Abstract
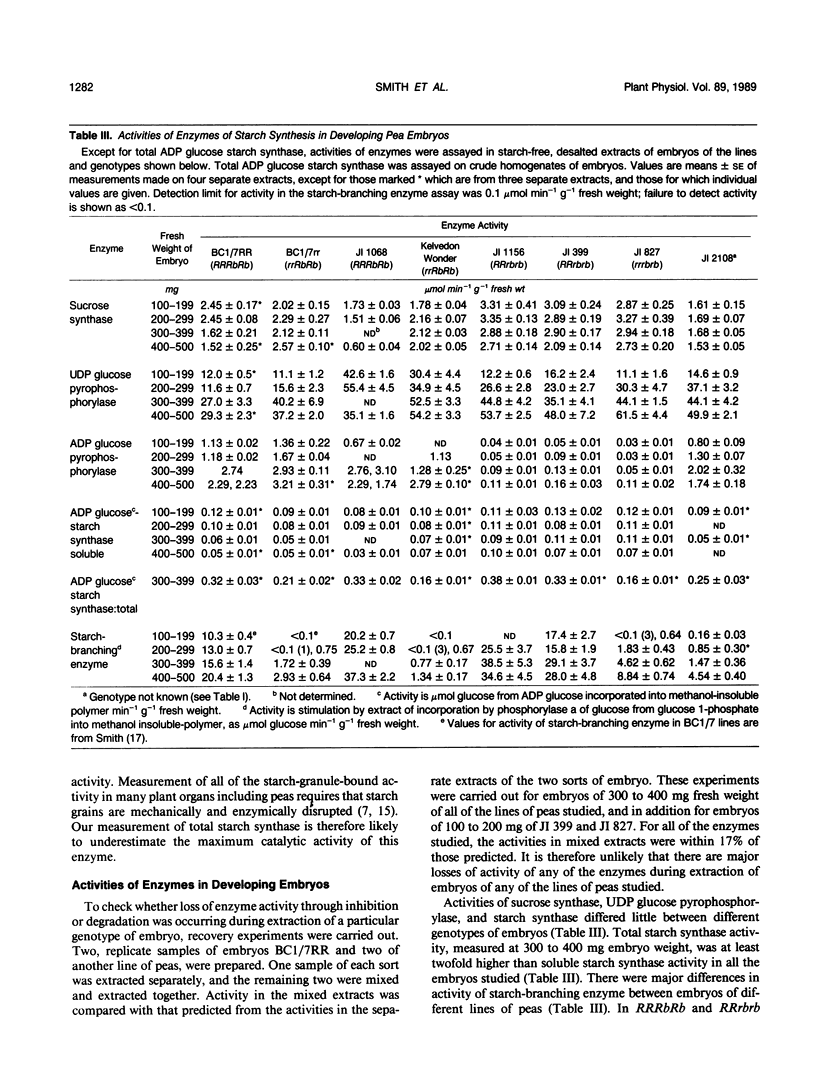
The aim of this work was to discover whether the rb locus of peas (Pisum sativum L.) affects seed starch content through action on an enzyme of starch synthesis in the developing embryo. The phenotypic effects of this locus are like those of the better characterised, unlinked r locus, which affects seed starch content through action on starch-branching enzyme. Embryos recessive at one or both of these loci (RRrbrb, rrRbRb, rrrbrb) have lower starch contents from an early stage of development than embryos dominant at these loci (RRRbRb). Maximum catalytic activities of enzymes of the pathway from sucrose to starch (sucrose synthase EC 2.4.1.13, UDP glucose pyrophosphorylase EC 2.7.7.9, ADP glucose pyrophosphorylase EC 2.7.7.27, ADP glucose-starch synthase EC 2.4.1.21, starch-branching enzyme EC 2.4.1.18) were compared in developing embryos of three lines of rbrb peas and four lines of RbRb peas. The only consistent difference between the two sorts of embryo was in the activity of ADP glucose pyrophosphorylase, which was at least tenfold lower in rbrb than in RbRb embryos. The activity in rbrb embryos was in most cases less than the estimated rate of starch synthesis of RRRbRb embryos. We conclude that the effect of the rb locus on the starch content of pea seeds is mediated through an alteration in the activity of ADP glucose pyrophosphorylase in the developing embryo.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Frydman R. B., Cardini C. E. Studies on the biosynthesis of starch. II. Some properties of the adenosine diphosphate glucose:starch glucosyltransferase bound to the starch granule. J Biol Chem. 1967 Jan 25;242(2):312–317. [PubMed] [Google Scholar]
- Ghosh H. P., Preiss J. Adenosine diphosphate glucose pyrophosphorylase. A regulatory enzyme in the biosynthesis of starch in spinach leaf chloroplasts. J Biol Chem. 1966 Oct 10;241(19):4491–4504. [PubMed] [Google Scholar]
- Hannah L. C., Nelson O. E. Characterization of adenosine diphosphate glucose pyrophosphorylases from developing maize seeds. Plant Physiol. 1975 Feb;55(2):297–302. doi: 10.1104/pp.55.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawker J. S., Ozbun J. L., Ozaki H., Greenberg E., Preiss J. Interaction of spinach leaf adenosine diphosphate glucose alpha-1,4-glucan alpha-4-glucosyl transferase and alpha-1,4-glucan, alpha-1,4-glucan-6-glycosyl transferase in synthesis of branched alpha-glucan. Arch Biochem Biophys. 1974 Feb;160(2):530–551. doi: 10.1016/0003-9861(74)90430-5. [DOI] [PubMed] [Google Scholar]
- Keeling P. L., Wood J. R., Tyson R. H., Bridges I. G. Starch Biosynthesis in Developing Wheat Grain : Evidence against the Direct Involvement of Triose Phosphates in the Metabolic Pathway. Plant Physiol. 1988 Jun;87(2):311–319. doi: 10.1104/pp.87.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald F. D., Preiss J. Solubilization of the starch-granule-bound starch synthase of normal maize kernels. Plant Physiol. 1983 Sep;73(1):175–178. doi: 10.1104/pp.73.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos-Scheperkeuter G. H., de Boer W., Visser R. G., Feenstra W. J., Witholt B. Identification of granule-bound starch synthase in potato tubers. Plant Physiol. 1986 Oct;82(2):411–416. doi: 10.1104/pp.82.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]


