Abstract
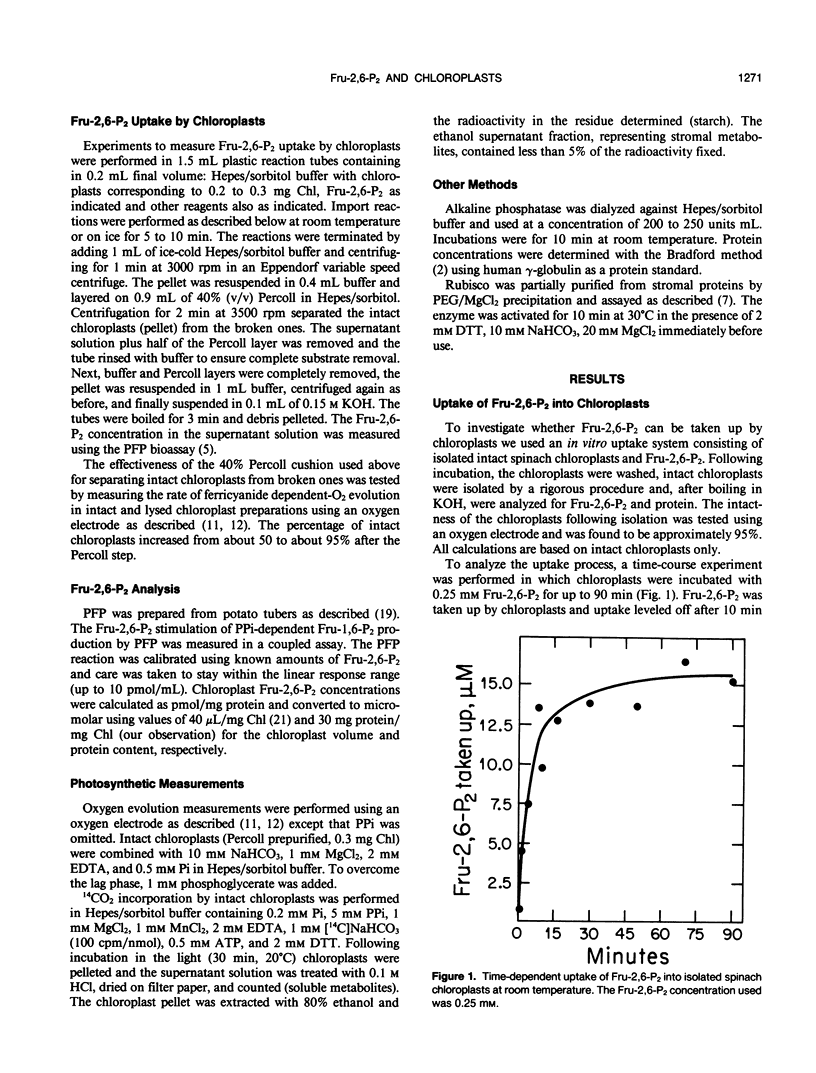
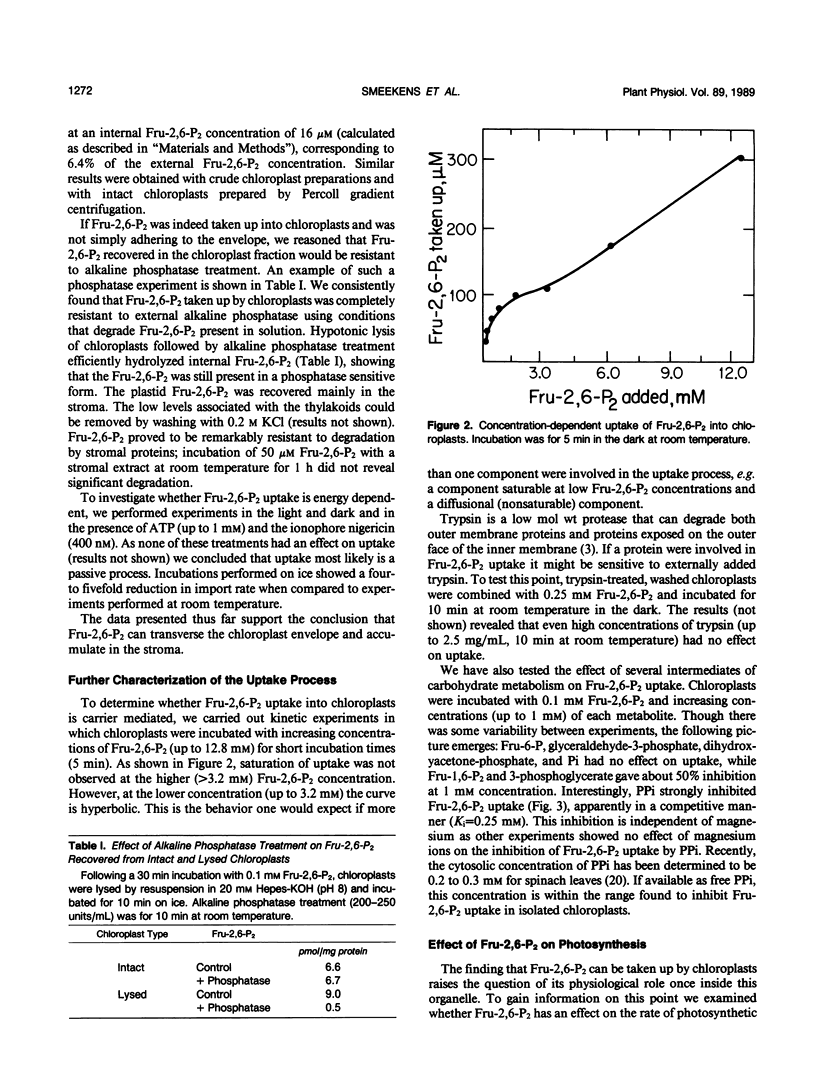
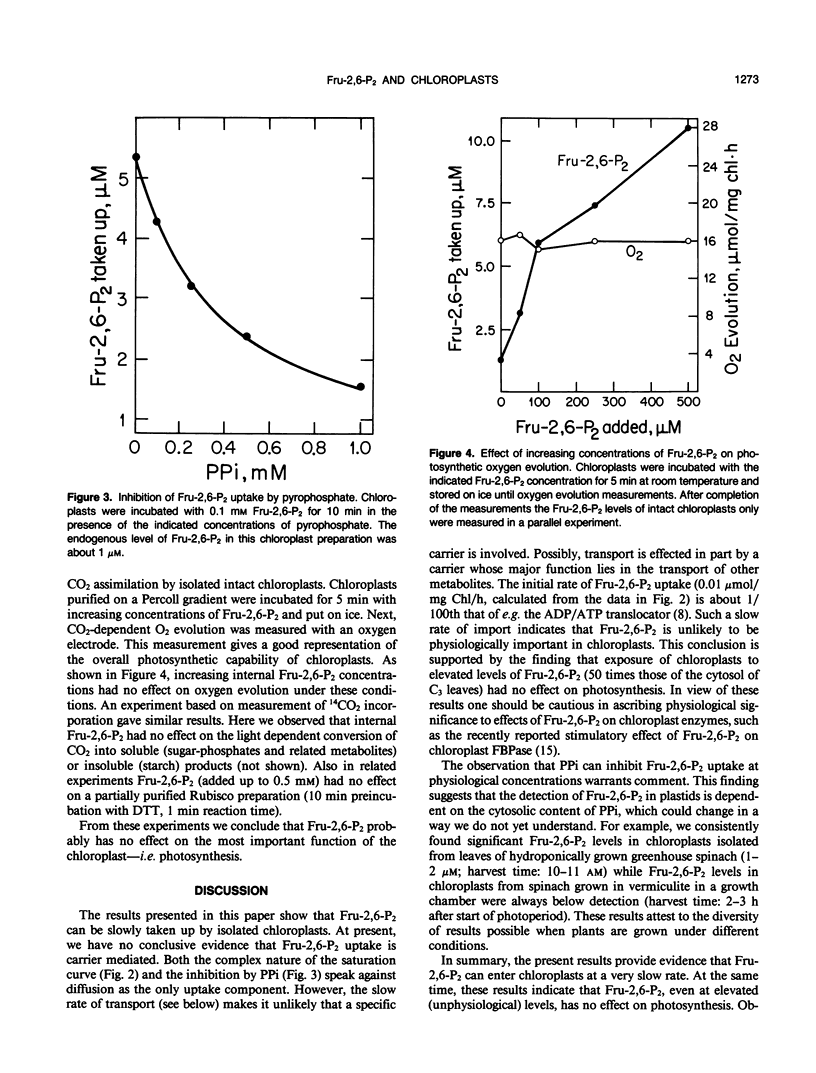
The regulatory metabolite fructose-2,6-bisphosphate (Fru-2,6-P2) has an important function in controlling the intermediary carbon metabolism of leaves. Fru-2,6-P2 controls two cytosolic enzymes involved in the interconversion of fructose-6-phosphate and fructose-1,6-bisphosphate (fructose-1,6-bisphosphatase and pyrophosphate, fructose-6-phosphate 1-phosphotransferase) and thereby controls the partitioning of photosynthate between sucrose and starch. It has been demonstrated that Fru-2,6-P2 is present mainly in the cytosol. Here we present evidence that Fru-2,6-P2 can be taken up by isolated intact chloroplasts but at a very slow rate (about 0.01 micromoles per milligram of chlorophyll per hour). This uptake is time and concentration dependent and is inhibited by PPi. When provided a physiological concentration of Fru-2,6-P2 (10 micromolar), chloroplasts accumulated up to 0.6 micromolar Fru-2,6-P2 in the stroma. Elevated plastid Fru-2,6-P2 levels had no effect on overall photosynthetic rates of isolated chloroplasts. The results indicate that, while Fru-2,6-P2 enters isolated chloroplasts at a sluggish rate, caution should be exercised in ascribing physiological importance to effects of Fru-2,6-P2 on chloroplast enzymes.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cline K., Werner-Washburne M., Andrews J., Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984 Jul;75(3):675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cséke C., Weeden N. F., Buchanan B. B., Uyeda K. A special fructose bisphosphate functions as a cytoplasmic regulatory metabolite in green leaves. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4322–4326. doi: 10.1073/pnas.79.14.4322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrendorf T., Holtum J. A., Mukherjee U., Latzko E. Fructose 2,6-bisphosphate, carbohydrate partitioning, and crassulacean Acid metabolism. Plant Physiol. 1987 May;84(1):182–187. doi: 10.1104/pp.84.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzog B., Stitt M., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : III. Properties of the Cytosolic Fructose 1,6-Bisphosphatase. Plant Physiol. 1984 Jul;75(3):561–565. doi: 10.1104/pp.75.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F. D., Chou Q., Buchanan B. B. Ion-exchange chromatography separates activities synthesizing and degrading fructose 2,6-bisphosphate from C3 and C4 leaves but not from rat liver. Plant Physiol. 1987;85:13–16. doi: 10.1104/pp.85.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F. D., Cséke C., Chou Q., Buchanan B. B. Activities synthesizing and degrading fructose 2,6-bisphosphate in spinach leaves reside on different proteins. Proc Natl Acad Sci U S A. 1987 May;84(9):2742–2746. doi: 10.1073/pnas.84.9.2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. Fructose 2,6-bisphosphate and plant carbohydrate metabolism. Plant Physiol. 1987 Jun;84(2):201–204. doi: 10.1104/pp.84.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Herzog B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : I. Coordination of CO(2) Fixation and Sucrose Synthesis. Plant Physiol. 1984 Jul;75(3):548–553. doi: 10.1104/pp.75.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Kürzel B., Heldt H. W. Control of Photosynthetic Sucrose Synthesis by Fructose 2,6-Bisphosphate : II. Partitioning between Sucrose and Starch. Plant Physiol. 1984 Jul;75(3):554–560. doi: 10.1104/pp.75.3.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Wirtz W., Stitt M., Heldt H. W. Enzymic determination of metabolites in the subcellular compartments of spinach protoplasts. Plant Physiol. 1980 Jul;66(1):187–193. doi: 10.1104/pp.66.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]


