Abstract
Purpose
Mesenchymal stem cells (MSCs) present a valuable treatment option for knee osteoarthritis with promising results. The purpose of the present study was to systematically review the clinical and functional outcomes following mesenchymal stem cell application focusing on early to moderate knee osteoarthritis.
Methods
A systematic search was done using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines in Pubmed, Scopus, Web of Science, and Cochrane Library databases. All Studies published between 2017 and March 2023 on patients treated with single mesenchymal stem cell injection for Kellgren-Lawrence grade I—III knee osteoarthritis reported on clinical and functional outcomes were included.
Results
Twelve articles comprising 539 patients and 576 knees treated with a single intraarticular injection of MSCs for knee osteoarthritis were included in the current systematic review. In eligible studies, the reported outcomes were improved concerning patient-reported outcomes measures, knee function, pain relief, and quality of patient's life.
Conclusion
Based on high-level evidence studies, single intraarticular injection of MSCs is a safe, reliable, and effective treatment option for Kellgren-Lawrence grade I—III knee osteoarthritis. However, the lack of homogeneity in the included studies and the variance in MSCs sources and preparations should be noted.
Level of evidence
III.
Keywords: Knee osteoarthritis, Outcomes, PROMs, Mesenchymal stem cells, MSCs, Regenerative medicine, Intraarticular injection
Introduction
Pain and functional limitations associated with knee osteoarthritis (OA) are frequent and negatively influence patients' quality of life [11]. Available conservative treatments include, among others, intraarticular injections of corticosteroids, hyaluronic acid (HA), blood derivatives products such as platelet-rich plasma (PRP), or the administration of human cells, known as cell therapy (CT). Indeed, cell therapy has gained more attention in recent decades and features an increasingly accepted treatment modality for knee OA [18].
Mesenchymal stem cells (MSCs) hold a great place in this direction and play a crucial role in tissue homeostasis, repair, and regeneration. Mesenchymal stem cells are characterized by their potential for self-renewal, plasticity, and the aptitude to differentiate into specific tissue cell types, including cartilage and bone cells [23]. More specifically, stem cells could act through different mechanisms, decreasing inflammation, recruiting cells, regulating the immune response, reducing apoptosis, and stimulating angiogenesis [17].
The scientific literature demonstrated growing evidence of efficacy in pain relief and increased function for chondral defects and OA using MSCs [19]. There are different sources of autologous MSCs in clinical practice; they are obtained most often from the bone marrow in the form of bone marrow aspirate concentrate (BMAC) or from the adipose tissue as a stromal vascular fraction (SVF) [15, 31]. Some authors used the same sources after culture expansion to achieve a higher number of stem cells again with promising results either as an injectable treatment or after surgical implantation [5, 12–14].
Several studies have reported decreased pain and improved functional scores after stem cell administration through intraarticular injection in cases of advanced OA [15]. However, the evidence of this challenging topic is constantly increasing and changing. This research systematically reviews the literature for recent studies focusing on autologous minimally processed MSCs administrated with a single intraarticular injection for early to moderate knee OA. It aims to expose post-injection patient-reported outcome measures (PROMs), radiological evaluation, and complications and give an overall use.
Materials and methods
The present systematic review was conducted following the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [16].
Search strategy
A comprehensive literature search was performed, by two investigators (Τ.Κ., C.P.), using the Medline (PubMed), Web of Science, Scopus, and Cochrane Central databases on March 2023. Firstly, the titles and abstracts of the identified studies were assessed for eligibility, followed by the full-text articles screening. Moreover, the references of the included articles were screened. A third researcher (M.I.) helped resolve any disagreement and provide a consensus.
Eligibility criteria
All studies that satisfied the following criteria were included in the present systematic review: i) studies on adult human subjects treated with intra-articular injections of MSCs recording PROMS, ii) OA classified as Kellgren-Lawrence I-IIΙ, iii) single injection treatment, iv) peer-reviewed articles published within the last six years and v) English language published studies.
The exclusion criteria were: i) studies used culture-expanded MSCs, ii) studies performed on cartilage defects, iii) intra-articular injections in combination with surgical procedures, iv) studies reporting incomplete data, and v) abstract, review articles, meta-analyses, or in vitro studies/animal studies.
Level of evidence and quality of studies
Each study's evidence level was assessed using the modified criteria of the Oxford Centre for Evidence‐Based Medicine Working Group (OCEBM). The studies were qualitatively assessed using the revised and validated version of the MINORS (Methodological Index for Non-Randomized Studies) score for the non-randomized studies and the MJS (Modified Jaded Scale) for the randomized control trials.
Data extraction and analysis
Data were extracted and recorded from each study as follows: first author, year of publication, study type, age, gender, sample size (number of patients and/or number of knees), grade of OA, MSCs quantity and source, Body Mass Index (B.M.I.), mean follow-up. The collected outcome measures that were available consisted of the Visual Analogue Scale (VAS) for pain, the Western Ontario Macmaster University Osteoarthritis Index (W.O.M.A.C.), the Knee Injury and Osteoarthritis Outcome Score (K.O.O.S), the Knee Society Score (K.S.S.) clinical and functional, the Lysholm Knee Scoring Scale, the Japanese Knee Osteoarthritis Measure (JKOM), the Numeric Rating Scale (NAS), the EQ-5D-5L questionnaire, complications, and radiographic analysis. A pooled analysis and a meta-analysis of the clinical outcomes were considered inappropriate due to the heterogeneity of the included studies and the significant risk of bias.
Results
Literature search and study identification
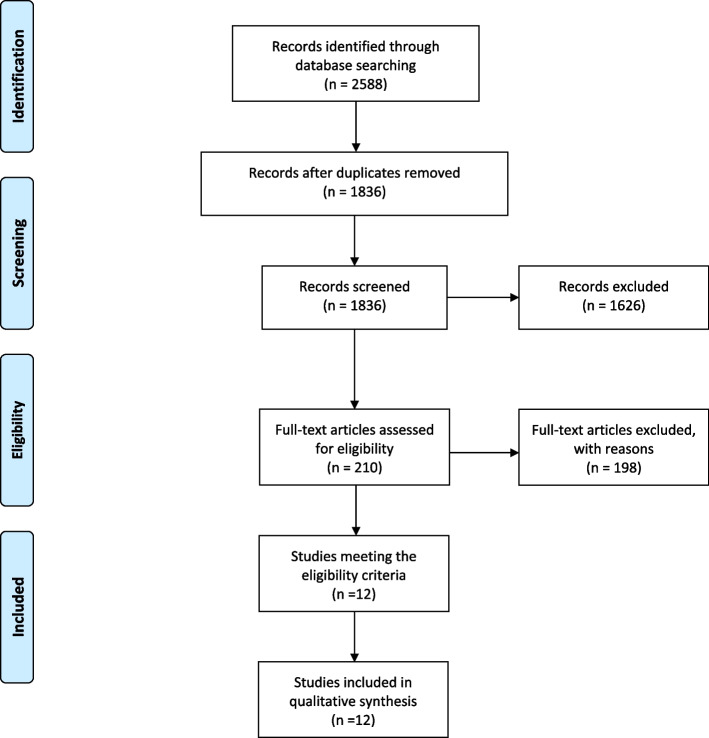
The literature search yielded 2558 potentially relevant studies. A total of 722 duplicate articles were excluded; thus, after the removal, 1836 were identified. The screening of the titles and abstracts demonstrated 1626 irrelevant articles. Two hundred ten records were retrieved for full-text evaluation. Based on inclusion and exclusion criteria, a further 198 articles were excluded. The remaining 12 studies were eligible for inclusion. The PRISMA flowchart (Fig. 1) illustrates the whole selection process.
Fig. 1.
PRISMA flow diagram for search results. From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. doi:10.1371/journal.pmed1000097
Characteristics of the eligible studies and patients
A total of 539 patients and 576 knees treated with intraarticular injection of stem cells were included in this systematic review. The mean reported age in the included studies ranged from 54—73 years, and the mean B.M.I. from 25.1 – 27 kg/m. The follow-up ranged from 3 months to at least 5 years. All the included studies were level 1 or 2. Characteristics of the included studies, study period, type of the study, level of evidence, quality of studies, and patient demographics are summarised in Table 1. The risk of bias in the analysed studies was low based on the MINORS (range 12/16—14/16) and the MJS (range 5/8 – 8/8).
Table 1.
Level of evidence (LoE), Quality of Study and demographics of patients included in the 12 studies
| Author | Study Type | LoE | Quality of study | N patients (Knees) | Lost FU | MSCs | Control Group | Gender (M/F) | Mean Age (years) | BMI (kg/m2) | Mean FU (months) | FU (range) | Study Period |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Garay-Mendoza 2017 [7] | PCS | 3 | 14/16 | 30 (30) | 4 | BMAC | Acetaminophen (31) | 7/23 | 55.7 | 29.5 | 6 | n.a | |
| Tsubosaka 2020 [21] | PCoS | 3 | 13/16 | 60 (60) | 3 | SVF | n.a | 69.4 | 25.1 | 13.7 | 11.7–15.7 | September 2017 – March 2018 | |
| Wells 2020 [24] | PCoS | 3 | 13/16 | 10 (13) | BMAC | 4/6 | 55.3 | n.a | 12 | April 2017—December 2018 | |||
| Varady 2020 [22] | PCoS | 3 | 12/16 | 17 (17) | BMAC | 7/10 | 54 | n.a | 12 | March 2018—March 2019 | |||
| Estrada 2020 [3] | PCS | 3 | 13/16 | 33 (33) | SVF | PRP (29) | n.a | 54.7 | 25.5 | 12 | March 2012—July 2019 | ||
| 27 (27) | BMAC | n.a | 54.7 | 25.5 | 12 | ||||||||
| Garza 2020 [8] | RCT | 2 | 7/8 | 13 (13) | SVF | Placebo | 4/9 | 60.5 | 27.6 | 12 | July 2016 – September 2017 | ||
| 13 (13) | SVF DD | Placebo | 7/6 | 59.5 | 28.8 | 12 | |||||||
| Kaszynski 2022 [10] | RCT | 2 | 6/8 | 20 (20) | SVF | PRP (20) Control (20) | 55 | 27 | 12 | n.a | |||
| Yokota 2022 [27] | POC | 3 | 14/16 | 38 (69) | SVF | Culture ASC (72) | 7/31 | 73 | 25 | 24 | November 2016 – April 2017 | ||
| Zhang 2022 [28] (Stem Cell Research & Therapy) | RCT | 2 | 8/8 | 56 (56) | 5 | SVF | HA (64) | 14/42 | 54 | 23.7 | 60 | May 2013—July 2015 | |
| Zhang 2022 [29] (BioMed Research International) | RCT | 2 | 6/8 | 50 (53) | SVF | HA (51) | 18/29 | 50.8 | 22.7 | 12 | January 2018—May 2021 | ||
| Altetto 2022 [1] | PCoS | 3 | 12/16 | 123 (123) | SVF | 57/66 | 57 | 27 | 6 | June 2019—November 2020 | |||
| Anz 2022 [2] | RCT | 3 | 5/8 | 49 (49) | 4 | BMAC | PRP (39) | 27/18 | 55.8 | 27.7 | 24 | n.a |
BMAC Bone Marrow Aspirate Concentrate, SVF Stromal Vascular Fraction. SVF DD Stromal Vascular Fraction Double Dose, PCS Prospective Comparative Study, PCoS Prospective Cohort Study, RCT Randomised Control Trial, POC Parallel Observation Cohort, ASC Adipose Stromal Cells
MSCs sources and site of injection
All the included studies used autologous-derived MSCs. Seven studies used MSCs provided by the abdominal adipose tissue [1, 10, 21, 27–29], four used bone marrow from the iliac crest [2, 7, 22, 24], and one from both sources [3]. Different reported devices were used for harvesting adipose or bone marrow-derived MSCs (Celution, Lipogems, Lipocell, GID SVF-2, PureBMC) [2, 8, 10, 21, 27, 28]. The most often superolateral approach was used, and some authors injected [2, 21, 22, 24] it under ultrasound guidance. Cell number was calculated in four studies [8, 21, 24, 28].
Clinical outcomes
Table 2 summarizes all reported outcomes that were improved in all eligible studies. Most studies evaluated pain relief with the VAS score [1, 7, 10, 21, 22, 27, 29]. Several PROMs were used to quantify clinical outcomes. Six studies reported the Western Ontario Macmaster University Osteoarthritis Index (WOMAC) [2, 7, 8, 10, 21, 28], five the Knee Injury and Osteoarthritis Score (KOOS) [1, 10, 21, 22, 27], two the International Knee Documentation Committee (IKDC) [3, 10], and from one study the Knee Society Score (KSS) clinical and functional [3], the Japanese Knee Osteoarthritis Measure (JKOM) [21], and the Lysholm score [22]. One study used the EQ-5D-5L to assess health-related quality of life. The same research estimates patients' mobility with the time up and go, five times sit to stand, and 10 min walk tests [10].
Table 2.
A summary of the mean outcome scores from the various publications
| Score | Author | Year | Number of knees | Modality | K-L Grade | Pre-injection score | Post-injection score | Improvement points | Time of post injection score | Notes (p-values) |
|---|---|---|---|---|---|---|---|---|---|---|
| VAS | Garay-Mandoza [7] | 2017 | 26 | BMAC | 2–3 | 5.3 | 0.9 | 4.4 | 6 months | Improved (n.a.) |
| Tsubosaka [21] | 2020 | 11 | SVF | 2 | 3.6 | 2.1 | 1.5 | 12 months | Improved (n.a.) | |
| Tsubosaka [21] | 2020 | 36 | SVF | 3 | 5.2 | 3.7 | 1.5 | 12 months | Improved (n.a.) | |
| Varady [22] | 2020 | 17 | BMAC | 1–3 | 2.7 | 2.1 | 0.6 | 3 months | Improved (p = 0.003) | |
| Yokota [27] | 2022 | 30 | SVF | 2–3 | 7.2 | 5 | 2.2 | 2 years | Improved (p < 0.05) | |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 5.1 | 2.8 | 2.3 | 12 months | Improved (p < 0.001) | |
| Aletto [1] | 2022 | 123 | SVF | 1–3 | 6.5 | 2 | 4.5 | 6 months | Improved (p < 0.05) | |
| Zhang [28] (Stem Cell Research & Therapy) | 2022 | 56 | SVF | 2–3 | 4 | 1.8 | 2.2 | 5 years | Improved (p < 0.001) | |
| Zhang [29] (BioMed Research International) | 2022 | 29 | SVF | 2 | 4.3 | 0.9 | 3.4 | 12 months | Improved (p < 0.001) | |
| Zhang [29] (BioMed Research International) | 2022 | 24 | SVF | 3 | 6 | 0.8 | 5.2 | 12 months | Improved (p < 0.001) | |
| WOMAC | Garay-Mandoza [7] | 2017 | 26 | BMAC | 2–3 | 62.6 | 91.7 | 30.1 | 6 months | Improved (n.a.) |
| Tsubosaka [21] | 2020 | 11 | SVF | 2 | 27.7 | 11.2 | 16.5 | 12 months | Improved (n.a.) | |
| Tsubosaka [21] | 2020 | 36 | SVF | 3 | 35.1 | 26 | 9.1 | 12 months | Improved (n.a.) | |
| Garza [8] | 2020 | 13 | SVF | 2–3 | 56.2 | 21.8 | 34.4 | 12 months | Improved (n.a.) | |
| Garza [8] | 2020 | 13 | SVFx2 | 2–3 | 47.1 | 13.2 | 33.9 | 12 months | Improved (n.a.) | |
| Anz [2] | 2022 | 45 | BMAC | 1–3 | 35.3 | 20.8 | 14.5 | 2 years | Improved (p < 0.01) | |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 63.9 | 83.6 | 19.7 | 12 months | Improved (p < 0.001) | |
| Zhang [28] (Stem Cell Research & Therapy) | 2022 | 56 | SVF | 2–3 | 33.2 | 27 | 6.2 | 5 years | Improved (p < 0.001) | |
| WOMAC Pain | Anz [2] | 2022 | 45 | BMAC | 1–3 | 7 | 3.8 | 3.2 | 2 years | Improved (p < 0.01) |
| Zhang [29] (BioMed Research International) | 2022 | 29 | SVF | 2 | 9,4 | 2,7 | 6,7 | 12 months | Improved (p < 0.001) | |
| WOMAC Physical Function | Anz [2] | 2022 | 45 | BMAC | 1–3 | 22.9 | 13.2 | 9.7 | 2 years | Improved (p < 0.01) |
| Zhang [29] (BioMed Research International) | 2022 | 29 | SVF | 2 | 24.7 | 10.1 | 14.6 | 12 months | Improved (p < 0.001) | |
| WOMAC Stiffness | Anz [2] | 2022 | 45 | BMAC | 1–3 | 3.8 | 2.2 | 1.6 | 2 years | Improved (p < 0.01) |
| Zhang [29] (BioMed Research International) | 29 | SVF | 2 | 2.8 | 0.9 | 1.9 | 12 months | Improved (p < 0.001) | ||
| KOOS | Tsubosaka [21] | 2020 | 11 | SVF | 2 | 52.9 | 68.5 | 15.6 | 12 months | Improved (n.a.) |
| Tsubosaka [21] | 2020 | 36 | SVF | 3 | 48.6 | 56.3 | 7.7 | 12 months | Improved (n.a.) | |
| Aletto [1] | 2022 | 52 | SVF | 1 | 55.4 | 89.8 | 34.4 | 6 months | Improved (p < 0.05) | |
| Aletto [1] | 2022 | 61 | SVF | 2 | 49.9 | 85.9 | 36 | 6 months | Improved (p < 0.05) | |
| Aletto [1] | 2022 | 10 | SVF | 3 | 45 | 84.1 | 39.1 | 6 months | Improved (p < 0.05) | |
| Yokota [27] | 2022 | 30 | SVF | 2–3 | 40 | 56 | 16 | 2 years | Improved (p < 0.05) | |
| KOOS pain | Varady [22] | 2020 | 17 | BMAC | 1–3 | 53.8 | 83 | 29.2 | 3 months | Improved p < 0.001 |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 57.8 | 78.9 | 21.1 | 12 months | Improved (p < 0.001) | |
| KOOS Symptoms | Varady [22] | 2020 | 17 | BMAC | 1–3 | 50.9 | 74.5 | 23.6 | 3 months | Improved (p = 0.053) |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 57.7 | 78.9 | 21.2 | 12 months | Improved (p < 0.001) | |
| KOOS ADL | Varady [22] | 2020 | 17 | BMAC | 1–3 | 61.1 | 89.3 | 28.1 | 3 months | Improved p < 0.001 |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 63.7 | 84 | 20.3 | 12 months | Improved (p < 0.001) | |
| KOOS Sports/Rec | Varady [22] | 2020 | 17 | BMAC | 1–3 | 36.9 | 72.6 | 35.7 | 3 months | Improved (p = 0.006) |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 35.5 | 66.1 | 30.6 | 12 months | Improved (p < 0.001) | |
| KOOS QOL | Varady [22] | 2020 | 17 | BMAC | 1–3 | 32.7 | 66.1 | 33.4 | 3 months | Improved (p = 0.003) |
| Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 38.8 | 62.2 | 23.4 | 12 months | Improved (p < 0.001) | |
| KOOS Index | Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 55.7 | 77.9 | 22.2 | 12 months | Improved (p < 0.001) |
| KOOS Joint Replacement | Wells [24] | 2020 | 13 | BMAC | 1–2 | 63.1 | 80.3 | 17.2 | 12 months | Improved (p < 0.001) |
| IKDC | Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 44.2 | 68.9 | 24.7 | 12 months | Improved (p < 0.001) |
| Estrada [3] | 2020 | 33 | SVF | 3 | 33.9 | 64.2 | 30.3 | 12 months | Improved (n.a.) | |
| Estrada [3] | 2020 | 27 | BMAC | 3 | 30.2 | 57.6 | 27.4 | 12 months | Improved (n.a.) | |
| Lysholm | Varady [22] | 2020 | 17 | BMAC | 1–3 | 55.5 | 77.3 | 21.8 | 3 months | Improved p = 0.009 |
| KSS-C | Estrada [3] | 2020 | 33 | SVF | 3 | 38.9 | 65.6 | 26.7 | 12 months | Improved (n.a.) |
| Estrada [3] | 2020 | 27 | BMAC | 3 | 33.8 | 56.7 | 22.9 | 12 months | Improved (n.a.) | |
| KSS-F | Estrada [3] | 2020 | 33 | SVF | 3 | 53.3 | 76.7 | 23.4 | 12 months | Improved (n.a.) |
| Estrada [3] | 2020 | 27 | BMAC | 3 | 52 | 75.6 | 23.6 | 12 months | Improved (n.a.) | |
| JKOM | Tsubosaka [21] | 2020 | 11 | SVF | 2 | 27.4 | 11.4 | 16 | 12 months | Improved (n.a.) |
| Tsubosaka [21] | 2020 | 36 | SVF | 3 | 37.4 | 31.6 | 5.8 | 12 months | Improved (n.a.) | |
| EQ-5D-5L | Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 70 | 80 | 10 | 12 months | Improved p < 0.01 |
| Time Up & Go (sec) | Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 6.9 | 5.6 | 1.3 | 12 months | Improved (p < 0.001) |
| 5 Times Sit to Stand (sec) | Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 10.8 | 8.2 | 2.6 | 12 months | Improved (p < 0.001) |
| 10 min walk test | Kaszynski [10] | 2022 | 20 | AAT | 2–3 | 6.2 | 4.8 | 1.4 | 12 months | Improved (p < 0.001) |
Referred to data published in VAS Visual Analog Scale for pain, WOMAC Western Ontario Macmaster University Osteoarthritis Index, IKDC International Knee Documentation Committee, KOOS Knee Injury and Osteoarthritis Score, KSS-C Knee Society Score-Clinical, KSS-F Knee Society Score-Functional, JKOM Japanese Knee Osteoarthritis Measure, n.a. not applicable
Radiological outcomes
Two studies analyzed radiological changes [28, 29]. One study used X-rays to determine the KL grade and the mechanical axis of the knee and MRI to evaluate the cartilage structure and volume, patella-femoral pathology, and bone marrow lesions and compared the pre-injection and 5-year follow-up status [28]. Based on the X-rays, the KL grade was increased by 15.7% and remained unchanged at 84.3%, and the varus mechanical axis was increased from 1,5° to 1,8ο. The MRI findings were as follows: the size of the full-thickness defect decreased by 5.9%, increased by 7.8%, and remained unchanged by 86.3%; the total cartilage volume decreased from 16.467,1 mm3 to 15.121,1 mm3; the rate of patella-femoral degeneration was increased from 49% to 58.8%; and the bone marrow lesion size was decreased from 123,5 mm2 to 90,3 mm2. The other study used the Whole-Organ Magnetic Resonance Imaging Score (WORMS) to assess the knee, while the MOCART score was used to examine cartilage repair [29]. Between the baseline and the 12-month follow-up, the WORMS improved from 54.9 to 40.5 in the KL2 group and from 75.7 to 57.5 in the KL3 group. Between the 6- and 12-month evaluation, the MOCART score was improved from 52.9 to 62.1 in patients with KL2 and from 46.5 to 57.1 in patients with KL3.
Complications
In total, three studies reported complications. One of them noted weakness in the knee (2 knees), pain around the injection site (1 knee), minor bleeding from the aspiration site (1 knee), and minor redness or swelling around the injection site (3 knees) [24]. The two other post-injection swelling in one knee [2, 7].
Discussion
The most important finding of this systematic review was that a single intraarticular administration of MSCs is a safe and efficient treatment option with good clinical results for dealing with early to moderate knee joint OA.
The recent literature has demonstrated encouraging results in managing knee OA. For instance, Song et al. [20], in a meta-analysis of 15 randomized control trials, including 584 patients with knee OA, found that the injection of MSCs has been associated with a significant decrease in both VAS and WOMAC scores at 12 months and six months follow-up, respectively, compared to controls. In another most recent meta-analysis of 43 studies, Zhao et al. [30] found an improvement in pain and functional scores at six but not 12 months after MSCs injection. Similarly, Shoukrie et al. [19] systematically reviewed ten studies. They concluded that MSCs injection significantly improved VAS, WOMAC, and KOOS scores and featured better post-injection MRI findings in patients with osteoarthritic knees. However, this study is differentiated from the present systematic review as it includes all grades of K-L classification.
Nevertheless, some authors question such studies' reliability and risk of bias [6, 25, 26]. In light of the above hesitation, the present systematic review tries to cover this question and contributes to the evidence of this challenging topic. This systematic review included 12 high-level evidence studies, treating 539 patients and 576 knees with a single intraarticular injection of MSCs. The reported outcomes showed that intraarticular administration of MSCs is an efficient and safe procedure associated with reduced pain and increased function in patients with early to moderate knee OA.
The VAS was mainly used to evaluate pain relief, and eight studies [1, 7, 10, 21, 22, 27–29] assessed it at baseline and after administration. The follow-up period was ranged between 3 months to 5 years, and the improvement varied between 0.9 to 5.2 points. It is essential to note that all the studies included patients with KL grade 1 to 3 OA, but only two studies [21, 29] presented the results of each degree separately. Thus, evaluating the improvement and efficacy in different severities of knee OA is difficult.
On the other hand, six studies [2, 7, 8, 10, 21, 28] described the clinical evaluation of the patients before and after the administration of the MSCs with the WOMAC score. One study [7] had a limited follow-up period of 6 months, three studies [8, 10, 21] reported the results after 12 months, and two studies had more than one-year evaluation, precisely 2 [2] and 5 [28] years, respectively. Again, all analyses presented an improvement. However, almost all studies have shown short-term clinical outcomes, and thus the interpretation of these results should be made cautiously. One of the studies showed the K-L grade 2 and 3 OA results separately and demonstrated more remarkable progress in the case of grade 2 arthritis which was predictable [21]. Another study [8] reported the results in two groups of thirteen patients receiving low and high doses of SVF and reported a dose-dependent improvement with the high-dose group to present better results. Again, these results are unsurprising as the literature has already demonstrated a clear relationship between the number of MSCs and better outcomes [9].
Two included studies were randomized control trials comparing the outcomes after SVF administration to hyaluronic acid. Zhang et al. [28] concluded that VAS and WOMAC scores in the SVF group were significantly better than in the HA group during the 5-year follow-up after treatment. Moreover, the average responsive time to SVF treatment (61.5 months) was significantly longer than the HA treatment (30.4 months) calculated by the Kaplan–Meier responsive curves. The other study [29] compared 53 knees with K-L grade 2 and 3 OA that received an intra-articular injection of SVF and 51 knees that received HA. The patient's VAS, WOMAC pain, stiffness, and physical function were evaluated at baseline and 1, 3, 6, and 12 months after injection with SVF and HA. SVF-treated knees showed significant improvement in clinical scores at both grades of OA till the final follow-up. On the contrary, in the control group, clinical scores were relieved one month after HA injection and amplified until the 12-month follow-up evaluation. Therefore, both studies demonstrated that using MSCs provides better clinical outcomes than HA. Moreover, it is a safe procedure as minor, or no adverse events were recorded in these studies.
In the last few years, there has also been a constantly increasing interest in platelet-rich plasma (PRP) injections. The PRP is derived after autologous blood centrifugation and contains several growth factors to cope with musculoskeletal disorders, mainly knee OA [4]. This systematic review includes two randomized control trials comparing the clinical outcomes of MSCs to that of platelet-rich plasma. Interestingly enough, both studies do not demonstrate the superiority of MSCs on PRP.
More precisely, Anz et al. [2] compared the efficacy of BMAC and PRP on pain and function in patients with knee OA up to 24 months after injection. Ninety symptomatic participants (KL grades 1–3) were randomized into PRP and BMAC injection groups. Both groups completed the WOMAC and subjective IKDC questionnaire before and after a single intra-articular leukocyte-rich PRP or BMAC injection. Both groups had significantly improved from baseline to 24 months after the injection; however, no difference was found at any time during the evaluation. In the other study, Estrada et al. [3] compared PRP, BMAC, and adipose-derived MSCs injections in treating OA of the knee using functional scores. Again, a statistically significant improvement was observed in the three groups at all time points during follow-up compared with baseline; nevertheless, without difference among treatment types.
The strengths of this review are the following: first, the fact of reporting the outcomes following intraarticular injection of autologous MSCs in early to moderate OA. Second, focusing on the recent literature as the included studies are published within the last six years. Third, all the included studies are of a high level of evidence. The clinical relevance of the present study is that it provides evidence to endorse the use of MSCs intraarticular injection for knee OA. Based on the promising outcomes, there should be part of the possible treatment algorithm during the decision-making in patients suffering from early to moderate knee OA.
This study should be considered on the subject of the following limitations. Firstly, the included studies lack homogeneity regarding PROMs, and thus the study was limited to presenting the data descriptively. Moreover, the diversity in the included studies and the variance in MSCs sources, preparations, and administration make the treatment not reproducible. Next, most of the included studies had a limited number of participants with different grades of O.A. Therefore, evaluating the treatment's effectiveness in each stage of O.A. is ambitious. Another potential limitation is that most included studies referred to a relatively short follow-up period. Nevertheless, ten of the twelve included studies had at least twelve months of follow-up, which is considered a sufficient period to evaluate the treatment's efficacy in this kind of research. However, further studies with a higher follow-up time are needed to validate these results. Last, the number of stem cells applied was not quantified to verify the required quantity to reach positive results. However, this is a common limitation in similar studies, as quantification of the cells is not routinely performed during the procedure.
Conclusion
Based on high-level evidence studies, the single intra-articular injection of MSCs is a safe, reliable, and effective treatment option for Kellgren-Lawrence grade I—III knee OA patients However, the lack of homogeneity in the included studies and the variance in MSCs sources and preparation should be noted.
Abbreviations
- BMAC
Bone Marrow Aspirate Concentrate
- BMI
Body Mass Index
- CT
Cell Therapy
- HA
Hyaluronic Acid
- IKDC
International Knee Documentation Committee
- JKOM
Japanese Knee Osteoarthritis Measure
- KL
Kellgren-Lawrence
- KOOS
Knee injury and Osteoarthritis Outcome Score
- KSS
Knee Society Score
- MINORS
Methodological Index for Non-Randomized Studies
- MJS
Modified Jaded Scale
- MOCART
Magnetic Resonance Observation of Cartilage Repair Tissue
- MSCs
Mesenchymal Stem Cells
- NAS
Numeric Rating Scale
- OA
Osteoarthritis
- OCEBM
Oxford Centre for Evidence‐Based Medicine Working Group
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis
- PROMs
Patient Reported Outcomes Measures
- PRP
Platelet-Rich-Plasma
- SVF
Stromal Vascular Fraction
- VAS
Visual Analogue Scale
- WOMAC
Western Ontario Macmaster University Osteoarthritis Index
- WORMS
Whole-Organ Magnetic Resonance Imaging Score
Authors’ contributions
T.K., C.P., and M.I., were involved in the systematic literature search and data extraction. The manuscript was prepared and critically revised by all authors and gave their approval prior to submission.
Funding
The project “Stem cells for the treatment of early to moderate osteoarthritis of the knee: a systematic review” was supported by a literature grant from the ON Foundation, Switzerland.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Aletto C, Giordano L, Quaranta M, Zara A, Notarfrancesco D, Maffulli N. Short-term results of intra-articular injections of stromal vascular fraction for early knee osteoarthritis. J Orthop Surg Res. 2022;17:310. doi: 10.1186/s13018-022-03196-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anz AW, Plummer HA, Cohen A, Everts PA, Andrews JR, Hackel JG. Bone marrow aspirate concentrate is equivalent to platelet-rich plasma for the treatment of knee osteoarthritis at 2 years: a prospective randomized trial. Am J Sports Med. 2022;50:618–629. doi: 10.1177/03635465211072554. [DOI] [PubMed] [Google Scholar]
- 3.Estrada E, Decima JL, Rodriguez M, Di Tomaso M, Roberti J. Patient-reported outcomes after platelet-rich plasma, bone marrow aspirate, and adipose-derived mesenchymal stem cell injections for symptomatic knee osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2020;13:1179544120931086. doi: 10.1177/1179544120931086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eymard F, Ornetti P, Maillet J, Noel E, Adam P, Legre-Boyer V, et al. Intra-articular injections of platelet-rich plasma in symptomatic knee osteoarthritis: a consensus statement from French-speaking experts. Knee Surg Sports Traumatol Arthrosc. 2021;29:3195–3210. doi: 10.1007/s00167-020-06102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Freitag J, Wickham J, Shah K, Tenen A. Real-world evidence of mesenchymal stem cell therapy in knee osteoarthritis: a large prospective two-year case series. Regen Med. 2022;17:355–373. doi: 10.2217/rme-2022-0002. [DOI] [PubMed] [Google Scholar]
- 6.Gadelkarim M, Abd Elmegeed A, Allam AH, Awad AK, Shehata MA, AbouEl-Enein A, et al. Safety and efficacy of adipose-derived mesenchymal stem cells for knee osteoarthritis: a systematic review and m-analysis. Joint Bone Spine. 2022;89:105404. doi: 10.1016/j.jbspin.2022.105404. [DOI] [PubMed] [Google Scholar]
- 7.Garay-Mendoza D, Villarreal-Martínez L, Garza-Bedolla A, Pérez-Garza DM, Acosta-Olivo C, Vilchez-Cavazos F, et al. The effect of intra-articular injection of autologous bone marrow stem cells on pain and knee function in patients with osteoarthritis. Int J Rheum Dis. 2018;21:140–147. doi: 10.1111/1756-185X.13139. [DOI] [PubMed] [Google Scholar]
- 8.Garza JR, Campbell RE, Tjoumakaris FP, Freedman KB, Miller LS, Santa Maria D, et al. Clinical Efficacy of Intra-articular Mesenchymal Stromal Cells for the Treatment of Knee Osteoarthritis: A Double-Blinded Prospective Randomized Controlled Clinical Trial. Am J Sports Med. 2020;48:588–598. doi: 10.1177/0363546519899923. [DOI] [PubMed] [Google Scholar]
- 9.Jo CH, Lee YG, Shin WH, Kim H, Chai JW, Jeong EC, et al. Intra-articular injection of mesenchymal stem cells for the treatment of osteoarthritis of the knee: a proof-of-concept clinical trial. Stem Cells. 2014;32:1254–1266. doi: 10.1002/stem.1634. [DOI] [PubMed] [Google Scholar]
- 10.Kaszyński J, Bąkowski P, Kiedrowski B, Stołowski Ł, Wasilewska-Burczyk A, Grzywacz K, Piontek T. Intra-Articular Injections of Autologous Adipose Tissue or Platelet-Rich Plasma Comparably Improve Clinical and Functional Outcomes in Patients with Knee Osteoarthritis. Biomedicines. 2022;10(3):684. doi: 10.3390/biomedicines10030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kyriakidis T, Asopa V, Baums M, Verdonk R, Totlis T. Unicompartmental knee arthroplasty in patients under the age of 60 years provides excellent clinical outcomes and 10-year implant survival: a systematic review : a study performed by the Early Osteoarthritis group of ESSKA-European Knee Associates section. Knee Surg Sports Traumatol Arthrosc. 2023;31:922–932. doi: 10.1007/s00167-022-07029-9. [DOI] [PubMed] [Google Scholar]
- 12.Kyriakidis T, Iosifidis M, Michalopoulos E, Melas I, Papadopoulos P, Stavropoulos-Giokas C. Matrix-induced adipose-derived mesenchymal stem cells implantation for knee articular cartilage repair Two years follow-up. Acta Orthop Belg. 2018;84:443–451. [PubMed] [Google Scholar]
- 13.Kyriakidis T, Iosifidis M, Michalopoulos E, Melas I, Stavropoulos-Giokas C, Verdonk R. Good mid-term outcomes after adipose-derived culture-expanded mesenchymal stem cells implantation in knee focal cartilage defects. Knee Surg Sports Traumatol Arthrosc. 2020;28:502–508. doi: 10.1007/s00167-019-05688-9. [DOI] [PubMed] [Google Scholar]
- 14.Lee WS, Kim HJ, Kim KI, Kim GB, Jin W. Intra-articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: a phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med. 2019;8:504–511. doi: 10.1002/sctm.18-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lopa S, Colombini A, Moretti M, de Girolamo L. Injective mesenchymal stem cell-based treatments for knee osteoarthritis: from mechanisms of action to current clinical evidences. Knee Surg Sports Traumatol Arthrosc. 2019;27:2003–2020. doi: 10.1007/s00167-018-5118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mousaei Ghasroldasht M, Seok J, Park H-S, Liakath Ali FB, Al-Hendy A. Stem cell therapy: from idea to clinical practice. Int J Mol Sci. 2022;23:2850. doi: 10.3390/ijms23052850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shang Z, Wanyan P, Zhang B, Wang M, Wang X. A systematic review, umbrella review, and quality assessment on clinical translation of stem cell therapy for knee osteoarthritis: ARe we there yet? Stem Cell Res Ther. 2023;14:91. doi: 10.1186/s13287-023-03332-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shoukrie SI, Venugopal S, Dhanoa RK, Selvaraj R, Selvamani TY, Zahra A, et al. Safety and efficacy of injecting mesenchymal stem cells into a human knee joint to treat osteoarthritis: a systematic review. Cureus. 2022;14:e24823. doi: 10.7759/cureus.24823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Song Y, Zhang J, Xu H, Lin Z, Chang H, Liu W, et al. Mesenchymal stem cells in knee osteoarthritis treatment: A systematic review and meta-analysis. J Orthop Translat. 2020;24:121–130. doi: 10.1016/j.jot.2020.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsubosaka M, Matsumoto T, Sobajima S, Matsushita T, Iwaguro H, Kuroda R. Comparison of Clinical and Imaging Outcomes of Different Doses of Adipose-Derived Stromal Vascular Fraction Cell Treatment for Knee Osteoarthritis. Cell Transplant. 2021;30:1–12. doi: 10.1177/09636897211067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varady NH, Cate G, Barghi A, Jobe N, Yakin D, Ylanan RC, et al. Positive early clinical outcomes of bone marrow aspirate concentrate for osteoarthritis using a novel fenestrated trocar. Knee. 2020;27:1627–1634. doi: 10.1016/j.knee.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 23.Vasanthan J, Gurusamy N, Rajasingh S, Sigamani V, Kirankumar S, Thomas EL, et al. Role of human mesenchymal stem cells in regenerative therapy. Cells. 2021;10:54. doi: 10.3390/cells10010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells K, Klein M, Hurwitz N, Santiago K, Cheng J, Abutalib Z, et al. Cellular and clinical analyses of autologous bone marrow aspirate injectate for knee osteoarthritis: a pilot study. PM R. 2021;13:387–396. doi: 10.1002/pmrj.12429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wiggers TG, Winters M, Van den Boom NA, Haisma HJ, Moen MH. Autologous stem cell therapy in knee osteoarthritis: a systematic review of randomised controlled trials. Br J Sports Med. 2021;55:1161–1169. doi: 10.1136/bjsports-2020-103671. [DOI] [PubMed] [Google Scholar]
- 26.Xia P, Wang X, Lin Q, Li X. Efficacy of mesenchymal stem cells injection for the management of knee osteoarthritis: a systematic review and meta-analysis. Int Orthop. 2015;39:2363–2372. doi: 10.1007/s00264-015-2785-8. [DOI] [PubMed] [Google Scholar]
- 27.Yokota N, Lyman S, Hanai H, Shimomura K, Ando W, Nakamura N. Clinical Safety and effectiveness of adipose-derived stromal cell vs stromal vascular fraction injection for treatment of knee osteoarthritis: 2-year results of parallel single-arm trials. Am J Sports Med. 2022;50:2659–2668. doi: 10.1177/03635465221107364. [DOI] [PubMed] [Google Scholar]
- 28.Zhang S, Xu H, He B, Fan M, Xiao M, Zhang J, et al. Mid-term prognosis of the stromal vascular fraction for knee osteoarthritis: a minimum 5-year follow-up study. Stem Cell Res Ther. 2022;13:105. doi: 10.1186/s13287-022-02788-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang Y, Bi Q, Luo J, Tong Y, Yu T, Zhang Q. The effect of autologous adipose-derived stromal vascular fractions on cartilage regeneration was quantitatively evaluated based on the 3D-FS-SPGR sequence: a clinical trial study. Biomed Res Int. 2022;2022:2777568. doi: 10.1155/2022/2777568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao D, Pan JK, Yang WY, Han YH, Zeng LF, Liang GH, et al. Intra-articular injections of platelet-rich plasma, adipose mesenchymal stem cells, and bone marrow mesenchymal stem cells associated with better outcomes than hyaluronic acid and saline in knee osteoarthritis: a systematic review and network meta-analysis. Arthroscopy. 2021;37:2298–2314 e2210. doi: 10.1016/j.arthro.2021.02.045. [DOI] [PubMed] [Google Scholar]
- 31.Zhu C, Wu W, Qu X. Mesenchymal stem cells in osteoarthritis therapy: a review. Am J Transl Res. 2021;13:448–461. [PMC free article] [PubMed] [Google Scholar]