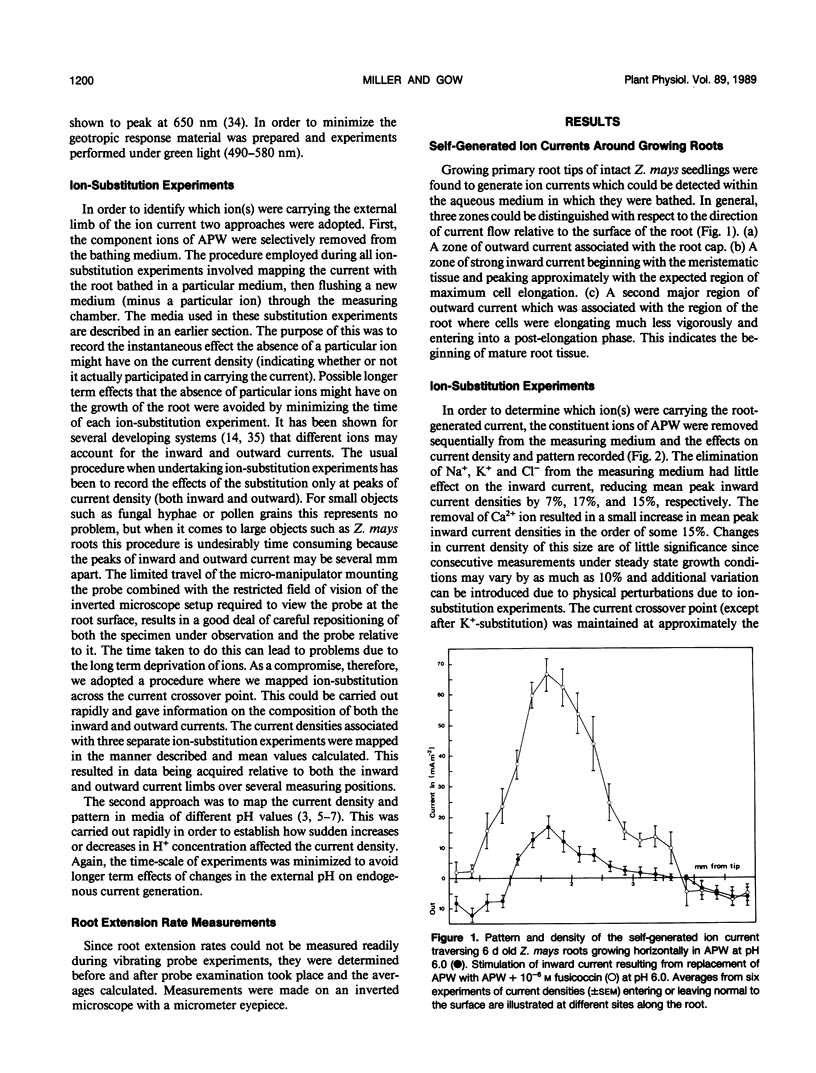
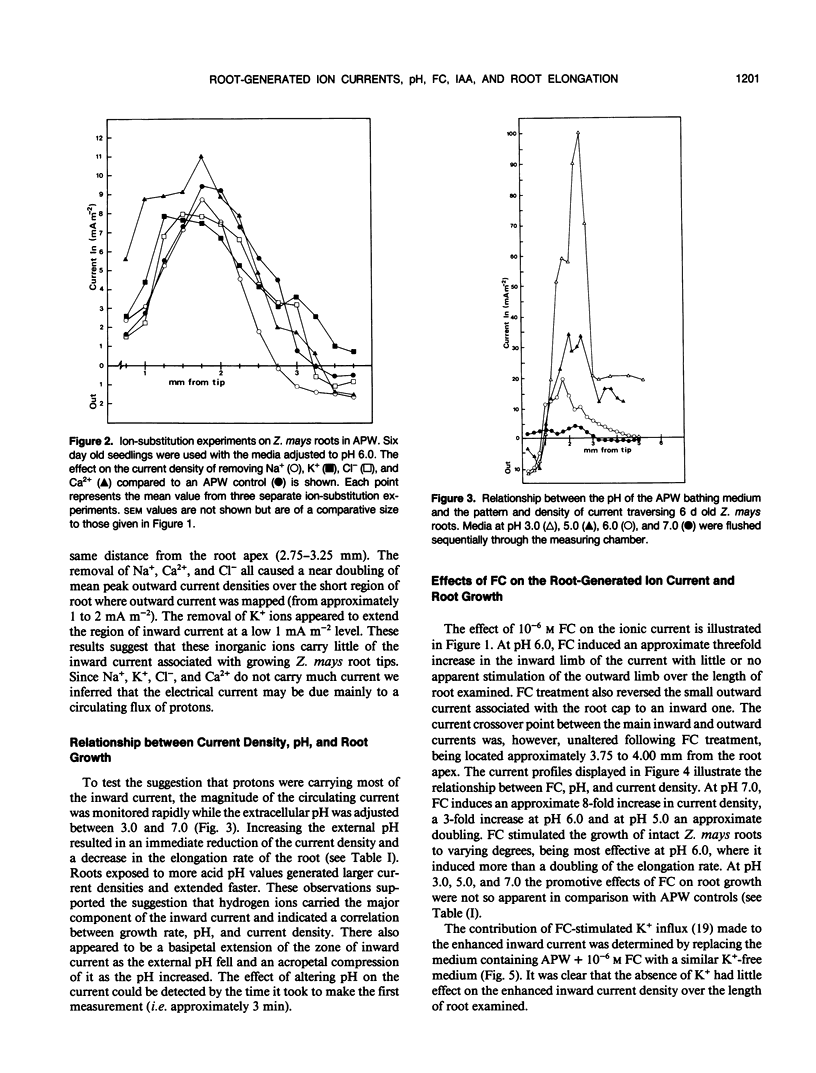
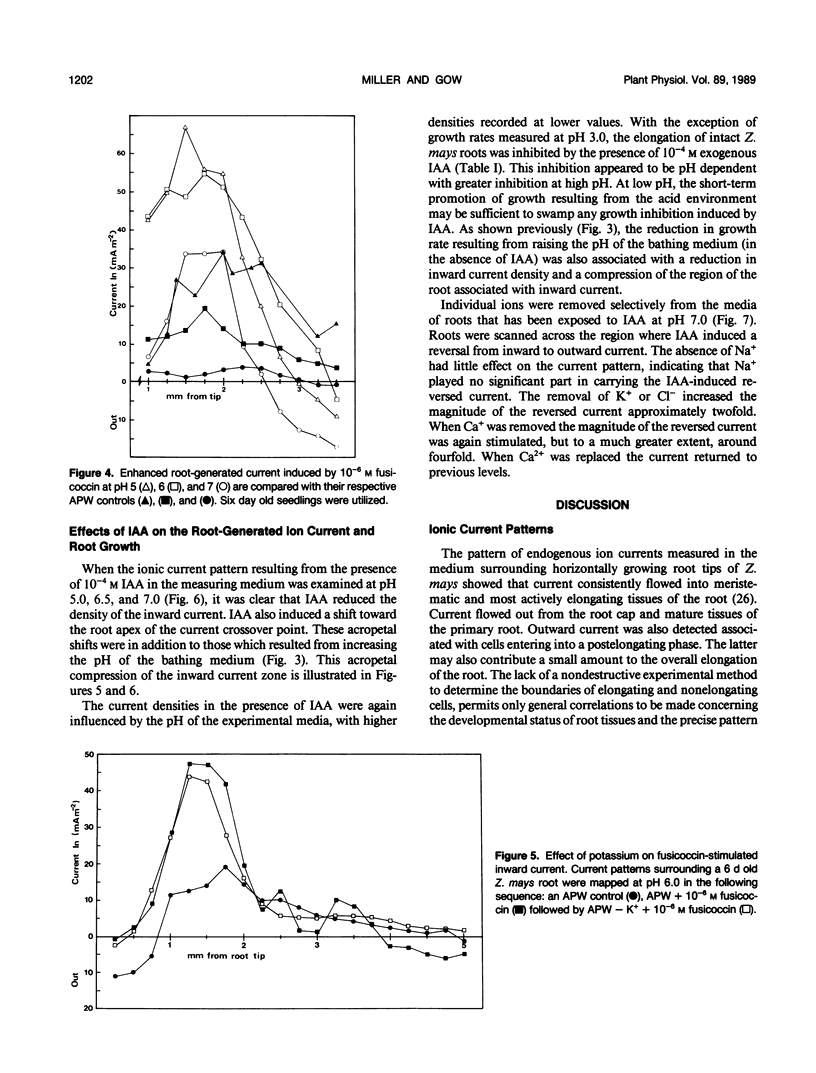
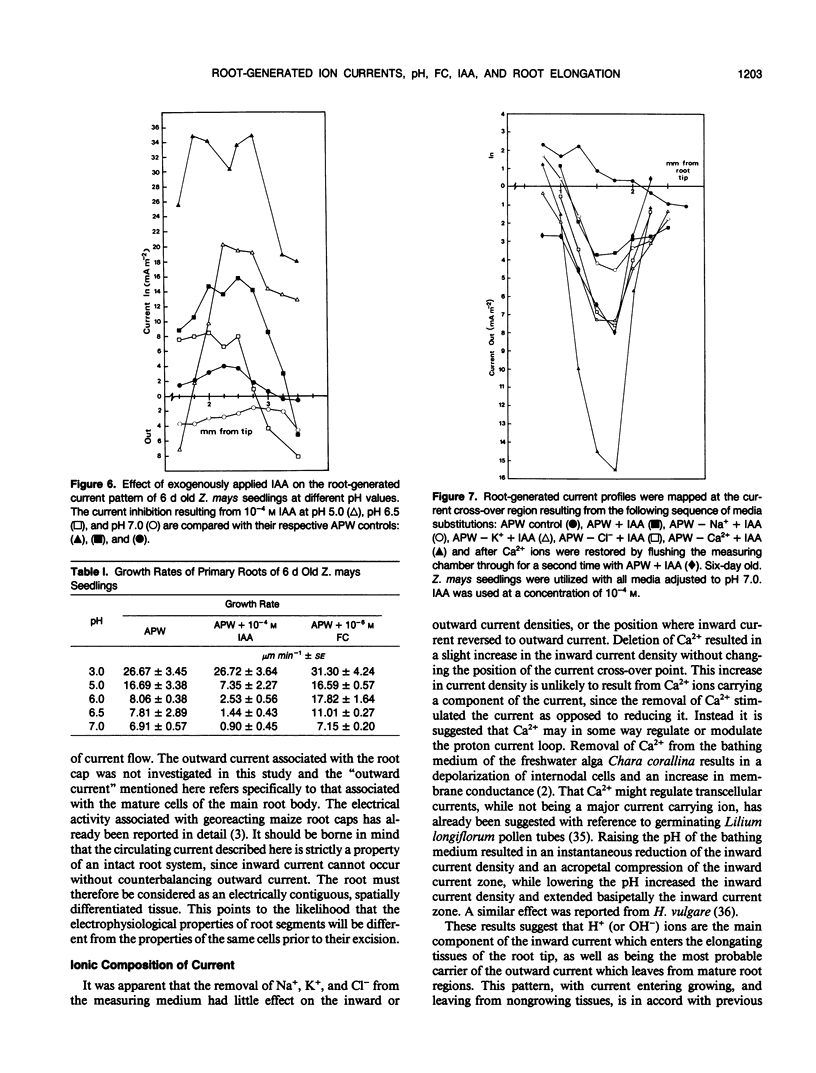
Correlations between root-generated ionic currents, extracellular pH, indoleacetic acid, fusicoccin, and growth were investigated. Current consistently entered the meristematic and elongating tissues of intact growing roots of Zea mays cv Golden Bantam. Mature root regions generated the outward limb of the current loop. Ion-substitution and pH-profile experiments suggested that the bulk of the ionic current was carried by H+. Calcium ions did not carry current, but calcium may regulate the proton circulation since the proton current density was slightly larger in calcium-depleted media. Increased root elongation at low pH was associated with increased current density and an extended zone of inward current. Conversely decreased elongation at high pH was associated with a reduced current density and a more restricted zone of inward current. The effect of the fungal toxin fusicoccin was to increase the current density of the inward limb of the ion current and to increase root extension. Concentrations of indoleacetic acid that reduced root growth, also reduced the density of the inward current and shortened the inward current zone. The results emphasize the point that roots are electrically contiguous over many millimeters and that the electrophysiology of root growth is best studied in intact root systems.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behrens H. M., Weisenseel M. H., Sievers A. Rapid Changes in the Pattern of Electric Current around the Root Tip of Lepidium sativum L. following Gravistimulation. Plant Physiol. 1982 Oct;70(4):1079–1083. doi: 10.1104/pp.70.4.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisson M. A. Calcium effects on electrogenic pump and passive permeability of the plasma membrane of Chara corallina. J Membr Biol. 1984;81(1):59–67. doi: 10.1007/BF01868810. [DOI] [PubMed] [Google Scholar]
- Björkman T., Leopold A. C. An electric current associated with gravity sensing in maize roots. Plant Physiol. 1987;84:841–846. doi: 10.1104/pp.84.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brawley S. H., Wetherell D. F., Robinson K. R. Electrical polarity in embryos of wild carrot precedes cotyledon differentiation. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6064–6067. doi: 10.1073/pnas.81.19.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronewald J. W., Cheeseman J. M., Hanson J. B. Comparison of the responses of corn root tissue to fusicoccin and washing. Plant Physiol. 1979 Feb;63(2):255–259. doi: 10.1104/pp.63.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe L. F., Nuccitelli R. An ultrasensitive vibrating probe for measuring steady extracellular currents. J Cell Biol. 1974 Nov;63(2 Pt 1):614–628. doi: 10.1083/jcb.63.2.614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin H. V., Pilet P. E. Effect of pH on IAA Uptake by Maize Root Segments. Plant Physiol. 1987 Feb;83(2):262–264. doi: 10.1104/pp.83.2.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkey T. J., Evans M. L. Geotropism in corn roots: evidence for its mediation by differential Acid efflux. Science. 1981 Apr 3;212(4490):70–71. doi: 10.1126/science.212.4490.70. [DOI] [PubMed] [Google Scholar]
- O'neill R. A., Scott T. K. Proton Flux and Elongation in Primary Roots of Barley (Hordeum vulgare L.). Plant Physiol. 1983 Sep;73(1):199–201. doi: 10.1104/pp.73.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilet P. E., Saugy M. Effect on Root Growth of Endogenous and Applied IAA and ABA: A Critical Reexamination. Plant Physiol. 1987 Jan;83(1):33–38. doi: 10.1104/pp.83.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitman M. G. Adaptation of barley roots to low oxygen supply and its relation to potassium and sodium uptake. Plant Physiol. 1969 Sep;44(9):1233–1240. doi: 10.1104/pp.44.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank A. R. CHANGES IN ELECTRICAL POLARITY IN THE AVENA COLEOPTILE AS AN ANTECEDENT TO HORMONE ACTION IN GEOTROPIC RESPONSE. Plant Physiol. 1945 Jan;20(1):133–136. doi: 10.1104/pp.20.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenseel M. H., Dorn A., Jaffe L. F. Natural H Currents Traverse Growing Roots and Root Hairs of Barley (Hordeum vulgare L.). Plant Physiol. 1979 Sep;64(3):512–518. doi: 10.1104/pp.64.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]


