Abstract
Evidence for the human health effects of pesticides is needed to inform risk assessment. We studied the relationship between occupational insecticide use and risk of non-Hodgkin lymphoma (NHL) by pooling data from nine case-control studies participating in the InterLymph Consortium, including 7909 cases and 8644 controls from North America, the European Union and Australia. Insecticide use was coded using self-report or expert assessment, for insecticide groups (eg, organophosphates, pyrethroids) and active ingredients (eg, malathion, permethrin). Associations with insecticides were estimated using logistic regression to produce odds ratios (ORs) and 95% confidence intervals (CI) for all NHL and NHL subtypes, with adjustment for study site, demographic factors and use of other pesticides. Occupational insecticide use, overall, was not associated with risk of NHL. Use of organophosphate insecticides was associated with increased risk of all NHL and the subtype follicular lymphoma, and an association was found with diazinon, in particular (ever use: OR = 2.05, 95%CI: 1.24-3.37). The carbamate insecticide, carbaryl, was associated with risk of all NHL, and the strongest associations were found with T-cell NHL for ever-use (OR = 2.44, 95%CI: 1.13-5.28) and longer duration (>8 years vs never: OR = 2.90, 95%CI: 1.02-8.25). There was no association of NHL with other broad groups of insecticides, including organochlorines and pyrethroids, and some inverse associations were estimated in relation to historical DDT use. Our findings contribute to the totality of evidence available to help inform risk decisions by public health and regulatory agencies of importance given continued, widespread use of organophosphate and carbamate insecticides.
Keywords: chronic lymphocytic leukemia, insecticide, multiple myeloma, non-Hodgkin lymphoma, pesticide
1 |. INTRODUCTION
Exposure to insecticides is widespread, both in agricultural and residential/community settings. Approximately 400 000 tons of insecticides are applied globally in agriculture each year, and this amount has remained relatively constant over the past two decades,1 even as restrictions have been imposed on certain insecticides due to ecological or health concerns. Several insecticides in current use are suspected to contribute to development of non-Hodgkin lymphoma (NHL) and have been prioritized in epidemiological research; however, for most insecticide active ingredients, the evidence from human studies remains limited for causal inference.2 These data deficiencies have hindered previous reviews of the potential carcinogenicity of some frequently used insecticides, such as permethrin,2 or necessitated heavy reliance on animal and mechanistic data in evidence conclusions (such as for dieldrin,3 parathion4 and carbaryl5).
General-population based studies often lack statistical power for investigation of insecticide-related risks, because of the relative rarity of pesticide-exposed jobs. Large occupational cohorts of participants with frequent pesticide exposures, such as the Agricultural Health Study (AHS) of over 50 000 licensed pesticide applicator participants,7 are advantageous, but few.8 Therefore, additional evidence from general-population based studies is desirable in the assessment of pesticide-related risks, within multiple, diverse populations and across the broad spectrum of jobs in which pesticides are used. A consortium-based approach to combine data from several general-population studies is valuable for enhancing statistical power beyond that achievable with any individual study—enabling well-powered analyses of less frequent exposures, as well as evaluation of etiological variation between NHL case subtypes.6 For example, the North American Pooled Project (NAPP), with a combined study population of 1690 NHL cases and over 5000 controls, reported increased risk of NHL in relation to use of several insecticides including lindane, malathion and carbaryl, and found that the associations were observed only for certain NHL subtypes.9,10
We previously assessed the association between lifetime occupational history and NHL risk using pooled data from 10 case-control studies participating in the InterLymph Consortium (>10 000 cases and >12 000 controls).11 As hypothesized, NHL risk was increased among participants with a history of work in farming. Crop farming was associated with increased risk of NHL in the pooled study, whereas animal farming and mixed (both crop and animal) farming were not. This observed heterogeneity of risk among farmers implies variation of exposures across those jobs, thus motivating research to characterize risk in relation to specific exposures, such as pesticides and the wide variety of active ingredients used in farming. In the InterLymph study, we also found increased risk of NHL among participants who worked in forestry or cleaning/janitorial jobs—suggesting possible pesticide-related effects in nonfarming occupations. To follow up on these findings, we conducted a study of occupational insecticide use in relation to NHL risk in an InterLymph pooled analysis of nine case-control studies that queried participants on pesticide exposures. Our goal was to identify past and current-use insecticides that may increase risk of NHL, and to evaluate associations with major NHL subtypes.
2 |. MATERIALS AND METHODS
2.1 |. Study population
The International Lymphoma Epidemiology Consortium (InterLymph) was formed in 2001 to facilitate intellectual exchange and collaborative research toward identifying preventable risk factors for lymphoid cancers. Data from case-control studies participating in InterLymph were pooled for this investigation of occupational insecticide exposures in relation to risk of non-Hodgkin lymphoid malignancies (NHL), including the major NHL subtypes diffuse large B-cell lymphoma (DLBCL) and follicular lymphoma (FL), as well as chronic lymphocytic leukemia (CLL)/small lymphocytic lymphoma/mantle cell lymphoma/prolymphocytic leukemia (hereafter referred to, collectively, as CLL), multiple myeloma (MM), other B-cell lymphomas (OBCL), T-cell lymphomas (TCL) and not otherwise specified/unknown NHL (NOS).12 Individual case-control studies were eligible for the pooled analysis if they collected information on occupational insecticide use, including specific types of insecticides.
A summary of the nine participating case-control studies is provided in Table 1.13-22 The individual studies included persons with histologically confirmed incident primary diagnosis of NHL that occurred during the respective enrollment periods, spanning from 1980 to 2013. Controls were identified in the general population or from participating hospitals/clinics and were frequency-or pair-matched to the cases by variables including age and sex, and in some studies, region or ethnicity. For evaluation of insecticides, the pooled database included 8644 controls and 7909 cases (1469 CLL, 1794 DLBCL, 1295 FL, 1355 MM, 1405 OBCL, 251 TCL and 340 NOS).
TABLE 1.
Case-control studies participating in the InterLymph study of insecticides
| Study abbreviation | LAMMCC13 | LANHL14 | Italian15 | Yale16,17 | Epilymph18 | NSW19 | ENGELA20 | Mayo21 | BCMM22 |
|---|---|---|---|---|---|---|---|---|---|
| Location(s) | USA: Los Angeles County | USA: Los Angeles County | Italy: Firenze, Forli, Imperia, Latina, Novara, Ragusa, Siena, Torino, Verona | USA: Connecticut | Europe: Czech Republic, France, Germany, Ireland, Italy, Spain | Australia: New South Wales and Australian Capital Territory | France: Bordeaux, Brest, Caen, Lille, Nantes, Toulouse | USA: Minnesota, Iowa, Wisconsin | Canada: British Columbia |
| Case diagnosis years | 1980-1990 | 1988-1991 | 1990-1994 | 1996-2002 | 1998-2003 | 1999-2001 | 2000-2004 | 2002-2012 | 2009-2013 |
| Source of controls | Population | Population | Population | Population | Population or Hospital | Population | Hospital | Clinic | Population |
| Control matching to cases | Pair-matched by age, sex, ethnic, neighborhood | Pair-matched by age, sex, ethnic, neighborhood | Frequency-matched by age, sex | Frequency-matched by age; females only | Pair-matched or frequency-matched by age, sex, region | Frequency-matched by age, sex, region | Pair-matched by age, sex, region | Frequency-matched by age, sex, region | Frequency-matched by age, sex |
| Participants queried regarding specific pesticides | All | All | Ever worked in agriculture | All | All | Ever worked as a farmer, pesticide applicator, or gardener | Ever worked as a farmer or gardener for at least 6 months | Ever worked on a farm or with pesticides for >1 year | Ever lived on a farm or worked in agriculture, gardening, parks, golf courses, or forestry |
| Basis for pesticide exposure classification | Self-report (closed-ended questions on pesticides exposed to at work) | Self-report (open-ended question on pesticides directly exposed to at work) | Expert-assessment (open-ended questions on products used at work and additional farming questionnaire; responses checked against a crop-exposure matrix) | Self-report (open-ended questions on exposures in work or leisure and additional farming questionnaire for pesticides handled, with provided list of frequently used pesticides as a prompt) | Expert-assessment (open-ended questions on products used at work and additional farming questionnaire; responses checked against a crop-exposure matrix) | Self-report (open-ended questions on specific pesticides personally mixed or applied) | Expert-assessment (open-ended questions about products used at work and additional farming questionnaire; responses checked against product availability dates, type and size of crops, geographic location, and treatment frequency) | Self-report (closed-ended questions on pesticides personally mixed or applied) | Self-report (open-ended questions on pesticides personally applied, mixed, or loaded at work or when living on a farm) |
| Included in the insecticides study (Ns) | |||||||||
| Cases | 275 | 368 | 1243 | 773 | 1869 | 688 | 404 | 1898 | 391 |
| Controls | 278 | 372 | 1142 | 706 | 2462 | 683 | 447 | 2183 | 371 |
| Case subtypes (% of all cases) | |||||||||
| Chronic lymphocytic leukemia/small lymphocytic lymphoma/mantle cell lymphoma/prolymphocytic leukemia (CLL) | 0% | 0% | 9.3% | 8.5% | 25.5% | 7.4% | 24.3% | 34.8% | 0% |
| Diffuse large B-cell lymphoma (DLBCL) | 0% | 35.9%a | 21.2% | 24.3% | 27.4% | 33.3% | 26.5% | 19.1% | 0% |
| Follicular lymphoma (FL) | 0% | 10.6% | 8.2% | 17.6% | 13.4% | 36.2% | 12.4% | 24.7% | 0% |
| Multiple myeloma (MM) | 100% | 0% | 14.2% | 23.2% | 14.8% | 0% | 13.9% | 0% | 100% |
| Other B-cell lymphoma (OBCL) | 0% | 27.2% | 2.6% | 7.6% | 18.6% | 15.4% | 14.9% | 10.2% | 0% |
| T-cell lymphoma (TCL) | 0% | 0.3% | 4.5% | 4.4% | 0% | 3.5% | 5.2% | 4.6% | 0% |
| NOS-unknown (NOS) | 0% | 26.1% | 39.9% | 14.4% | 0.3% | 4.2% | 3.0% | 6.6% | 0% |
| Control characteristics | |||||||||
| Age in years (mean [SD]) | 61.2 (9.0) | 51.1 (14.4) | 55.0 (13.7) | 61.3 (14.2) | 56.2 (16.0) | 56.3 (12.0) | 52.5 (13.5) | 61.6 (13.1) | 65.6 (8.0) |
| Male gender (%) | 54.7% | 49.2% | 55.4% | 0% | 53.6% | 57.7% | 100% | 53.3% | 57.4% |
| Non-white race or Hispanic ethnicity (%) | 32.0% | 23.9% | 0% | 8.1% | 1.5% | 12.9% | 0.4% | 2.4% | 9.4% |
| Low socioeconomic status (%) | 46.4% | 32.3% | 57.3% | 36.7% | 45.5% | 33.7% | 27.7% | 23.0% | 29.4% |
| Farming job, ever (%) | 10.4% | 6.5% | 31.4% | 2.1% | 17.1% | 15.1% | 18.1% | 15.6% | 7.0% |
| Occupational pesticide exposure, ever (%) | 5.0% | 8.3% | 26.7% | 11.9% | 8.5% | 10.3% | 10.5% | 14.2% | 5.1% |
| Occupational insecticide exposure, ever (%) | 2.2% | 3.8% | 6.3% | 8.2% | 2.0% | 8.2% | 8.3% | 12.1% | 3.8% |
Note: Studies are ordered in the table by the earliest case diagnosis year.
The LANHL study included intermediate- and high-grade NHL diagnosed in HIV-negative individuals, to correspond to a concurrent study protocol of HIV-related NHL.
We requested data from each study on occupational history, farming and pesticide use at work. Four of the studies queried all participants about pesticide use in any type of job, and five of the studies administered questions about pesticides to persons with a history in farming or who ever worked in other jobs with probable exposure, such as pest control, gardening and forestry. Only one questionnaire did not distinguish personal handling of chemicals between work and at home (Yale). Variables that were already harmonized for previous InterLymph studies were obtained from the Data Coordinating Center (DCC) of InterLymph (the Mayo Clinic, Rochester, MN, USA), including age, sex, race, Hispanic ethnicity, socioeconomic status (SES, based on education and/or income in the studies), NHL subtypes coded according to the 2008 World Health Organization (WHO) classification23,24 and occupational title coded according to the International Standard Classification of Occupations (ISCO) 1968.25
2.2 |. Pesticide exposure assessment
Occupational use of insecticides and other pesticides were coded directly from questionnaire responses (self-report, six studies) or from reviews conducted by local experts in the individual studies (expert assessment, three studies). Self-reported pesticide use was based on responses to either closed-ended13,21 or open-ended14,16,17,19,22 questions (Table 1); these were coded as a specific insecticide if either the active ingredient, or a product that contained the active ingredient, was named. Only personal handling of pesticides (ie, mixed/loaded/applied) was coded as exposed, from the questionnaires that provided this level of detail. Self-reported responses to open-ended questions were reviewed (blinded to case status) by the principal investigator (AJD) and an industrial hygienist (TH) and were coded as insecticide groups and active ingredients by matching reported pesticide names to information from product labels, EPA registration materials, manufacturer documentation, and pesticide classification databases; the few discrepancies between AJD and TH were resolved through discussion and reevaluation of information. Self-reported pesticides that were not coded as insecticide groups and/or active ingredients of interest were coded in broad categories as other insecticides, herbicides, other noninsecticide pesticides or “any pesticide” (where information was lacking for more specific coding). Expert assessment of pesticide use, previously conducted by three of the studies as described in previous publications,15,18,20 involved review (blinded to case status) of participant reports of pesticide use and cross-checking of responses against information such as product availability dates, geographic location and crops/animals (ie, crop-exposure matrix, for example,26). Based on the expert review, a participant’s originally reported pesticide use may have been coded by the expert as either exposed or unexposed. In addition, participants who did not report pesticide use might have been coded by experts as exposed based on their responses regarding farming, crops, and animals.
The insecticides coded for the pooled study were chosen based on a priori interest and frequency in the pooled data; they included a broad grouping of any type of insecticide, organochlorines (group), DDT, chlordane, lindane, dieldrin, organophosphates (group), malathion, chlorpyrifos, parathion, diazinon, pyrethroids (group), permethrin, carbamates (group) and carbaryl. Studies were included in the pooled data for each insecticide if there was any participant in the study that ever used the particular type of insecticide. For each insecticide, variables were created for ever-use and duration of use (years), by summarizing information across all jobs held by a participant. In addition, based on the first year of the insecticide use, lagged exposure variables were coded, limited to use that occurred earlier than 10 years before the reference date (diagnosis date for cases or corresponding reference date for controls). Duration and lagged-use duration variables were categorized for statistical analyses based on percentiles (p) among those exposed to each insecticide, in two exposure categories (≤50p, >50p), three categories (≤50p, >50p-75p, >75p), and four categories (≤50p, >50p-75p, >75p-90p, >90p).
2.3 |. Statistical analysis
2.3.1 |. Pooled analysis
All pooled analyses were conducted using SAS v. 9.4 (Cary, NC, USA). Logistic regression was used to estimate the odds ratio (OR) and 95% CI for the association between each insecticide and risk of NHL. Exposure was modeled as ever-use, duration in categories and lagged versions of the variables, each with never-use as the reference category. The trend in NHL risk across categories of duration was evaluated by the P-value from modeling the median of each duration category as a continuous variable. Several variables were selected, a priori, to adjust for potential confounding, including (all coded as indicator terms) the study center (eg, specific city or hospital acting as a data collection center for a particular study), participant age (years in categories of <45, 45-54, 55-64, 65-74, ≤75), gender and SES (low, medium, high). We also assessed several additional variables as potential confounders based on comparison of the unadjusted and adjusted estimates for insecticide use, including race/ethnicity (non-Hispanic white or assumed based on region/ethnicity [<2% of pooled population], non-white or Hispanic, missing), farming occupation (ever/never), “high-probability exposed occupation” (ever/never worked in any job we considered, a priori, as posing relatively high probability of pesticide use, including jobs in farming/fishing/forestry, gardener/groundskeeper, cleaner/janitor/building maintenance worker [including pest control], or general laborer), herbicide use (ever/never) and other noninsecticide pesticide use (ever/never, for example, fungicides, nematicides, rodenticides, etc.). Based on our assessment of covariate adjustment, we included herbicide use and other noninsecticide pesticide use as additional covariates in all analyses.
Etiologic heterogeneity was evaluated by fitting polytomous logistic regression models for the NHL subtypes, including CLL, DLBCL, FL, MM, OBCL and TCL, with estimation of the OR and 95% CI for each subtype-specific association vs a common control group. A P-value was computed for heterogeneity of the association with each insecticide exposure among the six subtypes (P-heterogeneity).
We presented as our main results the associations with ever and lagged use (yes/no) exposure with at least five exposed cases, and the associations with duration variables for the most fine categorization (2, 3 or 4 duration categories) that contained at least 10 exposed cases in the highest exposure category (we presented two-category duration as the default if none of the categorizations had 10 exposed cases in the highest category).
2.3.2 |. Sensitivity analyses
Several additional analyses of the pooled data were conducted to assess sensitivity of the main results to alternate specifications of the model covariates, exposure assessment, population subgroups or case definition, including (a) adjustment for farming occupation; (b) adjustment for insecticide groups, including organochlorines, organophosphates, carbamates or pyrethroids (differing based on the main insecticide of interest in the model), in addition to the main model covariates for herbicides and other noninsecticide pesticides; (c) dropping the covariates for herbicides and other noninsecticide pesticides; (d) dropping the covariate for SES; (e) fitting separate models for studies with exposures coded according to self-report or expert assessment; (f) considering as exposed, only persons with assessed exposure who also ever worked in a high-probability exposed occupation (as above, farming/fishing/forestry, gardener/groundskeeper, cleaner/janitor/building maintenance [including pest control] or general laborer); (g) limiting the population to participants who ever lived or worked on a farm, to assess the impact of hypothesized higher baseline risk of NHL in farmers27; (h) fitting separate models for males and females; (i) excluding the largest study with the highest frequency of insecticide exposure (Mayo); (j) defining cases according to an NHL classification that included lymphomas originating from B cells and T cells, but excluded CLL and MM (similar to the 1982 Working Formulation).28
2.3.3 |. Meta-analysis
Random effects meta-analysis was conducted on study-specific ORs to assess comparability of findings from the pooled analysis to those from an alternate approach to analysis of data from multiple studies, as well as to test heterogeneity of the estimated effect among studies by the I2 statistic. Meta-analyses were conducted using StataSE v. 15 (StataCorp LLC, College Station, TX, USA).
3 |. RESULTS
The nine case-control studies participating in the pooled analysis were conducted in North America and Europe from 1980 to 2013 (Table 1). Two of the studies focused only on MM (LAMMCC, BCMM), and an additional four studies included MM among other NHL subtypes (Italian, Yale, Epilymph, ENGELA). CLL was included as an NHL subtype in three studies (Epilymph, ENGELA, Mayo), whereas other studies included the histologically similar small lymphocytic lymphoma, but not CLL. Two studies conducted from 1988 to 1994 had relatively large proportions of cases with NOS-unknown pathology (LANHL [26.1%], Italian [39.9%]) and the NOS subtype declined over later study periods. One study included only women (Yale) and another included only men for the analysis of occupational insecticides (ENGELA); populations of the other studies were reasonably similar with regards to age and gender. There were very few non-Hispanic white participants in the European studies, compared to the North American and Australian studies. The individual study controls differed with regards to history of work in farming, as at least one study focused on agricultural regions (Italian, 31.4% ever held farming job) and other studies were conducted in large urban areas (LAMMCC [10.4% farming], LANHL [6.5% farming], BCMM [7.0% farming]). The frequency with low SES was highest in the study focused on agricultural areas (Italian, 57.3%) and was lowest in studies conducted during later time periods. Use of pesticides and insecticides appeared to correlate with the proportion of controls who ever worked in farming. One exception (Yale) reported low farming history (2.1%) among their all-female population in Connecticut, USA but relatively high insecticide use (8.2%); this was the only study that prompted for exposures in leisure, as well as at work. The study with the lowest coded insecticide use among controls (Epilymph, 2.0%) reported moderate farming history (17.1%); however, experts in that study assessed only certain classes of insecticides (organochlorines, organophosphates, carbamates). Frequencies of the insecticide classes and active ingredients used in each study are shown in Table S1. The highest numbers of exposed controls were observed in the relatively large Mayo and Italian studies. The proportion exposed was generally highest in the Mayo study, which collected data by closed-end questions specifying each pesticide.
Characteristics of the 7909 cases and 8644 controls in the pooled dataset are shown in Table 2. Although most of the individual studies matched by demographic factors, a higher proportion of cases than controls were older, male and of low SES. Cases and controls were fairly similar with regard to race/ethnicity; over 93% of participants were non-Hispanic white/assumed. Cases were slightly more likely than controls to have ever worked in farming (17.4% vs 16.2%) and to have ever been occupationally exposed to any type of pesticide (14.0% vs 12.6%) or insecticide (7.4% vs 6.6%). The 570 control participants with occupational insecticide use were more likely to have ever worked in any of the occupations considered a priori as having high probability of pesticide use (77.2%) than exclusively in other occupations (22.8%). Specifically, controls with insecticide use had frequently worked in farming (72.5%) and forestry (2.1%), or as gardeners/groundskeepers (5.0%), cleaners/janitors/building maintenance workers (6.7%) and general laborers (4.9%). However, when considering all controls who ever worked in farming, only 29.5% had ever used insecticides.
TABLE 2.
Characteristics of cases and controls in the InterLymph study of insecticides (n [%])
| Controls N = 8644 |
Cases N = 7909 |
|
|---|---|---|
| Age | ||
| <45 years | 1541 (17.8) | 1051 (13.3) |
| 45-54 years | 1541 (17.8) | 1433 (18.1) |
| 55-64 years | 2242 (26.0) | 2218 (28.1) |
| 65-74 years | 2470 (28.6) | 2422 (30.6) |
| ≥75 years | 850 (9.8) | 785 (9.9) |
|
| ||
| Gender | ||
| Female | 4139 (47.9) | 3620 (45.8) |
| Male | 4505 (52.1) | 4289 (54.2) |
|
| ||
| Race/Hispanic ethnicitya | ||
| White, non-Hispanic | 8156 (94.4) | 7361 (93.1) |
| Black | 113 (1.3) | 136 (1.7) |
| Other non-white or Hispanic | 337 (3.9) | 367 (4.6) |
| Missing | 38 (0.4) | 45 (0.6) |
|
| ||
| Socioeconomic status | ||
| Low | 3249 (37.6) | 3296 (41.7) |
| Medium | 2854 (33.0) | 2318 (29.3) |
| High | 2461 (28.5) | 1851 (23.4) |
| Missing | 80 (0.9) | 444 (5.6) |
|
| ||
| Farming occupation | ||
| Never | 7245 (83.8) | 6530 (82.6) |
| Ever | 1399 (16.2) | 1379 (17.4) |
|
| ||
| Occupational pesticide useb | ||
| Never | 7555 (87.4) | 6799 (86.0) |
| Ever | 1089 (12.6) | 1110 (14.0) |
|
| ||
| Occupational insecticide use, any | ||
| Never | 8074 (93.4) | 7323 (92.6) |
| Ever | 570 (6.6) | 586 (7.4) |
White category includes “assumed white”, based on region and/or ethnicity (<2% of study population).
Occupational use of any type of pesticide including insecticides, herbicides, fungicides, fumigants, rodenticides and so on.
Use of any type of insecticide was not associated with risk of all NHL for ever-use (OR = 1.01, 95%CI: 0.84-1.18), lagged use or with longer duration (Table 3 shows main results for all NHL; results from additional duration categorizations are shown in Table S2). Organochlorine insecticide use, as a group, was not associated with risk of all NHL (ever, OR = 0.92, 95% CI: 0.76-1.12). The most frequently used organochlorine, DDT, was inversely associated with all NHL for ever-use (OR = 0.78, 95% CI: 0.61-1.00), but without a trend by duration. Elevated, nonstatistically significant ORs were observed in association with the less frequently used organochlorine insecticides chlordane, lindane and dieldrin, such as increased risk of all NHL in association with lagged-use of lindane with duration longer than 8 years (OR = 2.13, 95%CI: 0.89-5.10, P-trend = .13). Use of organophosphate insecticides, as a group, was associated with risk of all NHL (ever-use, OR = 1.22, 95%CI: 1.01-1.47), and risk increased with longer duration (>31 years, OR = 1.49, 95%CI: 0.90-2.47, P-trend = .05). Of the organophosphate active ingredients we evaluated, diazinon was associated with increased risk of all NHL, with general trends of stronger associations with longer duration, but similar estimated effects for ever and lagged use. There were no clear associations with chlorpyrifos, malathion or parathion. The carbamate insecticide, carbaryl, was associated with increased risk of all NHL (ever, OR = 1.34, 95%CI: 1.03-1.75). The risk associated with carbaryl increased from the lowest duration category to duration >50th to ≤75th percentile for both ever use (OR = 1.97, 95%CI: 1.12, 3.45) and lagged use (OR = 2.12, 95%CI: 1.26, 3.56), but declined across longer durations. The insecticide groups, carbamates and pyrethroids, were not associated with risk of all NHL.
TABLE 3.
Association of occupational insecticide use with risk of NHL
| Ever-usea | Controls | Cases | OR (95% CI)b | Duration of use (years) | Controls | Cases | OR (95% CI) | Lagged use, Duration (years)b,c | Controls | Cases | OR (95% CI) | Studies included |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Insecticides, any | ||||||||||||
| Never | 8074 | 7323 | 1 (Referent) | |||||||||
| Ever | 570 | 586 | 1.00 (0.84, 1.18) | ≤10 | 302 | 285 | 0.92 (0.75, 1.14) | ≤10 | 265 | 267 | 0.99 (0.80, 1.24) | Nine studies: BCMM, ENGELA, Epilymph, Italian, LAMMCC, LANHL, Mayo, NSW, Yale |
| >10-22 | 126 | 147 | 1.15 (0.88, 1.52) | >10-20 | 118 | 126 | 1.03 (0.77, 1.38) | |||||
| Lagged | 503 | 523 | 1.00 (0.84, 1.20) | >22-32 | 91 | 82 | 0.81 (0.58, 1.13) | >20-30 | 73 | 72 | 0.92 (0.65, 1.32) | |
| >32 | 49 | 63 | 1.16 (0.78, 1.73) | >30 | 46 | 56 | 1.04 (0.68, 1.58) | |||||
| P-trend = .72d | P-trend = .96 | |||||||||||
|
| ||||||||||||
| Organochlorine insecticides | ||||||||||||
| Never | 8329 | 7604 | 1 (Referent) | |||||||||
| Ever | 315 | 305 | 0.92 (0.76, 1.12) | ≤8 | 190 | 175 | 0.88 (0.69, 1.12) | ≤8 | 182 | 171 | 0.90 (0.70, 1.14) | Nine studies: BCMM, ENGELA, Epilymph, Italian, LAMMCC, LANHL, Mayo, NSW, Yale |
| >8-15.5 | 52 | 43 | 0.76 (0.50, 1.16) | >8-15.5 | 55 | 46 | 0.78 (0.52, 1.18) | |||||
| Lagged | 301 | 288 | 0.90 (0.74, 1.10) | >15.5-26 | 35 | 50 | 1.36 (0.87, 2.15) | >15.5-25.5 | 29 | 43 | 1.35 (0.83, 2.21) | |
| >26 | 33 | 27 | 0.75 (0.44, 1.26) | >25.5 | 31 | 24 | 0.71 (0.41, 1.23) | |||||
| P-trend = .57 | P-trend = .46 | |||||||||||
|
| ||||||||||||
| DDT | ||||||||||||
| Never | 5535 | 5467 | 1 (Referent) | |||||||||
| Ever | 200 | 169 | 0.78 (0.61, 1.00) | ≤6.5 | 96 | 82 | 0.78 (0.56, 1.09) | ≤7 | 99 | 83 | 0.76 (0.55, 1.05) | Seven studies: BCMM, Italian, LAMMCC, LANHL, Mayo, NSW, Yale |
| >6.5-13 | 55 | 35 | 0.56 (0.36, 0.89) | >7-13 | 49 | 32 | 0.58 (0.36, 0.94) | |||||
| Lagged | 195 | 161 | 0.76 (0.59, 0.97) | >13-22 | 30 | 26 | 0.77 (0.45,1.34) | >13-20 | 29 | 21 | 0.65 (0.36, 1.16) | |
| >22 | 13 | 19 | 1.48 (0.71, 3.07) | >20 | 13 | 21 | 1.55 (0.76, 3.17) | |||||
| P-trend = .49 | P-trend = .48 | |||||||||||
|
| ||||||||||||
| Chlordane | ||||||||||||
| Never | 3791 | 3573 | 1 (Referent) | |||||||||
| Ever | 59 | 61 | 1.23 (0.82, 1.82) | ≤3.5 | 37 | 34 | 1.14 (0.69, 1.88) | ≤3.5 | 35 | 31 | 1.11 (0.66, 1.87) | Four studies: LAMMCC, Mayo, NSW, Yale |
| >3.5 | 22 | 24 | 1.19 (0.64, 2.21) | >3.5 | 21 | 24 | 1.25 (0.67, 2.34) | |||||
| Lagged | 56 | 55 | 1.17 (0.77, 1.76) | P-trend = .48 | P-trend = .42 | |||||||
|
| ||||||||||||
| Lindane | ||||||||||||
| Never | 4373 | 4273 | 1 (Referent) | |||||||||
| Ever | 29 | 32 | 1.23 (0.72, 2.09) | ≤8 | 20 | 13 | 0.73 (0.35, 1.51) | ≤8 | 19 | 13 | 0.77 (0.37, 1.62) | Four studies: BCMM, Italian, Mayo, Yale |
| >8 | 9 | 16 | 1.91 (0.82, 4.43) | >8 | 8 | 16 | 2.13 (0.89, 5.10) | |||||
| Lagged | 27 | 29 | 1.19 (0.68, 2.06) | P-trend = .21 | P-trend = .13 | |||||||
|
| ||||||||||||
| Dieldrin | ||||||||||||
| Never | 4211 | 4008 | 1 (Referent) | |||||||||
| Ever | 10 | 17 | 1.64 (0.72, 3.75) | ≤3.5 | 6 | 8 | 1.43 (0.46, 4.39) | ≤3.5 | 6 | 8 | 1.43 (0.46, 4.38) | Five studies: BCMM, LAMMCC, Mayo, NSW, Yale |
| >3.5 | 4 | 8 | 1.85 (0.54, 6.29) | >3.5 | 3 | 8 | 2.45 (0.64, 9.43) | |||||
| Lagged | 9 | 16 | 1.79 (0.76, 4.24) | P-trend = .27 | P-trend = .16 | |||||||
|
| ||||||||||||
| Organophosphate insecticides | ||||||||||||
| Never | 8251 | 7457 | 1 (Referent) | |||||||||
| Ever | 393 | 452 | 1.22 (1.01, 1.47) | ≤10 | 205 | 225 | 1.17 (0.93, 1.49) | ≤9 | 180 | 198 | 1.17 (0.92, 1.51) | Nine studies: BCMM, ENGELA, Epilymph, Italian, LAMMCC, LANHL, Mayo, NSW, Yale |
| >10-20 | 100 | 109 | 1.18 (0.88, 1.60) | >9-16 | 83 | 99 | 1.25 (0.91, 1.73) | |||||
| Lagged | 341 | 398 | 1.23 (1.01, 1.50) | >20-31 | 58 | 72 | 1.27 (0.87, 1.84) | >16-26 | 47 | 62 | 1.34 (0.90, 2.01) | |
| >31 | 27 | 41 | 1.49 (0.90, 2.47) | >26 | 31 | 39 | 1.27 (0.77, 2.08) | |||||
| P-trend = .05 | P-trend = .07 | |||||||||||
|
| ||||||||||||
| Malathion | ||||||||||||
| Never | 5549 | 5434 | 1 (Referent) | |||||||||
| Ever | 186 | 202 | 1.12 (0.87, 1.45) | ≤8 | 123 | 138 | 1.15 (0.85, 1.54) | ≤8 | 107 | 120 | 1.12 (0.82, 1.52) | Seven studies: BCMM, Italian, LAMMCC, LANHL, Mayo, NSW, Yale |
| >8-15.5 | 36 | 33 | 0.95 (0.57, 1.59) | >8-12 | 10 | 15 | 1.60 (0.70, 3.64) | |||||
| Lagged | 165 | 172 | 1.06 (0.81, 1.38) | >15.5-24 | 4 | 9 | 2.12 (0.64, 7.09) | >12-21 | 31 | 22 | 0.72 (0.40, 1.30) | |
| >24 | 21 | 17 | 0.89 (0.46, 1.73) | >21 | 16 | 14 | 0.92 (0.44, 1.94) | |||||
| P-trend = .96 | P-trend = .68 | |||||||||||
|
| ||||||||||||
| Chlorpyrifos | ||||||||||||
| Never | 4631 | 4529 | 1 (Referent) | |||||||||
| Ever | 83 | 73 | 0.87 (0.60, 1.25) | ≤3.5 | 45 | 45 | 0.98 (0.62, 1.55) | ≤3.5 | 43 | 40 | 0.91 (0.57, 1.47) | Four studies: Italian, Mayo, NSW, Yale |
| >3.5-8 | 26 | 13 | 0.47 (0.23, 0.98) | >3.5-8 | 21 | 12 | 0.53 (0.24, 1.14) | |||||
| Lagged | 75 | 63 | 0.81 (0.55, 1.19) | >8 | 11 | 13 | 1.16 (0.50, 2.68) | >8 | 10 | 10 | 0.94 (0.37, 2.36) | |
| P-trend = .47 | P-trend = .32 | |||||||||||
|
| ||||||||||||
| Parathion | ||||||||||||
| Never | 3528 | 3326 | 1 (Referent) | |||||||||
| Ever | 75 | 90 | 1.13 (0.80, 1.60) | ≤15 | 38 | 47 | 1.14 (0.71, 1.81) | ≤11 | 39 | 35 | 0.80 (0.49, 1.32) | Three studies: Italian, LAMMCC, Mayo |
| >15-22 | 20 | 18 | 0.89 (0.46, 1.74) | >11-19 | 12 | 25 | 2.10 (1.03, 4.28) | |||||
| Lagged | 66 | 80 | 1.13 (0.79, 1.64) | >22 | 16 | 23 | 1.34 (0.68, 2.61) | >19 | 15 | 20 | 1.25 (0.62, 2.51) | |
| P-trend = .53 | P-trend = .21 | |||||||||||
|
| ||||||||||||
| Diazinon | ||||||||||||
| Never | 5291 | 5175 | 1 (Referent) | |||||||||
| Ever | 72 | 93 | 1.38 (0.98, 1.95) | ≤8 | 56 | 56 | 1.07 (0.72, 1.61) | ≤7 | 29 | 38 | 1.29 (0.77, 2.17) | |
| >8-15 | 2 | 8 | 3.01 (0.62, 14.7) | >7-12 | 15 | 19 | 1.40 (0.69, 2.84) | |||||
| Lagged | 54 | 77 | 1.50 (1.02, 2.21) | >15 | 13 | 26 | 2.32 (1.16, 4.64) | >12 | 10 | 20 | 2.32 (1.05, 5.13) | Six studies: BCMM, Italian, LAMMCC, Mayo, NSW, Yale |
| P-trend = .007 | P-trend = .02 | |||||||||||
|
| ||||||||||||
| Pyrethroid insecticides | ||||||||||||
| Never | 5406 | 5289 | 1 (Referent) | |||||||||
| Ever | 126 | 108 | 0.83 (0.62, 1.10) | ≤8 | 69 | 54 | 0.80 (0.54, 1.17) | ≤8 | 56 | 47 | 0.86 (0.57, 1.30) | Six studies: BCMM, ENGELA, Italian, Mayo, NSW, Yale |
| >8-16 | 29 | 24 | 0.78 (0.45, 1.37) | >8-15 | 20 | 15 | 0.63 (0.31, 1.27) | |||||
| Lagged | 99 | 82 | 0.81 (0.59, 1.12) | >16 | 27 | 25 | 0.82 (0.47, 1.45) | >15 | 22 | 20 | 0.89 (0.48, 1.67) | |
| P-trend = .27 | P-trend = .34 | |||||||||||
|
| ||||||||||||
| Permethrin | ||||||||||||
| Never | 5045 | 4956 | 1 (Referent) | |||||||||
| Ever | 40 | 37 | 0.96 (0.59, 1.54) | ≤8 | 23 | 21 | 1.01 (0.54, 1.88) | ≤6.75 | 15 | 14 | 0.91 (0.42, 1.95) | Five studies: BCMM, Italian, Mayo, NSW, Yale |
| >8 | 15 | 14 | 0.84 (0.39, 1.80) | >6.75 | 15 | 14 | 0.91 (0.42, 1.97) | |||||
| Lagged | 30 | 29 | 0.95 (0.55, 1.63) | P-trend = .66 | P-trend = .76 | |||||||
|
| ||||||||||||
| Carbamate insecticides | ||||||||||||
| Never | 7309 | 6608 | 1 (Referent) | |||||||||
| Ever | 238 | 254 | 1.08 (0.86, 1.35) | ≤8 | 147 | 138 | 0.97 (0.73, 1.29) | ≤8 | 137 | 133 | 0.98 (0.73, 1.30) | Six studies: BCMM, Epilymph, Italian, Mayo, NSW, Yale |
| >8-15.5 | 41 | 60 | 1.47 (0.96, 2.25) | >8-13.5 | 21 | 30 | 1.39 (0.77, 2.49) | |||||
| Lagged | 209 | 221 | 1.07 (0.84, 1.35) | >15.5-25.5 | 31 | 33 | 1.06 (0.63, 1.79) | >13.5-20 | 29 | 36 | 1.35 (0.81, 2.26) | |
| >25.5 | 18 | 15 | 0.72 (0.35, 1.47) | >20 | 21 | 21 | 0.86 (0.45, 1.65) | |||||
| P-trend = .65 | P-trend = .52 | |||||||||||
|
| ||||||||||||
| Carbaryl | ||||||||||||
| Never | 4939 | 4817 | 1 (Referent) | |||||||||
| Ever | 146 | 176 | 1.34 (1.03, 1.75) | ≤8 | 98 | 100 | 1.18 (0.86, 1.64) | ≤6.5 | 71 | 68 | 1.04 (0.71, 1.51) | Five studies: BCMM, Italian, Mayo, NSW, Yale |
| >8-15.5 | 21 | 38 | 1.97 (1.12, 3.45) | >6.5-13 | 25 | 45 | 2.12 (1.26, 3.56) | |||||
| Lagged | 128 | 150 | 1.29 (0.97, 1.72) | >15.5-25.5 | 17 | 20 | 1.22 (0.62, 2.42) | >13-22 | 18 | 23 | 1.44 (0.75, 2.75) | |
| >25.5 | 9 | 11 | 1.10 (0.43, 2.80) | >22 | 13 | 14 | 1.02 (0.45, 2.31) | |||||
| P-trend = .10 | P-trend = .12 | |||||||||||
Note: Odds ratios (OR) and 95% confidence intervals (CI) from logistic regression models, with adjustment for study center, age, gender, socioeconomic status (SES), ever-use of herbicides, and ever-use of other noninsecticide pesticides.
Ever-use of insecticides, with separate models for ever-use, overall (in any time period) and lagged ever-use (occurring more than 10 years before the reference date).
Duration variable results are shown for the most fine exposure categorization (2 categories, 3 categories, or 4 categories) containing at least 10 exposed cases in the highest exposure category, or two-category duration as the default.
Duration of lagged use (occurring more than 10 years before the reference date).
P-value for a continuous variable with values set as the median of each duration category.
Subtype-specific associations between insecticide use and NHL risk are shown in Table 4 for ever-use and by duration for selected insecticides in Figure 1 (all subtype results of our main analysis are included in Table S2). Elevated ORs for the association between any type of insecticide and risks of the subtypes FL and TCL were not statistically significant. Substantial heterogeneity was observed among the subtypes for the association with ever-use of organochlorines (P-het = .005), with increased risk of FL (OR = 1.62, 95%CI: 1.14-2.31) but no trend by duration (Table S2), an inverse association with TCL based on small numbers, and no evidence of an association with the other subtypes. There were no statistically significant increased risks with DDT use, although elevated ORs were observed for MM with longer duration of use (Table S2); inverse associations were observed for CLL. As with all NHL, nonstatistically significant elevated ORs were estimated for the subtypes in association with chlordane, lindane and dieldrin. The observed association between organophosphates and all NHL (P-het = .06) was particularly reflected in the results for FL, which was strongly associated with organophosphates for ever-use (OR = 2.01, 95%CI: 1.41-2.84), without a clear trend by duration (Figure 1). Diazinon use was associated with increased risk of several subtypes and increases were generally highest with longer duration (Figure 1), such as with longer than 8 years for DLBCL (OR = 3.16, 95%CI: 1.22, 8.20, P-trend = .03) and FL (OR = 3.13, 95%CI: 1.24-7.89, P-trend = .006). Risk increases were also seen in association with parathion use for several subtypes, such as with lagged-use duration >11 years for MM (Table S2, 11 cases OR = 2.24, 95%CI: 1.00-5.03, P-trend = .08) and OBCL (17 cases, OR = 2.42, 95%CI: 1.23-4.74, P-trend = .01). Moderate heterogeneity was observed among the subtypes for the association with ever-use of carbaryl (P = .03), including strong associations with TCL and FL and no association with CLL. The subtype associations with carbaryl were generally strongest with longer duration (Figure 1) and when lagged, such as with lagged-use duration longer than 6.5 years (Table S2) for FL (18 cases, OR = 2.02, 95%CI: 1.10-3.72, P-trend = .02), OBCL (16 cases, OR = 1.87, 95%CI: 1.01-3.49, P-trend = .06) and TCL (6 cases, OR = 3.37, 95%CI: 1.25-9.13, P-trend = .01).
TABLE 4.
Association between ever-use of insecticides and risk of NHL subtypes
| Exposed cases | OR (95% CI) | P-heterogeneitya | |
|---|---|---|---|
| Insecticides, any type | .37 | ||
| CLL | 141 | 0.96 (0.69, 1.33) | |
| DLBCL | 114 | 0.97 (0.73, 1.29) | |
| FL | 123 | 1.30 (0.95, 1.78) | |
| MM | 69 | 0.85 (0.60, 1.21) | |
| OBCL | 93 | 0.99 (0.72, 1.34) | |
| TCL | 25 | 1.46 (0.78, 2.74) | |
|
| |||
| Organochlorine insecticides | .005 | ||
| CLL | 77 | 0.80 (0.58, 1.11) | |
| DLBCL | 53 | 0.93 (0.66, 1.31) | |
| FL | 65 | 1.62 (1.14, 2.31) | |
| MM | 40 | 0.79 (0.52, 1.19) | |
| OBCL | 54 | 0.94 (0.66, 1.34) | |
| TCL | 6 | 0.38 (0.16, 0.94) | |
|
| |||
| DDT | .24 | ||
| CLL | 43 | 0.59 (0.39, 0.89) | |
| DLBCL | 27 | 0.76 (0.48, 1.20) | |
| FL | 34 | 1.00 (0.64, 1.57) | |
| MM | 27 | 1.07 (0.64, 1.81) | |
| OBCL | 30 | 0.78 (0.50, 1.21) | |
|
| |||
| Chlordane | .61 | ||
| CLL | 14 | 0.86 (0.45, 1.65) | |
| DLBCL | 12 | 1.43 (0.73, 2.82) | |
| FL | 16 | 1.48 (0.80, 2.73) | |
| MM | 7 | 1.49 (0.54, 4.13) | |
| OBCL | 9 | 1.47 (0.68, 3.18) | |
|
| |||
| Lindane | .99 | ||
| CLL | 8 | 1.05 (0.46, 2.39) | |
| DLBCL | 5 | 1.23 (0.46, 3.28) | |
| FL | 6 | 1.34 (0.53, 3.37) | |
| OBCL | 6 | 1.31 (0.52, 3.27) | |
|
| |||
| Dieldrin | .85 | ||
| FL | 5 | 1.66 (0.52, 5.28) | |
|
| |||
| Organophosphate insecticides | .06 | ||
| CLL | 120 | 1.28 (0.92, 1.77) | |
| DLBCL | 78 | 1.09 (0.79, 1.51) | |
| FL | 95 | 2.01 (1.41, 2.84) | |
| MM | 44 | 1.06 (0.70, 1.60) | |
| OBCL | 82 | 1.13 (0.82, 1.56) | |
| TCL | 18 | 1.02 (0.52, 2.00) | |
|
| |||
| Malathion | .14 | ||
| CLL | 61 | 0.88 (0.58, 1.32) | |
| DLBCL | 32 | 1.03 (0.65, 1.63) | |
| FL | 54 | 1.72 (1.12, 2.62) | |
| MM | 12 | 1.25 (0.59, 2.68) | |
| OBCL | 27 | 0.87 (0.53, 1.41) | |
| TCL | 9 | 1.06 (0.46, 2.44) | |
|
| |||
| Diazinon | .22 | ||
| CLL | 23 | 1.06 (0.62, 1.82) | |
| DLBCL | 14 | 1.23 (0.66, 2.28) | |
| FL | 29 | 2.05 (1.24, 3.37) | |
| OBCL | 16 | 1.69 (0.92, 3.09) | |
|
| |||
| Parathion | .07 | ||
| CLL | 17 | 1.04 (0.57, 1.92) | |
| DLBCL | 11 | 0.83 (0.41, 1.67) | |
| MM | 15 | 1.20 (0.61, 2.34) | |
| OBCL | 35 | 1.94 (1.20, 3.16) | |
|
| |||
| Chlorpyrifos | .88 | ||
| CLL | 24 | 0.67 (0.40, 1.14) | |
| DLBCL | 12 | 0.87 (0.45, 1.69) | |
| FL | 16 | 0.78 (0.43, 1.42) | |
| OBCL | 11 | 0.90 (0.45, 1.80) | |
| TCL | 5 | 1.35 (0.47, 3.83) | |
|
| |||
| Pyrethroid insecticides | .24 | ||
| CLL | 21 | 0.56 (0.34, 0.95) | |
| DLBCL | 28 | 1.20 (0.76, 1.89) | |
| FL | 18 | 0.70 (0.41, 1.21) | |
| MM | 11 | 0.78 (0.39, 1.56) | |
| OBCL | 21 | 0.99 (0.60, 1.66) | |
|
| |||
| Permethrin | .57 | ||
| CLL | 12 | 0.93 (0.46, 1.88) | |
| DLBCL | 7 | 1.09 (0.47, 2.52) | |
| OBCL | 8 | 1.28 (0.57, 2.85) | |
|
| |||
| Carbamate insecticides | .84 | ||
| CLL | 75 | 1.00 (0.70, 1.43) | |
| DLBCL | 40 | 0.93 (0.62, 1.40) | |
| FL | 49 | 1.05 (0.70, 1.57) | |
| MM | 14 | 1.00 (0.53, 1.87) | |
| OBCL | 48 | 1.24 (0.83, 1.83) | |
| TCL | 14 | 1.45 (0.70, 3.02) | |
|
| |||
| Carbaryl | .03 | ||
| CLL | 41 | 0.83 (0.54, 1.27) | |
| DLBCL | 31 | 1.41 (0.89, 2.26) | |
| FL | 47 | 1.89 (1.23, 2.90) | |
| MM | 8 | 1.13 (0.51, 2.54) | |
| OBCL | 30 | 1.40 (0.87, 2.25) | |
| TCL | 12 | 2.44 (1.13, 5.28) | |
Note: Odds ratios (OR) and 95% confidence intervals (CI) from polytomous logistic regression models, with adjustment for study center, age, gender, socioeconomic status (SES), ever-use of herbicides and other noninsecticide pesticides; results for subtypes with <5 exposed cases not shown.
P-value for heterogeneity of the association among subtypes.
FIGURE 1.
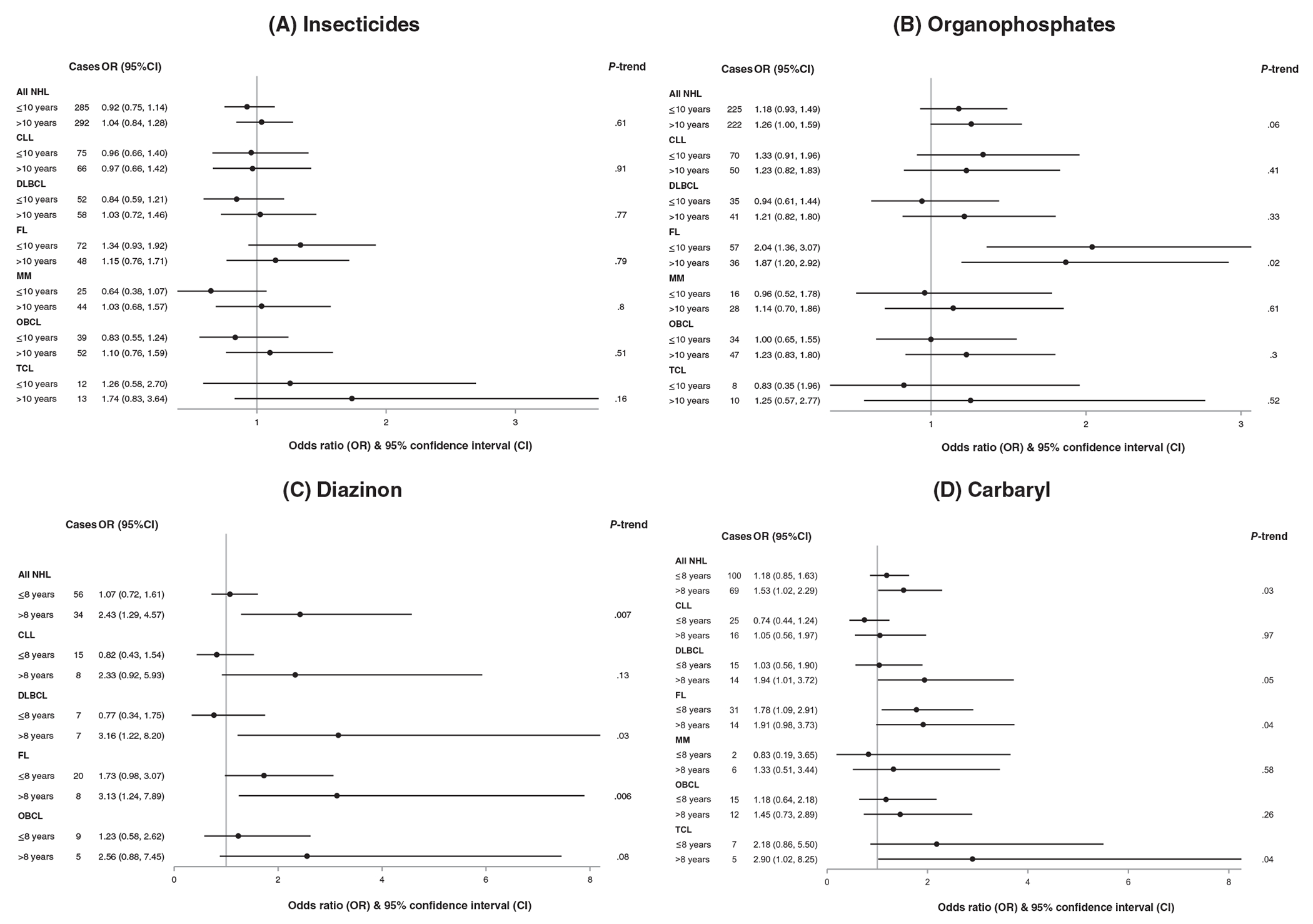
Association of occupational insecticide use duration (vs never-use) with risk of all NHL and NHL subtypes. Odds ratio (OR) and 95% confidence interval (CI) with adjustment for study center, age, gender, socioeconomic status (SES), herbicides, and other noninsecticide pesticides
Our main findings were generally robust to sensitivity analyses, particularly for subtype-specific effects (Figure 2). Results were similar with adjustment for farming occupation, when dropping the covariates for herbicides and other noninsecticide pesticides, or without adjustment for SES. Adjustment for organochlorines resulted in higher risk estimates for organophosphates and carbaryl (ie, negative confounding) in association with all NHL and some subtypes; for example, we observed increased risk of CLL in association with ever-use of organophosphates after adjustment for organochlorines (OR = 1.43, 95%CI: 1.01-2.01, not shown in figures). Associations with diazinon were similar following adjustment for organochlorines or carbamates. Carbaryl associations were somewhat diminished with adjustment for organophosphates; nevertheless, the increased risks remained statistically significant in association with TCL and for all NHL with longer lagged duration of use (data not shown). Risk estimates were generally elevated in studies with expert assessment of exposure and were higher (albeit less precise) than in those based on self-report (Figure 2 and Figure S1), but ORs were elevated with both types of exposure assessment for organophosphates, diazinon, and carbaryl. Based on expert assessment alone, there was a statistically significant association with ever use of lindane (OR = 3.14, 95%CI: 1.09-9.06). Consideration of exposure only among persons who ever worked in a high-probability exposed occupation resulted in similar risk estimates for organophosphates as a group and for parathion (data not shown), somewhat reduced risk estimates for diazinon with loss of statistical significance, and higher estimates for carbaryl. Estimates were slightly attenuated for all NHL when the analysis was limited to participants who ever lived or worked on a farm, but estimates remained elevated for the subtypes. Elevated risks of all NHL in association with ever-use of organophosphates, diazinon and carbaryl were only observed among males; however, findings for FL were observed in both males and females. In analyses limited to males, an estimated increased risk of TCL in association with any type of insecticide use (21 cases, OR = 2.52, 95%CI: 1.12-5.68, not shown in figures) increased with longer duration (>10 years, 11 cases, OR = 3.03, 95%CI: 1.21-7.62; P-trend = .04). Estimated risks were also higher in association with an NHL classification that excluded CLL and MM, than with the WHO NHL classification used in our main analysis. Exclusion of the Mayo study from the pooled population resulted in a large drop in exposure frequencies, but for most insecticides, did not result in large changes in the size of risk estimates or our conclusions. Results of random-effects meta-analysis of the study-specific ORs largely aligned with the main results (Figure 2), and there was little evidence for heterogeneity of effect between the individual studies for our main findings (Figure S2).
FIGURE 2.
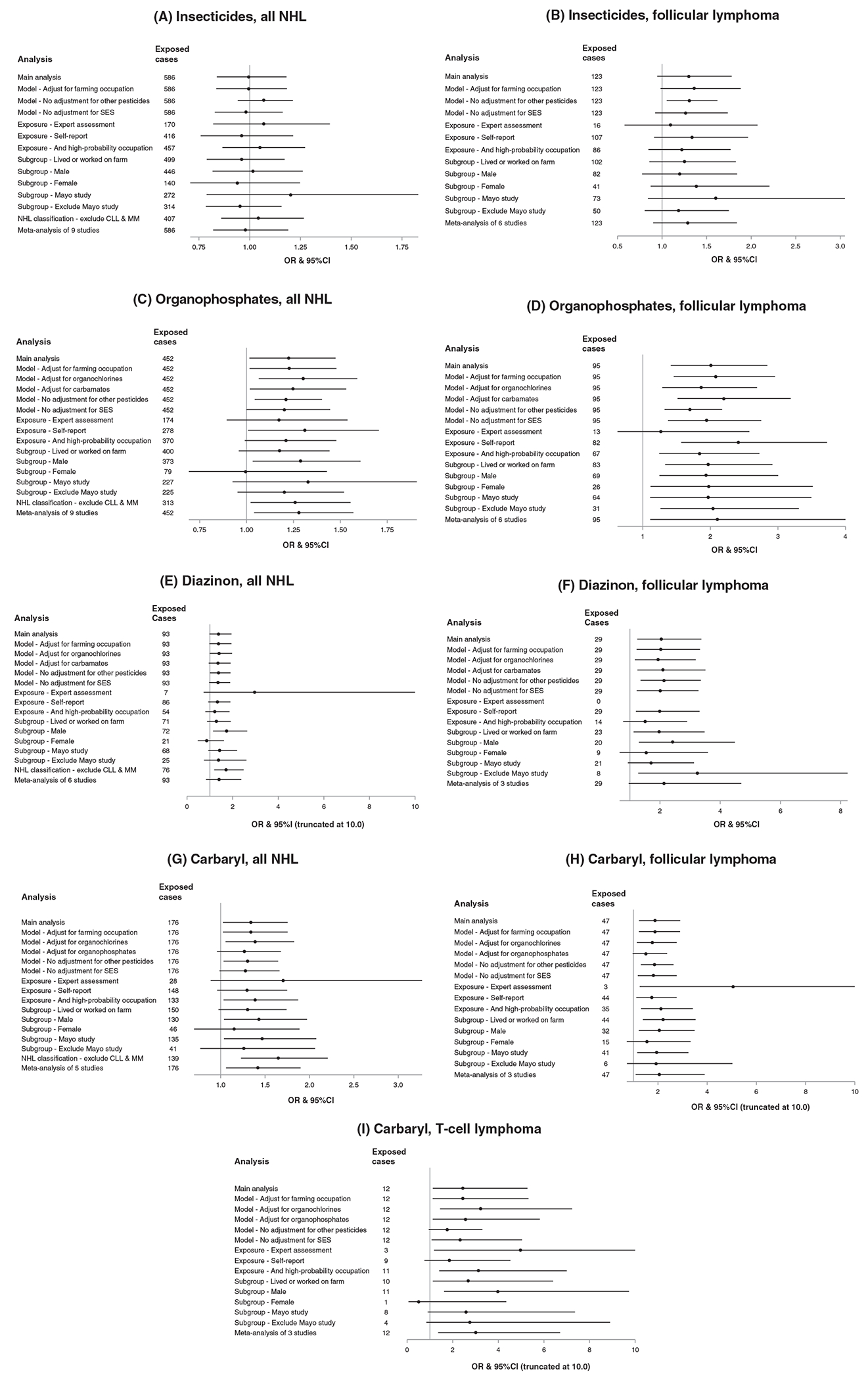
Comparison of main analysis, sensitivity analyses and meta-analysis results for occupational insecticide use, for the association between ever-use (vs never-use) and risk of all NHL and NHL subtypes. Odds ratio (OR) and 95% confidence interval (CI) with adjustment in the main analysis for study center, age, gender, socioeconomic status (SES), herbicides and other noninsecticide pesticides
4 |. DISCUSSION
In our pooled analysis of nine case-control studies, occupational insecticide use, overall, was not associated with risk of NHL. We found evidence for increased NHL risk in association with use of organophosphate insecticides as a group, and notably with diazinon, as well as with the carbamate insecticide, carbaryl. There was no association of NHL with other broad groups of insecticides, including organochlorines and pyrethroids. The associations with diazinon and carbaryl were generally stronger with longer duration of use, and the association with carbaryl was somewhat stronger with at least 10 years latency (ie, lagged use). We found negligible heterogeneity among the individual studies in the associations with these insecticides, and there was fair consistency in results across sensitivity analyses.
Our consortium-based pooling approach produced a large study population for analysis of specific insecticides in relation to NHL. We had excellent statistical power to investigate previously reported associations with organophosphate insecticides, carbamates and pyrethroids, among others. As in any study, we expect that a number of statistically significant associations would occur by chance alone; however, we do not believe chance explains our main findings. If we consider the proportion of statistically significant results expected by chance to be equal to our set alpha level of .05, we would expect roughly eight chance findings from the number of tests performed to generate Table 3 results (156 tests); nevertheless, we found double that number (16) of statistically significant results. Furthermore, we did not interpret all results (ie, all tests) as independent findings—rather, we considered our results, in whole, for interpretation of the association with any one insecticide (such as by looking for a trend across duration categories, assessing the importance of an exposure lag and comparing the association for all NHL and subtypes), and reported as our main findings those results with strength of evidence across analyses.
The size of our dataset enabled estimation of insecticide associations with the most common NHL subtypes. Among our novel findings were strong associations of carbaryl with TCL, and any type of insecticide with TCL among male participants. It is important to note that TCL, itself, is a heterogeneous group of neoplasms, and we did not have the statistical power to evaluate specific TCL subtypes. However, a previous InterLymph pooled study found increased risk of the cutaneous T-cell subtypes mycosis fungoides and Sezary syndrome (MF/SS) in association with a history of farming occupation, but no association with peripheral T-cell lymphoma, suggesting possible TCL subtype-specific associations with insecticides.6 Across the various results in our study, FL and OBCL subtypes were most frequently associated with insecticide exposures, such as prominent associations of FL with all organophosphates, diazinon and carbaryl. Of note, we found little evidence that CLL risk was associated with the insecticides we studied. The earlier studies, which originally classified NHL subtypes based on the Working Formulation (LANHL, Italian), had higher proportions of cases with NOS-unknown pathology, even after InterLymph harmonization of subtypes according to WHO. This disharmonization of subtype classification between earlier and later studies may have caused bias if the types of cases with NOS classification had differing etiology from other subtypes (ie, more or less strongly associated with insecticide exposure). Nonetheless, our main subtype-specific results (such as the association of carbaryl with FL or TCL) were similar between studies with large (Italian) and small (Mayo) proportions of NOS-unknown cases.
Organophosphates are one of the most widely used groups of insecticides in the world, although use has declined in recent years with restrictions and increasing use of other types of insecticides, including pyrethroids and neonicotinoids.29 Our observed associations between organophosphate insecticides and increased risk of NHL are supported by previous studies. In IARC’s most recent evaluation of organophosphate insecticides in 2015, parathion was classified as “possibly carcinogenic to humans” (Group 2B), and diazinon and malathion as “probably carcinogenic to humans” (Group 2A), based on limited human evidence, with some studies of NHL reporting positive associations.4,30 In the AHS cohort,7 there was a significant association between lifetime days of diazinon use and FL (>median vs never-use, RR = 3.8, 95%CI: 1.2-11.4) with a significant exposure-response trend across lifetime days (P = .02), as well as nonsignificant increased risk with high lifetime use for CLL/SLL (RR = 1.9, 95%CI: 1.0-3.6); the AHS found no association with other current-use organophosphate insecticides such as chlorpyrifos and malathion. These results largely align with our findings for organophosphates, although we also reported risk increases with DLBCL and OBCL subtypes. Our results differ from the NAPP analysis of pooled case-control studies from Canada and the midwestern region of the US (which did not include any overlap with the studies pooled in our study), which reported increased risks of NHL in association with malathion and diazinon exposures, and attenuation of the diazinon association when adjusted for other insecticides.10 Our results for diazinon were robust to adjustment for other insecticides.
Carbaryl, the active ingredient in the product Sevin, is a frequently used insecticide in food and nursery crops, and on turf. The USEPA has recognized carbaryl as “likely to be carcinogenic in humans”, based on data showing increased risk of hemangiosarcomas in mice.5 Our findings of carbaryl-related risks for all NHL and several subtypes agree with increased risk of FL observed in association with high lifetime days of use in the AHS (>median vs never-use, OR = 2.8, 95%CI: 1.0-7.4) and no association with CLL/SLL/MCL.7 Increased risk associated with longer duration of carbaryl use in the NAPP (≥6 years vs never-use, OR = 1.75, 95%CI: 1.13-2.70) was reduced with adjustment for exposure to other insecticides (OR = 1.24, 95% CI: 0.78-1.99), and similar attenuation was observed for the FL subtype association,10 whereas our lagged duration and subtype results were robust to such adjustments. The strongest associations with carbaryl in our study were with TCL; neither the AHS nor the NAPP reported on TCL. We cannot attribute meaning to the trend observed in our study of rising estimated risks across the first two carbaryl duration categories and declining risks with longer duration, but one possibility for this type of pattern is bias due to competing causes of death (ie, persons with longer duration of use died earlier from illnesses caused by long-term insecticide use, and therefore were not diagnosed with NHL during the study period). Carbamate insecticides, as a group, were not consistently associated with NHL risk, suggesting that a potential effect of carbaryl may occur through a mode of action that does not involve the common toxicological mechanism of carbamates.
Many organochlorine insecticides have been banned from use in the US and Europe for over 30 years. While we observed elevated risks in association with several organochlorines, including lindane, a recognized carcinogen,31 these estimates were imprecise based on small numbers of exposed participants. Given that most organochlorine exposures occurred in the distant past, it may not be feasible to detect the effects of these exposures in our pooled study if individual studies were conducted (ie, new NHL case identification) after the hypothetical latency period for organochlorine exposure causation of NHL. The inverse (protective) associations seen for CLL and TCL in relation to all organochlorines and DDT could reflect this type of limitation. A similar inverse association with organochlorine insecticide use was seen in association with risk of all NHL (RR = 0.86, 95%CI: 0.74-0.99) in a pooled analysis of three agricultural worker cohorts.8 The estimated reduced risks could also stem from unidentified, residual confounding.
We found no evidence of an association with pyrethroids, a large group of frequently used insecticides, which have largely replaced organophosphates in residential applications.29 Risk estimates for the specific active ingredient permethrin were based on very small numbers and were, therefore, impossible to fully characterize, even in this consortium setting. The AHS reported a strong association between permethrin and MM,7 but our study did not have enough exposed MM cases to evaluate this association. Given increasing use, further research on pyrethroids is needed—particularly with permethrin and other specific active ingredients.
Of note, we did not study neonicotinoids, another group of insecticides whose use is increasing rapidly.29 Neonicotinoids were either not queried or were infrequently reported in the individual studies of our pooled analysis—most likely due to the years when the studies were conducted.
Our study expanded on resources already developed within Inter-Lymph, including harmonization of case pathology according to the WHO classification12 and assignment of standardized occupational and industry codes to work histories.11 In addition, we capitalized on detailed pesticide exposure assessment conducted by local experts in three of the individual studies.15,18,20 Nevertheless, exposure coding for most of the studies was based on self-reported use by study participants, which likely resulted in underreporting/low sensitivity of exposure assessment (particularly for specific active ingredients). Recall bias is another concern with self-reported exposure in retrospective case-control studies, but there was little indication of such bias in our pooled analysis, as most of the insecticides we evaluated were not associated with increased risk of NHL, and ORs for studies with self-reported exposure were considerably lower than those estimated for expert-assessed exposure. Expert assessment of exposure, with comparison of detailed participant-reported pesticide use to additional information such as crop-exposure matrices likely increased the specificity of exposure coding, also suggested by the higher ORs we estimated for expert-assessed exposures vs selfreport. However, none of the studies assessed the validity of their expert-based assessment by comparison to “truth”, or compared the expert assessment to self-report alone. Exposure misclassification from any source (resulting from either expert- or self-assessment) may have caused the observed higher insecticide-related risks for males than for females, if we assume no biological difference in toxicity and the possibility that more frequent and intense exposures in males (based on gender differences in work tasks) would result in more accurate recall. Our pooled study may also be susceptible to selection bias, given that participation in the studies was low to moderate (generally between 40% and 70%32), eligible cases generally are more likely to participate than eligible controls,33 and participation in research among older adults has been reported to correlate with higher education and income.34,35 Due to these patterns in study participation and the knowledge that farming and occupational insecticide use is associated with lower SES (including in our data), we suspect that selection bias, if it occurred, served to bias any positive insecticide associations toward the null.
Evaluation of risks associated with insecticide chemical classes (eg, organophosphates, carbamates) can shed light on toxicologic mechanisms that lead to development of disease. Our results, like those of other studies, suggest a toxicological mechanism common to organophosphate insecticides that contributes to development of several NHL subtypes. Results from our study contribute to the totality of evidence available to support further carcinogenic classification and regulatory review of diazinon and carbaryl. Continued generation of data for informing regulation is particularly important given the widespread, current use of insecticides in agricultural and residential community settings.
Supplementary Material
What’s new?
The role of occupational pesticide use in non-Hodgkin’s lymphoma (NHL) remains unclear, despite many studies over the years. These authors analyzed pooled data from nine case-control studies, including 7909 cases and 8644 controls from North America, the European Union, and Australia. Occupational insecticide use overall did not increase cancer risk. Organophosphate insecticides as a group were associated with increased risk of NHL, and associations were also seen with the specific ingredients diazinon and carbaryl. Estimated risks with these current-use pesticides provide evidence for supporting public health measures.
ACKNOWLEDGMENTS
This research was funded through a grant from the National Cancer Institute (NCI) of the National Institutes of Health (NIH) (R03CA199515). The Mayo case-control study of NHL was also funded through grants from the NCI/NIH (R01CA92153 and P50CA97274).
Abbreviations:
- AHS
Agricultural Health Study
- CI
confidence interval
- CLL
chronic lymphocytic leukemia
- DLBCL
diffuse large B-cell lymphoma
- FL
follicular lymphoma
- MM
multiple myeloma
- NAPP
North American Pooled Project
- NHL
non-Hodgkin lymphoma
- NOS
not otherwise specified/unknown NHL
- OBCL
other B-cell lymphoma
- OR
odds ratio
- SES
socioeconomic status
- TCL
T-cell lymphoma
Footnotes
CONFLICT OF INTEREST
All authors disclosed no financial or personal relationships with other people and organizations that could inappropriately influence their work.
ETHICS STATEMENT
Participants of the individual case-control studies provided informed consent for use of their data in research. The pooled study was approved by the Institutional Review Board of the Drexel University Office of Research (IRB ID: 1504003599).
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
DATA AVAILABILITY STATEMENT
Original investigators of the individual case-control studies may be approached for collaborative use of the data included in the pooled analysis. Further information is available from the corresponding author upon request.
REFERENCES
- 1.Food and Agriculture Organization of the United Nations (FAO). Pesticide Use database. Statistics Division (ESS), Environment Statistics Team, FAO, 2018. Available at: http://www.fao.org/faostat/en/#data/RP (accessed December 6, 2020). [Google Scholar]
- 2.IARC. Occupational exposures in insecticide application, and some pesticides. IARC working group on the evaluation of carcinogenic risks to humans. Lyon, 16-23 October 1990. IARC Monogr Eval Carcinog Risks Hum. 1991;53:5–586. [PMC free article] [PubMed] [Google Scholar]
- 3.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. DDT, Lindane, and 2,4-D. Lyon, France: International Agency for Research on Cancer; 2018. [Google Scholar]
- 4.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Some Organophosphate Insecticides and Herbicides. Lyon, France: International Agency for Research on Cancer; 2017. [PubMed] [Google Scholar]
- 5.USEPA. Amended Reregistration Eligibility Decision (RED) for Carbaryl. Revised August 2008. EPA-738-R-08-010, August 2008. Office of Prevention, Pesticides and Toxic Substances (7508P), United States Environmental Protection Agency. [Google Scholar]
- 6.Morton LM, Slager SL, Cerhan JR, et al. Etiologic heterogeneity among non-Hodgkin lymphoma subtypes: the InterLymph non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. 2014;2014(48):130–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alavanja MC, Hofmann JN, Lynch CF, et al. Non-hodgkin lymphoma risk and insecticide, fungicide and fumigant use in the Agricultural Health Study. PLoS One. 2014;9(10):e109332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leon ME, Schinasi LH, Lebailly P, et al. Pesticide use and risk of non-Hodgkin lymphoid malignancies in agricultural cohorts from France, Norway and the USA: a pooled analysis from the AGRICOH consortium. Int J Epidemiol. 2019;48(5):1519–1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kachuri L, Beane Freeman LE, Spinelli JJ, et al. Insecticide use and risk of non-Hodgkin lymphoma subtypes: a subset meta-analysis of the North American Pooled Project. Int J Cancer. 2020;147(12):3370–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Koutros S, Harris SA, Spinelli JJ, et al. Non-Hodgkin lymphoma risk and organophosphate and carbamate insecticide use in the North American Pooled Project. Environ Int. 2019;127:199–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.‘t Mannetje A, De Roos AJ, Boffetta P, et al. Occupation and risk of non-Hodgkin lymphoma and its subtypes: a pooled analysis from the InterLymph consortium. Environ Health Perspect. 2016;124(4):396–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turner JJ, Morton LM, Linet MS, et al. InterLymph hierarchical classification of lymphoid neoplasms for epidemiologic research based on the WHO classification (2008): update and future directions. Blood. 2010;116(20):e90–e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nuyujukian DS, Voutsinas J, Bernstein L, Wang SS. Medication use and multiple myeloma risk in Los Angeles County. Cancer Causes Control. 2014;25(9):1233–1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin’s lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res. 1992;52(19 Suppl):5510s–5515s. [PubMed] [Google Scholar]
- 15.Miligi L, Costantini AS, Bolejack V, et al. Non-Hodgkin’s lymphoma, leukemia, and exposures in agriculture: results from the Italian multicenter case-control study. Am J Ind Med. 2003;44(6):627–636. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Y, Holford TR, Leaderer B, et al. Hair-coloring product use and risk of non-Hodgkin’s lymphoma: a population-based case-control study in Connecticut. Am J Epidemiol. 2004;159(2):148–154. [DOI] [PubMed] [Google Scholar]
- 17.Koutros S, Baris D, Bell E, et al. Use of hair colouring products and risk of multiple myeloma among US women. Occup Environ Med. 2009;66(1):68–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cocco P, Satta G, Dubois S, et al. Lymphoma risk and occupational exposure to pesticides: results of the Epilymph study. Occup Environ Med. 2013;70(2):91–98. [DOI] [PubMed] [Google Scholar]
- 19.Fritschi L, Benke G, Hughes AM, et al. Occupational exposure to pesticides and risk of non-Hodgkin’s lymphoma. Am J Epidemiol. 2005;162(9):849–857. [DOI] [PubMed] [Google Scholar]
- 20.Orsi L, Delabre L, Monnereau A, et al. Occupational exposure to pesticides and lymphoid neoplasms among men: results of a French case-control study. Occup Environ Med. 2009;66(5):291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cerhan JR, Fredericksen ZS, Wang AH, et al. Design and validity of a clinic-based case-control study on the molecular epidemiology of lymphoma. Int J Mol Epidemiol Genet. 2011;2(2):95–113. [PMC free article] [PubMed] [Google Scholar]
- 22.Weber L, Song K, Boyle T, et al. Organochlorine levels in plasma and risk of multiple myeloma. J Occup Environ Med. 2018;60(10):911–916. [DOI] [PubMed] [Google Scholar]
- 23.Jaffe ES, Harris NL, Diebold J, Müller-Hermelink HK. World Health Organization classification of lymphomas: a work in progress. Ann Oncol. 1998;9(Suppl 5):S25–S30. [DOI] [PubMed] [Google Scholar]
- 24.Swerdlow SH, Campo E, Harris NL, et al. , eds. World Health Organization; Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC Press; 2008. [Google Scholar]
- 25.International Labour Office (ILO). International standard classification of occupations (Revised Edition 1968). Geneva: ILO; 1981. [Google Scholar]
- 26.Miligi L, Settimi L, Masala G, et al. Pesticide exposure assessment: a crop exposure matrix. The working group on pesticide exposure assessment. Int J Epidemiol. 1993;22(Suppl 2):S42–S45. [DOI] [PubMed] [Google Scholar]
- 27.Lerro CC, Koutros S, Andreotti G, et al. Cancer incidence in the Agricultural Health Study after 20 years of follow-up. Cancer Causes Control. 2019;30(4):311–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg SA, Berard CW, Brown BW, et al. National Cancer Institute sponsored study of classifications of non-Hodgkin’s lymphomas. Summary and description of a working formulation for clinical usage. Cancer. 1982;49(10):2112–2135. [DOI] [PubMed] [Google Scholar]
- 29.Atwood D, Paisley-Jones C. Pesticides Industry Sales and Usage: 2008-2012 Market Estimates. Washington, DC: USEPA; 2017. [Google Scholar]
- 30.Guyton KZ, Loomis D, Grosse Y, et al. Carcinogenicity of tetrachlorvinphos, parathion, malathion, diazinon, and glyphosate. Lancet Oncol. 2015;16(5):490–491. [DOI] [PubMed] [Google Scholar]
- 31.Loomis D, Guyton K, Grosse Y, et al. Carcinogenicity of lindane, DDT, and 2,4-dichlorophenoxyacetic acid. Lancet Oncol. 2015;16(8):891–892. [DOI] [PubMed] [Google Scholar]
- 32.Morton LM, Sampson JN, Cerhan JR, et al. Rationale and Design of the International Lymphoma Epidemiology Consortium (InterLymph) non-Hodgkin lymphoma subtypes project. J Natl Cancer Inst Monogr. 2014;48:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sritharan J, Luo Y, Harris MA. Trends in participation rates in case-control studies of occupational risk factors 1991-2017. Occup Environ Med. 2020;77(10):659–665. [DOI] [PubMed] [Google Scholar]
- 34.Barrett NJ, Rodriguez EM, Iachan R, et al. Factors associated with biomedical research participation within community-based samples across 3 National Cancer Institute-designated cancer centers. Cancer. 2020;126(5):1077–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Enzenbach C, Wicklein B, Wirkner K, Loeffler M. Evaluating selection bias in a population-based cohort study with low baseline participation: the LIFE-adult-study. BMC Med Res Methodol. 2019;19(1):135. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Original investigators of the individual case-control studies may be approached for collaborative use of the data included in the pooled analysis. Further information is available from the corresponding author upon request.


