Abstract
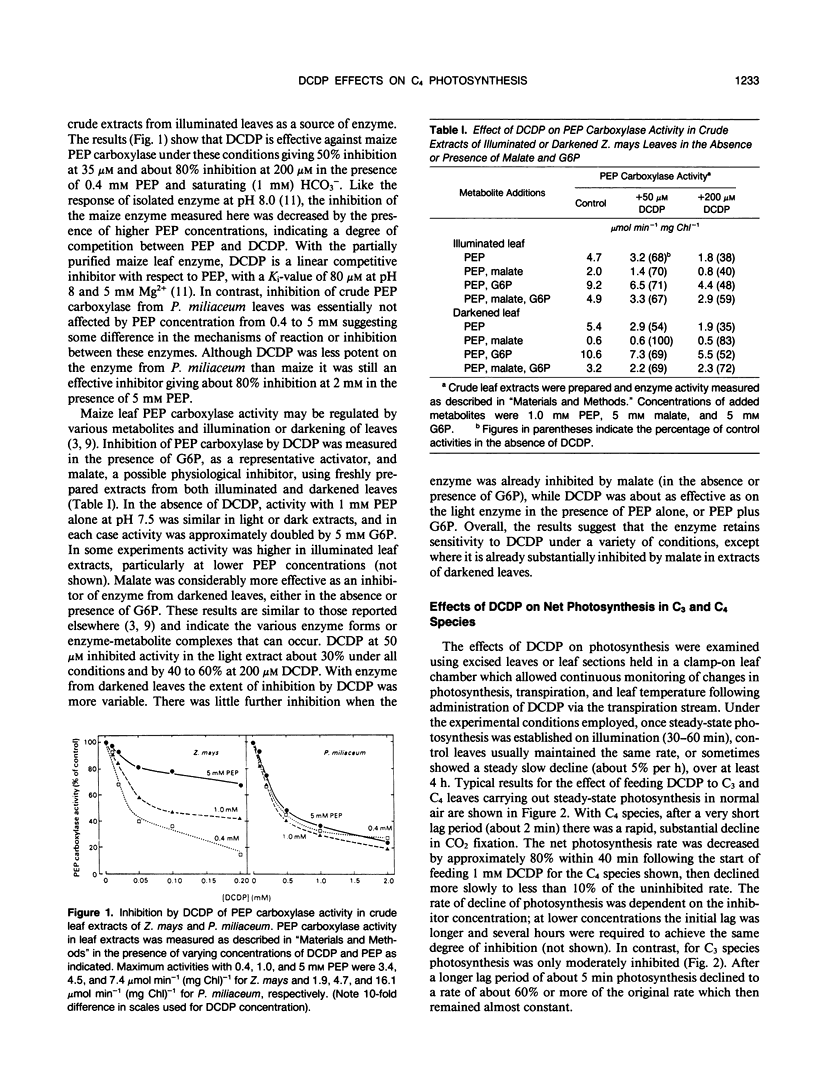
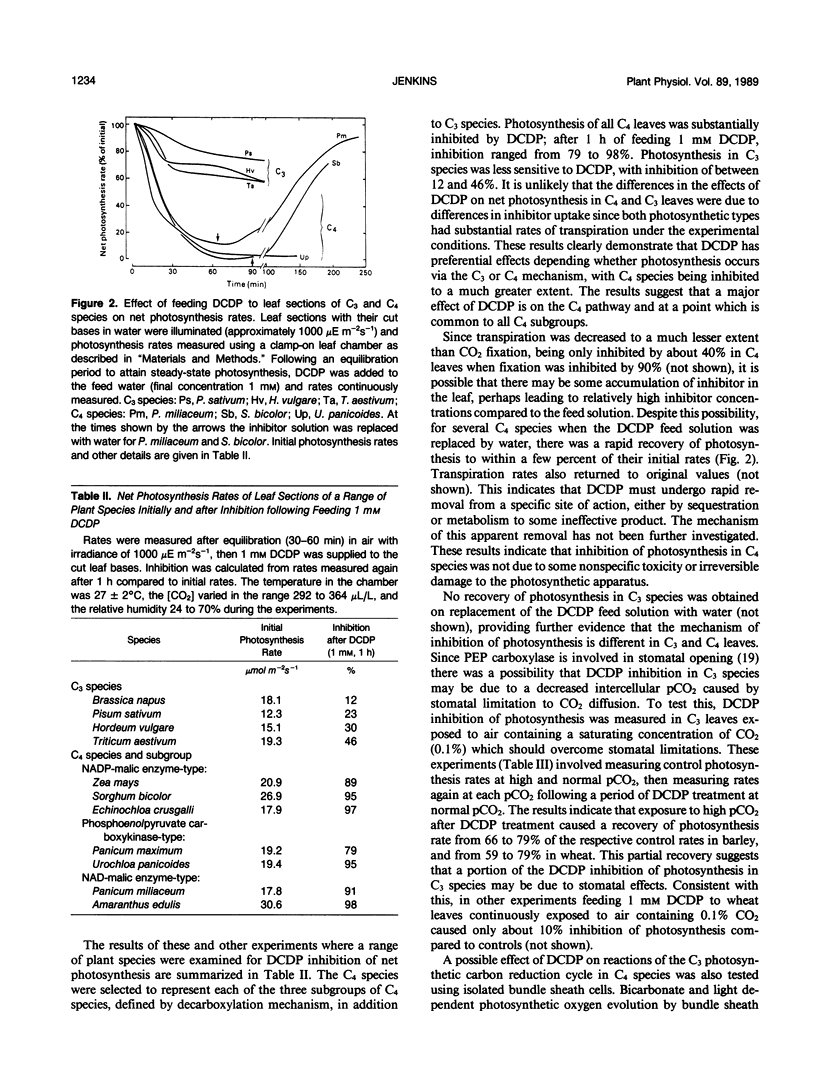
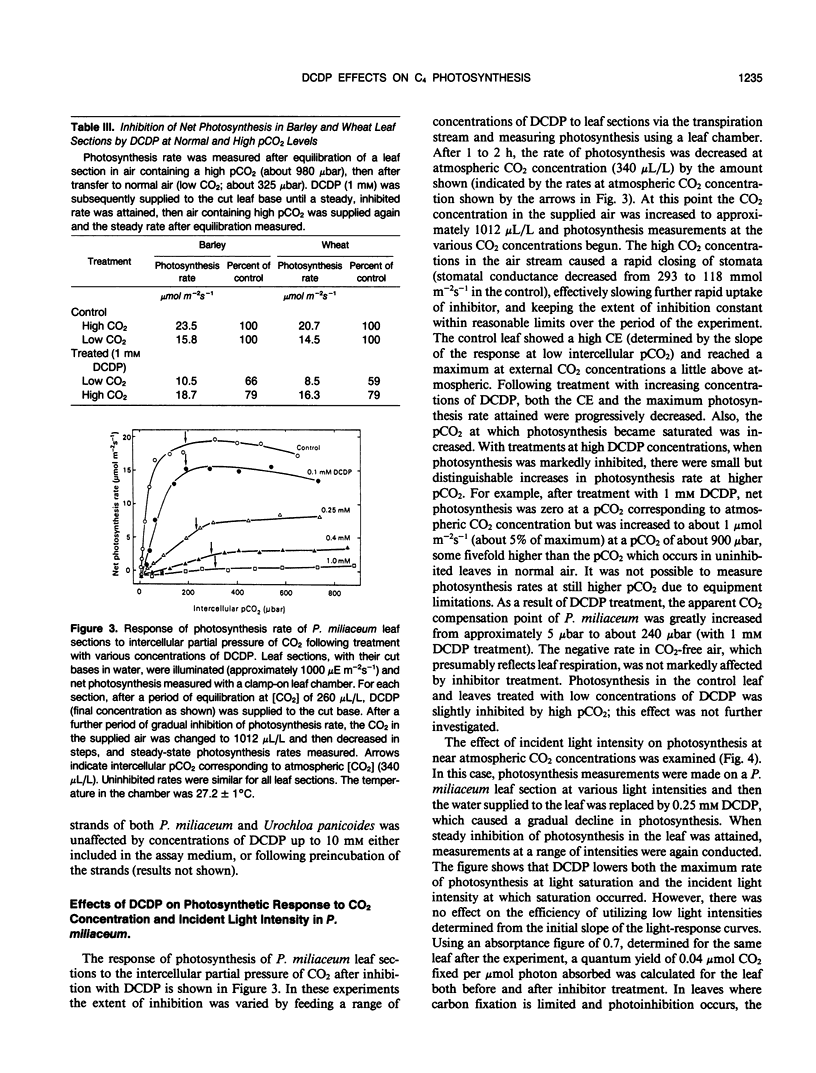
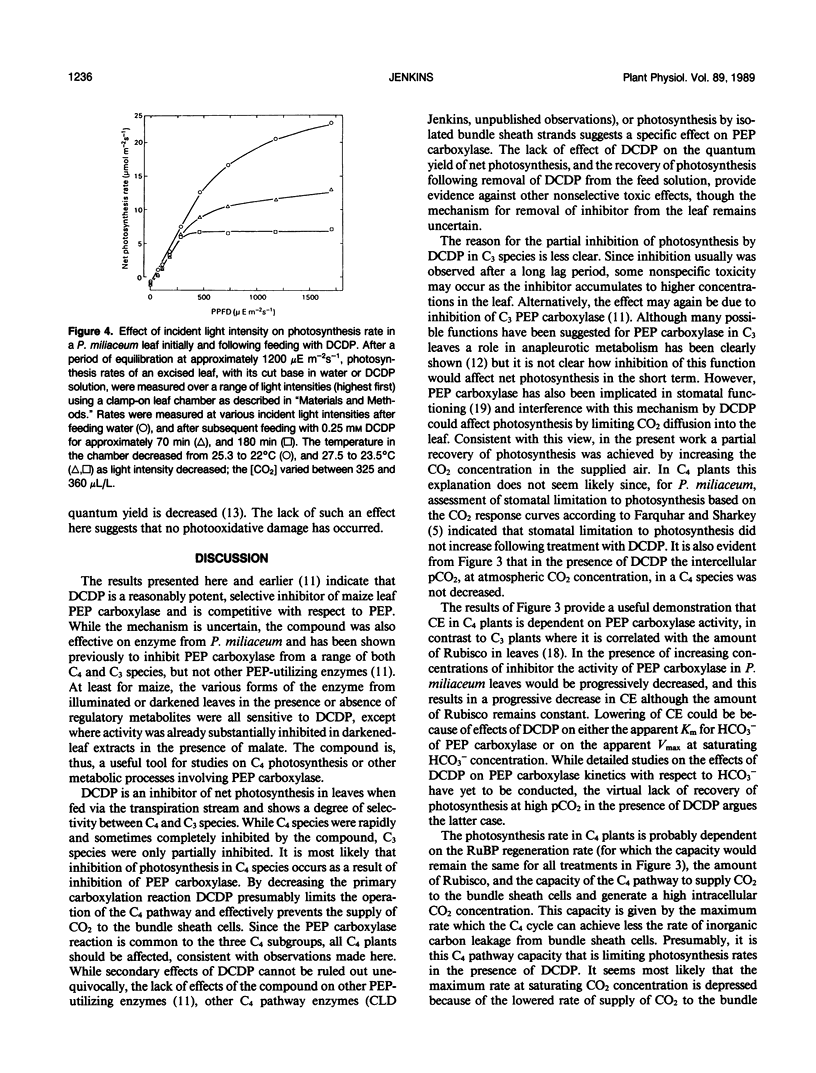
The effect of 3,3-dichloro-2-(dihydroxyphosphinoylmethyl)-propenoate (DCDP), an analog of phosphoenolpyruvate (PEP), on PEP carboxylase activity in crude leaf extracts and on photosynthesis of excised leaves was examined. DCDP is an effective inhibitor of PEP carboxylase from Zea mays or Panicum miliaceum; 50% inhibition was obtained at 70 or 350 micromolar, respectively, in the presence of 1 millimolar PEP and 1 millimolar HCO3−. When fed to leaf sections via the transpiration stream, DCDP at 1 millimolar strongly inhibited photosynthesis in C4 species (79-98% inhibition for a range of seven C4 species), but only moderately in C3 species (12-46% for four C3 species), suggesting different mechanisms of inhibition for each photosynthetic type. The response of P. miliaceum (C4) net photosynthesis to intercellular pCO2 showed that carboxylation efficiency, as well as the CO2 saturated rate, are lowered in the presence of DCDP and supported the view that carboxylation efficiency in C4 species is directly related to PEP carboxylase activity. A fivefold increase in intercellular pCO2 over that occurring in P. miliaceum under normal photosynthesis conditions only increased net photosynthesis rate in the presence of 1 millimolar DCDP from zero to about 5% of the maximal uninhibited rate. Therefore, it seems unlikely that direct fixation of atmospheric CO2 by the bundle sheath cells makes any significant contribution to photosynthetic CO2 assimilation in C4 species. The results support the concept that C4-selective herbicides may be developed based on inhibitors of C4 pathway reactions.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Burnell J. N., Hatch M. D. Photosynthesis in phosphoenolpyruvate carboxykinase-type C4 plants: photosynthetic activities of isolated bundle sheath cells from Urochloa panicoides. Arch Biochem Biophys. 1988 Jan;260(1):177–186. doi: 10.1016/0003-9861(88)90439-0. [DOI] [PubMed] [Google Scholar]
- Doncaster H. D., Leegood R. C. Regulation of phosphoenolpyruvate carboxylase activity in maize leaves. Plant Physiol. 1987 May;84(1):82–87. doi: 10.1104/pp.84.1.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank R. T., Hatch M. D. Mechanism of c(4) photosynthesis: the size and composition of the inorganic carbon pool in bundle sheath cells. Plant Physiol. 1987 Dec;85(4):958–964. doi: 10.1104/pp.85.4.958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber S. C., Sugiyama T. Changes in Sensitivity to Effectors of Maize Leaf Phosphoenolypyruvate Carboxylase during Light/Dark Transitions. Plant Physiol. 1986 Jun;81(2):674–677. doi: 10.1104/pp.81.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer E., O'leary M. H. Anapleurotic CO(2) Fixation by Phosphoenolpyruvate Carboxylase in C(3) Plants. Plant Physiol. 1987 May;84(1):58–60. doi: 10.1104/pp.84.1.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R. F., Pearcy R. W., Seemann J. R. The Nitrogen Use Efficiency of C(3) and C(4) Plants : III. Leaf Nitrogen Effects on the Activity of Carboxylating Enzymes in Chenopodium album (L.) and Amaranthus retroflexus (L.). Plant Physiol. 1987 Oct;85(2):355–359. doi: 10.1104/pp.85.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama T., Mizuno M., Hayashi M. Partitioning of Nitrogen among Ribulose-1,5-bisphosphate Carboxylase/Oxygenase, Phosphoenolpyruvate Carboxylase, and Pyruvate Orthophosphate Dikinase as Related to Biomass Productivity in Maize Seedlings. Plant Physiol. 1984 Jul;75(3):665–669. doi: 10.1104/pp.75.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]


