Abstract
The emergence of clinical isolates of methicillin-resistant Staphylococcus aureus with reduced susceptibility to vancomycin has prompted a search for new and novel therapeutic agents active against S. aureus. Lysostaphin, a peptidase produced by Staphylococcus simulans, specifically cleaves the glycine-glycine bonds unique to the interpeptide cross-bridge of the S. aureus cell wall. The effectiveness of various regimens of dosing with intravenous lysostaphin was compared to that of vancomycin in the rabbit model of aortic valve endocarditis caused by a clinical methicillin-resistant S. aureus isolate. All animals were treated for a total of 3 days. The most active regimen, lysostaphin given three times daily, produced sterile vegetations in 10 of 11 treated rabbits, with a mean reduction in vegetation bacterial counts of 8.5 log10 CFU/g compared to the counts in the untreated controls. In contrast, vancomycin given twice daily sterilized no vegetations and reduced vegetation bacterial counts by only 4.8 log10 CFU/g. Lysostaphin given once daily was less effective, reducing mean vegetation bacterial counts by only 3.6 log10 CFU/g, but the combination of lysostaphin once daily and vancomycin twice daily reduced the mean vegetation bacterial density by 7.5 log10 CFU/g, a result that was significantly better than that for either regimen alone (P < 0.05). Lysostaphin was well tolerated by the rabbits, with no evidence of immunological reactions following up to 9 weeks of intravenous administration. We conclude that lysostaphin given alone or in combination with vancomycin is more effective in the treatment of experimental methicillin-resistant S. aureus aortic valve endocarditis than vancomycin alone.
Vancomycin is the treatment of choice for serious methicillin-resistant Staphylococcus aureus (MRSA) infections. However, despite the susceptibility in vitro of most clinical isolates of MRSA to vancomycin, failure of vancomycin therapy has been reported in 14 to 35% of patients with endocarditis caused by this organism (29, 42). The high vancomycin failure rate and the lack of alternative therapeutic agents has prompted a search for newer antimicrobial agents with activity against MRSA. The emergence of vancomycin-resistant strains of enterococci (12) and the recent discovery of strains of MRSA with decreased susceptibility to vancomycin (2, 4, 25, 38, 43) emphasize this need.
Lysostaphin is a 27-kDa peptidase produced by Staphylococcus simulans and was isolated in 1960 by Schindler and Schuhardt, as described previously (39, 41, 45). Lysostaphin specifically cleaves the pentaglycine cross-links unique to the cell wall of S. aureus and lyses cells in all metabolic states (growing, resting, or heat killed). Because staphylococci are highly resistant to lysis with such standard agents as lysozyme or detergents, lysostaphin has been widely used in research laboratories as a staphylolytic agent. Lysostaphin was studied in the 1960s and 1970s as a potential therapeutic agent in numerous animal models and in a single human patient (3, 15, 21, 23, 24, 33, 40, 44, 45). However, although its antimicrobial properties appeared promising, development of lysostaphin as a therapeutic agent was abandoned. Some of the reasons for failure to pursue the clinical use of lysostaphin included the availability of antistaphylococcal antibiotics, fears concerning the potential immunogenicity of a parenterally administered protein, and the impurity of lysostaphin preparations. The availability of recombinant lysostaphin that is >90% pure and that can be produced in quantity from Bacillus sphaericus (37) has provided an opportunity to assess the efficacy of lysostaphin in an animal model of S. aureus endocarditis and to compare it to the efficacy of standard therapy with vancomycin. We assessed the susceptibility of MRSA to lysostaphin in vitro, determined its therapeutic efficacy in the rabbit model of aortic valve MRSA endocarditis, and evaluated its toxicity and immunogenicity after long-term administration.
MATERIALS AND METHODS
Bacterial strains.
S. aureus 27619, a homotypically methicillin-resistant isolate, was used to challenge rabbits. Additional MRSA strains were taken from a collection of clinical strains maintained at the Medical College of Virginia as described previously (13). Two S. aureus isolates with reduced susceptibility to vancomycin were kindly provided by Fred Tenover, Centers for Disease Control and Prevention (4, 38, 43). A final S. aureus strain with reduced susceptibility to vancomycin was an isogenic derivative of S. aureus 27619, designated 27619VR, which was produced through stepwise passage in the presence of increasing concentrations of glycopeptides. MICs were determined by a broth microdilution method in cation-adjusted Mueller-Hinton broth (Becton Dickinson, Cockeysville, Md.) according to standards of the National Committee for Clinical Laboratory Standards with a final inoculum of 105 CFU/ml (31). The MICs of lysostaphin were determined following the addition of 0.1% bovine serum albumin (Sigma). Bovine serum albumin prevents the absorption of lysostaphin to polystyrene microtiter wells. The lowest concentration of antibiotic yielding no visible growth after incubation at 37°C for 24 h was taken as the MIC.
Experimental infection.
The rabbit model of aortic valve endocarditis, as described previously (35), was used to evaluate antibiotic treatment regimens. Seventy-two hours after transcarotid placement of a polyethylene catheter across the aortic valve, rabbits were injected intravenously through the marginal ear vein with 1 ml of an overnight culture containing 107 CFU of MRSA strain 27619 per ml. Blood samples for culture were obtained 24 h later, and the rabbits were randomly assigned to one of the following treatment groups: lysostaphin at 5 mg/kg of body weight given intravenously (i.v.) every 8 h (t.i.d.), lysostaphin at 5 mg/kg given i.v. once a day (q.d.), vancomycin at 30 mg/kg given i.v. every 12 h (b.i.d.), lysostaphin at 5 mg/kg given i.v. q.d. plus vancomycin at 30 mg/kg given i.v. b.i.d., or no treatment (control group). The surviving animals were killed by i.v. administration of pentobarbital after a total of 3 days of antibiotic treatment. Rabbits with negative blood cultures at 24 h after infection were excluded from subsequent analysis. To reduce the possibility of antibiotic carryover, rabbits were killed at least 18 h after administration of the last dose. The heart and kidneys were aseptically removed from each rabbit. Aortic valve vegetations were removed from each rabbit’s heart and weighed, and serial dilutions of vegetation homogenates were made. Kidneys were examined, and areas of abscess or infarct were removed, weighed, homogenized in saline, and serially diluted. Dilutions were plated on Mueller-Hinton agar, and colonies were counted after 48 h of incubation at 37°C. Titers of bacteria were expressed as log10 CFU per gram of vegetation or kidney tissue. Sterile vegetation cultures contained ≤2 log10 CFU/g (the limit of detection).
Antibiotics.
Lysostaphin (Ambicin L) was supplied by AMBI, Inc., Tarrytown, N.Y. Lysostaphin powder was stored at 4°C, and fresh solutions were prepared daily in 0.05 M Tris HCl–0.145 M NaCl. Vancomycin was obtained from Abbott Laboratories, Chicago, Ill.
Inclusion criteria.
For the final analysis, animals that fulfilled the following criteria were included: (i) cultured blood samples were positive for MRSA 27619, the test organism, at 24 h; (ii) the rabbits survived at least 24 h of antibiotic treatment; (iii) the catheter was properly placed across the aortic valve at necropsy, with macroscopic evidence of aortic valve endocarditis (visible vegetations); and (iv) the aortic valve vegetation and kidney tissue either were sterile or yielded pure cultures of MRSA 27619.
Bacteremia clearance.
Six rabbits with experimentally induced aortic valve endocarditis as described above were used. Blood samples for culture were obtained 24 h following infection with S. aureus 27619 (time zero). The rabbits then received a single i.v. dose of either lysostaphin at 15 mg/kg or vancomycin at 60 mg/kg. Blood samples for culture were obtained serially from all rabbits at 2, 4, 6, 8, 10, 12, 24, 30, and 48 h after administration of the single dose of antibiotics. Blood collected from the rabbits was serially diluted and plated onto Mueller-Hinton agar for quantitative bacterial count determinations. Sterile blood cultures contained <10 CFU/ml of blood (the limit of detection).
Long-term effects of lysostaphin administration.
Two female New Zealand White rabbits (weight, 2 to 3 kg) received a weekly i.v. injection of lysostaphin at 15 mg/kg for 9 weeks. Animals were closely observed for the development of any signs of allergic or anaphylactic reactions. Urine was tested for proteinuria with urinalysis reagent strips (Multistix; Bayer Inc., Etobicoke, Ontario, Canada), and at the end of 9 weeks rabbits underwent autopsy, with removal of the kidneys for pathological study. Serum collected at 9 weeks was examined for evidence of neutralizing antibodies, and serum bactericidal titers were determined. A decreased lytic activity of lysostaphin was used as evidence for the presence of neutralizing antibodies against lysostaphin. A modification of the broth microdilution method was used to determine the effect of serum on lysostaphin activity. Lysostaphin was serially diluted in microtiter wells in a volume of 50 μl, and then 25 μl of serum from immunized rabbits was added. Finally, 25 μl of the test organism, S. aureus 27619, was added at a concentration that resulted in a final inoculum of 105 CFU/ml. Pooled commercial rabbit serum served as a negative control. The MIC was that concentration of lysostaphin yielding no visible growth after 24 h of incubation at 37°C.
Statistical analysis.
The mean numbers of bacteria per gram of vegetation and kidney tissue in all treatment groups were compared by analysis of variance. Sterile cultures were entered as 2 log10 CFU/g, the limit of detection. The Student-Newman-Keuls test was used to adjust for multiple comparisons. For analysis of the rate of sterilization of valve vegetations, we used Fischer’s exact test (two-tailed) with the permutation-style adjustment to adjust for multiple comparisons. A P value of <0.05 was considered statistically significant for all tests.
RESULTS
In vitro studies.
The MICs of antibacterial agents for S. aureus 27619, the isolate used in the rabbit endocarditis model, were as follows: oxacillin, >100 μg/ml; vancomycin, 1 μg/ml; and lysostaphin, 0.03 μg/ml. For 16 additional MRSA strains, the MICs of lysostaphin ranged from 0.007 to 0.125 μg/ml. Lysostaphin demonstrated similar activity (MICs, 0.015 to 0.030 μg/ml) against three MRSA isolates with reduced susceptibility (MIC, 8 μg/ml) to vancomycin.
Serum bactericidal activity and long-term tolerability.
Lysostaphin exhibited excellent serum bactericidal activity in rabbits. To assess the long-term tolerability and activity of lysostaphin, uninfected rabbits received weekly i.v. injections of lysostaphin (15 mg/kg) for a total of 9 weeks. At 9 weeks, rabbits demonstrated serum bactericidal levels of 1:128 4 h following i.v. dosing. In addition, measurable bactericidal activity was still present 12 h postdosing.
Although rabbits still demonstrated high levels of lysostaphin in serum following 9 weeks of treatment, as evidenced by serum bactericidal activity, there was evidence of neutralizing antibody formation. Serum obtained from treated rabbits did appear to inhibit the lytic activity of lysostaphin in broth microdilution MIC determinations. The addition of serum from rabbits treated with lysostaphin for 9 weeks raised the MICs at least eightfold compared to the MICs determined in the presence of nonimmune rabbit serum.
Lysostaphin was well tolerated by the rabbits during long-term dosing. There was no evidence of hypersensitivity reactions during the 9-week dosing period. Rabbits did not develop proteinuria. Finally, both rabbits underwent autopsy and pathological examination of the kidneys at the end of the 9-week dosing trial. The kidneys from the first rabbit were normal, and the other rabbit demonstrated nonspecific plasma cellular interstitial nephritis on examination.
Bacterial clearance in the rabbit model of endocarditis.
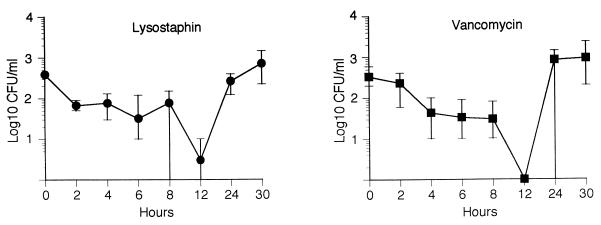
The results of the bacterial clearance studies are presented in Fig. 1. In the treatment of endocarditis, both lysostaphin and vancomycin had similar effects on bacterial clearance. Following the administration of a single dose of vancomycin (60 mg/kg) or lysostaphin (15 mg/kg) to rabbits with experimental endocarditis, there were similar rates of bacterial clearance for both agents. Twelve hours after the administration of a single dose, blood from all three rabbits treated with vancomycin and two of three rabbits treated with lysostaphin were sterile on culture. All rabbits had a return to positive blood cultures by 30 h postdosing.
FIG. 1.
Clearance of bacteremia in the rabbit model of experimental aortic valve endocarditis following the administration of single doses of lysostaphin (•) and vancomycin (▪). For the three rabbits in each group, means and ranges of bacterial counts in blood are given.
Endocarditis.
The results obtained from the 3-day antibiotic treatment regimen used to treat experimental endocarditis caused by MRSA 27619 are presented in Table 1. A total of 56 rabbits infected with MRSA 27619 were assigned to the various treatment regimens. Control rabbits had a mean aortic valve vegetation bacterial count of 10.79 ± 1.58 (standard deviation [SD]) log10 CFU/g, which is comparable to those reported previously from trials of MRSA endocarditis (1, 5, 6, 9, 10, 13, 14, 34).
TABLE 1.
Outcome of 3-day treatment of experimental methicillin-resistant S. aureus endocarditis
| Regimen | No. of rabbits sterile at the following site/total no. of rabbits:
|
Mean ± SD log10 CFU/g of tissue
|
||
|---|---|---|---|---|
| Valve vegetation | Kidney | Valve vegetation | Kidney | |
| None (controls) | 0/9 | 0/9 | 10.73 ± 1.58 | 7.85 ± 1.77 |
| Lysostaphin t.i.d. | 10/11a | 8/11 | 2.26 ± 0.85b,c | 2.26 ± 1.70b |
| Lysostaphin q.d. | 2/10 | 3/10 | 7.08 ± 3.74b | 4.32 ± 2.97b |
| Lysostaphin-vancomycin | 3/11 | 7/10 | 3.23 ± 1.41b,c | 2.69 ± 2.09b |
| Vancomycin b.i.d. | 0/15 | 10/15 | 5.89 ± 1.61b | 2.27 ± 1.56b |
P = 0.019 versus vancomycin at 30 mg/kg given i.v. b.i.d.–lysostaphin at 5 mg given q.d. by Fisher’s exact test.
P ≤ 0.05 for all regimens versus controls.
P ≤ 0.5 versus vancomycin at 30 mg/kg given i.v. b.i.d. or lysostaphin at 5 mg/kg given i.v. q.d.
All treatment regimens reduced aortic valve vegetation bacterial counts significantly (P < 0.05) compared with the counts in the vegetations from the controls. The most effective treatment arm was lysostaphin at 5 mg/kg given i.v. t.i.d., with a mean aortic valve vegetation bacterial count of 2.26 ± 0.85 (SD) log10 CFU/g. That is a mean reduction of 8.5 log10 CFU/g compared with the counts in the controls (P < 0.05). The second most effective treatment arm was the combination of lysostaphin at 5 mg/kg given i.v. q.d. with vancomycin at 30 mg/kg given i.v. b.i.d., with a mean aortic valve vegetation bacterial count of 3.23 ± 1.41 (SD) log10 CFU/g, which was a mean reduction of 7.5 log10 CFU/g compared with the counts in the controls (P < 0.05). The combination of lysostaphin at 5 mg/kg given i.v. q.d. with vancomycin at 30 mg/kg given i.v. b.i.d. was significantly better than either regimen alone (vancomycin b.i.d. or lysostaphin q.d.) at reducing mean aortic valve bacterial counts (P < 0.05). Lysostaphin at 5 mg/kg given i.v. q.d. also achieved a statistically significant (P < 0.05) reduction in the mean aortic valve vegetation bacterial counts compared with the counts in the controls (mean reduction, 3.6 log10 CFU/g). Rabbits treated with lysostaphin at 5 mg/kg given i.v. t.i.d. had significantly lower mean bacterial titers than those treated with vancomycin b.i.d. Mean aortic valve vegetation bacterial counts were 3.48 log10 CFU/g lower in the group given lysostaphin t.i.d. than in the vancomycin treatment group (2.26 versus 5.74 log10 CFU/g; P < 0.05). Although mean bacterial counts were lower for rabbits treated with lysostaphin t.i.d. (2.26 log10 CFU/g) than the combination of lysostaphin q.d. with vancomycin b.i.d. (3.23 log10 CFU/g), these differences were not statistically significant.
Rates of sterilization of aortic valve vegetations were significantly better in rabbits treated with regimens containing lysostaphin. All three lysostaphin regimens produced sterile aortic valve vegetation cultures, while no sterile aortic valve vegetation cultures were seen for rabbits treated with vancomycin alone. Lysostaphin at 5 mg/kg given i.v. t.i.d. sterilized the cultures of aortic valve vegetations from 91% (10 of 11) of the treated rabbits after 3 days of treatment. Although lysostaphin at 5 mg/kg given i.v. t.i.d. was not significantly better than the combination of lysostaphin with vancomycin in reducing mean aortic valve vegetation bacterial counts, the rate of sterilization (10 of 11 [91%] versus 3 of 11 [27%]) was significantly better (P = 0.019).
Table 1 depicts the results of kidney tissue cultures for the different treatment groups. Similar rates of kidney tissue abscess sterilization were seen with vancomycin (67%), lysostaphin at 5 mg/kg given i.v. t.i.d. (72%), and the combination of lysostaphin q.d. with vancomycin b.i.d. (70%). All treatment regimens reduced mean bacterial counts in the kidneys compared with the counts in the kidneys of the controls (P < 0.05), and no statistically significant difference was observed among the treated groups.
DISCUSSION
The treatment of serious infections caused by MRSA requires prolonged i.v. administration of bactericidal antibiotics. Since these strains are resistant to all β-lactams, glycopeptides such as vancomycin or teicoplanin are considered the treatments of choice. Despite the universal susceptibility of S. aureus to vancomycin, clinical failures in patients with severe MRSA infections are not uncommon (29, 42). Relapse rates of up to 15% have been reported following the treatment of endocarditis due to S. aureus with vancomycin (42). Many investigators have also suggested that the duration of S. aureus bacteremia is prolonged with vancomycin treatment compared to that with β-lactam treatment (29). Although the effectiveness of vancomycin in animal models of endocarditis can be improved with the addition of aminoglycosides and rifampin (1, 34), studies demonstrating effective alternative therapeutic agents in the treatment of MRSA endocarditis have been lacking.
In the studies reported above, we investigated the efficacy of lysostaphin compared to that of vancomycin in the treatment of experimental endocarditis due to MRSA. Lysostaphin given t.i.d. was significantly more effective than vancomycin at reducing the mean vegetation bacterial counts, producing sterile vegetations in 91% of rabbits, while vancomycin sterilized the vegetations in none of the rabbits. Our experiments did not examine rabbits for evidence of relapse, although the high rate of sterilization would argue against a risk for relapse among rabbits treated with lysostaphin t.i.d. However, in rabbits treated with vancomycin or lower doses of lysostaphin, in which vegetation bacterial counts were higher, there may be a significantly higher risk of relapse. Lysostaphin was also an effective adjunctive treatment when it was given in combination with vancomycin. The addition of lysostaphin q.d. to vancomycin reduced the mean aortic valve vegetation counts by 2.5 log10 CFU/g compared to those after treatment with vancomycin alone (3.23 versus 5.91 log10 CFU/g).
The reductions in aortic valve vegetation counts and rates of sterilization seen with lysostaphin in our study are the highest reported for single agents in the rabbit model of MRSA endocarditis (Table 2). The most effective single-agent regimens used to treat experimental MRSA endocarditis in the rabbit have been glycopeptides, either teicoplanin or vancomycin, with aortic valve sterilization rates of 27 to 80% reported for teicoplanin and sterilization rates of 0 to 87% reported for vancomycin. Although vancomycin is considered the best agent in this model, it is relatively ineffective at sterilizing aortic valve vegetations, results that mirror the lack of efficacy reported for vancomycin therapy of human S. aureus endocarditis. Of 15 studies of experimental MRSA aortic valve endocarditis with vancomycin doses and treatment durations similar to those used in our study, 6 reported no sterilization of aortic valve vegetations (5, 7, 13, 16, 18, 19), while 4 described sterilization of fewer than half of the vegetations (1, 6, 10, 32). Five remaining studies (11, 14, 26–28) reported aortic valve sterilization rates of 55 to 87%, although three of those studies used an MRSA strain that appears to have been more easily eradicated by a variety of antimicrobial agents in this model (26–28). Overall, 67% of studies with vancomycin for the treatment of experimental endocarditis due to MRSA have reported sterilization of aortic valve vegetations for fewer than half of treated animals.
TABLE 2.
Comparison of effectiveness of antimicrobial agents in the treatment of experimental MRSA aortic valve endocarditis in the rabbit
| Antimicrobial agenta | Mean reductions in aortic valve vegetations (log10 CFU/g)b | Sterilization rates (%)c | Reference(s) |
|---|---|---|---|
| Vancomycin | 1–6.96 | 0–87 | 5–7, 9–11, 13, 14, 16, 18, 26, 28, 32 |
| Teicoplanin | 4.2–6.06 | 11–80 | 8, 10, 14, 28 |
| Ampicillin-sulbactam | 0–4.8 | 0–31 | 7, 11 |
| Cephalothin | 1.1 | 0 | 6, 9 |
| Ciprofloxacin | 5.08 | 52 | 26 |
| Enoxacin | 4.2 | 0 | 20 |
| Fleroxacin | 6.1 | 67 | 27 |
| FK037 | 3.3–6.24 | 9–37 | 13 |
| Fusidic acid | 1.1 | 0 | 16 |
| Gentamicin | 3.6 | 9 | |
| Imipenem | 1–4.9 | 0–33 | 5, 11, 13 |
| L-695,256 (carbapenem) | 3.7 | 0 | 5 |
| Minocycline | 3.4 | 32 | |
| Nafcillin | 2.9 | 14 | 11 |
| Penicillin | 0–4.73 | 0–25 | 5, 19 |
| Rifampin | 0–2.45 | 0–10 | 1, 7 |
| RP 59500 | 1.5–3.1 | 0 | 17, 18 |
| Ticarcillin-clavulanate | 1.5 | 11 | 11 |
| Trimethoprim-sulfamethoxazole | 0.83 | 0 | 14 |
| Lysostaphin | 8.47 | 91 | Present study |
Treatment regimens were completed over 3 to 4 days with the rabbit model of experimental aortic valve endocarditis.
Mean reductions in counts (log10 CFU per gram of vegetation) compared to the counts for untreated control animals.
Rates of sterilization of aortic valve vegetation material.
Lysostaphin was first tested in animal models of staphylococcal infection in the 1960s (15, 21, 23, 24, 40, 41). Most of that work was with mice, and acute intraperitoneal infection or renal abscess models and standard penicillin-susceptible S. aureus strains were used. Lysostaphin was clearly efficacious in those models. Although the doses were as high as 100 mg/kg, the purity or potency of the lysostaphin preparations used was not always reported, so the true doses of the active ingredient may have been significantly lower in some cases. In one study (24), a very rapid reduction in kidney bacterial loads was demonstrated with single lysostaphin doses as low as 1.5 or 3 mg/kg; among the other agents tested, only penicillin G benzathine (Bicillin; at 25 or 100 mg/kg) gave results comparable to those achieved with lysostaphin. There is also a report of therapy of endocarditis in dogs caused by a penicillinase-producing S. aureus strain (21). That study was of an exploratory nature, and only one or a few dogs with documented endocarditis were treated with any given regimens of lysostaphin. Some evidence of efficacy was seen with dosages of 50 mg/kg/treatment or greater.
In humans, a lysostaphin spray was shown to eradicate S. aureus from the nares of 80% of carriers (30). One human patient has been treated with a single 500-mg i.v. dose of lysostaphin (44). The patient was suffering from acute myelocytic leukemia with profound pancytopenia and severe daunorubicin-induced cardiomyopathy. During his period of pancytopenia, he developed MRSA pneumonia and abscesses of the buttocks, thighs, and arms. He failed to respond to 3 weeks of treatment with antistaphylococcal antibiotics including nafcillin, methicillin, vancomycin, and cephalothin. Prior to the i.v. dosing, the patient demonstrated a 5-mm erythematous reaction to the intracutaneous administration of lysostaphin. A brief episode of flushing, hypotension, and tachycardia was noted following i.v. dosing, but the episode resolved with diphenhydramine and epinephrine. Unfortunately, the patient died of progressive heart failure 3 days after the lysostaphin infusion. Prior to death and at autopsy there was no evidence of staphylococcal infection, with negative cultures of samples of blood, lungs, and abscess sites. There was no examination for the presence of antibody directed against lysostaphin. The investigators concluded that further trials of lysostaphin treatment as adjunctive treatment for severe staphylococcal infections were warranted.
Since those studies were reported, rabbit and rat models of MRSA endocarditis have been established and are widely used to assess new therapeutic regimens. Our studies with the rabbit indicate that lysostaphin may be an effective therapeutic agent for the treatment of serious staphylococcal infections, despite concerns about potential immunogenicity. Lysostaphin was well tolerated by the rabbits, even with prolonged treatment for up to 9 weeks. In particular, there was no evidence of serum sickness, as assessed by the absence of fever, weight loss, proteinuria, joint swelling, or histologic renal lesions, following prolonged dosing. Rabbits demonstrated evidence of the presence of neutralizing antibodies following extended dosing, as indicated by an eightfold reduction of the lytic action of lysostaphin in the presence of immune serum. Despite the presence of neutralizing antibodies, high levels of serum bactericidal activity persisted. This is in concordance with earlier studies with the rabbit, in which Schaeffner et al. (40) demonstrated the presence of neutralizing and precipitating antibodies in the rabbit following repeated i.v. dosing. However, as in our own study, no adverse reactions were seen by those investigators following the administration of multiple doses.
Data on the immunogenicity of lysostaphin in human subjects is largely limited to studies evaluating its topical use. Among patients treated with topical lysostaphin in attempts to eradicate nasal staphylococcal carriage, there has been little evidence of sensitization or induced antibody formation (22, 36). Protein products such as thrombolytic enzymes (streptokinase) have been used with success for some time to treat humans with a low rate of medically manageable hypersensitivity reactions. These observations, in conjunction with previous data from studies with animals, would indicate that short-term or adjunctive therapy with lysostaphin may be possible in humans.
These studies also highlight the potential for the use of peptides as antimicrobial agents. Although lysostaphin is a large protein of approximately 27 kDa, its effectiveness in the endocarditis model indicates that the level of permeation into vegetations is adequate and that large protein products can exert potent antimicrobial activity in vivo.
In summary, lysostaphin may be an effective antimicrobial agent for the treatment of severe MRSA infections. Lysostaphin also demonstrates potent in vitro activity against S. aureus strains with reduced susceptibility to vancomycin. Additional studies with rabbits evaluating the activity of lysostaphin in the treatment of experimental endocarditis due to S. aureus with intermediate susceptibility to vancomycin are planned. These studies may have a significant impact as the search for alternative therapeutic agents in the treatment of serious MRSA infections continues.
ACKNOWLEDGMENTS
We thank Geri Hale-Cooper for technical assistance. We also thank Richard Novick for advice and encouragement in completing these experiments.
This work was supported in part by a grant from AMBI, Inc., and Public Health Service grant R37 AI35705.
REFERENCES
- 1.Bayer A S, Lam K. Efficacy of vancomycin plus rifampin in experimental aortic-valve endocarditis due to methicillin-resistant Staphylococcus aureus: in vitro-in vivo correlations. J Infect Dis. 1985;151:157–165. doi: 10.1093/infdis/151.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Boyce J M, Medeiros A A. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Clinical isolates of methicillin-resistant Staphylococcus aureus from the United States with subpopulations of cells with reduced susceptibility to vancomycin, abstr. LB-15; p. 6. [Google Scholar]
- 3.Bramley A J, Foster R. Effects of lysostaphin on Staphylococcus aureus infections of the mouse mammary gland. Res Vet Sci. 1990;49:120–121. [PubMed] [Google Scholar]
- 4.Centers for Disease Control and Prevention. Update: Staphylococcus aureus with reduced susceptibility to vancomycin—United States, 1997. Morbid Mortal Weekly Rep. 1997;46:813–815. [PubMed] [Google Scholar]
- 5.Chambers H F. In vitro and in vivo antistaphylococcal activities of L-695,256, a carbapenem with high affinity for the penicillin-binding protein PBP 2a. Antimicrob Agents Chemother. 1995;39:462–466. doi: 10.1128/aac.39.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers H F, Hackbarth C J, Drake T A, Rusnak M G, Sande M A. Endocarditis due to methicillin-resistant Staphylococcus aureus in rabbits: expression of resistance to β-lactam antibiotics in vivo and in vitro. J Infect Dis. 1984;149:894–903. doi: 10.1093/infdis/149.6.894. [DOI] [PubMed] [Google Scholar]
- 7.Chambers H F, Kartalija M, Sande M. Ampicillin, sulbactam, and rifampin combination treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis in rabbits. J Infect Dis. 1995;171:897–902. doi: 10.1093/infdis/171.4.897. [DOI] [PubMed] [Google Scholar]
- 8.Chambers H F, Kennedy S. Effects of dosage, peak and trough concentrations in serum, protein binding, and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob Agents Chemother. 1990;34:510–514. doi: 10.1128/aac.34.4.510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chambers H F, Miller M H. Emergence of resistance to cephalothin and gentamicin during combination therapy for methicillin-resistant Staphylococcus aureus endocarditis in rabbits. J Infect Dis. 1987;155:581–585. doi: 10.1093/infdis/155.3.581. [DOI] [PubMed] [Google Scholar]
- 10.Chambers H F, Sande M A. Teicoplanin versus nafcillin and vancomycin in the treatment of experimental endocarditis caused by methicillin-susceptible or -resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1984;26:61–64. doi: 10.1128/aac.26.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chambers H F, Sachdeva M, Kennedy S. Binding affinity for penicillin-binding protein 2a correlates with in vivo activity of β-lactam antibiotics against methicillin-resistant Staphylococcus aureus. J Infect Dis. 1990;162:705–710. doi: 10.1093/infdis/162.3.705. [DOI] [PubMed] [Google Scholar]
- 12.Clark N C, Cooksey R C, Hill B C, Swenson J M, Tenover F C. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob Agents Chemother. 1993;37:2311–2317. doi: 10.1128/aac.37.11.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Climo M W, Markowitz S M, Williams D S, Hale-Cooper C G, Archer G L. Comparison of in-vitro and in-vivo efficacy of FK037, vancomycin, imipenem and nafcillin against staphylococcal species. J Antimicrob Chemother. 1997;40:59–66. doi: 10.1093/jac/40.1.59. [DOI] [PubMed] [Google Scholar]
- 14.De Gorgolas M, Aviles P, Verdejo C, Fernandez Guerrero M L. Treatment of experimental endocarditis due to methicillin-susceptible or methicillin-resistant Staphylococcus aureus with trimehoprim-sulfamethoxazole and antibiotics that inhibit cell wall synthesis. Antimicrob Agents Chemother. 1995;39:953–957. doi: 10.1128/aac.39.4.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dixon R E, Goodman J S, Koenig M G. Lysostaphin: an enzymatic approach to staphylococcal disease. III. Combined lysostaphin-methicillin therapy of established staphylococcal abscesses in mice. Yale J Biol Med. 1968;41:62–68. [PMC free article] [PubMed] [Google Scholar]
- 16.Fantin B, Leclercq R, Duval J, Carbon C. Fusidic acid alone or in combination with vancomycin for therapy of experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1993;37:2466–2469. doi: 10.1128/aac.37.11.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fantin B, Leclercq R, Merle Y, Saint-Julien L, Veyrat C, Duval J, Carbon C. Critical influence of resistance to streptogramin B-type antibiotics on activity of RP 59500 (quinupristin-dalfopristin) in experimental endocarditis due to Staphylococcus aureus. Antimicrob Agents Chemother. 1995;39:400–405. doi: 10.1128/aac.39.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fantin B, Leclercq R, Ottaviani M, Vallois J-M, Maziere B, Duval J, Pocidalo J-J, Carbon C. In vivo activities and penetration of the two components of the streptogramin RP 59500 in cardiac vegetations of experimental endocarditis. Antimicrob Agents Chemother. 1994;38:432–437. doi: 10.1128/aac.38.3.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fantin B, Pierre J, Castela-Papin N, Saint-Julien L, Drugeon H, Farinotti R, Carbon C. Importance of penicillinase production for activity of penicillin alone or in combination with sulbactam in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1996;40:1219–1224. doi: 10.1128/aac.40.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilbert M, Boscia J A, Kobasa W D, Kaye D. Enoxacin compared with vancomycin for the treatment of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1986;29:461–463. doi: 10.1128/aac.29.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goldberg L M, DeFranco J M, Watanakunakorn C, Hamburger M. Studies in experimental staphylococcal endocarditis in dogs. VI. Treatment with lysostaphin. 1967. pp. 45–53. . Antimicrob. Agents Chemother. 1966. [PubMed] [Google Scholar]
- 22.Harris R L, Nunnery A W, Riley H D., Jr Effects of lysostaphin on staphylococcal carriage in infants and children. Antimicrob Agents Chemother. 1967;7:110–112. doi: 10.1128/AAC.7.1.110. [DOI] [PubMed] [Google Scholar]
- 23.Harrison E F, Cropp C B. Therapeutic activity of lysostaphin in experimental staphylococcal infections. Can J Microbiol. 1967;13:93–97. [Google Scholar]
- 24.Harrison E F, Zygmunt W A. Lysostaphin in experimental renal infections. J Bacteriol. 1967;93:520–524. doi: 10.1128/jb.93.2.520-524.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguru T, Tenover F C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–146. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 26.Kaatz G W, Barriere S L, Schaberg D R, Fekety R. Ciprofloxacin versus vancomycin in the therapy of experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1987;31:527–530. doi: 10.1128/aac.31.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaatz G W, Seo S M, Barriere S L, Albrecht L M, Rybak M J. Efficacy of fleroxacin in experimental methicillin-resistant Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1989;33:519–521. doi: 10.1128/aac.33.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaatz G W, Seo S M, Reddy V N, Bailey E M, Rybak M J. Daptomycin compared with teicoplanin and vancomycin for therapy of experimental Staphylococcus aureus endocarditis. Antimicrob Agents Chemother. 1990;34:2081–2085. doi: 10.1128/aac.34.11.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levine D P, Fromm B S, Reddy B R. Slow response to vancomycin or vancomycin plus rifampin in methicillin-resistant Staphylococcus aureus endocarditis. Ann Intern Med. 1991;115:674–680. doi: 10.7326/0003-4819-115-9-674. [DOI] [PubMed] [Google Scholar]
- 30.Martin R R, White A. The selective activity of lysostaphin in vivo. J Lab Clin Med. 1967;70:1–8. [PubMed] [Google Scholar]
- 31.National Committee for Clinical Laboratory Standards. Methods for dilution antimicrobial susceptibility test for bacteria that grow aerobically: Approved standard M7-A4. 4th edition. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 32.Nicolau D P, Freeman C D, Nightingale C H, Coe C J, Quintiliani R. Minocycline versus vancomycin for treatment of experimental endocarditis caused by oxacillin-resistant Staphylococcus aureus. Antimicrob Agents Chemother. 1994;38:1515–1518. doi: 10.1128/aac.38.7.1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oldham E R, Daley M J. Lysostaphin: use of a recombinant bactericidal enzyme as a mastitis therapeutic. J Dairy Sci. 1991;74:4175–4182. doi: 10.3168/jds.S0022-0302(91)78612-8. [DOI] [PubMed] [Google Scholar]
- 34.Perdikaris G, Giamarellou H, Pefanis A, Donta I, Karayiannakos P. Vancomycin or vancomycin plus netilmicin for methicillin- and gentamicin-resistant Staphylococcus aureus aortic valve experimental endocarditis. Antimicrob Agents Chemother. 1995;39:2289–2294. doi: 10.1128/aac.39.10.2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlman B B, Freedman L R. Experimental endocarditis. II. Staphylococcal infection of the aortic valve following placement of a polyethylene catheter in the left side of the heart. Yale J Biol Med. 1971;44:206–213. [PMC free article] [PubMed] [Google Scholar]
- 36.Quickel K E, Jr, Selden R, Caldwell J R, Nora N F, Schaffner W. Efficacy and safety of topical lysostaphin treatment of persistent nasal carriage of Staphylococcus aureus. Appl Microbiol. 1971;22:446–450. doi: 10.1128/am.22.3.446-450.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Recsei, P. A. June 1990. U.S. patent 4,931,390.
- 38.Robinson-Dunn B, Jennings G, Mitchell J, Ionescu M, Farnaz D, Donabedian S, Perri M B, Thal L A, Sunstrum J, Chow J W, Smith T, Zervos M J. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Characterization of a unique isolate of vancomycin-intermediate Staphylococcus aureus. abstr. LB-14; p. 6. [Google Scholar]
- 39.Schaffner W, Melly M A, Hash J H, Koenig M G. Lysostaphin: an enzymatic approach to staphylococcal disease. I. In vitro studies. Yale J Biol Med. 1967;39:215–229. [PMC free article] [PubMed] [Google Scholar]
- 40.Schaffner W, Melly M A, Koenig M G. Lysostaphin: an enzymatic approach to staphylococcal disease. II. In vivo studies. Yale J Biol Med. 1967;39:230–244. [PMC free article] [PubMed] [Google Scholar]
- 41.Schuhardt V T, Schindler C A. Lysostaphin therapy in mice infected with Staphylococcus aureus. J Bacteriol. 1964;88:815–816. doi: 10.1128/jb.88.3.815-816.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Small P M, Chambers H F. Vancomycin for Staphylococcus aureus endocarditis in intravenous drug users. Antimicrob Agents Chemother. 1990;34:1227–1231. doi: 10.1128/aac.34.6.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith T L, Pearson M, Wilcox K, Robinson-Dunn B, Hill B, Lancaster M, Rodgers G, Ruble C, Flegel E, McCallister S, Miller J M, Ellis H, Campbell C, Jarvis W R. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Staphylococcus aureus with reduced susceptibility to vancomycin, United States, 1997, abstr. LB-16; p. 6. [Google Scholar]
- 44.Stark F R, Thornsvard C, Flannery E P, Artenstein M S. Systemic lysostaphin in man. Apparent antimicrobial activity in a neutropenic patient. N Engl J Med. 1974;291:239–240. doi: 10.1056/NEJM197408012910507. [DOI] [PubMed] [Google Scholar]
- 45.Zygmunt W, Tavormina P A. Lysostaphin: model for a specific enzymatic approach to infectious disease. Prog Drug Res. 1972;16:309–333. doi: 10.1007/978-3-0348-7081-8_7. [DOI] [PubMed] [Google Scholar]