Abstract
S-1153 is a new imidazole compound that inhibits human immunodeficiency virus (HIV) type 1 (HIV-1) replication by acting as a nonnucleoside reverse transcriptase inhibitor (NNRTI). This compound inhibits replication of HIV-1 strains that are resistant to nucleoside and nonnucleoside reverse transcriptase inhibitors. S-1153 has a 50% effective concentration in the range of 0.3 to 7 ng/ml for strains with single amino acid substitutions that cause NNRTI resistance, including the Y181C mutant, and also has potent activity against clinical isolates. The emergence of S-1153-resistant variants is slower than that for nevirapine, and S-1153-resistant variants contained at least two amino acid substitutions, including F227L or L234I. S-1153-resistant variants are still sensitive to the nucleoside reverse transcriptase inhibitors zidovudine (AZT) and lamivudine. In a mouse and MT-4 (human T-cell line) in vivo HIV replication model, S-1153 and AZT administered orally showed a marked synergy for the inhibition of HIV-1 replication. S-1153 shows a significant accumulation in lymph nodes, where most HIV-1 infection is thought to occur. S-1153 may be an appropriate candidate for two- to three-drug combination therapy for HIV infection.
The reverse transcriptase (RT) of human immunodeficiency virus (HIV) is a suitable target for anti-HIV agents because RT is unique to retroviruses and no known homolog is expressed in human cells. Nucleoside RT inhibitors (NRTIs) such as zidovudine (AZT) and ddI inhibit RT selectively but are considerably toxic to cellular and mitochondrial DNA synthesis (29). In this regard, nonnucleoside RT inhibitors (NNRTIs) are attractive because these compounds are highly specific and no nucleoside-related toxicity is expected. However, the most serious disadvantage of the NNRTI compounds is the rapid emergence of resistant variants and the cross-resistance of one mutation to several NNRTIs (17).
Because of HIV’s high rate of mutation, treatment with a single anti-HIV drug selects for the rapid enrichment of resistant variants. Combination therapy seeks to avoid this problem by demanding that the virus become simultaneously resistant to several agents. Also, by reducing the viral population size, combination therapy reduces the probability of the emergence of resistant variants. For example, an infected person with a high viral load may contain 1010 to 1011 viruses (25). The forward mutation rate for a specific base substitution in HIV is about 10−5 (7). Thus, if resistance to a compound can arise by a single mutation, about 105 resistant viruses may be preexisting in an untreated person, and there may be double-mutant viruses that are resistant to a combination of two drugs. These considerations suggest that drugs will be particularly useful against HIV if resistance can be achieved only by multiple mutations.
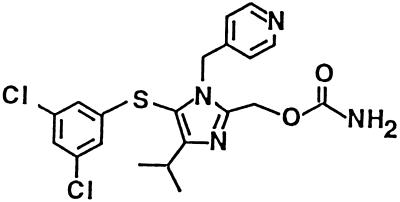
A second consideration for HIV therapy is cross-resistance. During the early development of S-1153, the problem of resistance to NNRTIs became widely known (15, 17, 27). Thus, if a patient’s virus becomes resistant, this entire class of compounds becomes unusable. Our initial lead compounds were similar to other NNRTIs in that resistant variants of HIV-1 could arise by the same mutations that cause resistance to other members of this compound group (12a). We therefore tested derivatives for their activities against HIV type 1 (HIV-1) strains that were resistant to known NNRTIs. On the basis of this and other criteria, such as the higher concentration of S-1153 in the lymph nodes than in plasma after oral administration to rats and the safety profile of the drug, S-1153 was chosen from among a large number of imidazole-containing compounds for further study. This compound, S-1153 (Fig. 1), is more potent than other approved drugs in this class, is active in a mouse model of HIV infection, and requires at least two mutations for RT to become resistant.
FIG. 1.
Chemical structure of S-1153.
MATERIALS AND METHODS
Compounds.
S-1153, 5-(3,5-dichlorophenyl)thio-4-isopropyl-1-(4-pyridyl)methyl-1H-imidazol-2-ylmethyl carbamate, was synthesized in our laboratories. The chemical structure of S-1153 is shown in Fig. 1. A detailed description of the synthesis and structure-activity data will be published elsewhere. AZT, ddI, and ddC were purchased from Sigma (St. Louis, Mo.). Nevirapine (21), loviride (24), delavirdine (10), lamivudine (3TC) (6), saquinavir (8), indinavir (16), ritonavir (20), and nelfinavir (22) were synthesized in our laboratories.
Cells and viruses.
MT-4, MT-2 (13), and M8166, a subclone of C8166 (4), are human T-cell lines established by human T-cell leukemia virus type I-induced transformation and are highly sensitive to HIV-1, producing a rapid and strong cytopathic effect (CPE). The cells were cultured in RPMI 1640 medium supplemented with 10% fetal calf serum and antibiotics in a humidified incubator with a 5% CO2 atmosphere at 37°C. The HIV strains used in this study were HIV-1 IIIB (13), HIV-1 SF33 (19), HIV-1 SF-2 (30), HIV-2 Rod (5), and HIV-1 NL432 derived from the infectious molecular clone pNL432 (1). HIV stocks were stored as cell-free culture supernatants at −80°C.
In vitro RT inhibition assay.
HIV-1 RT activity was measured in a reaction mixture containing 50 mM Tris-HCl (pH 8.3), 150 mM KCl, 10 mM MgCl2, 0.1% Nonidet P-40, 3 mM dithiothreitol, 10 μg of poly(rA) per ml as a template, 1 μg of oligo(dT) per ml as a primer, various concentrations of dTTP and [3H]dTTP as substrates, and 0.045 U of recombinant HIV-1 RT enzyme (Seikagaku Corp., Tokyo, Japan) per ml. After 10 or 60 min of incubation at 37°C, the reaction mixtures were chilled on ice and collected with a Skatron Harvestor (Skatron, Inc., Sterling, Va.) on a DEAE-Filtermat (LKB-Pharmacia, Turku, Finland). After washing with 5% Na2HPO4 and H2O, the amount of radioactivity was determined by LKB Beta Plate scintillation spectroscopy.
Construction of RT mutants.
In vitro mutagenesis was applied to the RT-coding region of the infectious molecular clone pNL432 to obtain drug-resistant mutants (see Table 5). The mutant clones were transfected to SW480 cells as described previously (1). The virus stocks were prepared from cell-free supernatants at 2 to 3 days posttransfection.
TABLE 5.
Sensitivities of molecular clones with RT gene mutations to S-1153 and other anti-HIV agentsa
| Virus mutation | EC50 (ng/ml)
|
|||
|---|---|---|---|---|
| S-1153 | Nevirapine | Delavirdine | Loviride | |
| Wild type (strain NL432) | 0.31 ± 0.07 | 6.8 ± 2.1 | 5.9 ± 2.1 | 6.3 ± 0.5 |
| L100I | 0.94 ± 0.18 | 14 ± 12 | >500 | 5.4 ± 3.4 |
| K103N | 0.31 ± 0.14 | 300 ± 170 | 460 ± 80 | 140 ± 60 |
| V106A | 1.4 ± 0.9 | >500 | 150 ± 50 | 100 ± 40 |
| Y181C | 4.2 ± 1.9 | >500 | >500 | >500 |
| Y188C | 0.46 ± 0.17 | >500 | 32 ± 7 | 230 ± 140 |
| G190A | 0.34 ± 0.07 | >500 | 1.3 ± 0.3 | 250 ± 110 |
| F227C | 2.4 ± 1.1 | 87 ± 32 | 9.6 ± 5.1 | 58 ± 29 |
| F227L | 0.46 ± 0.05 | 28 ± 5 | 0.78 ± 0.48 | 23 ± 7 |
| L234I | 6.8 ± 4.2 | 3.5 ± 1.5 | 13 ± 8 | 0.45 ± 0.24 |
| P236L | 1.1 ± 0.1 | 21 ± 1 | >500 | 2.8 ± 0.4 |
| V106A + Y181C | 2.5 ± 0.2 | >500 | >500 | >500 |
| V106A + F227L | 120 ± 10 | >500 | 37 ± 2 | >500 |
Each value represents the mean ± standard deviation for at least three experiments, each of which was performed in duplicate.
In vitro antiviral assays.
The anti-HIV activity of each compound in vitro was assayed by determining the level of inhibition of virus-induced CPE in the cells essentially as described previously (23).
The levels of growth inhibition of the drug-resistant mutants were assayed by measuring viral RT activity in the culture supernatant. MT-4 cells were infected with the corresponding virus and were incubated for 4 days in the absence or presence of serial dilutions of the compounds. Then, the RT activities in the culture supernatants were assayed as described previously (28). The dose of the compound required to inhibit HIV RT activity in the culture by 50% (IC50) or 90% (IC90) of that required to inhibit the activity of the control was calculated.
Clinical isolates were tested in human peripheral blood mononuclear cell (PBMC) cultures by the following procedure. PBMCs were activated with phytohemagglutinin for 2 days. Compounds were added, and the cultures were incubated for 15 h. Virus was added at a multiplicity of infection (MOI) of 0.001, and the culture was incubated for 8 h. Virus and compound were removed, and the cells were incubated for 15 days. Virus production was determined by measuring the level of p24 core antigen production with the NEK-060 kit (Du Pont).
All data in Table 1 are mean values for at least three independent experiments. The data in Tables 2, 3, 5, and 6 are mean values for at least two independent experiments.
TABLE 1.
In vitro anti-HIV activities of S-1153 and other anti-HIV agents compared by the MTT assaya
| Inhibitor | CC50 (ng/ml) | EC50 (ng/ml) | EC90 (ng/ml) |
|---|---|---|---|
| S-1153 | 11,000 ± 1,000 | 1.4 ± 0.6 | 2.7 ± 1.5 |
| Nevirapine | 70,000 ± 6,000 | 18 ± 5 | 47 ± 6 |
| Loviride | 49,000 ± 21,000 | 11 ± 4 | 21 ± 11 |
| Delavirdine | 21,000 ± 4,000 | 11 ± 2 | 34 ± 8 |
| AZT | 13,000 ± 5,000 | 1.9 ± 0.6 | 4.3 ± 1.9 |
| ddI | >100,000 | 550 ± 150 | 1,700 ± 900 |
| ddC | 3,200 ± 400 | 18 ± 3 | 42 ± 7 |
| 3TC | >100,000 | 39 ± 10 | 98 ± 19 |
| Saquinavir | 8,500 ± 500 | 3.7 ± 0.6 | 8.6 ± 1.7 |
| Indinavir | >100,000 | 14 ± 6 | 41 ± 23 |
| Ritonavir | 13,000 ± 2,000 | 26 ± 3 | 56 ± 11 |
| Nelfinavir | 4,600 ± 100 | 10 ± 2 | 24 ± 4 |
Each value represents the mean ± standard deviation for at least three experiments, each of which was performed in triplicate.
TABLE 2.
In vitro anti-HIV activities of S-1153, AZT, and nevirapine compared by the MTT assaya
| Virus/cell | EC50 (ng/ml)
|
||
|---|---|---|---|
| S-1153 | AZT | Nevirapine | |
| HIV-1 IIIB/MT-4 | 1.4 ± 0.6 | 1.9 ± 0.6 | 18 ± 5 |
| HIV-1 IIIB/MT-2 | 1.2 ± 0.4 | 51 ± 7 | 24 ± 7 |
| HIV-1 IIIB/M8166 | 2.1 ± 0.9 | 67 ± 22 | 53 ± 14 |
| HIV-1 SF33/MT-4 | 1.0 ± 0.2 | 14 ± 5 | 38 ± 4 |
| HIV-1 SF33/M8166 | 1.3 ± 0.3 | 150 ± 10 | 53 ± 1 |
| HIV-1 SF2/M8166 | 0.90 ± 0.02 | 5.0 ± 0.6 | 51 ± 5 |
| HIV-1 NL432/MT-4 | 0.51 ± 0.02 | 1.6 ± 0.1 | 13 ± 1 |
| HIV-2 Rod/M8166 | >CC50 | 2.6 ± 0.5 | >CC50 |
Each value represents the mean ± standard deviation for at least three experiments, each of which was performed in triplicate.
TABLE 3.
In vitro activities of S-1153 and AZT against clinical isolates of HIVa
| Virus/cell | S-1153
|
AZT
|
||
|---|---|---|---|---|
| EC50 (ng/ml) | EC90 (ng/ml) | EC50 (ng/ml) | EC90 (ng/ml) | |
| RF/MT-2 | 3.1, <1 | 9.0, 8.0 | 46, 43 | 110, 120 |
| A018Cb/PBMC | <1, <1 | 6.6, 6.6 | 390, 590 | 3,500, 5,300 |
| CH910499/PBMC | 4.5, 4.8 | 9.8, 9.6 | 180, 190 | 530, 540 |
| CH900029/PBMC | 1.1, <1 | 9.9, 8.1 | 5.5, 4.3 | 78, 74 |
| CH900285/PBMC | <1, <1 | 8.0, 8.0 | 2.1, 5.9 | 51, 77 |
| NIH301593/PBMC | 2.0, 3.1 | 9.7, 9.4 | 9.0, 14 | 90, 100 |
| NIH302054/PBMC | 4.0, 3.4 | 10, 9.1 | 6.4, 9.3 | 170, 120 |
The values from two independent experiments are presented and are separated by a comma; each experiment was performed once.
AZT-resistant strain.
TABLE 6.
Sensitivities of molecular clones with RT gene mutations to S-1153 and other anti-HIV agentsa
| Virus mutation | S-1153
|
AZT
|
3TC
|
|||
|---|---|---|---|---|---|---|
| EC50 (ng/ml) | EC90 (ng/ml) | EC50 (ng/ml) | EC90 (ng/ml) | EC50 (ng/ml) | EC90 (ng/ml) | |
| Wild type (NL432) | 0.31 ± 0.07 | 1.1 ± 0.2 | 1.2 ± 0.5 | 5.6 ± 0.9 | 11 ± 7 | 67 ± 31 |
| Y181C | 4.2 ± 1.9 | 14 ± 3 | 0.90 ± 0.26 | 3.6 ± 0.6 | 20 ± 5b | 62 ± 12b |
| F227C | 2.4 ± 1.1 | 9.8 ± 1.6 | 0.14 ± 0.01 | 1.1 ± 0.7 | 17 ± 6b | 88 ± 24b |
| L234I | 6.8 ± 4.2 | 11 ± 6 | 0.63 ± 0.34 | 2.3 ± 1.1 | 27 ± 6b | 98 ± 10b |
| V106A + F227L | 120 ± 10 | 270 ± 80 | 0.64 ± 0.11 | 5.1 ± 3.3 | 14 ± 11b | 54 ± 29b |
| M184V | 0.25 ± 0.08 | 1.2 ± 0.1 | 0.84 ± 0.41 | 4.2 ± 1.1 | >500 | >500 |
| D67N + K70R | 0.31 ± 0.06 | 1.1 ± 0.2 | 2.4 ± 1.6 | 30 ± 21 | 18 ± 16b | 200 ± 130b |
| T215Y | 0.17 ± 0.10 | 0.40 ± 0.08 | 5.6 ± 1.7 | 37 ± 9 | 60 ± 14b | 240 ± 60b |
| T215Y + L234I | 0.61 ± 0.07 | 3.0 ± 0.3 | 0.89 ± 0.61 | 4.6 ± 1.9 | 30 ± 3b | 180 ± 20b |
Each value represents the mean ± standard deviation for at least three experiments, each of which was performed in duplicate unless indicated otherwise.
Each value represents the mean ± standard deviation for two experiments, each of which was performed in duplicate.
Selection of S-1153-resistant HIV-1 variants.
M8166 cells were infected with a highly infectious HIV-1 clone, HIV-1 IIIB clone 3 (11), at a high MOI (MOI, 0.2 to 0.5) and were exposed to S-1153 or nevirapine initially at two- to fivefold the 50% effective concentration (EC50). Infected cells were incubated for 4 days, the RT activities in the culture supernatants were assayed, and then the cells were passaged 1:4 in fresh medium containing the antiviral agents. The doses of S-1153 were modified according to the CPE observed in the cells. If the virus was replicating (a visible CPE or a high level of RT activity), the dose of the agent was increased two- to fivefold in the following passage. Cells were passaged as described above twice a week.
Analysis of RT mutation.
The RT regions in the resistant variants were sequenced with a double-stranded DNA cycle sequencing system (GIBCO BRL).
In vivo efficacy of S-1153.
The in vivo efficacy of S-1153 was monitored with the mouse–MT-4 cell model as described previously (28). AZT and S-1153 were dissolved or suspended in 0.5% methylcellulose and were administered orally immediately after the injection of 5 × 107 HIV-infected MT-4 cells in 0.3 ml of phosphate-buffered saline into the peritoneal cavity of a BALB/c mouse. The percent inhibition of viral growth by the drugs 24 h after transplantation is the mean value for three mice.
Animal welfare.
The evaluation of S-1153 with the mouse–MT-4 model was approved by the Shionogi Animal Use and Care Committee and was conducted in accordance with institutional guidelines.
RESULTS
In vitro activity of S-1153 against RT.
S-1153 showed potent activity against purified RT. In a standard assay with poly(rA) and oligo(dT), the IC50s of S-1153 and nevirapine were 0.45 and 6.51 μM, respectively. The Km for dTTP was 2.62 μM, and the Ki for S-1153 was 1.63 μM. When the concentration of the dTTP substrate was varied, a mixed type of inhibition was observed (data not shown).
In vitro activity of S-1153 against HIV replication.
The anti-HIV activities of S-1153 and the other anti-HIV agents were assayed by inhibition of virus-induced CPE on MT-4 cells, based on a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with 96-well microplates. Anti-HIV data are reported as the EC50 or EC90 for virus-induced cell killing (Table 1). S-1153 showed the most potent activity among the agents tested, with an EC50 of 1.4 ng/ml. Nevirapine was 13-fold weaker than S-1153 in this assay, consistent with the inhibitory activities of the drugs against purified RT. The cytotoxicity of S-1153 to PBMCs, measured by determining the IC50s for cell growth inhibition and cell death, were 15 and 17.5 μg/ml, respectively, which were similar to the 50% cytotoxic concentration (CC50) for MT-4 cells (Table 1). By these measurements, the chemotherapeutic index is more than 10,000.
The activity of S-1153 against different HIV strains and in different cell lines was tested (Table 2). S-1153 had similar antiviral activities against HIV-1 IIIB (a T-cell-tropic strain), HIV-1 SF-33 (a dual-tropic strain), HIV-1 SF-2 (a T-cell-tropic strain), and HIV-1 NL432 (a molecular clone) in MT-4, MT-2, or M8166 cells. The activity of AZT was significantly cell dependent, which may have been due to variation in the ability of different cells to phosphorylate AZT (2, 9). The antiviral activity of S-1153 appeared to be specific to HIV-1: no activity against HIV-2 Rod or against influenza A virus was observed (Table 2; data not shown).
S-1153 also exerted strong inhibitory activity against clinical isolates of HIV, as well as laboratory strains (Table 3), with EC50s of less than 10 ng/ml. AZT-resistant and -sensitive HIV strains were equally susceptible to S-1153. Two AZT-resistant variants were tested, and both were inhibited by nanomolar amounts of S-1153. The concentrations of AZT required for inhibition of most clinical isolates were 10 times higher than those of S-1153.
Selection of HIV-1 variants resistant to S-1153.
The emergence of variants resistant to an anti-HIV agent may be inevitable if the agent is used as a monotherapy. To understand how a particular anti-HIV drug might be used in a combination therapy, it is desirable to know what type of resistant mutants might evolve, whether such mutants have a growth disadvantage, and whether such mutants are more resistant or more sensitive to other anti-HIV drugs. To address these questions, we isolated S-1153-resistant mutants from HIV-1 IIIB clone 3. In an initial attempt, M8166 cells were infected with HIV-1 IIIB clone 3 and passaged six times in the presence of 10 ng of S-1153 per ml. This experiment was repeated three times, and no resistant variants were obtained. In these experiments, each plate was initially infected with 1 × 104 to 2.5 × 104 PFU of virus, and there might have been no S-1153-resistant variants in this population. However, nevirapine-resistant mutants were consistently isolated after fewer passages, which means that there might have been some nevirapine-resistant variants in this initial population. In a second experiment, cells and viruses were incubated in the presence of 1 or 5 ng of S-1153 per ml. At 5 ng/ml, virus replication was almost completely blocked, but at 1 ng/ml, virus replication was observed because 1 ng of S-1153 per ml was below the EC50 for strain IIIB clone 3. Then, the dose of S-1153 was increased by two- to fivefold in the following passage, and S-1153-resistant variants were isolated after more than 31 days in the presence of S-1153 at a concentration of 50 ng/ml (Table 4). The RT-coding regions from two S-1153-resistant clones were sequenced, and in both cases more than one amino acid substitution mutation was present. Clone 1 contained the mutations V106A and F227L and the EC50 for the clone was 740 ng/ml, while clone 2 had the mutations K103T, V106A, and L234I and the EC50 for the clone was 37 ng/ml in the MTT assay.
TABLE 4.
Isolation of S-1153 and nevirapine resistant variants in vitro
| Drug | Emergence of resistant mutants
|
Amino acid substitution in RT | |
|---|---|---|---|
| No. of passages | No. of days of culture | ||
| S-1153 | >9 | >31 | K103T + V106A + L234I, V106A + F227L |
| Nevirapine | 2–3 | 7–10 | Y181C, V106A |
Susceptibilities of various HIV-1 RT mutants to RT inhibitors.
On the basis of the sequences of the S-1153-resistant strains and other mutations known to cause resistance to other NNRTIs, we constructed a series of single and double mutants with mutations in the RT gene (Tables 5 and 6). HIV-1 cDNA molecular clone pNL432 was used as a starting point for in vitro mutagenesis, so that the resistant mutations would be compared in the same genetic background. Mutant clones with single amino acid substitutions at residues 100, 103, 106, 181, 190, 227, and 236 showed only minimally reduced sensitivities to S-1153 (less than 10-fold), while the clone with the mutation L234I was 22-fold less sensitive. The concentration of S-1153 required to inhibit any of these viruses with single mutations was equal to or less than the concentrations of nevirapine, delavirdine, or loviride required to inhibit the parental HIV-1 strain. In contrast, several of the viruses with single mutations were more than 50-fold less sensitive to nevirapine, delavirdine, or loviride. The clone with the mutation Y181C was resistant to all of these drugs (EC50s, >500 ng/ml) but was still sensitive to S-1153 (EC50, 4.2 ng/ml). It was previously found that high doses of NNRTI combinations selected for the V106A plus Y181C double mutant (3), but this strain is still sensitive to S-1153 (EC50, 2.5 ng/ml). Conversely, the V106A plus F227L double mutant, which is resistant to S-1153 (EC50, 120 ng/ml), is still somewhat sensitive to delavirdine (EC50, 37 ng/ml), and the F227L single mutant is more sensitive to delavirdine than the parent strain. Similarly, the L234I variant, which has the most S-1153-resistant single mutation, is more sensitive to loviride than the parent strain. This information could be useful in designing combination therapies with NNRTIs. The patterns of cross-resistance and sensitivity were also examined for S-1153 and the NRTIs AZT and 3TC (Table 6). No cross-resistance was observed. However, there may be genetic interactions in which different mutations mutually reduce the resistance that they confer. The activities of AZT and 3TC were unchanged or greater against clones with the mutations Y181C, F227C, L234I, and V106A plus F227L, which cause weak or strong resistance to S-1153. Conversely, the mutations M184V (which causes 3TC resistance), T215Y, or D67N plus K70R (which causes AZT resistance) had no effect on sensitivity to S-1153. Interestingly, when the mutation L234I causing resistance to S-1153 and the mutation T215Y causing resistance to AZT were combined, the HIV strain with both mutations had wild-type sensitivities to both drugs.
In vivo efficacy of S-1153.
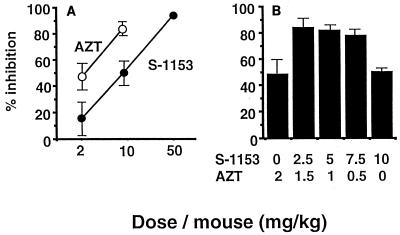
The in vivo efficacy of S-1153 was demonstrated in the mouse–MT-4 cell model, which was previously established in our laboratory (Fig. 2). In this model, HIV-infected MT-4 cells are injected into the peritoneal cavity and anti-HIV agents are administered orally. This model measures the ability of the test compound to be absorbed, circulate in the blood, and reach the target tissue for a long enough period of time to cause inhibition. In this model, there was dose-dependent inhibition of HIV-1 replication for both AZT and S-1153 (Fig. 2A). The combination of AZT and S-1153 is quite effective against HIV replication, suggesting a synergistic action of these two compounds in vivo (Fig. 2B). This is an important observation because the drugs are metabolized in vivo and do not have a fixed concentration like they did in the in vitro experiments. These findings support the potential usefulness of S-1153 in a combination therapy.
FIG. 2.
Inhibition of HIV-1 replication by S-1153 in the mouse–MT-4 cell model. The method used was the same as that published previously (28). AZT and S-1153 were dissolved or were suspended in 0.5% methylcellulose and were administered orally immediately after the injection of 5 × 107 HIV-infected MT-4 cells in 0.3 ml of phosphate-buffered saline into the peritoneal cavities of BALB/c mice. The percent inhibition of viral growth by the drugs 24 h after transplantation is the mean for three mice. (A) Dose-dependent inhibition of HIV-1 replication by S-1153 or AZT alone. (B) Inhibition of HIV-1 replication by the combination of S-1153 and AZT.
DISCUSSION
Combination therapy has become the standard for the treatment of HIV infection. For a drug to be included in a combination therapy, several factors must be considered, including the rate of evolution of resistant variants, the ability of such variants to be cross-resistant to other drugs, and the side-effect profile of the drug. NNRTIs are attractive because they cause minimal side effects. However, the usefulness of these compounds is limited by the fact that HIV-1 RT can often become resistant to these drugs as a result of a single mutation.
We sought to identify an improved NNRTI by screening for compounds that would inhibit HIV-1 strains that are resistant to known NNRTIs or to AZT. From a set of derivatives of an initial NNRTI lead compound, S-1153 was chosen because of its potency and its ability to inhibit existing NNRTI-resistant HIV strains and because resistance to this compound appears to require at least two mutations.
S-1153 is more potent than other NNRTIs. When tested against purified RT, S-1153 had an IC50 of 0.45 μM and was 15-fold more potent than nevirapine (see Results). Similarly, S-1153 was 10- to 15-fold more potent than nevirapine, loviride, or delavirdine in its ability to inhibit HIV-1 replication in vitro (Tables 1, 2, and 5). The inhibition of HIV-1 replication did not depend on the HIV-1 strain or the host cell used (Table 2). In addition, S-1153 had a low level of cytotoxicity, because its chemotherapeutic index was more than 10,000.
Many NNRTIs and protease inhibitors have high-level protein binding characteristics which affect their antiviral activities. Among the human serum proteins albumin has the greatest ability to bind to S-1153 (unpublished data). In the presence of 50 mg of human serum albumin per ml and 1 mg of alpha-1-acid glycoprotein per ml, S-1153 showed ninefold less activity than its activity without albumin, while nevirapine showed fourfold less activity than its activity without albumin (data not shown). Taking into account these data, S-1153 may have sixfold greater antiviral activity than nevirapine in human plasma.
HIV-1 variants that are resistant to S-1153 arise much more slowly than variants resistant to nevirapine (Table 4). This is most likely due to the fact that two mutations are required to confer high-level resistance to S-1153. Single mutations can confer partial resistance to this drug, increasing the EC50 by up to 22-fold. However, the resulting EC50 of S-1153 for these viruses with single mutations is then similar to the EC50s of nevirapine, delavirdine, and loviride for wild-type HIV-1. The two S-1153-resistant clones that were characterized had V106A-F227L and K103T-V106A-L234I mutations. All of these mutations line the pocket in which other NNRTIs are known to bind (12, 14).
How can S-1153’s enhanced potency and relative insensitivity to mutation be explained? The three-dimensional structures of several different RT-NNRTI complexes have been solved (12, 14, 26). The NNRTIs all lie in a pocket, and mutations that confer resistance to S-1153 and other NNRTIs affect the amino acids that surround this pocket. A large number of variants resistant to other NNRTIs have been sequenced. On the basis of these structures and mutational studies, Hopkins et al. (14) noted that most mutations conferring NNRTI resistance are the result of the replacement of a larger amino acid with a smaller one. As a result, a contact is lost and the affinity is reduced. Those investigators suggest that mutations that increase the size of a pocket amino acid might be lethal to the polymerase. As a result, no mutations cause steric interference with NNRTI binding but allow full polymerase activity. This is a fortunate situation for the design of anti-HIV drugs.
S-1153, with a molecular weight of 451, is larger than most NNRTIs. It is possible that this compound simply makes more contacts with the RT than other such compounds and therefore binds more tightly. The results of our mutational analysis are consistent with the idea that mutations in the NNRTI-binding pocket cause the loss of a contact but do not cause steric interference. Mutational loss of one contact may reduce the binding somewhat, but S-1153 is still potent enough that inhibitory levels of the compound should be achievable in vivo.
Some S-1153-resistant mutants with single and double mutations are cross-resistant to other NNRTIs. However, the mutations that confer resistance to delavirdine are not particularly resistant to S-1153, and the double mutant that is resistant to S-1153 is only about sixfold more resistant to delavirdine. AZT- and 3TC-resistant mutants are sensitive to S-1153, and vice versa (Table 6). In addition, when the mutation (L234I) conferring the strongest S-1153 resistance is combined with the mutation (T215Y) conferring AZT resistance, the virus with the double mutation is almost as sensitive to both drugs as wild-type HIV. This interference phenomenon has been noted for other combinations of mutations conferring NRTI and NNRTI resistance (17, 18). These drug-mutation interactions may be useful in designing combination therapies with S-1153 and other compounds that act on RT.
Young et al. (31) suggested that another NNRTI, DMP-266, also requires two mutations in the RT gene to acquire resistance. We have not compared S-1153 in parallel with DMP-266, but the single mutation Y188L caused a 1,000-fold decrease in sensitivity to DMP-266, and L100I and K103N each caused 67-fold decreases in sensitivity (31).
In a simple mouse model of HIV infection, S-1153 can block virus replication (Fig. 2). HIV-infected MT-4 cells were implanted into the peritoneal cavity by a standard procedure (28). Oral dosing with S-1153, AZT, or S-1153 plus AZT inhibited viral growth, with 50 mg of S-1153 per kg of body weight showing complete inhibition. Combinations of S-1153 and AZT were particularly effective and at relatively low doses nearly completely inhibited virus replication, suggesting that these compounds act synergistically, although this has not been confirmed in vivo. In addition, the concentration of S-1153 in lymph nodes is two to seven times higher than that in plasma after oral administration to rats (unpublished results), and this drug showed little toxicity in studies with animals (unpublished results). Taken together, the properties of S-1153 described above indicate that this drug may be useful after the failure of therapy with the originally used NNRTIs but could be especially useful in combination with nucleoside analogs or protease inhibitors in drug-naive patients. A synergistic effect between S-1153 and AZT against HIV in an in vitro anti-HIV assay was observed (data not shown). However, the complementary effects of combinations of anti-HIV agents for the prevention of the emergence of resistant variants may be more important than the simple expectation of synergistic activity for clinical purposes. Clinical trials are under way to evaluate the safety, pharmacokinetic profile, and anti-HIV activity of S-1153 and the emergence of variants of HIV resistant to S-1153 in humans.
ACKNOWLEDGMENTS
We thank S. Kawauchi, M. Nishimura, Y. Ogura, and A. Suyama for technical assistance. We thank J. Way for reviewing the manuscript.
REFERENCES
- 1.Adachi A, Gendelman H E, Koenig S, Folks T, Willey R, Rabson A, Martin M A. Production of acquired immunodeficiency syndrome-associated retrovirus in human and non-human cells transfected with an infectious molecular clone. J Virol. 1986;59:284–291. doi: 10.1128/jvi.59.2.284-291.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini J, Herdewijn P, De Clercq E. Differential patterns of intracellular metabolism of 2′,3′-didehydro-2′,3′-dideoxythymidine and 3′-azido-2′,3′-dideoxythymidine, two potent anti-human immunodeficiency virus compounds. J Biol Chem. 1989;264:6127–6133. [PubMed] [Google Scholar]
- 3.Balzarini J, Karlsson A, Perez-Perez M-J, Camarasa M J, Tarpley W G, De Clercq E. Treatment of human immunodeficiency virus type 1 (HIV-1)-infected cells with combinations of HIV-1-specific inhibitors results in a different resistance pattern than does treatment with single-drug therapy. J Virol. 1993;67:5353–5359. doi: 10.1128/jvi.67.9.5353-5359.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clapham P R, Weiss R A, Dalgleish A G, Exley M, Whitby D, Hogg N. Human immunodeficiency virus infection of monocytic and T-lymphocytic cells: receptor modulation and differentiation induced by phorbol ester. Virology. 1987;158:44–51. doi: 10.1016/0042-6822(87)90236-4. [DOI] [PubMed] [Google Scholar]
- 5.Clavel F, Guetard D, Brun-Vezinet F, Chamaret S, Rey M-A, Santos-Ferreira M O, Laurent A G, Dauguet C, Katlama C, Rouzioux C, Klatzmann D, Champalimaud J L, Montagnier L. Isolation of a new human retrovirus from west African patients with AIDS. Science. 1986;233:343–346. doi: 10.1126/science.2425430. [DOI] [PubMed] [Google Scholar]
- 6.Coates J A V, Cammack N, Jenkinson H J, Jowett A J, Jowett M I, Pearson B A, Penn C R, Rouse P L, Viner K C, Cameron J M. (−)-2′-Deoxy-3′-thiacytidine is a potent, highly selective inhibitor of human immunodeficiency virus type 1 and type 2 replication in vitro. Antimicrob Agents Chemother. 1992;36:733–739. doi: 10.1128/aac.36.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffin J M. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science. 1995;267:483–489. doi: 10.1126/science.7824947. [DOI] [PubMed] [Google Scholar]
- 8.Craig J C, Duncan I B, Hockley D, Grief C, Roberts N A, Mills J S. Antiviral properties of Ro31-8959, an inhibitor of human immunodeficiency virus (HIV) proteinase. Antivir Res. 1991;16:295–305. doi: 10.1016/0166-3542(91)90045-s. [DOI] [PubMed] [Google Scholar]
- 9.Dhawan R K, Kharbanda S, Nakamura M, Ohno T, Kufe D. Effects of granulocyte-macrophage colony-stimulating factor on 3′-azido-3′-deoxythymidine uptake, phosphorylation and nucleotide retention in human U-937 cells. Biochem Pharmacol. 1990;40:2695–2700. doi: 10.1016/0006-2952(90)90589-d. [DOI] [PubMed] [Google Scholar]
- 10.Dueweke T J, Pushkarskaya T, Poppe S M, Swaney S M, Zhao J Q, Chen I S Y, Stevenson M, Tarpley W G. A mutation in reverse transcriptase of bis(heteroaryl)piperazine-resistant human immunodeficiency virus type 1 that confers increased sensitivity to other nonnucleoside inhibitors. Proc Natl Acad Sci USA. 1993;90:4713–4717. doi: 10.1073/pnas.90.10.4713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Farrash M A, Kuroda M, Kitazaki T, Masuda T, Kato K, Hatanaka M, Harada S. Generation and characterization of a human immunodeficiency virus type 1 (HIV-1) mutant resistant to an HIV-1 protease inhibitor. J Virol. 1994;68:233–239. doi: 10.1128/jvi.68.1.233-239.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Esnouf R-M, Ren J, Hopkins A L, Ross C K, Jones E Y, Stammers D K, Stuart D I. Unique features in the structure of the complex between HIV-1 reverse transcriptase and the bis(heteroaryl)piperazine (BHAP) U-90152 explain resistance mutations for this nonnucleoside inhibitor. Proc Natl Acad Sci USA. 1997;94:3984–3989. doi: 10.1073/pnas.94.8.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12a.Fujiwara, T., et al. Unpublished results.
- 13.Harada S, Koyanagi Y, Yamamoto N. Infection of HTLV-III/LAV in HTLV-I-carrying cells MT-2 and MT-4 and application in a plaque assay. Science. 1985;229:563–566. doi: 10.1126/science.2992081. [DOI] [PubMed] [Google Scholar]
- 14.Hopkins A L, Ren J, Esnouf R M, Willcox B E, Jones E Y, Ross C, Miyasaki T, Walker R T, Tanaka H, Stammers D K, Stuart D I. Complexes of HIV-1 reverse transcriptase with inhibitors of the HEPT series reveal conformational changes relevant to the design of potent nonnucleoside inhibitors. J Med Chem. 1996;39:1589–1600. doi: 10.1021/jm960056x. [DOI] [PubMed] [Google Scholar]
- 15.Kimberlin D W, Coen D M, Biron K K, Cohen J I, Lamb R A, McKinlay M, Emini E A, Whitley R J. Molecular mechanisms of antiviral resistance. Antivir Res. 1995;26:369–401. doi: 10.1016/0166-3542(95)00027-j. [DOI] [PubMed] [Google Scholar]
- 16.Lam P Y S, Jadhav P K, Eyermann C J, Hodge C N, Ru Y, Bacheler L T, Meek J L, Otto M J, Rayner M M, Wong Y N, Chang C-H, Weber P C, Jackson D A, Sharpe T R, Erickson-Viitanen S. Rational design of potent, bioavailable, nonpeptide cyclic ureas as HIV protease inhibitors. Science. 1994;263:380–383. doi: 10.1126/science.8278812. [DOI] [PubMed] [Google Scholar]
- 17.Larder B A. 3′-Azido-3′-deoxythymidine resistance suppressed by a mutation conferring human immunodeficiency virus type 1 resistance to nonnucleoside reverse transcriptase inhibitors. Antimicrob Agents Chemother. 1992;36:2664–2669. doi: 10.1128/aac.36.12.2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larder B A. Interactions between drug resistance mutations in human immunodeficiency virus type 1 reverse transcriptase. J Gen Virol. 1994;75:951–957. doi: 10.1099/0022-1317-75-5-951. [DOI] [PubMed] [Google Scholar]
- 19.Mackewics C E, Ortega H, Levy J A. Effect of cytokine on HIV replication in CD4+ lymphocytes: lack of identity with the CD8+ cell antiviral factor. Cell Immunol. 1994;153:329–343. doi: 10.1006/cimm.1994.1032. [DOI] [PubMed] [Google Scholar]
- 20.Markowitz M, Mo H, Kempf D J, Norbeck D W, Bhat T N, Erickson J W, Ho D. Selection and analysis of human immunodeficiency virus type 1 variants with increased resistance to ABT-538, a novel protease inhibitor. J Virol. 1995;69:701–706. doi: 10.1128/jvi.69.2.701-706.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Merluzzi V J, Hargrave K D, Labadia M, Grozinger K, Skoog M, Wu J C, Shih C-K, Eckner K, Hattox S, Adams J, Rosehthal A S, Faanes R, Eckner R J, Koup R A, Sullivan J L. Inhibition of HIV-1 replication by a nonnucleoside reverse transcriptase inhibitor. Science. 1990;250:1411–1413. doi: 10.1126/science.1701568. [DOI] [PubMed] [Google Scholar]
- 22.Patick A K, Mo H, Markowitz M, Appelt K, Wu B, Musick L, Kalish V, Kaldor S, Reich S, Ho D, Webber S. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob Agents Chemother. 1996;40:292–297. doi: 10.1128/aac.40.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pauwels R, Balzarini J, Baba M, Snoeck R, Schols D, Herdewijn P, Desmyter J, De Clercq E. Rapid and automated tetrazolium-based colorimetric assay for the detection of anti-HIV compounds. J Virol Methods. 1988;20:309–321. doi: 10.1016/0166-0934(88)90134-6. [DOI] [PubMed] [Google Scholar]
- 24.Pauwels R, Andries K, Debyser Z, Van Daele P, Schols D, Stoffels P, De Vreese K, Woestenborghs R, Vandamme A-M, Janssen C G M, Anne J, Cauwenbergh G, Desmyter J, Heykants J, Janssen M A C, De Clercq E, Janssen P A J. Potent and highly selective human immunodeficiency virus type 1 (HIV-1) inhibition by a series of α-anilinophenylacetamide derivatives targeted at HIV-1 reverse transcriptase. Proc Natl Acad Sci USA. 1993;90:1711–1715. doi: 10.1073/pnas.90.5.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perelson A S, Neumann A U, Markowitz M, Leonard J M, Ho D D. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271:1582–1586. doi: 10.1126/science.271.5255.1582. [DOI] [PubMed] [Google Scholar]
- 26.Ren J, Esnouf R, Garman E, Somers D, Ross C, Kirby I, Keeling J, Darby G, Stuart D, Stammers D. High resolution structures of HIV-1 RT from four RT-inhibitor complexes. Nature Struct Biol. 1995;2:293–302. doi: 10.1038/nsb0495-293. [DOI] [PubMed] [Google Scholar]
- 27.Richman D D, Havlir D, Corbeil J, Looney D, Ignacio C, Spector S A, Sullivan J, Cheeseman S, Barringer K, Pauletti D, Shih C-K, Myers M, Griffin J. Nevirapine resistance mutations of human immunodeficiency virus type 1 selected during therapy. J Virol. 1994;68:1660–1666. doi: 10.1128/jvi.68.3.1660-1666.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sato A, Kodama M, Abe K, Miki S, Nishimura M, Suyama A, Ogata M, Toyoda T, Sugimoto H, Yoshie O, Fujiwara T. A simple and rapid method for preliminary evaluation of in vivo efficacy of anti-HIV compounds in mice. Antivir Res. 1995;27:151–163. doi: 10.1016/0166-3542(95)00004-6. [DOI] [PubMed] [Google Scholar]
- 29.Schinazi R F, Mead J R, Feorino P M. Insights into HIV chemotherapy. AIDS Res Hum Retroviruses. 1992;8:963–990. doi: 10.1089/aid.1992.8.963. [DOI] [PubMed] [Google Scholar]
- 30.Shioda T, Levy J A, Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991;349:167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- 31.Young S D, Britcher S F, Tran L O, Payne L S, Lumma W C, Lyle T A, Huff J R, Anderson P S, Olsen D B, Carroll S S, Pettibone D J, O’brien J A, Ball R G, Balani S K, Lin J H, Chen I-W, Schleif W A, Sardana V V, Long W J, Byrnes V W, Emini E A. L-743,726 (DMP-266): a novel, highly potent nonnucleoside inhibitor of the human immunodeficiency virus type 1 reverse transcriptase. Antimicrob Agents Chemother. 1995;39:2602–2605. doi: 10.1128/aac.39.12.2602. [DOI] [PMC free article] [PubMed] [Google Scholar]