Abstract
Epidemiological and experimental evidence indicated that processed meat consumption is associated with colorectal cancer risks. Several studies suggest the involvement of nitrite or nitrate additives via N-nitroso-compound formation (NOCs). Compared to the reference level (120 mg/kg of ham), sodium nitrite removal and reduction (90 mg/kg) similarly decreased preneoplastic lesions in F344 rats, but only reduction had an inhibitory effect on Listeria monocytogenes growth comparable to that obtained using the reference nitrite level and an effective lipid peroxidation control. Among the three nitrite salt alternatives tested, none of them led to a significant gain when compared to the reference level: vegetable stock, due to nitrate presence, was very similar to this reference nitrite level, yeast extract induced a strong luminal peroxidation and no decrease in preneoplastic lesions in rats despite the absence of NOCs, and polyphenol rich extract induced the clearest downward trend on preneoplastic lesions in rats but the concomitant presence of nitrosyl iron in feces. Except the vegetable stock, other alternatives were less efficient than sodium nitrite in reducing L. monocytogenes growth.
Subject terms: Gastrointestinal cancer, Physiology
Introduction
In 2015, the state of epidemiological and experimental knowledge led the International Agency for Research on Cancer (IARC) to classify the consumption of processed meat (meat processed by salting, curing by nitrite or nitrate salts, fermentation, smoking, etc.) as carcinogenic to humans (Group 1, in particular for colorectal cancer risk (CRC))1,2. The starting point for this classification was based on the experimental and epidemiological findings obtained since the 1990s, which have highlighted a positive association between processed meat consumption and CRC risk (World Cancer Research Fund - WCRF, 1997)3. Since then, these first epidemiological data have been consolidated by meta-analyses, including those carried out by the World Cancer Research Fund4. Moreover, experimental studies performed in a CRC animal model have demonstrated that consumption of cooked ham model and commercial hot dog sausages promoted colon preneoplastic lesions5,6.
In the context of human health, nitrite or nitrate salts and linked nitrogen species such as nitric oxide (NO) are the subject of growing scientific debate. Indeed, the ingestion of nitrate or nitrite salts under conditions that result in endogenous nitrosation is classified as “probably carcinogenic” to humans (Group 2 A) by IARC from 20107. Beyond the mechanistic hypotheses involving heme iron, the carcinogenic properties of N-nitroso compounds (NOCs) are particularly suspected to explain the positive association between processed meat consumption and CRC risk8. Nitrosation and nitrosylation, the two main reactions leading to the formation of NOCs in meat product and during digestion, are due to the frequent addition of the food additives E249 to E252 (potassium nitrite-E249, sodium nitrite-E250, sodium nitrate-E251 and potassium nitrate-E252) in processed meats9. Experimental toxicological data highlight the genotoxicity and carcinogenicity of NOCs. Among this important family of compounds, N-nitrosodimethylamine (NDMA) is identified as having the highest carcinogenic potential (SCCS 2011)10. The nitrosonium ion can also react with free thiol groups (R-SH), through an S-nitrosation reaction, to form nitrosothiols.
The nitrite and nitrate salts are used as food additives in various processed meat products in order to prevent or reduce the growth of pathogenic bacteria (e.g. Clostridium botulinum, Clostridium perfringens, Listeria monocytogenes (L. monocytogenes), Salmonella spp., to extend shelf life, to limit oxidation and to contribute to the color and taste of processed products (organoleptic functions)11. Regarding foodborne pathogens, Lebrun et al.12 found that incorporation rates of sodium nitrite at a concentration of at least 30 mg/kg prevented the outgrowth and toxinogenesis of psychotropic Clostridium botulinum Group II type B in a cooked ham model. Furthermore, L. monocytogenes is one of the most common foodborne pathogens and its detection in the processed meat products accounted for 32% of the recalls registered for these products in the last year in France (data collected on the rappel.conso.gouv website of the French government). Concerning carcinogenesis, several experimental and epidemiological studies conducted specifically on the food additives nitrate and nitrite suggested a role of food additive-induced nitrosation and nitrosylation in CRC promotion. Indeed, regarding epidemiology, two meta-analyses had recently highlighted positive associations between nitrate salts (but not nitrite salt) from the diet and the risk of colorectal13 and ovarian cancer14. But authors did not distinguish between natural nitrite/nitrate salts from water, vegetables and nitrite/nitrate salts supplied as food additives. However, a recent study conducted on the NutriNet-Santé cohort allowed to distinguish the different dietary sources of nitrate and nitrite salts (natural food sources or food additives) and their respective association with cancer risk15. Compared to non-consumers, high consumers of nitrated and nitrited food additives had a higher risk of several cancers: the food additives nitrate and nitrite salts were positively associated with breast and prostate cancer risks, respectively, while no association was observed for nitrite/nitrate from natural sources. Regarding experimental data, we previously demonstrated that a diet based on cooked meat product without nitrite salt limited the promotion of CRC in a rodent model pre-treated with azoxymethane as opposed to a diet composed of cooked processed meat with 120 mg/kg of sodium nitrite16. Despite this clear effect on CRC promotion in this animal model, the absence of sodium nitrite was associated in this study to an increased endogenous lipid peroxidation along with a high toxic alkenal (such as 4-hydroxynonenal (HNE)) absorption as reflected by increased urinary di-hydroxynonane mercapturic acid (DHN-MA) excretion16. The production and absorption of those toxic alkenals could induce deleterious effects in extra-intestinal locations as proposed by Boléa et al.17.
Interestingly, these studies in a rat model did not demonstrate any association between CRC promotion, consumption of processed meats and total NOCs18,19, but highlighted a positive association with a subcategory of NOCs: the nitrosylated heme iron originated from the chemical reaction between heme iron and nitrite salt. Nitrosyl heme is formed within the processed meat products (responsible for the typical pink color of cooked processed meats) but also during digestion in the small intestine. Indeed, due to the alkaline conditions in the lower part of the gastrointestinal tract, S-nitrosothiols are degraded and the released nitric oxide may also react with heme to form nitrosyl heme20. The numerous studies on nitrosamines carcinogenicity and our previous studies proposing an association, in the rat model of CRC, between nitrosylated iron and CRC development highlight the need to limit population exposure to these food additives.
This study provides an original multidisciplinary approach considering food safety, technological properties and toxicological aspects to examine the effect of reducing or removing ingoing sodium nitrite, but also of current or future nitrite salt replacement strategies including antioxidants, vitamins and natural phenolic compounds on (i) endogenous reactions (nitrosation, nitrosylation and peroxidation), (ii) colonic ecosystem (microbiota and colon mucosal detoxification capacities), (iii) promotion of colon carcinogenesis in a CRC rat model, (iv) growth potential of L. monocytogenes on a sliced cooked ham model. This study could provide relevant and useful highlights to food regulation agencies and to implement appropriate short-term strategies for nitrite reduction or replacement in the meat processing sector.
Results
Impacts of sodium nitrite concentrations on cooked ham model products, microbiological risk, colon ecosystem and colorectal carcinogenesis
Regarding consequences of sodium nitrite concentrations on nitrosylation, nitrosation and lipid peroxidation in the cooked ham model, we observed that, compared to the reference cooked ham model produced with 120 mg/kg of sodium nitrite (Ni-120), the reduction of the food additive to 90 mg/kg (Ni-90) did not induce a decrease of nitrosylation of heme iron, with an equal percentage of nitrosylation. In the same way, reduction from 120 to 90 mg/kg did not result in a significant modification of total non-volatile N-nitrosamines and nitrosothiols but induced however a significant reduction (p < 0.05) of residual nitrite and nitrate (Table 1). Compared to Ni-120, the removal of the food additive resulted in a strong and significant decrease of nitrosylated iron in the cooked ham model with a percentage of nitrosylation falling from 58 to 9%. The absence of added sodium nitrite also induced a disappearance of nitrosothiols, residual nitrite and a significant decrease in residual nitrate but a strong increase of lipid peroxidation (thiobarbituric acid reactive substances (TBARS)) in comparison to Ni-120 (Table 1). Change from 90 to 0 mg/kg of sodium nitrites reduced also significantly the nitrosylation of heme iron, the presence of non-volatile N-nitrosamines, residual nitrite and nitrate and increased lipid peroxidation (p < 0.05; Table 1).
Table 1.
Impact of sodium nitrite levels on biochemical characteristics of cooked ham models (0 vs 90 vs 120 mg/kg).
| Ni-120 | Ni-90 | Ni-0 | |
|---|---|---|---|
| Total heme iron (µM) | 189 ± 40α | 146 ± 5α | 111 ± 9b |
| Nitrosylated iron (µM) | 110 ± 11α | 83 ± 6b | 10 ± 1c |
| % Nitrosylated iron | 58 | 57 | 9 |
| TBARS (µg MDA/g) | 0.041 ± 0.006α | 0.042 ± 0.004α | 0.303 ± 0.002b |
| Total free iron (mg/kg) | 4.92 ± 0.04α | 6.45 ± 0.04b | 5.97 ± 0.01c |
| Total non volatile N-nitrosamines (mg/kg) | 2.09 ± 1.02αb | 4.08 ± 0.28α | 1.27 ± 0.17b |
| Nitrosothiols (mg/kg) | 2.05 ± 0.25α | 1.38 ± 0.21α | 0.00 ± 0.00α |
| Residual nitrites (mg/kg) | 9.36 ± 0.23α | 6.76 ± 0.11b | 0.00 ± 0.00c |
| Residual nitrates (mg/kg) | 24.95 ± 1.07α | 14.59 ± 0.97b | 4.51 ± 0.01c |
Mean values with unlike letters were significantly different (p ≤ 0.05). Data are mean ± SD, n = 3 for total heme, nitrosylated iron and TBARS and n = 6 for residual nitrites, residual nitrates, total non volatile N-nitrosamines and nitrosothiols.
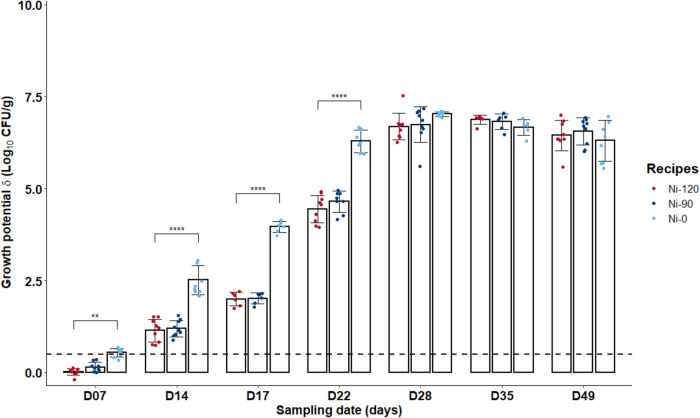
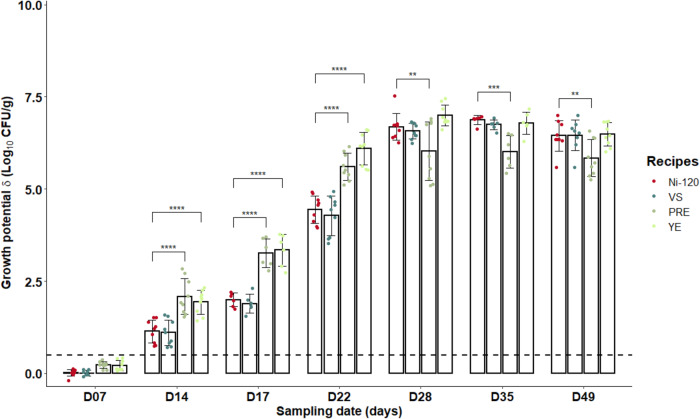
In terms of microbiological risk and under the storage conditions tested, L. monocytogenes was able to grow in sliced cooked ham model samples with added sodium nitrite salt up to 120 mg/kg (δ > 0.5 Log10 CFU/g by day 14 for the three tested recipes, Fig. 1). However, pathogen growth was significantly reduced during the first 3 weeks in samples prepared with 90 or 120 mg sodium nitrite per kg of meat compared to those without added nitrite salt (p ≤ 0.01) with, in addition, no significant difference between samples containing 90 or 120 mg of NaNO2/kg (p > 0.05). On the contrary, after 28 days of storage, no difference was observed between the three nitrite levels (90 or 120 but also 0 mg per kg).
Fig. 1. Growth potentials (δ) of Listeria monocytogenes at several sampling times during shelf-life of the sliced cooked ham model products with different sodium nitrite levels (0 vs 90 or 120 mg/kg).
Data were represented using scatter plots with bar (mean ± SD, n = 3), significance was determined by ANOVA test followed pairwise comparison using the estimated marginal means. **p ≤ 0.01, ****p ≤ 0.0001. The dashed line represents the limit value of 0.5 Log10 CFU/g above which the cooked ham model samples support the growth of L. monocytogenes.
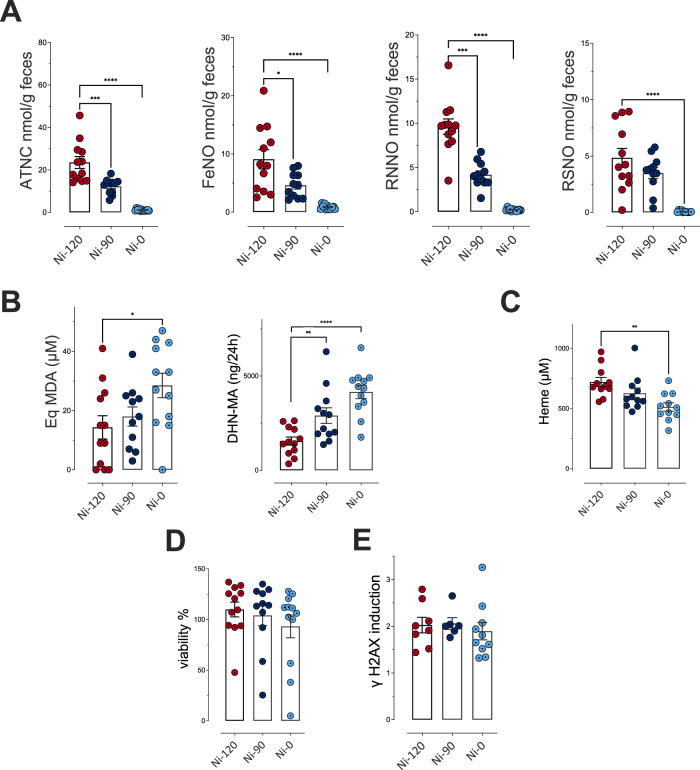
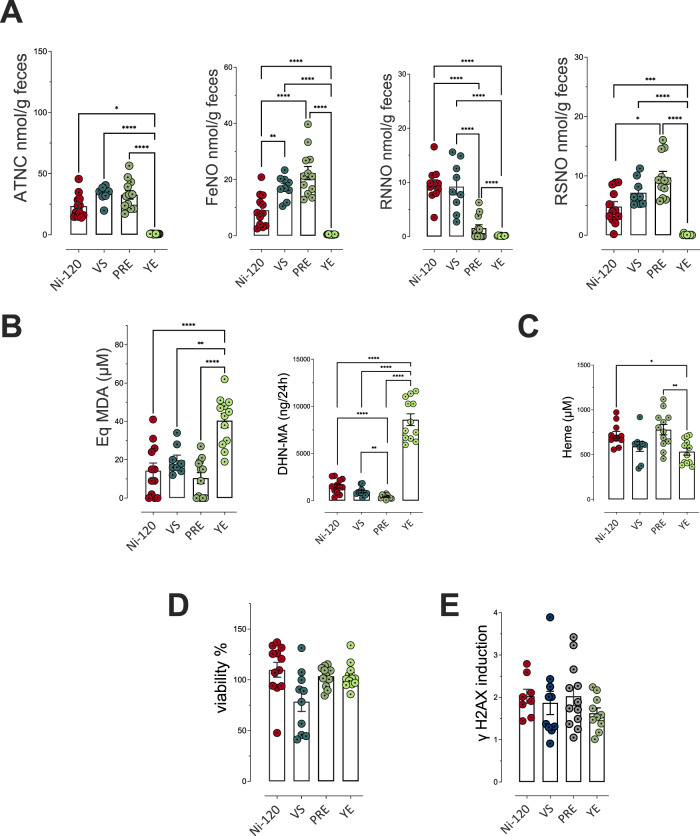
Concerning endogenous formations and fecal activities, the reduction from 120 to 90 mg/kg and removal of sodium nitrite induced a dose-dependent decrease in the formation of total nitroso compounds (ATNC) and the three subcategories measured in the rat feces. Compared to the reference dose (Ni-120, the reduction (Ni-90) indeed induced a significant decrease (p ≤ 0.05) for 3 biomarkers of endogenous nitrosation and nitrosylation (ATNC, FeNO and residual N-nitroso compounds (RNNO)) and the removal induced a near disappearance of these fecal markers with an almost total absence of fecal total NOCs, nitrosylated iron, RNNO and S-nitrosothiols (RSNO, Fig. 2A). Conversely, we observed a dose-dependent significant increase in luminal lipid peroxidation measured in fecal waters (Fig. 2B, TBARS) and a dose-dependent increase in urinary excretion of DHN-MA (Fig. 2B), the major metabolite of HNE indicating an increase in HNE absorption, probably due to an abundant presence of this lipid oxidation product in intestinal lumen. These consequences of sodium nitrite reduction or removal are not associated with higher levels of heme iron, a peroxidation catalyst (Fig. 2C). These modifications in the fecal contents of N-nitroso compounds and alkenals were not associated with a change in the cytotoxic and genotoxic activities of fecal waters (Fig. 2D, E).
Fig. 2. Impact of sodium nitrite levels in cooked ham models (0 vs 90 vs 120 mg/kg) on fecal and urinary biomarkers of lipid peroxidation and NOC formation.
A Nitroso-compounds in fecal water measured as apparent total NOCs (ATNC), as nitrosyl iron (FeNO), N-nitroso compounds (RNNO) and S-nitrosothiols (RSNO) (nmol/g of feces). B Lipid peroxidation measured as TBARS (MDA equivalents, µM) in fecal water and DHN-MA in urine of 24 h (ng/vol of 24 h). C Heme in fecal water (µM). D Cytotoxic activity of fecal water as % of cellular viability. E Genotoxic activity of fecal water. Data were represented using scatter plots with bar (mean ± sem, n = 12, except if outliers are removed), significance was determined by ANOVA (Welch’s ANOVA for D) followed by a Dunnett’s mean comparison test, data from panel A and B (DHN-MA) were log transformed before ANOVA. *p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
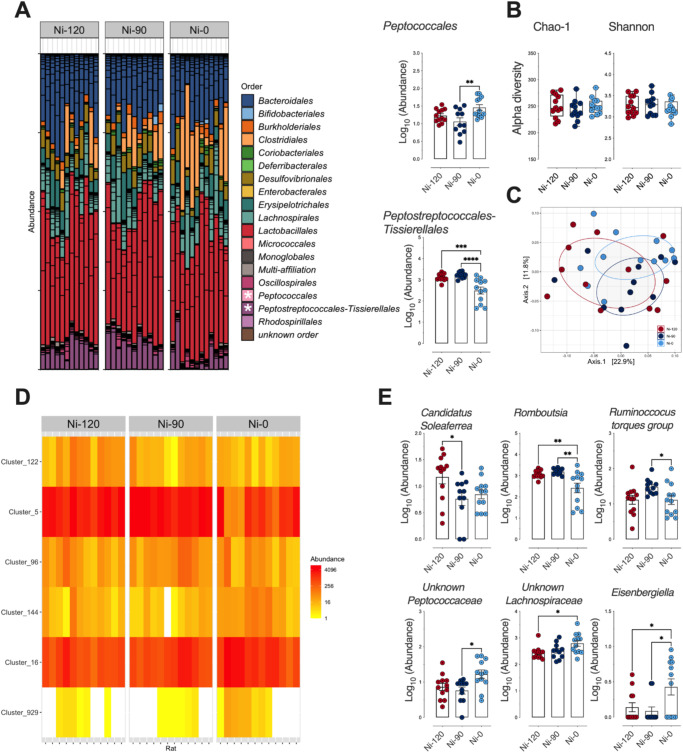
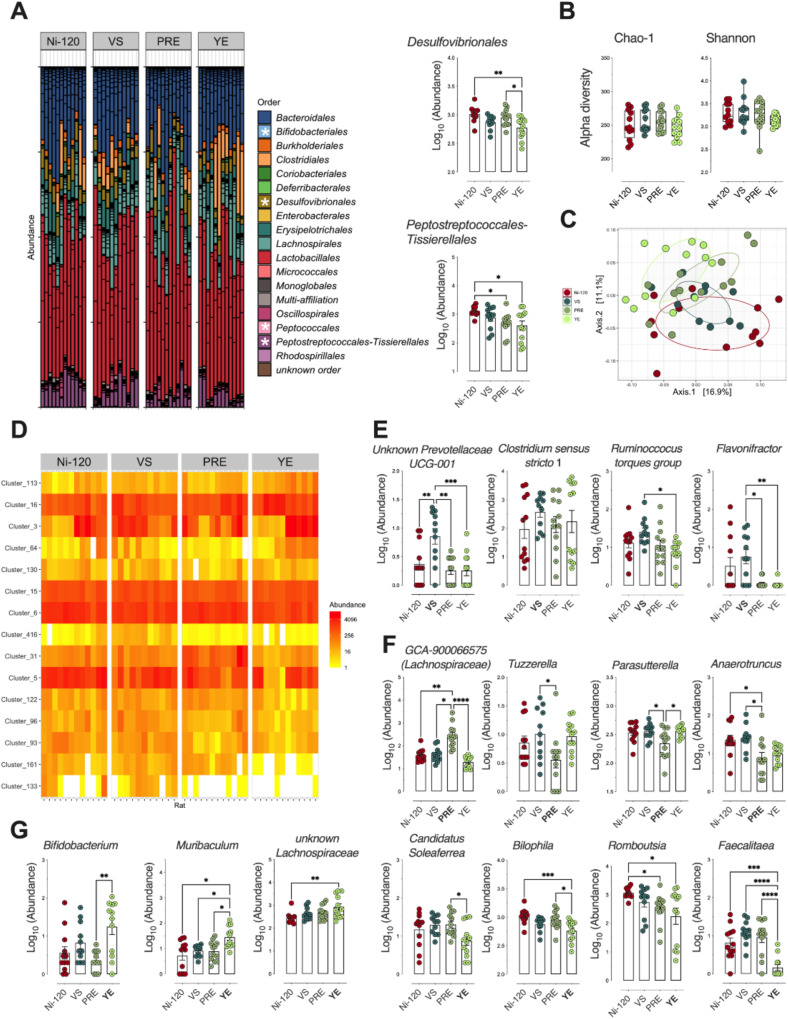
Compositional analysis of the fecal microbiota performed at the order level (Fig. 3A), as well as diversity characterization at a finer level (Fig. 3B, C), did not reveal major changes in the fecal microbiota in response to sodium nitrite reduction (Ni-90) in cooked ham model diet. By performing differential analysis at the order level, however, significant alterations in Peptococcales and Peptostreptococcales-Tissierellales were detected in the microbiota of rats fed the processed meat in which sodium nitrite was removed (Ni-0) (Fig. 3A). At the genus level (Fig. 3D), 6 bacterial communities underwent significative variations with sodium nitrite content and showed that reduced sodium nitrite intake was associated with a decrease in Candidatus Soleaferrea, Romboutsia, and genera belonging to the Ruminoccocus torques group, and an enrichment of Eisenbergiella and unknown genera belonging to Peptococcaceae and Lachnospiraceae (Fig. 3E).
Fig. 3. Impact of sodium nitrite levels in cooked ham models (0 vs 90 vs 120 mg/kg) on fecal microbiota.
A Distribution of bacterial communities at the order level. * Significant impact on Peptococccales and Peptostreptococcales-Tissierellales using differential abundance analysis at the order level (Deseq2, Padj ≤ 0.05): Normalized Log10 abundances were represented using scatter plots with bar (mean ± sem, n = 12). B Alpha diversity: No significant impact seen on richness (Chao-1) or eveness (Shannon). Individual values are represented using box and whiskers ( + mean). C Beta diversity (Unifrac distances, manova p > 0.05). No significant difference between microbiota of rats fed the 3 cooked ham model diets. D Heatmap of clusters agglomerated at the genus level affected by sodium nitrite content in ham-based diets. Padj ≤ 0.05 using differential abundance analysis (Deseq2). E Normalized abundance of the 6 clusters displaying dose effects as a function of dietary sodium nitrite content in (D). Clusters are agglomerated at the genus level and normalized Log10 abundances were represented using scatter plots with bar (mean ± sem, n = 12), significance was determined by a Kruskal-Wallis followed by Dunn’s mean comparison test.**p ≤ 0.05; **p ≤ 0.01.
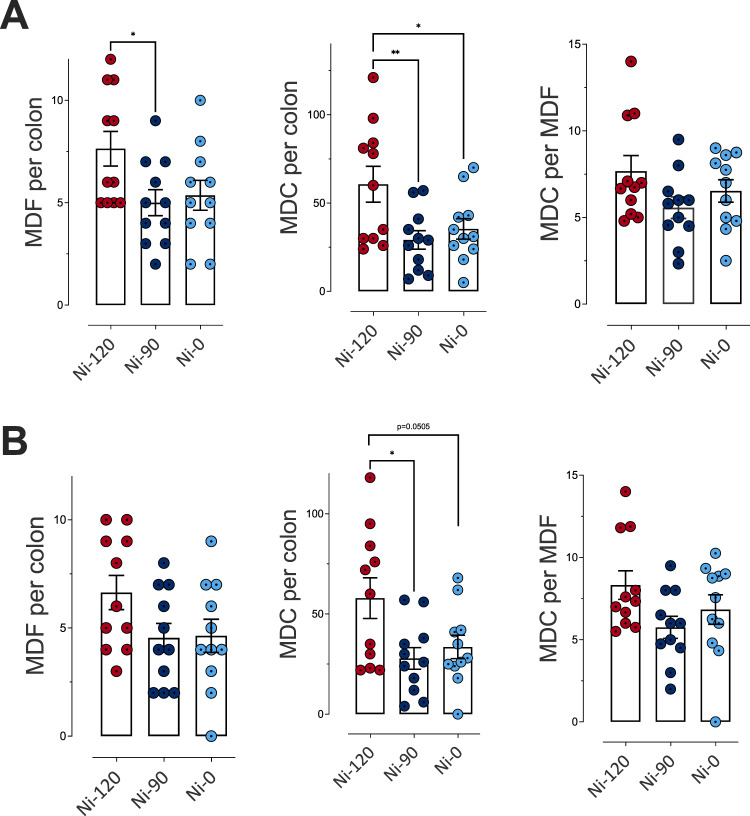
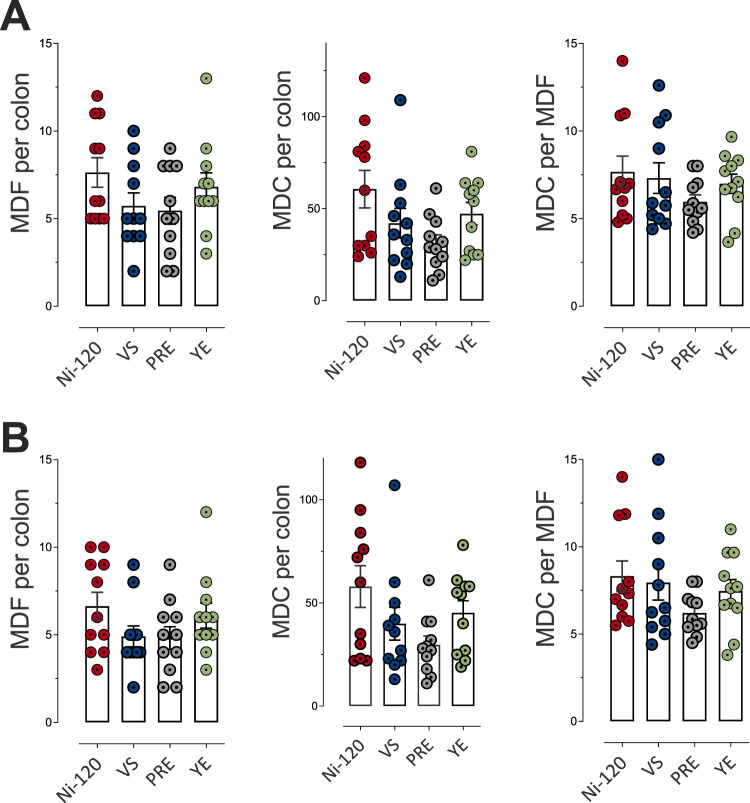
About promotion of colon carcinogenesis in the rat model, we noted that compared to the cooked ham model produced with 120 mg/kg of sodium nitrite (Ni-120), the reduction of sodium nitrite to 90 mg/kg (Ni-90) induced a reduction in the number of the preneoplastic lesions MDF (mucin depleted foci) per colon, while the removal (Ni-0) induced an effect very close to significance (p = 0.069) (Fig. 4A). Nitrite salt reduction and removal induced a clear and significant reduction of the total number of crypts depleted in mucin (Fig. 4A, B). Globally the same reducing effect was observed considering only bigger MDF lesions (Fig. 4B), with, a slightly less significant effect. However, nitrite salt removal that is protective against processed meat-induced promotion of carcinogenesis in comparison to Ni-120 was not more effective than Ni-90.
Fig. 4. Impact of sodium nitrite levels in cooked ham models (0 vs 90 vs 120 mg/kg) on MDF formation in rat colon.
A Number of MDF per colon, of mucin depleted crypts (MDC) per colon and crypts per focus for MDF with a multiplicity (i.e. the number of crypts forming each focus) higher than 2 crypts/MDF. B Number of MDF per colon, of MDC per colon and crypts per focus for MDF with a multiplicity higher than 4 crypts/MDF. Data were represented using scatter plots with bar (mean ± sem, n = 11), significance was determined by ANOVA followed by a Dunnett’s mean comparison test. *p ≤ 0.05; **p ≤ 0.01.
Impacts of three alternatives to nitrite salt on cooked ham model products, microbiological risk, colon ecosystem and colorectal carcinogenesis
In comparison to the reference cooked ham model with 120 mg/mg of sodium nitrite (Ni-120), alternatives to sodium nitrite salt had very different effects on the characteristics of processed meats (Table 2). The use of a vegetable stock (VS) induced an increase in the percentage of heme iron nitrosylation, the concentration of residual nitrite compared to the reference processed meat Ni-120 (p < 0.05). The VS alternative had no impact on the concentration of total non-volatile N-nitrosamines, lipid peroxidation or residual nitrates, while it decreased the concentration of nitrosothiols in comparison to Ni-120 (Table 2). The Polyphenol-Rich Extract (PRE) increased the concentration of total non-volatile N-nitrosamines (p ≤ 0.05, Table 2) and significantly decreased the concentration of nitrosothiols, residual nitrites and nitrates without impact on lipid peroxidation in comparison to Ni-120. The processed meat produced with the Lallemand solution (YE) presented significantly less nitrosylated heme iron, total non-volatile N-nitrosamines, nitrosothiols and residual nitrates or nitrites and a strong and significant increase of lipid peroxidation (TBARS) in comparison to the reference processed meat Ni-120 (Table 2).Regarding microbiological risk after a week of storage, L. monocytogenes was not able to grow on sliced cooked ham model products, regardless of the recipes (δ < 0.5 Log10 CFU/g, Fig. 5). Thereafter, all products supported the growth of the pathogen (δ > 0.5 Log10 CFU/g) until the end of storage. However, the products with polyphenol-rich extract (PRE) or Lallemand solution (YE) exhibited significantly higher growth potentials than those manufactured with the vegetables stock (VS) or with the amount of 120 mg/kg of sodium nitrite from day 14 to day 22 (p ≤ 0.0001, Fig. 5).
Table 2.
Impact of alternatives to nitrites on biochemical characteristics of cooked ham models.
| Ni-120 | VS | PRE | YE | |
|---|---|---|---|---|
| Total heme iron (µM) | 189 ± 40α | 153 ± 12α | 156 ± 18α | 142 ± 2α |
| Nitrosylated iron (µM) | 110 ± 11α | 108 ± 7α | NA | 20 ± 2b |
| % Nitrosylated iron | 58 | 70 | NA | 14 |
| TBARS (µg MDA/g ham) | 0.041 ± 0.006α | 0.032 ± 0.002α | 0.041 ± 0.001α | 0.158 ± 0.021b |
| Total free iron (mg/kg) | 4.92 ± 0.04α | 4.21 ± 0.01b | 2.42 ± 0.01c | 5.48 ± 0.03d |
| Total non volatile N-nitrosamines (mg/kg) | 2.09 ± 1.02α | 1.57 ± 0.88α | 7.09 ± 2.24b | 0.55 ± 0.14c |
| Nitrosothiols (mg/kg) | 2.05 ± 0.25α | 0.68 ± 0.47b | 0.98 ± 0.33b | 0.15 ± 0.03c |
| Residual nitrites (mg/kg) | 9.36 ± 0.23α | 13.56 ± 0.50b | 0.19 ± 0.03c | 0.08 ± 0.02c |
| Residual nitrates (mg/kg) | 24.95 ± 1.07α | 23.35 ± 1.04b | 2.79 ± 1.03c | 3.20 ± 0.10c |
Impact of 120 mg/kg of sodium nitrite or Vegetable Stock (VS), Polyphenol-rich Extract (PRE), Lallemand solution (YE). Data were mean ± SD, n = 3 for total heme, nitrosylated iron and TBARS and n = 6 for residual nitrites, residual nitrates, total non volatile N-nitrosamines and nitrosothiols. Mean values with unlike letters were significantly different (p ≤ 0.05). NA: data not available due to an interference with polyphenols contained in PRE (see supplementary data (Tables S5 and S6).
Fig. 5. Growth potentials (δ) of Listeria monocytogenes at several sampling times during shelf-life of the sliced cooked ham model products with alternatives to nitrites.
(120 mg/kg of nitrite or Vegetable Stock (VS), Polyphenol-rich Extract (PRE), Lallemand solution (YE)). Data were represented using scatter plots with bar (mean ± SD, n = 3), significance was determined by ANOVA test followed pairwise comparison using the estimated marginal means. **p < 0.01, ***p < 0.001, ****p < 0.0001. The dashed line represents the limit value of 0.5 Log10 CFU/g above which the cooked ham samples support the growth of L. monocytogenes.
Concerning endogenous formations and fecal activities, and as observed for the biochemical characteristics of cooked ham models, the three alternatives to sodium nitrite have different effects on endogenous reactions. In comparison to Ni-120, the use of vegetable stock (VS) induced a significant increase of the fecal endogenous formation of total nitroso compounds and nitrosyl iron (p ≤ 0.05, Fig. 6A) without altering the production of fecal RNNO and RSNO (Fig. 6A), luminal lipid peroxidation, urinary excretion of DHN-MA (Fig. 6B) or heme bioavailability (Fig. 6C). The replacement of sodium nitrite by polyphenol-rich extract (PRE) induced a significant increase of fecal endogenous formation of total nitroso compounds, nitrosyl iron and RSNO (p < 0.05, Fig. 6A) while significantly reducing RNNO levels (p ≤ 0.05, Fig. 6A). The consumption of PRE cooked ham model did not change luminal lipid peroxidation (TBARS) or heme bioavailability (Fig. 6C), but decreased significantly urinary excretion of DHN-MA (Fig. 6B).
Fig. 6. Impact of nitrite salt alternatives (Vegetable Stock (VS), Polyphenol-rich Extract (PRE), Lallemand solution (YE)) on fecal and urinary biomarkers of NOCs formation and lipid peroxidation.
A Nitroso-compounds in fecal water measured as total NOCs (ATNC), as nitrosyl iron (FeNO), N-nitroso compounds (RNNO) and S-nitrosothiols (RSNO) (nmol/g of feces). B Lipid peroxidation measured as TBARS (MDA equivalents, µM) in fecal water and DHN-MA in urine of 24 h (ng/vol of 24 h). C Heme in fecal water (µM). D Cytotoxic activity of fecal water as % of cellular viability. E Genotoxic activity of fecal water. Data were represented using scatter plots with bar (mean ± sem, n = 12, except if outliers are removed), significance was determined by ANOVA (Welch’s ANOVA for D) followed by Tukey’s mean comparison, data from (A) and (B) (DHN-MA) were log transformed before ANOVA. *p ≤ 0.05; **p ≤ 0.01.
With an opposite effect, the cooked ham model treated with the Lallemand solution (YE) induced a significant decrease of fecal endogenous formation of total nitroso compounds, nitrosyl iron, RNNO and RSNO (p < 0.05, Fig. 6A), but a high increase of luminal lipid peroxidation, and particularly of urinary excretion of DHN-MA (Fig. 6B) without modification of the heme bioavailability comparing to sodium nitrite use (Fig. 6C). These modifications in the fecal contents of N-nitroso compounds and alkenals were not associated with a change in the cytotoxic and genotoxic activities of fecal waters (Fig. 6D, E).
The exploration of effect of nitrite alternatives on distribution at the order level of the fecal microbiota, allowed to observe, as compared with microbiota of rats Ni-120 (Fig. 7A), changes in response to sodium nitrite alternatives within Bifidobacteriales (increase with YE only, Supplementary Fig. 7A), Desulfovibrionales (decrease mainly with YE), Peptococcales (increase with VS and PRE, Supplementary Fig. 7) and Peptostreptococcales-Tissierellales (decrease with PRE and YE, Supplementary Fig. 7). Regarding diversity, none of the indices related to α-diversity was altered by diet content (Fig. 7B), but dissimilarity analysis between groups (p = 0.0004, Fig. 7C) indicated that the difference in microbiota between Ni-120 and YE was significantly greater (p = 0.001) than that between Ni-120 and PRE (p = 0.017), whereas no significant difference was obtained between the microbiota of the Ni-120 and VS rat groups (p = 0.083). Differential analysis at the genus level supported these results (Fig. 7D, Supplementary Table 9B) and revealed distinct signatures depending on the alternative tested (Fig. 7E, F, G, Supplementary Fig. 7). Interestingly, abundance variations for some genera in response to the diet supplemented with VS (Fig. 7E), PRE (Fig. 7F) or YE (Fig. 7G) were similar to those observed in diets in which sodium nitrite content was reduced or removed (Supplementary Fig. 7B).
Fig. 7. Impact of alternatives to sodium nitrites 120 mg/kg (Vegetable Stock (VS), Polyphenol-rich Extract (PRE), Lallemand solution (YE) on fecal microbiota.
A Distribution of bacterial communities at the order level. *Main impact on Bifidobacteriales, Desulfovibrionales, Peptococccales and Peptostreptococcales-Tissierellales using differential abundance analysis at the order level (Deseq2, Padj ≤ 0.05): Normalized Log10 abundances were represented using scatter plots with bar (mean ± sem, n = 12). B Alpha diversity: No significant impact seen on richness (Chao-1) or eveness (Shannon). Individual values are represented using box and whiskers ( + mean). C Beta diversity (Unifrac distances, manova p ≤ 0.001). The rats fed the 4 meat-based diets clustered differentially according to their respective microbiota in terms of qualitative abundance and taxonomy of OTUs. D Heatmap of clusters agglomerated at the genus level affected by the meat-based diets (15 clusters). Padj ≤ 0.05 using differential abundance analysis (Deseq2). E Normalized abundance of the 4 clusters in D displaying specificities associated with the fermented Vegetable Stock diet (VS). Clusters are agglomerated at the genus level and normalized Log10 abundances were represented using scatter plots with bar (mean ± sem, n = 12) F Normalized abundance of the 4 clusters in D displaying specificities associated with the Polyphenol-rich Extract diet (PRE). Clusters are agglomerated at the genus level and normalized Log10 abundances were represented using scatter plots with bar (mean ± sem, n = 12). G Normalized abundance of the 7 clusters in D displaying specificities associated with the Lallemand solution (YE). Clusters are agglomerated at the genus level and normalized Log10 abundances were represented using scatter plots with bar (mean ± sem, n = 12), significance was determined by a Kruskal-Wallis followed by Dunn’s mean comparison test.*p ≤ 0.05; **p ≤ 0.01; ***p ≤ 0.001.
But, compared to the cooked ham model produced with 120 mg/kg of sodium nitrite (Ni-120), the various alternatives did not significantly modify the number of preneoplastic lesions (Fig. 8A, B), despite a decreasing trend for VS and PRE compared to Ni-120. If considering the number of mucin depleted crypts, both ANOVA reveal an almost significant effect (p = 0.064 and p = 0.071), for all lesions and for lesions with 4 or more crypts, respectively.
Fig. 8. Impact of alternatives to sodium nitrite (Vegetable Stock (VS), Polyphenol-rich Extract (PRE), Lallemand solution (YE)) on MDF formation in rat colon.
A Number of MDF per colon, of mucin depleted crypts (MDC) per colon and crypts per focus for MDF with a multiplicity (i.e. the number of crypts forming each focus) higher than 2 crypts/MDF. B Number of MDF per colon, of MDC per colon and crypts per focus for MDF with a multiplicity higher than 4 crypts/MDF. Data were represented using scatter plots with bar (mean ± sem, n = 11).
Discussion
This study was conducted as a multi-risk assessment of the consequences of the reduction, removal or substitution of sodium nitrite in a cooked ham model, regarding impacts on (i) the growth potential of L. monocytogenes, (ii) the meat product characterization, (iii) the promotion of colorectal carcinogenesis (at a preneoplastic stage) and (iv) the colonic ecosystem (endogenous neoformations of lipid peroxidation products and NOCs, and microbiota dysbiosis).
Our results clearly showed that nitrite salt reduction or removal in cooked model ham could modify most of the tested parameters in this work (Table 1, Figs. 1–4). As observed previously16, the results of the present experimentation confirmed that nitrite salt removal limited colorectal carcinogenesis promotion at the preneoplastic stage (Fig. 4). MDF, used in these studies to assess the impact on promotion of colorectal carcinogenesis, were identified in 2003 in a CRC animal model and in humans in 2008 by Caderni et al.21. MDF share dysplastic traits and mutational profiles with colonic tumors as β-catenin and frequent Apc mutations22. MDF are proposed as cancer precursors and usable endpoints in short term carcinogenesis study with a good predictive character for tumor stage effects21. In this way, the very recent results of Crowe et al.23. confirmed our previous results at the tumor stage, by demonstrating also in Min mice that a nitrite-free sausage protects against promotion of intestinal tumors in comparison to nitrited ones. The results of our team, including the present study, and the results of Crowe et al. support the role of nitrite salt food additive in colorectal cancer promotion associated to processed meat consumption. If these conclusions stemmed from animal studies at the preneoplastic stage of colon carcinogenesis cannot be directly extrapolated to humans, the data are supported by a recent epidemiological study. In agreement with the conclusions of our animal study, positive epidemiological associations between the intake of the nitrited and nitrated food additives and the development of cancers was reported in the NutriNet-Santé French cohort, including a positive trend for colorectal cancer15. If previous epidemiological studies did not differentiate natural vs food additive sources, the Nutrinet-Santé study was able to compare the effects of natural (water and vegetable source) and food additive nitrite intakes. In agreement with our study, this epidemiological associations emphasized the role of the food additives at least for prostate and breast cancers and with a tendance for colorectal cancer15.
Evaluated for the first time in the present study in an animal model of colorectal carcinogenesis, reduction of sodium nitrite from 120 to 90 mg/kg also induced a protective effect on colon preneoplastic lesions (Fig. 4). But, importantly, if the number of preneoplastic lesions was also decreased with the removal, we did not observe any difference between reduction and removal. As expected, residual nitrite and nitrate salts were dose-dependently decreased in the cooked ham models with the three levels of nitrites but also luminal nitrosylation and nitrosation. Nitrosation refers to the addition of a nitrosonium ion (NO+) to a nucleophilic group such as an amine, which generates nitrosamines or nitrosamides24. Nitrosamines, which can be formed throughout the digestive tract either at acidic pH in the stomach or at neutral pH and in the presence of heme iron in the intestine, can lead to the formation of DNA adducts25. In contrast, nitrosylation is a direct addition of NO to a reactant such as heme iron leading to the formation of nitrosyl heme9. Total nitroso compounds and the subcategories issued form nitrosation and nitrosylation were indeed found decreased in the feces of the rats fed the ham-rich diets (Fig. 2). However, the effect of this reduction or removal of sodium nitrite remains small on colon fecal microbiota, as revealed by the lack of structural difference in α and β diversity (Fig. 3B, C) as well as on gene expressions in colon mucosa (Supplementary Fig. 4), urinary excretion of 8-isoPGF2α (Supplementary Fig. 6A) fecal cytotoxic and genotoxic activities (Fig. 2D, E). Regarding fecal microbiota at the order level, we observed a decrease in Peptostreptococcales-Tissierellales (Fig. 3A), some members of which, such as Peptostreptococcus, are known to be enriched in fecal and mucosal samples from patients with CRC26. In our rat model, the decrease in Peptostreptococcales-Tissierellales was mainly due to the decrease in Romboutsia (Fig. 3D, E), the presence of which has found associated in mice with intestinal damage, increase of inflammatory cytokines, iNOS and AOM- dextran sodium sulfate -induced CRC27,28. But, as a consequence of nitrite salt removal, and as already reported by our group16, lipid peroxidation as measured by the TBARS index was increased in the cooked ham model (Table 1). The TBARS index in rat feces also was significantly increased when nitrite salt was removed from the cooked ham model, while the urinary lipid peroxidation biomarker DHN-MA was increased dose-dependently as nitrite salt decreased (Fig. 2B). Numerous studies of our team have proposed experimentally a role for lipid peroxidation products in the promotion of colorectal carcinogenesis18,29,30 Thus, despite the absence of fecal nitrosation and nitrosylation when sodium nitrite was removed (Fig. 2A), the lack of additional protection against cancer risk in comparison to the decrease at 90 mg/kg could be explained by this significant and concomitant increase in luminal lipid peroxidation. Moreover, the high level of DHN-MA observed in the present study reflected a significant intestinal absorption of those toxic alkenals and raised the question of a systemic impact. Indeed, the studies of Awada et al.31 and Bolea et al.17 demonstrated that an increase in luminal alkenal production is associated, as reflected in our study by the increase of the fecal TBARS index, to an increase in plasma concentrations of bioactive alkenals. The study of Bolea et al. also reported in mice that a high plasmatic level of alkenals caused dysfunction in peripheral tissues, particularly vascular dysfunction.
However, previous results from our team have demonstrated in a CRC animal model that enriching a cooked processed meat with antioxidants, such as vitamin E, red wine or pomegranate extracts, limited the processed meat-induced luminal lipid peroxidation and DHN-MA urinary excretion19,32. The efficacy of vitamin E supplementation has been verified also in healthy human volunteers32. Thus, on the basis of all these data, we propose that the protective efficiency of sodium nitrite removal on colon carcinogenesis could be improved by controlling luminal lipid peroxidation with natural antioxidants. Furthermore, by limiting the luminal formation of alkenals, their absorption will be limited and their effects on peripheral tissues could therefore be controlled. The decrease in DHN-MA urinary excretion after processed meat supplementation with red wine or pomegranate extracts supports this hypothesis19. Future studies need to focus on these points.
Regarding the microbiological risk, the behavior of L. monocytogenes during the shelf-life of such product with reduced amounts of nitrite salt or alternatives to replace nitrite salt has not been clearly described in previous studies; although a few studies have shown that added sodium nitrite might help control this pathogen in ready-to-eat meat products33–35. Cooked ham model samples used in the microbiological assays (challenge test assays on L. monocytogenes) exhibited typical features (more details in supplementary Information and Supplementary Tables 3 and 4) and the nitrate, nitrite and salt (NaCl) levels measured were consistent with the additive doses in corresponding recipes (see Table 3 and Supplementary Table 3). The present study showed that sodium nitrite added in the cooked ham products exerted an inhibitory effect against L. monocytogenes, as previously reported on various ready-to-eat cooked meat products33–36. Although sodium nitrite alone was not sufficient to prevent the growth of L. monocytogenes during actual shelf life of sliced cooked ham products, the amounts of sodium nitrite equal or greater than 90 mg NaNO2/kg led to a significant reduction of L. monocytogenes growth during the first 3 weeks of storage compared to the control without nitrite (Ni-0). Moreover, it should be noted that the presence of sodium ascorbate (0.3 g/kg) in the different levels (90 and 120 mg/kg) may have enhanced the anti listerial effect of sodium nitrite as previously described by others33,36.
Table 3.
List of the ingredients and additives (g/kg of cured meat) included in the 6 produced cooked ham models.
| Recipes | ||||||
|---|---|---|---|---|---|---|
| Ni-120 | Ni-90 | Ni-0 | VS | PRE | YE | |
| Sodium nitrite (NaNO2) | 0.12 | 0.09 | 0 | 0 | 0 | 0 |
| Nitrated broth NAT 223 | 0 | 0 | 0 | 4 | 0 | 0 |
| Starters CS300 | 0 | 0 | 0 | 0.25 | 0 | 0 |
| Polyphenol-rich / ascorbic acid rich extract | 0 | 0 | 0 | 0 | 10 | 0 |
| Yeast extract | 0 | 0 | 0 | 0 | 0 | 5 |
| Starters | 0 | 0 | 0 | 0 | 0 | 0.2 |
| Total sodium chloride | 18 | 18 | 18 | 20 | 18 | 20 |
| Sodium ascorbate | 0.3 | 0.3 | 0.3 | 0.3 | 0 | 0 |
| Dextrose | 5 | 5 | 5 | 5 | 5 | 0 |
| Saccharose | 0 | 0 | 0 | 0 | 0 | 1 |
| Water (mL/kg of meat) | 75 | 74 | 74 | 67 | 64 | 68 |
Among the three alternative products that were tested as nitrite salt replacement, the vegetable stock (VS) gave, for almost all the parameters tested, results that were very close to those of Ni-120, whatever the dose of the food additive. Indeed, results on luminal lipid peroxidation and ATNC formation with different concentrations of vegetable stock were similar to nitrites doses of 120, (Fig. 6A, B), 80 or 40 mg/kg (Supplementary Fig. 1A, B). The yeast/bacterial extract (YE) was unable to control lipid peroxidation in the processed meat product (Table 2) and in vivo (Fig. 6B). As such, this alternative can be compared to nitrite removal (Ni-0), with however a possible slight gain in the modulation of some microbiota specific species: regarding the commensal microbiota, YE helped to improve microbiota homeostasis by increasing the following bacterial genus (Bifidobacteria, Muribaculum and unknown Lachnospiraceae associated respectively with anti-cancer effects37, longevity38 and short-chain fatty acid production39) and decreasing genus associated with adverse effects (Bilophila, Romboutsia and Faecalitaea associated respectively with inflammation40, intestinal damage, increase of inflammatory cytokines28 and presence of adenoma in patients41). However, these modifications were not associated with a significant health positive effect on preneoplastic lesions and were correlated with very low impacts on gene expressions in colon mucosa (Supplementary Fig. 5), urinary excretion of 8-isoPGF2α (Supplementary Fig. 6B) and fecal cytotoxic and genotoxic activities (Fig. 6D, E). Ni-0 and YE formulations do not provide nitrosated compounds and do not improve the health effect that could be expected in comparison to nitrite reduction (Ni-90), most likely because of the lack of lipid peroxidation control. This is even more obvious for YE, in which there is no ascorbic acid, and this absence can explain an even greater increase in lipid peroxidation. These results underline the fact that it seems very important to control this process. The addition of antioxidants could represent a solution and the PRE product could provide a valuable alternative. However, the presence of a little concentration of non-volatile nitrosamines in the PRE-treated ham model and of some ATNC and nitrosyl iron in feces, makes us think that there is maybe a source of NO in this product. This was not expected as this curing product is not supposed to provide any NO source. It was not possible to determine unequivocally nitrosyl iron (FeNO) in PRE cooked ham model by the colorimetric method we used because interferences due to zinc protoporphyrin (ZnPP), known for giving Parm ham its pink/red colour, or to the presence of colored polyphenols cannot be excluded. However, Zn quantification in the nitrited (Ni-120) and PRE-treated ham did not reveal any difference (Supplementary Table 1). The percentage of FeNO detected increased when loading Ni-0 ham with the polyphenol epigallocathechine gallate known to bring a pink colour (Supplementary Table 5). This increase is even amplified when adding ferulic acid and ascorbic acid (FEA) (Supplementary Table 6). Interestingly, the determination of NO2, NO3, RSNO and RNNO using the colorimetric Griess method did not interfere with FEA (Supplementary Table 7). Nonetheless, concerning ATNC and FeNo detection in feces, the method we used is a published method that has been widely used and usually gives consistent results, to the best of our experience. This assay method has been used, for example, by the group of S. Bingham and G. Kuhnle, who are references in the field of endogenous formation of NOCs during consumption of meat products42,43. The CLD88 assay has also been used by this team to monitor the fecal excretion of NOCs in humans after consumption of processed red meat with standard or reduced levels of nitrite and added polyphenols44. However, this method relies on the release of NO from NOCs. The NO is then transferred out of the faecal matrix by an inert gas and detected by chemiluminescence. Since detection takes place outside the faecal matrix, the risk of interference is low, but we could not rule it out, particularly for the components present in the PRE product. To exclude the risk of bias due to the presence of such polyphenolic compounds, we overloaded Ni-0 fecal water with polyphenols and ascorbic acid and analyzed NOCs before and after this overloading (Supplementary Fig 3). No differences were observed, indicating the absence of interference and therefore of false positives attributable to polyphenols in the PRE formulation. Finally, the presence of non-volatile nitrosamines in the PRE cooked ham model, although in low concentration, led us to suspect a nitrosating and nitrosylating agent in this recipe, with thus a consequent risk on the formation of volatile nitrosamines considered carcinogenic. However, the determination of 5 volatile nitrosamines in the reference cooked ham model (Ni-120) and the three alternatives showed values below the quantification limit of these compounds (Supplementary Table 2).
Regarding the challenge test assays on L. monocytogenes, the use of the vegetable stock (VS) led to a similar reduction of L. monocytogenes growth during the first 3 weeks of storage compared to that obtained with the amount of 120 mg/kg of sodium nitrite (Ni-120). The inhibitory effect of the VS alternative (with high sodium nitrate content) against L. monocytogenes is not surprising since natural nitrate is reduced to nitrite (to reach an expected concentration of 80 mg NaNO2/kg in our assays) during food processing via the nitrate reductase activity of starter cultures45. Alternatively, certain plant active extracts were demonstrated to limit the growth of foodborne pathogens including L. monocytogenes on that kind of product, which is often attributed to high contents of polyphenolic compounds11,46–49. However, in our assays, the alternative PRE employed following the manufacturer’s recommended concentration was less efficient than sodium nitrite (≥90 mg/kg) or the VS alternative solution in reducing L. monocytogenes growth during the first 3 weeks of storage. A similar trend was obtained using the newly developed strategy Lallemand solution (YE) to replace or reduce nitrite salts.
Based on this study, the effects of reduction, removal and alternatives to sodium nitrite, the decrease of sodium nitrite salt level in cooked processed meat appears to be a short-term perspective for limiting population exposure to sodium nitrite additive, to nitrosated compounds and to colon carcinogenesis risk while slowing the L. monocytogenes growth and efficiently controlling lipid peroxidation. The lack of a greater effect on carcinogenesis risk with the removal of sodium nitrite provides an additional issue to solve with the increase in luminal lipid peroxidation. This conclusion is reinforced by the lack of protective effect of the YE which, if it is truly free of nitrites/nitrates or NO, induced a very high luminal lipid peroxidation.
In conclusion, if the removal of nitrite salts should not be excluded even in the short term in certain cooked meats, in particular those with a very short shelf life, it appears necessary to quickly evaluate if the enrichment with antioxidants of low-nitrited or non-nitrited cooked processed meat (like the cooked ham model of this study) would allow a greater protection than with the reduction to 90 ppm. This work also highlights the need for an evaluation of alternatives on endogenous formations and colon carcinogenesis promotion before introduction on the market.
Methods
Production, microbiological and biochemical characteristics of cooked ham model products for the study on microbiological risk and colon carcinogenesis in rats
For the production of the experimental cured meat, we chosen an air-exposed ham model called DCNO (for Dark Cooked meat with sodium Nitrite, Oxidized) in our previous studies because it promotes colon carcinogenesis in rats16. It was used as the reference and positive control in this study to test potentially protective effects of reduction, removal or alternatives to sodium nitrite. It was produced with the usual sodium nitrite concentration in France, i.e. 120 mg/kg. Dark red meat was obtained from fresh defatted, derinded, denerved and deboned pork shoulders. The product without sodium nitrite was the negative control of this study.
For the carcinogenesis study, six experimental cooked ham models were made in a ham factory according to a process allowing a homogeneous curing treatment. Each model is composed of 80 kg ground pork (10-12 mm) mixed with appropriate concentrations of ingredients/additives as describe in Table 3. Three concentrations in sodium nitrite were added in brine, at the final rate in finished products: 120 mg/kg (Ni-120) the maximum authorized by the French Code of Practice, 90 mg/kg (Ni-90) for the reduced level and 0 mg/kg (Ni-0) for the cooked ham model without sodium nitrite. Three alternatives were added in the brine without sodium nitrite, at a concentration recommended by suppliers: a vegetable stock (VS) that contained different ingredients: sugar, a powdered juice of chards, dehydrated carrot (Bouillon Nat 223, DAT-Schaub France, Thiais, France), an aroma rich in polyphenol and ascorbic acid called “polyphenol-rich extract” (PRE), and a yeast extract (savor lyfe NR01) and Staphylococcus xylosus culture (Lalcult Carne Rose 01) from Lallemand SAS (Blagnac, France) called YE. After curing, the ground pork was tumbled for 18 h with a total of 2 700 rotations. Then, the cured ground pork was transferred into moulds and packed into heat shrink bags under vacuum condition. Vacuum packed products were steam cooked up to a core temperature of 68 °C for 990 min, with a temperature stage at 50 °C for 60 min, for the development of both the YE and VS starters.
After this thermal processing, 685 min were required in a cold room at 4 °C to fall the temperature from 67 °C to 4 °C (representative of a pasteurization value P70/10 of 90 min). Cooked ham models were unpacked, sliced into a 15 mm thickness and 60 kg of sliced cooked ham were packed in 350 g top sealed trays under protective atmosphere (50% CO2 and 50% N2). Some of these samples were stored at −20 °C (D02: day 2 post packaging) for the carcinogenesis study and the other ones were stored 14 days at 4 °C and 35 days at 8 °C for the biochemical analyses. Samples of ham models were frozen before being ground in liquid nitrogen to avoid any oxidation before biochemical characterizations. Total heme iron, lipid peroxidation, nitrosylheme content and concentration of total non-heme iron, Fe2 + , and Fe3+ were assessed9.
Briefly, total heme iron content was assessed in the form of acid hematin and was extracted in acidic acetone as previously described9. Nitrosylheme content was assessed by diluting the samples in an acetone/water mixture. Samples were then mixed in the dark for 15 minutes and filtered through 0.45 µm filters (Interchim®, France) and nitrosylheme absorbance was measured at 540 nm using an absorption coefficient of 11.3 mM−1cm−150. Nitrosylation was expressed as the percentage of nitrosylated iron to total heme iron. The concentration of total non-heme iron, Fe2+, and Fe3+ was determined using the ferrozine method, according to Stolze, Dadak, Liu and Nohl51. In addition, the trace elements Fe, and Zn were quantified by inductively coupled plasma spectrometry (ICP-AES) following the Poitevin method52. The results are expressed in mg/kg. Lipid oxidation was assesed by the TBARS method according to Bonifacie et al.9 Nitroso-compounds were quantified as described previously9, and results of ATNC, nitrosothiol, non-volatile N-nitrosamine and nitrosamide contents were expressed in mg/kg. To check whether polyphenol present in the PRE formulation may interfere with nitrosyl heme and total heme assays, Ni-0 ham (Ni-0) and the same ham loaded with different concentrations of epigallocatechine gallate (EGCG) (5 mg/mL, 10 mg/mL, 20 mg/mL) were used to measure impacts on these two assays. 1 g of Ni-0 ham was grounded with 1 mL of water (Ni-0) and 1 g of the same ham was grounded in 1 mL solution containing 100 µL EGCG 5 mg/mL in H2O or 100 µL EGCG 10 mg/mL in H2O or 100 µl EGCG 20 mg/mL in H2O + 650 µL H2O (EGCG5, EGCG10, EGCG20).
Nitrite and nitrate ion contents were then determined in the filtrates using the Griess reaction with a Sigma-Aldrich colorimetric assay kit (23479-1KT-F). Absorbance was measured on a microplate at 540 nm on a MULTISKAN SPECTRUM spectrophotometer from Thermo Scientific (Waltham, USA). Residual nitrite and nitrate were expressed in mg/kg.
Regarding volatile nitrosamines, assays were carried out and quantified by the Eurofins company (https://www.eurofins.fr/). Five nitrosamines (N-Nitrosodimethylamine (NDMA), N-Nitrosomethylethylamine (NMEA), N-Nitrosodiethylamine (NDEA), N-nitrosodiisobutylamine (NDiBA), N-Nitrosodibutylamine (NDBA)) were quantified in triplicate on cooked ham models stored at −80 °C until analysis. Quantification was performed by LC-(APCI)MS/MS.
Global physicochemical features of cooked ham models were presented in Supplementary Table 3.
Growth potential of Listeria monocytogenes in cooked ham model products
Growth potential of L. monocytogenes was followed at different sampling times during shelf-life of sliced cooked ham model products, specifically produced for this assay, including the 6 different recipes (Table 3). Three specific and independent batches of sliced cooked ham model products were used for this assay. They were produced following similar processes to those employed in the meat processing industry. Briefly, these products were composed of 2.7 kg ground pork supplemented with a specific brine solution as described below. The sliced cooked ham model products were made in a pilot-scale unit (IFIP, France). They were prepared with fresh boned and derinded pork shoulders. Ground pork was mixed with appropriate concentrations of sodium nitrite (0, 90 or 120 mg/kg) or alternatives, NaCl (18 or 20 g/kg), sodium ascorbate (0 or 300 mg/kg), dextrose (0 or 5 g/kg) and/or saccharose (0 or 1 g/kg) using a vacuum mixer (CDH, France) at a constant speed of 350 rpm for 10 min. The different starters used in the VS and YE recipes were stored at −20 °C and prepared according to the manufacturer’s instructions. In all recipes, water was added in order to have a 10% brine rate (Supplementary Table 3).
For each recipe, pH and aw values were determined according to the NF ISO 21807:2005 and NF V04–408 standards at D0 (after slicing), D22 and D49. Oxygen and carbon dioxide were measured using an O2 and CO2 head space gas analyzer, Checkmate 3 (Dansensor, France) at D0 and D49. At the same sampling dates, nitrite, nitrate and sodium contents analyses were performed at Actalia laboratory (Villers Bocage, France). Colour measurements were carried out with a CM 600 spectrophotometer (Minolta, Japan) configurated as follows: measurement area of 8 mm in diameter, illuminant D65, geometry d/8°. The value considered was the red hue angle (H*). Lipid oxidation was measured by the TBARS content using the method of Mercier et al.53 For each experimental assay and recipe, measurements were performed in triplicate on a pool of 3 cooked ham model samples from D49. Results are displayed in the Supplementary Table 3.
After processing, cooked ham products were cooled at 4°C and sliced using a TIP350 slicer (Simplex, France). Raw material samples tested in the present study were all negative for L. monocytogenes (data not shown) and sliced cooked ham samples were then surface inoculated with a cocktail of three L. monocytogenes strains, then packed under protective atmosphere (gas mixture including 50% CO2 and 50% N2) in polyamide (20 µm) and polyethylene (70 µm) bags (O2 transmission rate, 50 cm3/m2 in 24 h at 23 °C and 85% relative humidity; moisture vapor transmission rate, 2.60 g/m2 in 24 h at 23 °C and 85% relative humidity) (Soussana, France) by using the Multivac A300 packaging machine. These samples were stored under reasonably foreseeable conditions (time and temperature) that could be encountered along the supply chain i.e., 14 days at 4 °C (cold storage chamber) and 35 days at 8 °C (distributor shelving and domestic storage in refrigerator). The cocktail of three L. monocytogenes strains in equal proportions was prepared in saline solution : Lm176 (isolated from cooked ham, clonal complex 2), Lm212 (isolated from cooked ham, clonal complex 101) and Lm004 (isolated from “rillettes” involved in a previous outbreak of listeriosis, clonal complex 1) (IFIP collection). The use of several strains mixed in a cocktail is recommended by the ISO 20976-1 standard for estimation of growth potential so that variations among strains are considered. After a preculture in BHI broth (bioMérieux, France), the cocktail of the three L. monocytogenes strains in equal proportions in saline solution was used to inoculate 2 cooked ham slices per recipe (about 30 g each per sample) at 2.0 ± 0.12 log10 CFU/g (mean ± SD). At each sampling date (D0, D07, D14, D17, D22, D28, D35 and D49), three samples per recipe were analyzed for the enumeration of L. monocytogenes using the BRD 07/17-01/09 standard method. Briefly, each 60 g sample was four-fold diluted in buffered peptone water (Bio-Rad, France) and stomacher at medium speed for 1 min. One hundred microliters of this suspension and/or of the appropriate tenfold serial dilutions were plated on AL agar plates which were incubated at 30 °C for 48 h before counting colonies. The quantification limit of this analysis was 4 CFU/g. For each recipe, lactic acid bacteria were enumerated in triplicate (from 3 different samples/recipe) at D0 and D49 by plating appropriate dilutions on MRS medium (bioMérieux, France) after a 48 h-incubation at 30 °C. The quantification limit of this analysis was 10 CFU/g. For each independent experiment, growth potential was calculated according to the equation (adapted from the ISO 20976-1:2019 standard), where is the value obtained for one sample at a given sampling date and is the mean value obtained for the three replicate samples analyzed at D0. When δ was higher than 0.5 log10 CFU/g the sliced cooked ham model sample was considered permissive to the growth of L. monocytogenes. Results are shown in the Supplementary Table 4.
Study of carcinogenesis: animals, design, diets and preneoplastic lesions scoring
Animal experiment was approved by the local Ethical Committee (CE n°86), authorized by the French Ministry of Research (APAFIS 27180_2020091017209910_v2) and conducted in accordance with the European Council on Animals used in Experimental Studies and ARRIVE guidelines.
Male Fischer 344 (F344/DuCrl) rats were purchased from Charles River Laboratories, 12 rats/group, aged 5-6 weeks. After acclimatization, they received a single i.p. injection of azoxymethane (Sigma; 20 mg/kg) in NaCl (9 g/L water) to induce colon preneoplastic lesions. Seven days later, the rats were randomly assigned to six groups and fed the experimental diets daily ad libitum (at the end of the afternoon in order to avoid any important oxidative degradation before food intake) for 98-99 days before euthanasia. Colons were then removed, washed, opened, coded, and fixed in 10% buffered formalin (Sigma-Aldrich) before Mucin Depleted Foci (MDF) analysis. Formaline-fixed colons were stained with high iron diamine-Alcian blue procedure (HID-AB)21 for scoring the preneoplastic lesions. Scoring was achieved by a reader blinded for colon sample origin.
Body weight was monitored every week during the 4 first weeks, then every two weeks. Food and water intakes were measured on day 15 and day 85. Feces were collected during 3 days on days 72 to 74 or 79 to 81. Each rat was placed in a metabolic cage and urine was collected on day 72 or on day 79. Anal feces for microbiota analysis were collected at the end of the experimental diet period and kept at −80 °C before analysis. Fecal and urine samples were kept at −80 °C and −20 °C, respectively before analysis.
Experimental diets were based on powdered low-calcium, no-fat AIN-76 rodent diets (SAAJ, Jouy-en-Josas, France), supplemented with 5% safflower oil (MPBio), with 45% (dry weight) of experimental cured meats and 8% of gelatin. Model cooked ham trays were opened and stored at 4 °C for 3.5 days before rat diet preparation (every two weeks), to induce an oxidation step related to bad storage conditions. The diets were prepared by mixing ham, the AIN-76-based powder and the oil, and then frozen at −20 °C until use.
Urinary, fecal, mucosal and microbiota analysis
DHN-MA, the major urinary metabolite of HNE54 was measured using a Bertin Bioreagent kit (Montigny-le-Bretonneux, France) with diluted urines (1/1000) in the kit buffer and 8-isoPGF2α was assayed using Cayman ELISA kits (Bertin Technologies), according to the manufacturer’s instructions.
Fecal waters were prepared from feces collected during 24 h. Feces were added with ultrapure water (0.5 g/mL). To prevent oxidation, 50 µl/mL butylated hydroxytoluene (BHT) was added to water. The feces mixture was homogenized with a FastPrep (MPBiomedicals) 3 times for 30 seconds at 6 m/s in Lysing Matrix E tubes and then centrifuged at 3 200 g for 20 minutes at 4 °C, as described previously, with slight modifications55. Supernatants (fecal waters) were collected and kept at −80 °C until use. Heme was measured with the Heme Assay (Kit MAK316 – Sigma) according to the recommendations of the manufacturer; fecal TBARS were measured in fecal waters according to Ohkawa et al.56 For cytotoxicity and genotoxicity, fecal waters were tested on normal immortalized colon epithelial cells (Co cells), which were established as described previously57,58. Conditionally immortalized cells were seeded into 96-well plates at 15 × 103 cells per well in permissive conditions. After reaching subconfluence (70%), cells were transferred to 37 °C and treated for 24 h with filtered fecal waters in serum-free DMEM to avoid any reaction between serum and fecal water. Cytotoxicity was assessed using WST-1 kit as described earlier55 and genotoxicity was measured using the ɣ H2AX in-cell Western blot assay according to Khoury et al.59.
For the quantification of fecal N-nitroso compounds (NOCs), fecal water aliquots were transferred in amber reaction tubes and stored at −80 °C until further use. The following steps and analyses were done under reduced light conditions, while all samples and chemicals were kept on ice. Fecal water samples were analysed for NOCs using an Ecomedics CLD88 NO-Analyzer (Eco Physics GmbH, Hürth, Germany) as published recently with slight modifications60.
Each fecal water sample was measured in triplicate after three individual pretreatments incubated on ice: (I) 100 µL of diluted sample was mixed with 200 µL sulfanilamide (30 mg/mL in 1 M HCl), incubated for 5 min and further diluted with 100 µL ultrapure water; (II) 100 µL of diluted sample was mixed with 100 µL HgCl2 (14.5 mg/mL in ultrapure water) and incubated for 30 min, followed by the addition of 200 µL sulfanilamide and an incubation for 5 min; (III) 100 µL of diluted sample was mixed with 100 µL K3Fe(CN)6 (38 mg/mL in ultrapure water) and incubated for 30 min, followed by the addition of 200 µL sulfanilamide and an incubation for 5 min. After pretreatment, 100 µL of each sample was injected into the purge vessel, containing 10.5 mL of the following reaction solution: 2 mL KI (50 mg/mL in ultrapure water), 400 µL CuSO4 (50 mg/mL in ultrapure water), 8 mL acetic acid and 100 µL antifoam. The reaction solution was kept at 60 °C and changed regularly after measuring five pretreated samples in triplicate. The area under curve was quantified by sodium nitrite standards (10–250 nM) dissolved in ultrapure water. For differentiation of apparent total N-nitroso compounds (ATNC) in S-nitrosothiols (RSNO), nitrosyl iron (FeNO) and residual N-nitroso compounds (RNNO), the contents were calculated individually by the results of pretreatments. Pretreatment I allowed calculation of ATNC content. RSNO contents were obtained by subtraction of the results of pretreatment II from the results of pretreatment I. FeNO contents were calculated similarly by subtraction of the results of pretreatment III from the results of pretreatment I. Finally, RNNO contents were determined by the substraction of the sum of RSNO and FeNO from ATNC. All results are shown as nanomole per gram feces.
To check whether polyphenols and ascorbic acid present in the PRE formulation may interfere with the NOC assay and generate false-positive results, pooled fecal waters (100 µL) from Ni-0 ham fed rats were added with coktail called “FEA” (0.2 mg of epigallocatechine gallate (EGCG), 0.8 mg ferulic acid and 0.5 mg ascorbic acid (Sigma-Aldrich)) and analysed for Total NOCs with the method described above.
Regarding, gene expression assays in colon mucosa, total cellular RNA was extracted with Tri reagent (Molecular Research Center). Total RNA samples (1 µg) were then reverse-transcribed with the iScript™ Reverse Transcription Supermix (Bio-Rad) for real-time quantitative polymerase chain reaction (qPCR) analyses. The primers for Sybr Green assays are presented in Supplementary Table 8. Amplifications were performed on a ViiA 7 Real-Time PCR System (Applied Biosystems). The qPCR data were normalized to the level of the RNA Polymerase II Subunit A (POLR2A) messenger RNA (mRNA) and analyzed by the LinRegPCR v.11 software.
Concerning the high-throughput 16 S rRNA gene amplicon analysis for microbiota exploration, genomic DNA from snap-frozen fecal samples and the amplification of hypervariable V3-V4 regions of their 16 S rRNA gene were prepared as described previously61. Libraries preparation and sequencing (Illumina Miseq cartridge) were performed by the Genotoul facility (Get-Biopuces, Toulouse). Raw sequences were processed using the FROGS pipeline (Galaxy Version 3.2.3) and analyzed using the R package Phyloseq (v1.34.0) as follows62: Each pair-end valid denoised sequences were filtered, merged and clustered with the swarm fastidious option using a maximum aggregation distance of 163. Putative chimera were removed (vsearch) and clusters (i) whose abundance represented at least 0,005 % of all sequences, (ii) presents in at least 2 times in a minimum of 5% of total samples with a prevalence threshold of 5% of all samples, were retained, yielding to 318 final clusters. The silva 138.1_16 S reference database was used for cluster affiliation into Operational Taxonomic Units (OTUs) using Blast+with equal multihits, taxonomic multi-affiliations were checked manually. Within sample community richness and eveness (α diversity) were estimated using both the Chao-1 and Shannon indexes respectively, and examined by one-way ANOVA analysis and Tukey’s multiple comparisons test. Divergence of bacterial composition between samples (β diversity, Phyloseq) was explored using the Unifrac distance matrices, and statistically tested using permutational multivariate analysis of variance (MANOVA using Adonis test with 9999 permutations followed by pairwise multilevel comparisons). OTUs were agglomerated at the order and genus ranks to further analyze differential abundances according to diets using the Deseq2 package (v1.30.1). Detailed results of statistical analyses were reported in Supplementary Table 9 as supporting information. For graphical visualization purpose in parallel, raw 16S counts of taxa, whose abundance was significantly affected by diets, were normalized according to the mixMC pipeline64.
Impact of sodium nitrite concentrations and vegetable stock in a cooked ham model on fecal and urinary biomarkers of lipid peroxidation and on NOC formation: Production of cooked ham models and experimental design of the short-term nutritional study in F344 rats
For this short-term nutritional study, 4 experimental cooked ham models were made in a pilot-scale unit (IFIP, France) according to a process that permitted a homogeneous curing treatment. These products were composed of 2.5 kg ground pork supplemented with a specific brine solution as described below. They were prepared with fresh boned and derinded pork ham. Further trimming and cutting were performed, and the pieces of meat were ground through a 20 mm plate of a grinder (DRC 98, PSV, France). In each experiment, the ground pork was divided into 4 recipes, then mixed with appropriate concentrations of sodium nitrite (80, or 40 mg/kg) or alternative (vegetable stock with Nitrated broth NAT 223 (Soussana, France) and Starters CS300 (Soussana, France) to mimic 80 or 40 mg/kg of sodium nitrite in VS80 and VS40 with respectively 4 and 2 g of broth per kg of meat and 0.25 g of starter by kg of meat), NaCl (14 g/kg), sodium erythorbate (500 mg/kg), dextrose (5 g/kg) using a vacuum mixer (CDH, France) at a constant speed of 350 rpm for 10 min. The starter used in the VS recipes was stored at −20 °C and prepared according to the manufacturer’s instructions. In all recipes, water was added in order to have a 10% brine rate.
After processing, the 4 experimental cooked ham models were stored at −20 °C until distribution to rats. For this 14 day-long nutritional study, male Fischer 344 (F344/IcoCrl) rats (5 rats/group) were purchased from Charles River Laboratories at 5 weeks of age. After acclimatization, rats were randomly assigned to the experimental groups and given diets for two weeks. Experimental diets were based on powdered low-calcium, no-fat AIN-76 rodent diets (SAAJ, Jouy-en-Josas, France), supplemented with 5% safflower oil (MPBio) given in a separated feeder (7.5 g/d/rat) and 30 g of processed meat produced with nitrites at 40 or 80 mg/kg (respectively Ni-40 and Ni-80) or vegetable stock mimicking same sodium nitrite level (respectively VS-40 and VS-80) corresponding to 46-49% of dry matter of the diet. Feces were collected in plastic metabolic cages respectively at days 7-9 and 14 and urines were collected at days 7 and 14 to follow fecal and urinary biomarkers of peroxidation and fecal biomarkers of nitrosylation and nitrosation: heme iron, fecal TBARS, urinary DHN-MA (see above for methods) and ATNC, nitrosyl iron in feces. Fecal ATNC and nitrosyl iron were analyzed according to Kuhnle et al.25 with a CLD88 Exhalyzer (Ecomedics, Duernten, Switzerland). Sulfamic acid solution (500 µl, 5%) was added to 100 µl of fecal water to remove nitrite and samples were injected into a purged vessel kept at 60 °C and filled with a standard tri-iodide reagent (38 mg I2 was added to a solution of 108 mg KI in 1 mL water; to this mixture, 13.5 mL glacial acetic acid was added) to determine total ATNC. To determine mercury(II) stable compounds, 100 µL of 10 mM aqueous HgCl2 was added prior to analysis; to determine mercury(II) and ferricyanide stable compounds, 100 µL each of 10 mM aqueous HgCl2 and 10 mM aqueous K3Fe(CN)6 solution were added prior to analysis. Nitrosyl iron was determined as difference between mercury(II) stable ATNC and mercury(II) and K3Fe(CN)6 stable compounds. Data are concentrations (in µM), measured in triplicate in 100 µL of each sample.
Statistical analyses
For the animal experiment, once outliers were identified/removed by a ROUT test, and normality was checked, ANOVA was performed using GraphPad Prism software (version 9.5.0), followed by a Dunnett’s or a Tukey’s mean comparison test, in case of comparison to a control group (sodium nitrite effect), or comparison of all means (alternative effect), respectively. In case of heteroscedasticity (tested by Bartlett’s and Brown-Forsythe tests), data were log transformed before ANOVA (DHN-MA and NOCs). In case of non-normality of residuals, a nonparametric test (Kruskal-Wallis) was performed followed by Dunn’s mean comparison test (some RT-qPCR and bacterial taxa). Finally, if equal SD cannot be assumed despite data transformation, a Welch’s ANOVA was performed (cytotoxicity test). The growth potential values of L. monocytogenes were compared among the different recipes using the ANOVA test followed, when significant, by pairwise comparison using the estimated marginal means (Emmeans test) followed by Bonferroni correction. Two-side analyses were used throughout, and p values less than or equal to 0.05 were considered significant.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Supplementary information
Acknowledgements
The authors thank Xavier Blanc (UE 1298 SAAJ, Sciences de l’Animal & de l’Aliment, INRAE) for providing custom experimental diets and all members of the EZOP (Animal facility) for assistance with the animal experimentation. The authors thank the Genotoul bioinformatics platform Toulouse Occitanie and Sigenae group for providing help and storage resources thanks to Galaxy instance (https://galaxy-workbench.toulouse.inra.fr). The authors thank Bettina Seeger (University of Veterinary Medicine, Hannover) for providing the CLD88 et NO-Analyzer for NOC quantification. The authors thank G. Kuhnle (University of Reading) for fecal NOC analyses in the supplementary experiment. This study was co-financed (38%) by IFIP with a consortium of industries during the Subnitrites project (sub-part of the Adduits project).
Author contributions
F.G., F.P., V.S.-L., G.N. and A.P. designed the research studies, analyzed data, and wrote the manuscript. B.F. and M.O. acquired and analyzed data and wrote the manuscript. TK and GC acquired data, analyzed data, reviewed and edited the manuscript. C.B., N.N., E.F., J.D., E.K., N.P., V.B., L.A. and J.-L.M. conducted experiments, acquired and analyzed data. S.J. and L.T. analyzed data. V.T., P.P., C.H.-T. reviewed and edited the manuscript.
Data availability
The sequencing data of the 16 S rRNA gene have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB59736. Supporting Information is available from the author.
Competing interests
A.P., B.F., J.-L.M., S.J. and G.N. declare competing financial interests: they are employed by the French Pork Institute (IFIP). F.P., F.G. and V.S.-L. declare competing financial interests: some research projects from their academic research teams have been co-financed by the processed meat sector. C.B., N.N., E.F., V.B., J.D., P.P., C.H.T., L.T., E.K., N.P., L.A., V.T., M.O., G.C. and T.K. declare no competing financial or non-financial interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41538-023-00228-9.
References
- 1.Bouvard V, et al. Carcinogenicity of consumption of red and processed meat. Lancet Oncol. 2015;16:1599–1600. doi: 10.1016/S1470-2045(15)00444-1. [DOI] [PubMed] [Google Scholar]
- 2.IARC Working Group on the Evaluation of Carcinogenic Risk to Humans. Red Meat and Processed Meat. (International Agency for Research on Cancer, 2018). [PubMed]
- 3.WCRF/AICR. WCRF. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. (World Cancer Research Fund and American Institute for Cancer Research, 1997).
- 4.WCRF/AICR. Continuous Update Project Expert Report 2018. Meat, fish and dairy products and the risk of cancer. (World Cancer Research Fund and American Institute for Cancer Research, 2018).
- 5.Pierre FHF, et al. Freeze-dried ham promotes azoxymethane-induced mucin-depleted foci and aberrant crypt foci in rat colon. Nutr. Cancer. 2010;62:567–573. doi: 10.1080/01635580903532408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santarelli RL, et al. Calcium inhibits promotion by hot dog of 1,2-dimethylhydrazine-induced mucin-depleted foci in rat colon. Int J. Cancer. 2013;133:2533–2541. doi: 10.1002/ijc.28286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IARC. Ingested Nitrate and Nitrite, and Cyanobacterial Peptide Toxins.
- 8.Crowe W, Elliott CT, Green BD. A Review of the In Vivo Evidence Investigating the Role of Nitrite Exposure from Processed Meat Consumption in the Development of Colorectal Cancer. Nutrients. 2019;11:2673. doi: 10.3390/nu11112673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bonifacie A, et al. Chemical reactivity of nitrite and ascorbate in a cured and cooked meat model implication in nitrosation, nitrosylation and oxidation. Food Chem. 2021;348:129073. doi: 10.1016/j.foodchem.2021.129073. [DOI] [PubMed] [Google Scholar]
- 10.Directorate-General for Health and Consumers (European Commission) Now known as. Opinion on nitrosamines and secondary amines in cosmetic products. (Publications Office of the European Union, 2012).
- 11.Bernardo P, Patarata L, Lorenzo JM, Fraqueza MJ. Nitrate Is Nitrate: The Status Quo of Using Nitrate through Vegetable Extracts in Meat Products. Foods. 2021;10:3019. doi: 10.3390/foods10123019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lebrun S, et al. Influence of reduced levels or suppression of sodium nitrite on the outgrowth and toxinogenesis of psychrotrophic Clostridium botulinum Group II type B in cooked ham. Int J. Food Microbiol. 2020;334:108853. doi: 10.1016/j.ijfoodmicro.2020.108853. [DOI] [PubMed] [Google Scholar]
- 13.Hosseini F, et al. Nitrate-nitrite exposure through drinking water and diet and risk of colorectal cancer: A systematic review and meta-analysis of observational studies. Clin. Nutr. 2021;40:3073–3081. doi: 10.1016/j.clnu.2020.11.010. [DOI] [PubMed] [Google Scholar]
- 14.Khodavandi A, Alizadeh F, Razis AFA. Association between dietary intake and risk of ovarian cancer: a systematic review and meta-analysis. Eur. J. Nutr. 2021;60:1707–1736. doi: 10.1007/s00394-020-02332-y. [DOI] [PubMed] [Google Scholar]
- 15.Chazelas E, et al. Nitrites and nitrates from food additives and natural sources and cancer risk: results from the NutriNet-Santé cohort. Int J. Epidemiol. 2022;51:1106–1119. doi: 10.1093/ije/dyac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Santarelli RL, et al. Meat processing and colon carcinogenesis: cooked, nitrite-treated, and oxidized high-heme cured meat promotes mucin-depleted foci in rats. Cancer Prev. Res (Philos.) 2010;3:852–864. doi: 10.1158/1940-6207.CAPR-09-0160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolea G, et al. Digestive n-6 lipid oxidation, a key trigger of vascular dysfunction and atherosclerosis in the western diet: protective effects of apple polyphenols. Mol. Nutr. Food Res. 2021;65:e2000487. doi: 10.1002/mnfr.202000487. [DOI] [PubMed] [Google Scholar]
- 18.Bastide NM, et al. A central role for heme iron in colon carcinogenesis associated with red meat intake. Cancer Res. 2015;75:870–879. doi: 10.1158/0008-5472.CAN-14-2554. [DOI] [PubMed] [Google Scholar]
- 19.Bastide NM, et al. Red wine and pomegranate extracts suppress cured meat promotion of colonic mucin-depleted foci in carcinogen-induced rats. Nutr. Cancer. 2017;69:289–298. doi: 10.1080/01635581.2017.1263745. [DOI] [PubMed] [Google Scholar]
- 20.Steinberg P. Red meat-derived nitroso compounds, lipid peroxidation products and colorectal cancer. Foods. 2019;8:252. doi: 10.3390/foods8070252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Caderni G, et al. Identification of mucin-depleted foci in the unsectioned colon of azoxymethane-treated rats: correlation with carcinogenesis. Cancer Res. 2003;63:2388–2392. [PubMed] [Google Scholar]
- 22.Femia AP, et al. Frequent mutation of Apc gene in rat colon tumors and mucin-depleted foci, preneoplastic lesions in experimental colon carcinogenesis. Cancer Res. 2007;67:445–449. doi: 10.1158/0008-5472.CAN-06-3861. [DOI] [PubMed] [Google Scholar]
- 23.Crowe W, et al. Dietary inclusion of nitrite-containing frankfurter exacerbates colorectal cancer pathology and alters metabolism in APCmin mice. NPJ Sci. Food. 2022;6:60–68. doi: 10.1038/s41538-022-00174-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Mey E, De Maere H, Paelinck H, Fraeye I. Volatile N-nitrosamines in meat products: Potential precursors, influence of processing, and mitigation strategies. Crit. Rev. Food Sci. Nutr. 2017;57:2909–2923. doi: 10.1080/10408398.2015.1078769. [DOI] [PubMed] [Google Scholar]
- 25.Kuhnle GGC, et al. Diet-induced endogenous formation of nitroso compounds in the GI tract. Free Radic. Biol. Med. 2007;43:1040–1047. doi: 10.1016/j.freeradbiomed.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 26.Huybrechts I, et al. The human microbiome in relation to cancer risk: a systematic review of epidemiologic studies. Cancer Epidemiol. Biomark. Prev. 2020;29:1856–1868. doi: 10.1158/1055-9965.EPI-20-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee P-S, Teng C-Y, Kalyanam N, Ho C-T, Pan M-H. Garcinol reduces obesity in high-fat-diet-fed mice by modulating gut microbiota composition. Mol. Nutr. Food Res. 2019;63:e1800390. doi: 10.1002/mnfr.201800390. [DOI] [PubMed] [Google Scholar]
- 28.Xiong Q, et al. Regulation of a High-Iron Diet on Lipid Metabolism and Gut Microbiota in Mice. Anim. (Basel) 2022;12:2063–2077. doi: 10.3390/ani12162063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierre F, et al. Apc mutation induces resistance of colonic cells to lipoperoxide-triggered apoptosis induced by faecal water from haem-fed rats. Carcinogenesis. 2007;28:321–327. doi: 10.1093/carcin/bgl127. [DOI] [PubMed] [Google Scholar]
- 30.Surya R, et al. Red meat and colorectal cancer: Nrf2-dependent antioxidant response contributes to the resistance of preneoplastic colon cells to fecal water of hemoglobin- and beef-fed rats. Carcinogenesis. 2016;37:635–645. doi: 10.1093/carcin/bgw035. [DOI] [PubMed] [Google Scholar]
- 31.Awada M, et al. Dietary oxidized n-3 PUFA induce oxidative stress and inflammation: role of intestinal absorption of 4-HHE and reactivity in intestinal cells. J. Lipid Res. 2012;53:2069–2080. doi: 10.1194/jlr.M026179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pierre FHF, et al. Calcium and α-tocopherol suppress cured-meat promotion of chemically induced colon carcinogenesis in rats and reduce associated biomarkers in human volunteers. Am. J. Clin. Nutr. 2013;98:1255–1262. doi: 10.3945/ajcn.113.061069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duffy LL, Vanderlinde PB, Grau FH. Growth of Listeria monocytogenes on vacuum-packed cooked meats: effects of pH, aw, nitrite and ascorbate. Int J. Food Microbiol. 1994;23:377–390. doi: 10.1016/0168-1605(94)90164-3. [DOI] [PubMed] [Google Scholar]
- 34.Glass, K., McDonnell, L., Sawyer, C. & Claus, J. Minimum Nitrite Levels Required to Control Listeria monocytogenes on Ready-to-Eat Poultry Products Manufactured with Lactate and Diacetate. (2008).
- 35.Tsaloumi S, et al. Quantitative risk assessment of Listeria monocytogenes in ready-to-eat (RTE) cooked meat products sliced at retail stores in Greece. Food Microbiol. 2021;99:103800. doi: 10.1016/j.fm.2021.103800. [DOI] [PubMed] [Google Scholar]
- 36.King AM, Glass KA, Milkowski AL, Seman DL, Sindelar JJ. Modeling the Impact of Ingoing Sodium Nitrite, Sodium Ascorbate, and Residual Nitrite Concentrations on Growth Parameters of Listeria monocytogenes in Cooked, Cured Pork Sausage. J. Food Prot. 2016;79:184–193. doi: 10.4315/0362-028X.JFP-15-322. [DOI] [PubMed] [Google Scholar]
- 37.Asadollahi P, et al. Anti-cancer effects of Bifidobacterium species in colon cancer cells and a mouse model of carcinogenesis. PLoS One. 2020;15:e0232930. doi: 10.1371/journal.pone.0232930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sibai M, et al. Microbiome and longevity: high abundance of longevity-linked muribaculaceae in the gut of the long-living rodent spalax leucodon. OMICS. 2020;24:592–601. doi: 10.1089/omi.2020.0116. [DOI] [PubMed] [Google Scholar]
- 39.Sorbara MT, et al. Functional and genomic variation between human-derived isolates of lachnospiraceae reveals inter- and intra-species diversity. Cell Host Microbe. 2020;28:134–146.e4. doi: 10.1016/j.chom.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David LA, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bosch S, et al. The potential of fecal microbiota and amino acids to detect and monitor patients with adenoma. Gut Microbes. 2022;14:e2038863. doi: 10.1080/19490976.2022.2038863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lunn JC, et al. The effect of haem in red and processed meat on the endogenous formation of N-nitroso compounds in the upper gastrointestinal tract. Carcinogenesis. 2007;28:685–690. doi: 10.1093/carcin/bgl192. [DOI] [PubMed] [Google Scholar]
- 43.Joosen AMCP, et al. Effect of processed and red meat on endogenous nitrosation and DNA damage. Carcinogenesis. 2009;30:1402–1407. doi: 10.1093/carcin/bgp130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van Breda SG, et al. Replacement of nitrite in meat products by natural bioactive compounds results in reduced exposure to N-nitroso compounds: the PHYTOME project. Mol. Nutr. Food Res. 2021;65:e2001214. doi: 10.1002/mnfr.202001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alahakoon AU, Jayasena DD, Ramachandra S, Jo C. Alternatives to nitrite in processed meat: Up to date. Trends Food Sci. Technol. 2015;45:37–49. [Google Scholar]
- 46.De Niederhausern S, et al. Plant extracts for the control of listeria monocytogenes in meat products. Appl. Sci. -Basel. 2021;11:10820–10833. [Google Scholar]
- 47.Hao YY, Brackett RE, Doyle MP. Inhibition of Listeria monocytogenes and Aeromonas hydrophila by plant extracts in refrigerated cooked beef. J. Food Prot. 1998;61:307–312. doi: 10.4315/0362-028x-61.3.307. [DOI] [PubMed] [Google Scholar]
- 48.Ullah H, et al. Natural Polyphenols for the Preservation of Meat and Dairy Products. Molecules. 2022;27:1906–1920. doi: 10.3390/molecules27061906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zamuz S, et al. The role of phenolic compounds against Listeria monocytogenes in food. A review. Trends Food Sci. Technol. 2021;110:385–392. [Google Scholar]
- 50.Hornsey HC. The colour of cooked cured pork. I.—Estimation of the Nitric oxide-Haem Pigments. J. Sci. Food Agriculture. 1956;7:534–540. [Google Scholar]
- 51.Stolze K, Dadak A, Liu Y, Nohl H. Hydroxylamine and phenol-induced formation of methemoglobin and free radical intermediates in erythrocytes. Biochem. Pharmacol. 1996;52:1821–1829. doi: 10.1016/s0006-2952(96)00460-1. [DOI] [PubMed] [Google Scholar]
- 52.Poitevin E. Official Methods for the Determination of Minerals and Trace Elements in Infant Formula and Milk Products: A Review. J. AOAC Int. 2016;99:42–52. doi: 10.5740/jaoacint.15-0246. [DOI] [PubMed] [Google Scholar]
- 53.Mercier Y, Gatellier P, Viau M, Remignon H, Renerre M. Effect of dietary fat and vitamin E on colour stability and on lipid and protein oxidation in turkey meat during storage. Meat Sci. 1998;48:301–318. doi: 10.1016/s0309-1740(97)00113-7. [DOI] [PubMed] [Google Scholar]
- 54.Gueraud F, et al. Enzyme immunoassay for a urinary metabolite of 4-hydroxynonenal as a marker of lipid peroxidation. Free Radic. Biol. Med. 2006;40:54–62. doi: 10.1016/j.freeradbiomed.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 55.Martin OCB, et al. Targeting Colon Luminal Lipid Peroxidation Limits Colon Carcinogenesis Associated with Red Meat Consumption. Cancer Prev. Res (Philos.) 2018;11:569–580. doi: 10.1158/1940-6207.CAPR-17-0361. [DOI] [PubMed] [Google Scholar]
- 56.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 57.Forest V, Clement M, Pierre F, Meflah K, Menanteau J. Butyrate restores motile function and actin cytoskeletal network integrity in apc mutated mouse colon epithelial cells. Nutr. Cancer. 2003;45:84–92. doi: 10.1207/S15327914NC4501_10. [DOI] [PubMed] [Google Scholar]
- 58.Plaisancié P, et al. Study of the colonic epithelial-mesenchymal dialogue through establishment of two activated or not mesenchymal cell lines: Activated and resting ones differentially modulate colonocytes in co-culture. PLOS ONE. 2022;17:e0273858. doi: 10.1371/journal.pone.0273858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Khoury L, Zalko D, Audebert M. Complementarity of phosphorylated histones H2AX and H3 quantification in different cell lines for genotoxicity screening. Arch. Toxicol. 2016;90:1983–1995. doi: 10.1007/s00204-015-1599-1. [DOI] [PubMed] [Google Scholar]
- 60.Seiwert N, et al. Chronic intestinal inflammation drives colorectal tumor formation triggered by dietary heme iron in vivo. Arch. Toxicol. 2021;95:2507–2522. doi: 10.1007/s00204-021-03064-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Martin OCB, et al. Haem iron reshapes colonic luminal environment: impact on mucosal homeostasis and microbiome through aldehyde formation. Microbiome. 2019;7:72–90. doi: 10.1186/s40168-019-0685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Escudié F, et al. FROGS: Find, rapidly, OTUs with galaxy solution. Bioinformatics. 2018;34:1287–1294. doi: 10.1093/bioinformatics/btx791. [DOI] [PubMed] [Google Scholar]
- 63.Mahé F, Rognes T, Quince C, de Vargas C, Dunthorn M. Swarm: robust and fast clustering method for amplicon-based studies. PeerJ. 2014;2:e593. doi: 10.7717/peerj.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lê Cao K-A, et al. MixMC: a multivariate statistical framework to gain insight into microbial communities. PLoS One. 2016;11:e0160169. doi: 10.1371/journal.pone.0160169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequencing data of the 16 S rRNA gene have been deposited in the European Nucleotide Archive (ENA) at EMBL-EBI under accession number PRJEB59736. Supporting Information is available from the author.