Abstract
Melamine (Mel) was used as host matrix for liquid nitroglycerin (NG), to prepare Mel/NG solid powdered compounds containing up to 45 wt% of this explosive. The two preparation processes used for this purpose consisted in evaporating a solution of both components, either in ambient conditions or under reduced pressure by the Spray Flash-Evaporation (SFE) process. In Mel/NG materials, amorphous nitroglycerin is distributed in the crystallized melamine matrix as inclusions, which were found to be smaller in size in the material prepared by the SFE process. Mel/NG materials are not stable over time: they gradually lose the nitroglycerin they contain by evaporation.
Keywords: Melamine, Nitroglycerin, Explosive, Matrix, Inclusion, Composite
1. Introduction
Nitroglycerin is a liquid substance under ambient conditions, which has particularly high explosive strength and explosion heat [1]. Despite these interesting properties, nitroglycerin is dangerous to handle in liquid state owing to its particularly low sensitivity threshold to impact (∼0.2 J) [2]. The hazardous properties of nitroglycerin are related to its physical state. First, nitroglycerin is a viscous liquid [3] which traps the minute bubbles formed by its evaporation. The heat released by the adiabatic compression of these bubbles by an impact results in hot spots which easily activate the reaction of the explosive [4]. On the other hand, the thin layers of nitroglycerin formed by spilling the liquid on surfaces are more sensitive to percussion than the bulk explosive. Stabilising nitroglycerin in solid form is therefore necessary to handle this explosive safely and to use it in energetic compositions. Nitroglycerin is classically desensitized by the top-down approach invented and patented by Nobel, which consists in impregnating the explosive into inert porous substrates such as diatomaceous earth (kieselguhr), wood sawdust or charcoal [5] or to gelatinize it with nitrocellulose [6], to obtain dynamites. Nitroglycerin is currently used in ammonium nitrate-based industrial explosives to increase their explosive power, as well as plasticizer in double and triple-base propellants [7].
Contemporary research on nitroglycerin focuses mainly on its use for medicinal purposes [8,9], and work on its use as component for new energetic materials is fairly rare [10]. The sorption of nitroglycerin in polymers used to package tablets containing it [11] or in the polyvinyl chloride tubes used to administer it [12], and in nitrocellulose [13] shows that it can be trapped in certain materials for which it has an affinity. An orientation study carried out as part of this research, which involved investigating the interaction of nitroglycerin with a series of molecules from a qualitative point of view, showed that melamine had a particularly good affinity for nitroglycerin. The thermogravimetric study published by Costa and Camino shows that melamine exhibits good thermal stability, at least up to 300 °C [14]. The thermal stability range of melamine overlaps that of nitroglycerin, enabling it to be used as a host matrix for the latter. Another interesting property of melamine lies in its ability to form supramolecular architectures [15,16], in which nitroglycerin could be trapped in even greater quantities than in pure melamine. Stabilising nitroglycerin in the form of a fine-grained solid powder is essential for using it in solid detonating compositions such as NSTEX [17,18]. NSTEX are hybrid energetic materials which are formulated by mixing a nanothermite with a high explosive in fine powder. In these composite explosives, the fast combustion of the nanothermite activates the transition from deflagration to detonation of the explosive [19]. The use of nitroglycerin in these compositions could be a means of designing initiation dynamites that are insensitive to mechanical stress, but capable of detonating under the action of flame ignition.
The aim of this research was to trap nitroglycerin in a melamine host matrix by bottom-up approaches. For this purpose, two processes were used which consist to remove the solvent in which the both components are dissolved, either by spontaneous evaporation or by spray flash-evaporation (SFE) [20,21].
2. Materials and methods
2.1. Materials
The nitroglycerin was provided by Nitrochemie AG (Wimmis, Switzerland) in a methanol solution with a mass concentration of 5%. The solvent was evaporated under reduced pressure (T = 66 °C, P < 200 mbar) and its full removing was checked by infrared spectroscopy. Melamine (99%, lot MKCF8887) was received from Sigma-Aldrich. Absolute ethanol was from VWR Chemicals (99.98%, AnalaR NORMAPUR® ACS, Reag. Ph. Eur., lot 21A134010).
2.2. Preparation methods of inclusion compounds
2.2.1. Safety warning
Pure nitroglycerin is very sensitive to impact, especially when it contains gas bubbles or when it forms a thin layer on the surface of objects. Pure nitroglycerin as well as materials prepared from this explosive should be handled with care, in small quantities (typically 1–2 g), by experienced scientists and in accordance with local laws and regulations. The authors decline all responsibility for any misuse of the results reported in this article.
The first formulation method consists in weighing the appropriate amount of melamine and nitroglycerin, in a 1:1 M ratio, to obtain a final sample mass of less than half a gram. These compounds were dissolved in about 150 mL of absolute ethanol in a crystallizing dish. The inclusion compound, subsequently referred to as Mel/NG-SE, is formed by the spontaneous evaporation (SE) of ethanol under ambient conditions, which takes about one day.
The second formulation method used the Spray Flash-Evaporation (SFE) process which was described in Refs. [20,21]. The solution, which was composed of melamine (0.3 g) and nitroglycerin (0.5 g) dissolved in a mixture of ethanol (500 mL) and water (100 mL), was sprayed through a 100 μm nozzle kept at 100 °C, by applying an upstream pressure of 40 bar, in an atomization chamber maintained under dynamic vacuum (5 mbar). The temperature of the sprayed solution was set to obtain the highest possible pressure ratio between saturation pressure and chamber pressure [22], while minimizing nitroglycerin evaporation. The slight natural acidity of the melamine solution (pH = 5) and the very short heating time of the solution (a few seconds) do not allow the hydrolysis of nitroglycerin, which was observed by Halasz et al. in an aqueous alkaline medium (pH = 9–12) under the action of prolonged heating [23]. The sample, subsequently referred to as Mel/NG-SFE, was collected on a metallic filter.
Mel/NG samples were stored in airtight containers at room temperature (15–25 °C).
2.3. Characterization techniques
Fourier-transform infrared (FT-IR) transmission spectra were recorded with a Tensor 27 apparatus from Bruker. 3D Raman imaging was performed with confocal Raman microscope LabRAM HR Evolution from Horiba equipped with a solid state laser emitting at 532 nm. The morphology of the materials was studied using a FEI Nova NanoSEM 450 scanning electron microscope (SEM), after metallization of the samples by a thin layer of gold deposited by sputtering. Thermogravimetric (TGA) experiments were performed with a SDT 650 apparatus from TA Instruments, using 90 μL open alumina cups. The dynamic TGA analyses were carried out under nitrogen flow (100 mL/min) using a heating rate of 5 K/min. TGA experiments to study the desorption kinetics of nitroglycerin from the melamine matrix were performed in isothermal conditions from 60 °C to 100 °C in 10 °C steps on samples of 1–3 mg, under an Ar/O2 (98/2) flow. X-Ray Diffraction patterns of the materials were recorded with a D8 Advance AXS from Bruker. The sensitivity thresholds to impact were measured with the BFH-12 model of BAM fall hammer apparatus from OZM research, on 40 mm3 samples.
2.4. Study of the desorption kinetics
Nitroglycerin evaporation from melamine matrix was studied using a zero order mechanism (R1) [24] in which the mass fraction of evaporated nitroglycerin (0 < α < 1) increases linearly with time (α = k × t). The k value was used in an Arrhenius’ equation to represent graphically the evolution of ln k according to the reciprocal of isotherm temperature:
where:
-
▪
A is the pre-exponential factor,
-
▪
E is the activation energy of nitroglycerin evaporation (J/mol),
-
▪
R is the molar gas constant (≈8.314 J mol−1 K−1),
-
▪
T is the isotherm temperature (K).
The activation energy of nitroglycerin evaporation is finally calculated from the slope of the corresponding line.
3. Results and discussion
The composite materials formed by trapping nitroglycerin in melamine are powdered solids, in which the presence of both molecules is confirmed by infrared spectrometry in Fig. 1(a). The absence of the characteristic broad and intense band attributed to the stretching of hydroxyl functional groups in FT-IR spectra (3200-3700 cm−1) of Mel/NG-SE and Mel/NG-SFE materials also shows that they do not contain the solvents (ethanol and ethanol/water, respectively) which were used to formulate them. The bands observed in the nitroglycerin spectrum at 1265 cm−1 and 1630 cm−1, which respectively correspond to the symmetric (νs) and asymmetric stretching (νas) of -NO2 groups [25], are found in Mel/NG-SE and Mel/NG-SFE materials. While there is no significant wavenumber shift of these bands for Mel/NG-SE, shifts of ∼8 cm−1 and ∼6 cm−1 to higher and lower wavenumbers are respectively observed for symmetric and asymmetric stretching of –NO2 groups for Mel/NG-SFE. The changes in –NO2 stretching means that the melamine matrix influences the energy level of the liquid nitroglycerin trapped in the inclusions, meaning that the latter are very small in size. No shift of the stretch bands attributed to melamine –NH2 groups at 3468 cm−1, 3417 cm−1, 3325 cm−1 and 3122 cm−1 [26] is observed for Mel/NG-SE and Mel/NG-SFE materials, meaning that the trapping of nitroglycerin has no influence on the melamine matrix. Nitroglycerin has only interfacial contact with melamine and has no influence on the solid crystal lattice of melamine molecules. The 3D Raman spectra in Fig. 1(b) show that nitroglycerin is irregularly distributed throughout the volume of the melamine matrix, in small inclusions (areas in yellow/orange) and in larger micron-sized pockets (areas in green). The NG contained in a Mel/NG-SE sample (29 wt% NG) is highly desensitized to impact, with a sensitivity threshold increasing from less than 0.25 J to 5.5 J.
Fig. 1.
(a) FT-IR spectra of the materials and their components, (b) 3D Raman spectra of the Mel/NG-SE compound in which melamine is represented in red and nitroglycerin in green.
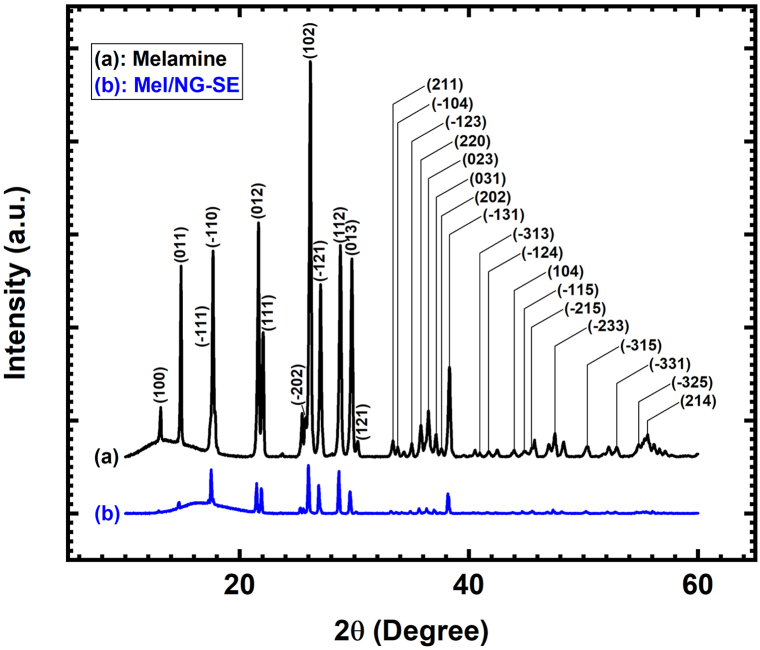
As shown in Fig. 2, the X-Ray diffraction peaks of melamine (Fig. 2(a); JCPDS card: 00-039-1950) are identified in the diffraction pattern of the Mel/NG-SE material (Fig. 2(b)). As no new diffraction peak is observed, the Mel/NG-SE material is neither a cocrystal nor a solvate, but a compound in which amorphous nitroglycerin is trapped in the crystallized melamine matrix. In other words, nitroglycerin, whose melting point (13.5 °C) is much lower than that of melamine (345 °C) [27], is trapped as liquid inclusions in the latter.
Fig. 2.
X-Ray diffraction patterns of (a) melamine and (b) the Mel/NG-SE compound.
The particles of commercial melamine observed by SEM in Fig. 3(a) are non-porous and have micrometric sizes. The Mel/NG-SE particles shown in Fig. 3(b and c) are larger; their surface is covered with open pores, whose diameter is ranging from submicron scale to several tens of microns. The morphology of the material suggests that it is formed by the slow crystallization of melamine from the solution around nitroglycerin droplets which act as template. The growth process of the melamine matrix around nitroglycerin inclusions is governed by the evaporation of ethanol. The Mel/NG-SFE material is composed of aggregates of angular particles whose internal porosity is essentially formed by interconnected cavities, which result from the coalescence of nitroglycerin bubbles which escape from the material during SFE processing. The size of elementary nitroglycerin inclusions in the melamine matrix (5–50 nm) was estimated from the imprints left by their evaporation during the formation of the material, which correspond to the smallest single cavities observed in Mel/NG-SFE particles (0.2–1 μm, Fig. 3(d)). The presence of nitroglycerin in Mel/NG particles observed by SEM was qualitatively detected by the degradation of the samples under the electron beam during observations performed at higher magnifications. The effect produced is a swelling of the observed area, which is attributed to the heat-induced release of nitroglycerin from the melamine matrix. Mel/NG materials are stable when observed at lower magnifications, such as those used to record SEM images of the materials (Fig. 3).
Fig. 3.
SEM images of (a) melamine and the composite materials: (b–c) Mel/NG-SE at low and higher magnification, (d) Mel/NG-SFE.
The TGA curves of the materials presented in Fig. 4(a) show that nitroglycerin evaporation and melamine sublimation occur in separated temperature ranges extending from 105 to 187 °C, and from 230 °C to 330 °C, respectively. This makes it possible to determine the mass content of nitroglycerin in Mel/NG-SE (41.3%) and Mel/NG-SFE (44.7%). Nitroglycerin evaporation is observed in Mel/NG materials, although at lower temperatures, as shown in Fig. 4(b), which means that nitroglycerin hosted in melamine evaporates more readily than in its pure liquid state. The lower evaporation temperature of nitroglycerin in Mel/NG-SFE material is attributed to the miniaturization of nitroglycerin inclusions. This was confirmed by calculating the activation energy of nitroglycerin evaporation from the experimental data obtained in TGA isothermal experiments for pure nitroglycerin (98 kJ/mol), Mel/NG-SE (82 kJ/mol) and Mel/NG-SFE (47 kJ/mol) materials. Interestingly, these values are of the same order as those reported by Sućeska (≈81.9 kJ/mol) [28] and Tompa (≈75.3 kJ/mol) [29] for desorption of nitroglycerin from double-base propellants. However, in Mel/NG materials, the activation energy of nitroglycerin evaporation is strongly influenced by the size of the explosive domains in the melamine matrix, as illustrated by the difference noticed between Mel/NG-SE and Mel/NG-SFE materials. The lowering of the melamine sublimation temperature observed for Mel/NG materials was attributed to its higher surface area. The volume initially occupied in the melamine matrix by nitroglycerin is released by the evaporation of the latter, resulting in a highly porous material. The lower sublimation temperature of the melamine formed by the decomposition of the Mel/NG-SFE material, shows that its porosity is more finely structured than the one of melamine formed by the degradation of Mel/NG-SE material, and that the size of the nitroglycerin inclusions in the former material is smaller than in the latter.
Fig. 4.
(a) TGA curves of the materials studied and (b) their temperature derived signals, (c) desorption times of nitroglycerin from the melamine matrix: experimental values are represented by squares (Mel/NG-SFE) and triangles (Mel/NG-SE), while the lines are trend curves obtained by extrapolating these values.
The time required to completely desorb nitroglycerin from the melamine matrix in Mel/NG materials, relative to sample mass, was studied isothermally by thermogravimetry at different temperatures between 60 °C and 100 °C and represented in Fig. 4(c). For both samples, desorption time evolves according to an exponential decay law with increasing temperature. It can be noticed that Mel/NG-SFE material is more stable along time than the material prepared by simple evaporation. As the nitroglycerin contents of both samples are similar, this means that the explosive is trapped more efficiently by the melamine matrix in the material prepared by SFE process. It can also be seen that the desorption time decreases more sharply with increasing temperature in the Mel/NG-SFE material, which is in good agreement with the fact that the activation energy for nitroglycerin evaporation is lower for this material than for that prepared by the simple evaporation process. Evaporation loss of nitroglycerin in Mel/NG materials is a relatively slow phenomenon, however, which can be limited by storing these materials in hermetically sealed containers, with a volume appropriate to that of the sample, and kept at room temperature.
4. Conclusions
Solid powdered materials in which nitroglycerin is trapped in a melamine matrix were prepared from solutions in ethanol by processes involving either a slow (SE) or a rapid (SFE) evaporation of the solvent. These compounds contain up to 45% by weight of nitroglycerin, which is distributed in the crystallized melamine matrix as liquid inclusions, which are smaller in size (typically nanoscale) in the material prepared by the SFE process. Melamine/nitroglycerin compounds are not stable over time as they lose nitroglycerin they contain by evaporation. This phenomenon is strongly accelerated by heating Mel/NG materials. The energetic performance of these new nitroglycerin based composite materials, should be enhanced by using energetic molecules as host matrices such as hydrazine sulphate or erythritol tetranitrate instead of melamine.
Author contribution statement
Cédric Schwartz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data.
Marc Comet: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Bastien Lallemand; Maxence Vince; Anna K. Ott; Fabien Schnell; Benjamin Bonnet: Performed the experiments; Contributed reagents, materials, analysis tools or data.
Denis Spitzer: Analyzed and interpreted the data.
Data availability statement
Data included in article/supplementary material/referenced in article.
Additional information
No additional information is available for this paper.
Funding statement
This research was carried out as part of Cédric Schwartz's doctoral thesis, co-funded by French-German Research Institute of Saint-Louis (ISL) and the Defence Innovation Agency of the Directorate General of Armament (DGA) (grant number: DGA 01D19024292 AID).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Meyer R., Köhler J., Homburg A. sixth ed. Wiley-VCH &Co. KGaA; 2007. Explosives. [Google Scholar]
- 2.Levine D., Boyars C. The sensitivity of nitroglycerin to impact. Combust. Flame. 1965;9:131–140. doi: 10.1016/0010-2180(65)90059-3. [DOI] [Google Scholar]
- 3.McNiff E.F., Yap P.S.K., Fung H.-L. Nitroglycerin. Anal. Profiles Drug Subst. 1981;9:519–541. doi: 10.1016/S0099-5428(08)60152-5. [DOI] [Google Scholar]
- 4.Bowden F.P., Mulcahy M.F.R., Vines R.G., Yoffe A., Rivett A.C.D., Robertson R. The detonation of liquid explosives by gentle impact. The effect of minute gas spaces. Proc. R. Soc. London, Ser. A. 1947;188:291–311. doi: 10.1098/rspa.1947.0010. [DOI] [Google Scholar]
- 5.A. Nobel “Improved explosive compound”. 1868 https://patents.google.com/patent/US78317 [Google Scholar]
- 6.A. Nobel “Improvement in gelatinated explosive compounds”. 1876 https://patents.google.com/patent/US175735 [Google Scholar]
- 7.Sabatini J.J., Johnson E.C. A short review of nitric esters and their role in energetic materials. ACS Omega. 2021;6:11813–11821. doi: 10.1021/acsomega.1c01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams J. Nitroglycerin and long-acting nitrates. N. Engl. J. Med. 1980;302(22):1234–1237. doi: 10.1056/NEJM198005293022205. https://www.nejm.org/doi/full/10.1056/NEJM198005293022205 [DOI] [PubMed] [Google Scholar]
- 9.Meunier M., Yammine A., Bettaieb A., Plenchette S. Nitroglycerin: a comprehensive review in cancer therapy. Cell Death Dis. 2023;14(5):323. doi: 10.1038/s41419-023-05838-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang Z., Wang B., Guo Y., Jin Y., Fei L., Huang S., Zhang W., Tang C., Zhang Q. From heart drug to propellant fuels: designing nitroglycerin-ionic liquid composite as green high-energy hypergolic fluids. Combust. Flame. 2021;233 doi: 10.1016/j.combustflame.2021.111597. [DOI] [Google Scholar]
- 11.Pikal M.J., Bibler D.A., Rutherford B. Polymer sorption of nitroglycerin and stability of molded nitroglycerin tablets in unit-dose packaging. J. Pharm. Sci. 1977;66(9):1293–1297. doi: 10.1002/jps.2600660922. [DOI] [PubMed] [Google Scholar]
- 12.Treleano A., Wolz G., Brandsch R., Welle F. Investigation into the sorption of nitroglycerin and diazepam into PVC tubes and alternative tube materials during application. Int. J. Pharm. 2009;369(1–2):30–37. doi: 10.1016/j.ijpharm.2008.10.024. [DOI] [PubMed] [Google Scholar]
- 13.Kuo D.T.F., Simini M., Allen H.E. Sorption and desorption kinetics of nitroglycerin and 2,4-dinitrotoluene in nitrocellulose and implications for residue-bound energetic materials. Water Res. 2018;128:138–147. doi: 10.1016/j.watres.2017.10.052. [DOI] [PubMed] [Google Scholar]
- 14.Costa L., Camino G. Thermal behaviour of melamine. J. Therm. Anal. 1988;34:423–429. doi: 10.1007/BF01913181. [DOI] [Google Scholar]
- 15.Zhang X.-L., Chen X.-M. Supramolecular architectures and helical water chains in cocrystals of melamine and aromatic carboxylic acids. Cryst. Growth Des. 2005;5(2):617–622. doi: 10.1021/cg0498251. [DOI] [Google Scholar]
- 16.Roy B., Bairi P., Nandi A.K. Supramolecular assembly of melamine and its derivatives: nanostructures to functional materials. RSC Adv. 2014;4:1708–1734. doi: 10.1039/C3RA44524K. [DOI] [Google Scholar]
- 17.Comet M., Martin C., Klaumünzer M., Schnell F., Spitzer D. Energetic nanocomposites for detonation initiation in high explosives without primary explosives. Appl. Phys. Lett. 2015;107(24) doi: 10.1063/1.4938139. [DOI] [Google Scholar]
- 18.Comet M., Martin C., Schnell F., Spitzer D. Nanothermites: a short review. Factsheet for experimenters, present and future challenges. Propellants, Explos. Pyrotech. 2019;44(1):18–36. doi: 10.1002/prep.201800095. [DOI] [Google Scholar]
- 19.Khasainov B., Comet M., Veyssière B., Spitzer D. Comparison of performance of fast-reacting nanothermites and primary explosives. Propellants, Explos. Pyrotech. 2017;42(7):754–772. doi: 10.1002/prep.201600181. [DOI] [Google Scholar]
- 20.Risse B., Spitzer D., Hassler D., Schnell F., Comet M., Pichot V., Muhr H. Continuous formation of submicron energetic particles by the flash-evaporation technique. Chem. Eng. J. 2012;203:158–165. doi: 10.1016/j.cej.2012.07.032. [DOI] [Google Scholar]
- 21.Risse B., Hassler D., Spitzer D. “Préparation de nanoparticules par evaporation flash”. 2013 https://patentscope.wipo.int/search/fr/detail.jsf?docId=WO2013117671 [Google Scholar]
- 22.Lobry E., Berthe J.-E., Spitzer D. Spray flash evaporation SFE process: identification of the driving parameters on evaporation to tune particle size and morphology. Chem. Eng. Sci. 2021;231 doi: 10.1016/j.ces.2020.116307. [DOI] [Google Scholar]
- 23.Halasz A., Thiboutot S., Ampleman G., Hawari J. Microwave-assisted hydrolysis of nitroglycerin (NG) under mild alkaline conditions: new insight into the degradation pathway. Chemosphere. 2010;79:228–232. doi: 10.1016/j.chemosphere.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Shirotani K., Sekiguchi K. Studies on the ethanol, n-propanol and isopropanol solvates of cortisone acetate. Chem. Pharm. Bull. 1981;29:2983–2992. doi: 10.1248/cpb.29.2983. [DOI] [Google Scholar]
- 25.Contini A.E., Flood N., McAteer D., Mai N., Akhavan J. Low hazard small-scale synthesis and chemical analysis of high purity nitroglycerine (NG) RSC Adv. 2015;5:87228–87232. doi: 10.1039/C5RA17951C. [DOI] [Google Scholar]
- 26.Zhu Heng, Xu Shi-ai. Preparation and fire behavior of rigid polyurethane foams synthesized from modified urea–melamine–formaldehyde resins. RSC Adv. 2018;8:17879–17887. doi: 10.1039/C8RA01846D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lide D.R., editor. CRC Handbook of Chemistry and Physics. CRC Press; Boca Raton, FL: 2005. Internet Version 2005. [Google Scholar]
- 28.Sućeska M., Mušanić S.M., Houra I.F. Kinetics and enthalpy of nitroglycerin evaporation from double base propellants by isothermal thermogravimetry. Thermochim. Acta. 2010;510:9–16. doi: 10.1016/j.tca.2010.06.014. [DOI] [Google Scholar]
- 29.Tompa A.S. Thermal analysis of liquid and solid propellants. J. Hazard Mater. 1980;4:95–112. doi: 10.1016/0304-3894(80)80026-4. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supplementary material/referenced in article.