Abstract
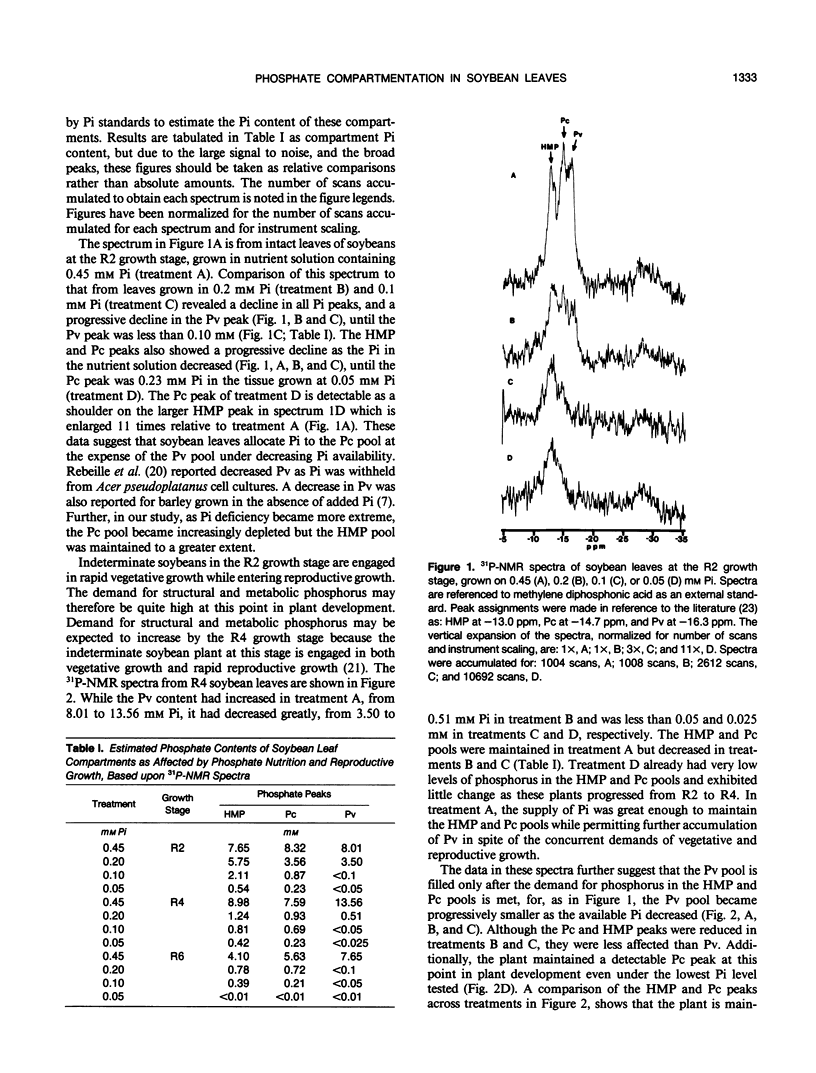
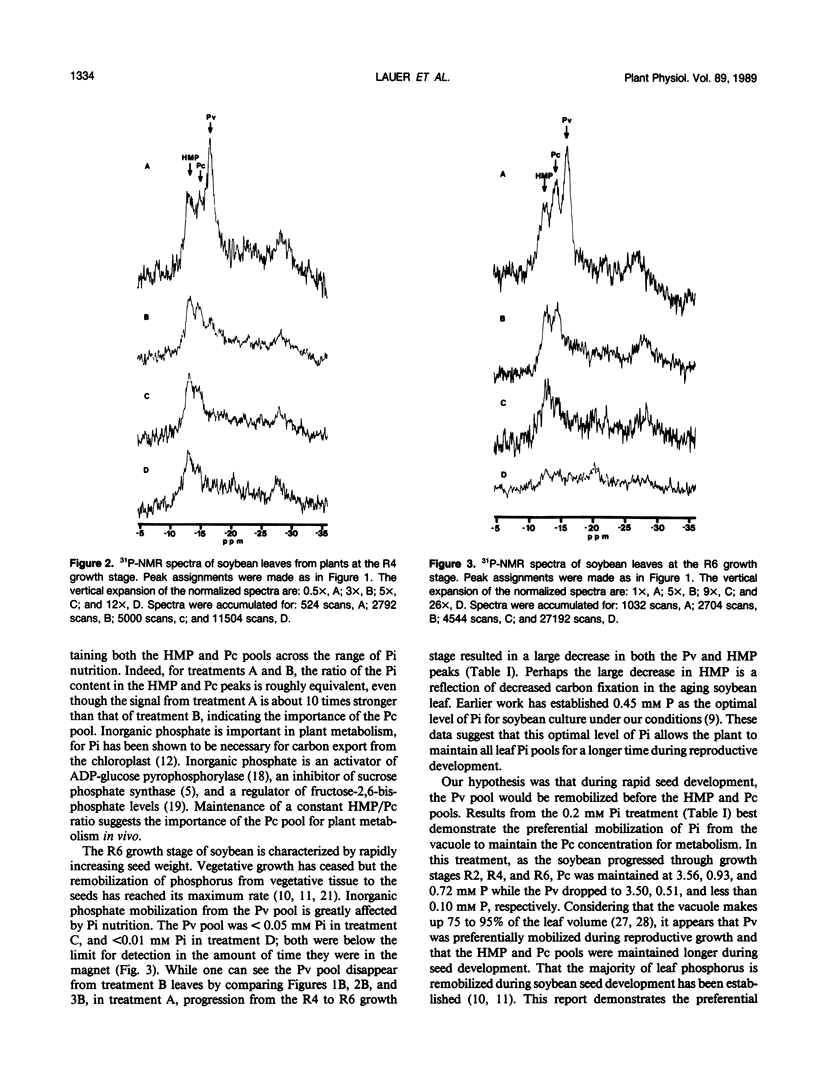
Most leaf phosphorus is remobilized to the seed during reproductive development in soybean. We determined, using 31P-NMR, the effect phosphorus remobilization has on vacuolar inorganic phosphate pool size in soybean (Glycine max [L.] Merr.) leaves with respect to phosphorus nutrition and plant development. Phosphate compartmentation between cytoplasmic and vacuolar pools was observed and followed in intact tissue grown hydroponically, at the R2, R4, and R6 growth stages. As phosphorus in the nutrient solution decreased from 0.45 to 0.05 millimolar, the vacuolar phosphate peak became less prominent relative to cytoplasmic phosphate and hexose monophosphate peaks. At a nutrient phosphate concentration of 0.05 millimolar, the vacuolar phosphate peak was not detectable. At higher levels of nutrient phosphate, as plants progressed from the R2 to the R6 growth stage, the vacuolar phosphate peak was the first to disappear, suggesting that storage phosphate was remobilized to a greater extent than metabolic phosphate. Under suboptimal phosphate nutrition (≤ 0.20 millimolar), the hexose monophosphate and cytoplasmic phosphate peaks declined earlier in reproductive development than when phosphate was present in optimal amounts. Under low phosphate concentrations (0.05 millimolar) cytoplasmic phosphate was greatly reduced. Carbon metabolism was coincidently disrupted under low phosphate nutrition as shown by the appearance of large, prominent starch grains in the leaves. Cytoplasmic phosphate, and leaf carbon metabolism dependent on it, are buffered by vacuolar phosphate until late stages of reproductive growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bieleski R. L. Effect of phosphorus deficiency on levels of phosphorus compounds in spirodela. Plant Physiol. 1968 Aug;43(8):1309–1316. doi: 10.1104/pp.43.8.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doehlert D. C., Huber S. C. Phosphate inhibition of spinach leaf sucrose phosphate synthase as affected by glucose-6-phosphate and phosphoglucoisomerase. Plant Physiol. 1984 Sep;76(1):250–253. doi: 10.1104/pp.76.1.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabau L. J., Blevins D. G., Minor H. C. P Nutrition during Seed Development : Leaf Senescence, Pod Retention, and Seed Weight of Soybean. Plant Physiol. 1986 Dec;82(4):1008–1012. doi: 10.1104/pp.82.4.1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt H. W., Chon C. J., Maronde D. Role of orthophosphate and other factors in the regulation of starch formation in leaves and isolated chloroplasts. Plant Physiol. 1977 Jun;59(6):1146–1155. doi: 10.1104/pp.59.6.1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy T. M., Matson G. B., Morrison S. L. Ultraviolet-Stimulated KHCO(3) Efflux from Rose Cells: Regulation of Cytoplasmic pH. Plant Physiol. 1983 Sep;73(1):20–24. doi: 10.1104/pp.73.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojalvo I., Rokem J. S., Navon G., Goldberg I. P NMR Study of Elicitor Treated Phaseolus vulgaris Cell Suspension Cultures. Plant Physiol. 1987 Nov;85(3):716–719. doi: 10.1104/pp.85.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer P. E., Tu S. I., Gerasimowicz W. V., Cavanaugh J. R. In VivoP NMR Studies of Corn Root Tissue and Its Uptake of Toxic Metals. Plant Physiol. 1986 Jan;80(1):77–84. doi: 10.1104/pp.80.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Martin J. B., Douce R. Relationship between the cytoplasm and the vacuole phosphate pool in Acer pseudoplatanus cells. Arch Biochem Biophys. 1983 Aug;225(1):143–148. doi: 10.1016/0003-9861(83)90017-6. [DOI] [PubMed] [Google Scholar]
- Roberts J. K., Jardetzky O. Monitoring of cellular metabolism by NMR. Biochim Biophys Acta. 1981 Nov 9;639(1):53–76. doi: 10.1016/0304-4173(81)90005-7. [DOI] [PubMed] [Google Scholar]
- Roby C., Martin J. B., Bligny R., Douce R. Biochemical changes during sucrose deprivation in higher plant cells. Phosphorus-31 nuclear magnetic resonance studies. J Biol Chem. 1987 Apr 15;262(11):5000–5007. [PubMed] [Google Scholar]