Abstract
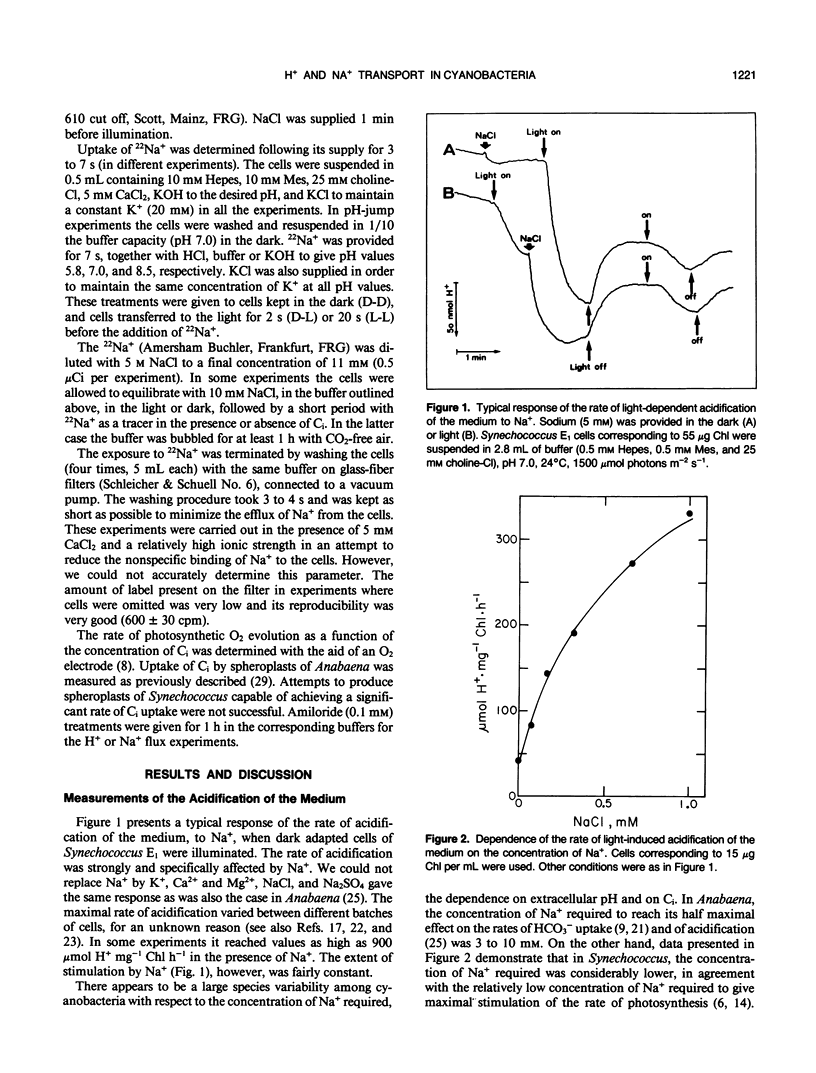
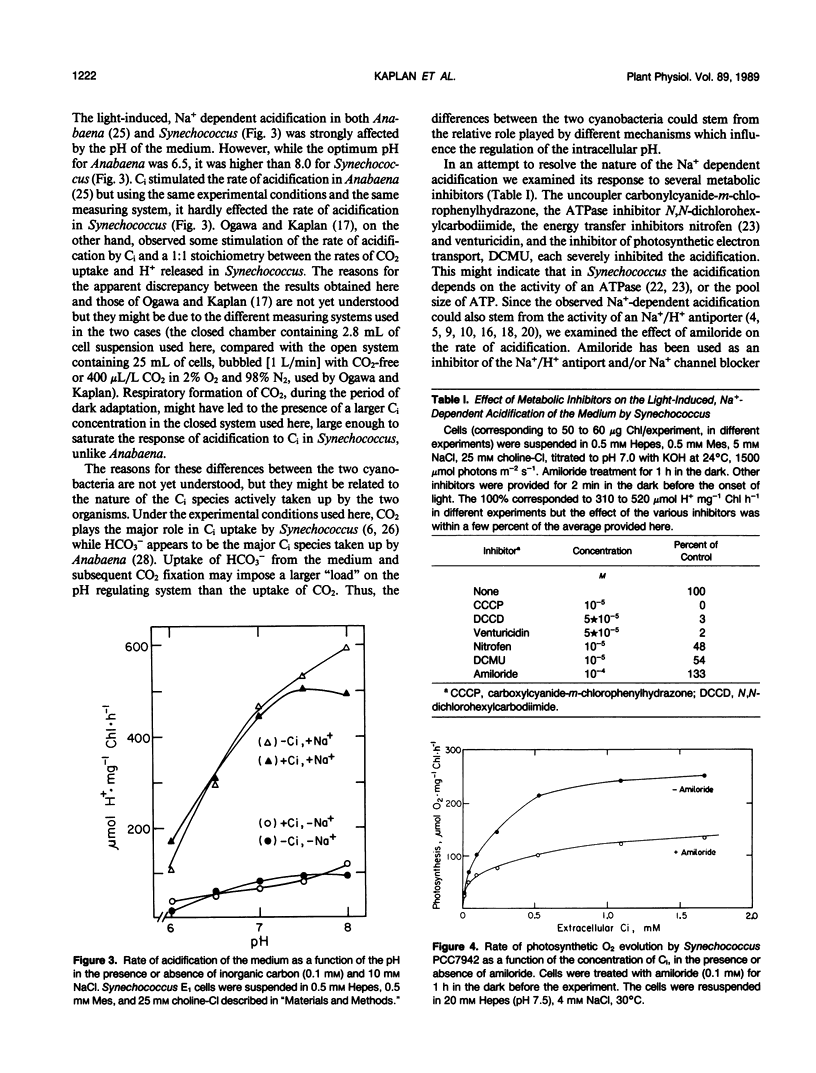
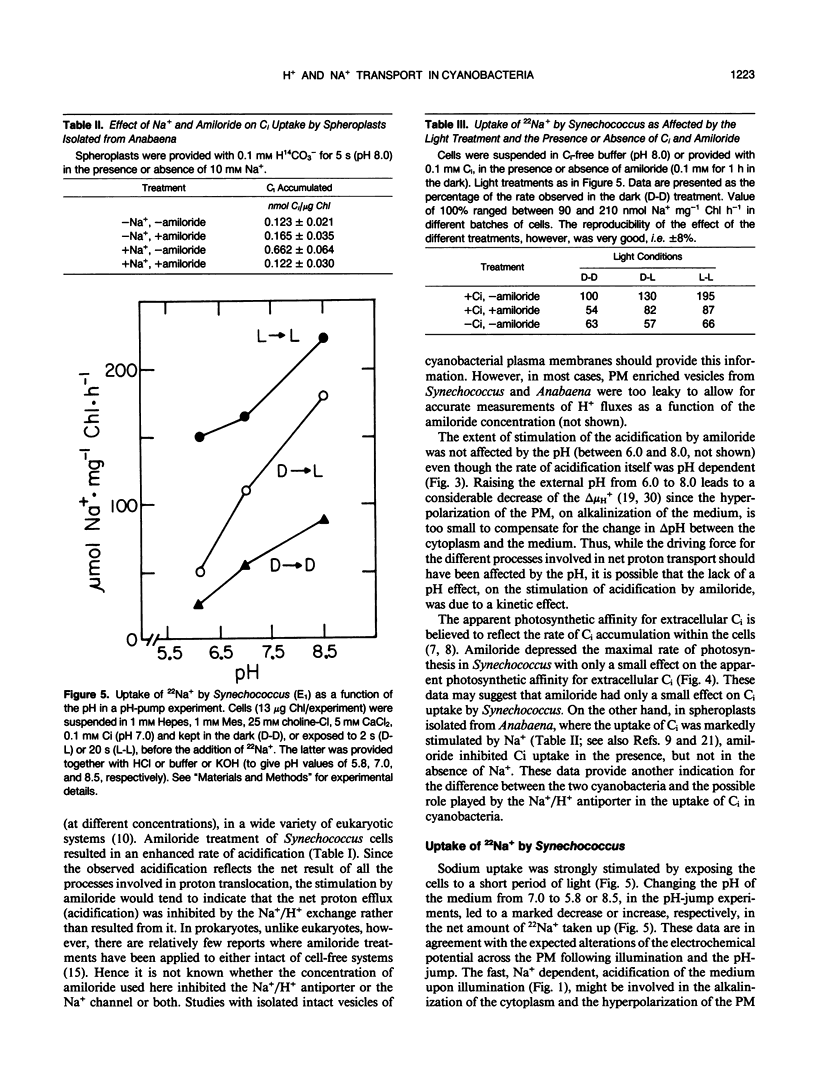
We investigated the nature of the light-induced, sodium-dependent acidification of the medium and the uptake of sodium by Synechococcus. The rate of acidification (net H+ efflux) was strongly and specifically stimulated by sodium. The rates of acidification and sodium uptake were strongly affected by the pH of the medium; the optimal pH for both processes being in the alkaline pH range. Net proton efflux was severely inhibited by inhibitors of adenosine triphosphatase activity, energy transfer, and photosynthetic electron transport, but was not affected by the presence of inorganic carbon (Ci). Light and Ci stimulated the uptake of sodium, but the stimulation by Ci was observed only when Ci was present at the time sodium was provided. Amiloride, a potent inhibitor of Na+/H+ antiport and Na+ channels, stimulated the rate of acidification but inhibited the rate of sodium uptake. It is suggested that acidification might stem from the activity of a light dependent proton excreting adenosine triphosphatase, while sodium transport seems to be mediated by both Na+/H+ antiport and Na+ uniport.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Andrews T. J. Photosynthesis and Inorganic Carbon Usage by the Marine Cyanobacterium, Synechococcus sp. Plant Physiol. 1982 Aug;70(2):517–523. doi: 10.1104/pp.70.2.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badger M. R., Bassett M., Comins H. N. A Model for HCO(3) Accumulation and Photosynthesis in the Cyanobacterium Synechococcus sp: Theoretical Predictions and Experimental Observations. Plant Physiol. 1985 Feb;77(2):465–471. doi: 10.1104/pp.77.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumwald E., Wolosin J. M., Packer L. Na+/H+ exchange in the cyanobacterium Synechococcus 6311. Biochem Biophys Res Commun. 1984 Jul 18;122(1):452–459. doi: 10.1016/0006-291x(84)90497-2. [DOI] [PubMed] [Google Scholar]
- Erber W. W., Nitschmann W. H., Muchl R., Peschek G. A. Endogenous energy supply to the plasma membrane of dark aerobic cyanobacterium Anacystis nidulans: ATPase-independent efflux of H+ and Na+ from respiring cells. Arch Biochem Biophys. 1986 May 15;247(1):28–39. doi: 10.1016/0003-9861(86)90529-1. [DOI] [PubMed] [Google Scholar]
- Espie G. S., Canvin D. T. Evidence for Na-Independent HCO(3) Uptake by the Cyanobacterium Synechococcus leopoliensis. Plant Physiol. 1987 May;84(1):125–130. doi: 10.1104/pp.84.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krulwich T. A. Na+/H+ antiporters. Biochim Biophys Acta. 1983 Dec 30;726(4):245–264. doi: 10.1016/0304-4173(83)90011-3. [DOI] [PubMed] [Google Scholar]
- Marcus Y., Schwarz R., Friedberg D., Kaplan A. High CO(2) Requiring Mutant of Anacystis nidulans R(2). Plant Physiol. 1986 Oct;82(2):610–612. doi: 10.1104/pp.82.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Canvin D. T. Na-Stimulation of Photosynthesis in the Cyanobacterium Synechococcus UTEX 625 Grown on High Levels of Inorganic Carbon. Plant Physiol. 1987 May;84(1):118–124. doi: 10.1104/pp.84.1.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. G., Colman B. Active transport and accumulation of bicarbonate by a unicellular cyanobacterium. J Bacteriol. 1980 Sep;143(3):1253–1259. doi: 10.1128/jb.143.3.1253-1259.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki-Oda N., Oosawa F. Amiloride-sensitive Na+-H+ antiporter in Escherichia coli. J Bacteriol. 1985 Jul;163(1):395–397. doi: 10.1128/jb.163.1.395-397.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T., Kaplan A. The Stoichiometry between CO(2) and H Fluxes Involved in the Transport of Inorganic Carbon in Cyanobacteria. Plant Physiol. 1987 Apr;83(4):888–891. doi: 10.1104/pp.83.4.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Schuldiner S. pH homeostasis in bacteria. Biochim Biophys Acta. 1981 Dec;650(2-3):151–166. doi: 10.1016/0304-4157(81)90004-6. [DOI] [PubMed] [Google Scholar]
- Paschinger H. DCCD induced sodium uptake by Anacystis nidulans. Arch Microbiol. 1977 Jun 20;113(3):285–291. doi: 10.1007/BF00492037. [DOI] [PubMed] [Google Scholar]
- Peschek G. A., Czerny T., Schmetterer G., Nitschmann W. H. Transmembrane Proton Electrochemical Gradients in Dark Aerobic and Anaerobic Cells of the Cyanobacterium (Blue-Green Alga) Anacystis nidulans: Evidence for Respiratory Energy Transduction in the Plasma Membrane. Plant Physiol. 1985 Sep;79(1):278–284. doi: 10.1104/pp.79.1.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Hinrichs I., Böger P. Effect of Monochromatic Light on Proton Efflux of the Blue-Green Alga Anabaena variabilis. Plant Physiol. 1986 Jul;81(3):939–941. doi: 10.1104/pp.81.3.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Riege H., Böger P. Light-Induced Proton Release by the Cyanobacterium Anabaena variabilis: Dependence on CO(2) and Na. Plant Physiol. 1988 Mar;86(3):769–772. doi: 10.1104/pp.86.3.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz R., Friedberg D., Kaplan A. Is there a role for the 42 kilodalton polypeptide in inorganic carbon uptake by cyanobacteria? Plant Physiol. 1988 Oct;88(2):284–288. doi: 10.1104/pp.88.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu C., Spiller H., Wynns G. C., Silverman D. N. Carbonic Anhydrase and the Uptake of Inorganic Carbon by Synechococcus sp. (UTEX-2380). Plant Physiol. 1987 Sep;85(1):72–77. doi: 10.1104/pp.85.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volokita M., Zenvirth D., Kaplan A., Reinhold L. Nature of the Inorganic Carbon Species Actively Taken Up by the Cyanobacterium Anabaena variabilis. Plant Physiol. 1984 Nov;76(3):599–602. doi: 10.1104/pp.76.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]


