Abstract
The comparative efficacies of amoxicillin and cefuroxime against acute otitis media caused by a penicillin-resistant (MIC, 2 μg/ml) Streptococcus pneumoniae strain were assessed in a gerbil model by challenging each ear with 107 bacteria through transbullar instillation. Each antibiotic was tested at two doses (5 and 20 mg/kg of body weight) administered at 2, 10, and 18 h postinoculation. Samples were obtained from the middle ear (ME) on days 3 and 7 postinoculation for determination of bacterial counts. Only amoxicillin, at both doses, was able to significantly halt the weight loss in animals, reducing both the number of culture-positive animals and the bacterial concentration in ME samples versus the values for untreated animals. Comparison of the efficacies between the antibiotics, determined by their ability to achieve culture-negative ME specimens, showed that amoxicillin at 5 mg/kg was significantly more active than cefuroxime at the same dose. The use of higher doses of either amoxicillin or cefuroxime did not produce significantly better results than those obtained with the lower dose but caused a greater inflammatory response. The more favorable results obtained with amoxicillin compared with those obtained with cefuroxime could be related to the antimicrobial susceptibility of the pneumococcal strain (MICs and minimum bactericidal concentrations of 1 and 1 μg/ml and 4 and 4 μg/ml for amoxicillin and cefuroxime, respectively) as well as to the better pharmacokinetic parameters obtained with amoxicillin.
Acute otitis media (AOM) is one of the most frequently diagnosed infectious diseases of childhood, and Streptococcus pneumoniae is responsible for up to 50% of cases of AOM (5, 24). On the other hand, the incidence of penicillin-insensitive S. pneumoniae strains is increasing worldwide (1), and in Spain up to 54% of pneumococci isolated from middle ears (MEs) are penicillin insensitive (6, 16). Although the antimicrobial treatment of AOM is still controversial, antibiotics are capable of producing a rapid microbiological cure and avoiding severe complications, such as meningitis or other infections (4, 14).
AOM induced in gerbils is a well-established model for studying AOM as well as for comparing the efficacies of different antimicrobial regimens (3, 10).
The aim of the present investigation was to compare the clinical and bacteriological efficacies of two antibiotics in a gerbil model of AOM produced by a penicillin-resistant S. pneumoniae strain. This organism is a rather frequent cause of AOM in children, who are usually empirically treated with either amoxicillin or cefuroxime.
Part of this work has been presented at the 8th European Congress of Clinical Microbiology and Infectious Diseases, Lausanne, Switzerland, 25 to 28 May 1997 [6a].)
MATERIALS AND METHODS
Bacteria.
A strain of S. pneumoniae type 23 isolated from a bacteremic patient was used. The MIC and minimum bactericidal concentration (MBC) of benzylpenicillin for the strain were both 2 μg/ml. Bacterial virulence was maintained by passage in mice.
Antibiotics.
The antibiotics used for in vitro studies were amoxicillin trihydrate (SmithKline Beecham Pharmaceuticals, Worthing, England) and cefuroxime sodium (Sigma Chemical Co., St. Louis, Mo.). For in vivo (therapeutic) use, the contents of commercial vials (Clamoxyl [SmithKline Beecham Pharmaceuticals, Toledo, Spain] and Curoxima [Glaxo, Madrid, Spain]) were reconstituted in nonpyrogenic sterile distilled water to the desired concentrations.
In vitro studies.
MICs and MBCs were determined by a broth microdilution method by previously described methods (19, 23). The median of five separate determinations was used to calculate the MIC and MBC of each antibiotic.
Animals.
Eight- to 9-week-old adult female Mongolian gerbils weighing 44 to 54 g each were purchased from B & K Universal G.J.S.L. (Grimston, United Kingdom). They were given free access to food and water and were housed in a protected unit with a slight negative pressure and with a 12-h light and 12-h dark cycle. Two weeks prior to the experiments, the anteroinferior part of the auricle was removed to facilitate access to the ear drum. For invasive procedures the animals were anesthetized with 50 mg of ketamine (Ketolar; Parke-Davis, Barcelona, Spain) per kg of body weight and 13 mg of xylazine (Rompun; Bayer, Leverkusen, Germany) per kg.
Experimental otitis.
An overnight culture of the organism was kept in aliquots at −70°C, and on each day of experimentation a freshly thawed aliquot was incubated for 4 h at 37°C in a 5% CO2 atmosphere in brain heart infusion broth enriched with 5% horse serum. The number of viable bacteria in this culture was determined by the colony counting method. The animals included in the different treatment or control groups were inoculated bilaterally with 20 μl of this bacterial culture (approximately 107 CFU), which was introduced directly into the ME bulla, on each day of the experiment. The tympanic membrane was left intact and swelled without rupture during the inoculation. A normal tympanic aspect and correct inoculation were verified with an operating microscope.
Treatment regimen and efficacy studies.
Each antibiotic was tested at two doses (5 and 20 mg/kg/dose) and was administered subcutaneously in 500 μl at 2, 10, and 18 h postinoculation (p.i.). The animals in the control group received apyrogenated sterile distilled water in the same way.
Treated and control animals were studied longitudinally for weight and behavior. The otoscopic aspect was evaluated on days 3 and 7 p.i., and ME samples for determination of the bacterial counts in the ME effusion were obtained on days 3 and 7 p.i. by washing the ME fossa with 20 μl of saline injected and withdrawn via the epitympanic membrane with a 0.33-mm needle.
Shortly after sampling, aliquots of serial 10-fold dilutions in saline were plated onto sheep blood agar. The plates were incubated for 18 h at 37°C in a 5% CO2 atmosphere. Bacterial counts are expressed as log10 CFU/20 μl of ear washing fluid; the lowest detectable bacterial count was 0.30 log10 CFU in 20 μl. To evaluate cell and bacterial contents, 3 μl of ME washing fluid was extended over a 6-cm2 slide surface to be Gram stained and observed under a high-field high-power (×1,000) microscope. The inflammatory response was evaluated by examination for the presence of polymorphonuclear leukocytes. For cell counts, the mean number of cells in 10 fields was calculated and was expressed as no cells (<1 cell), few cells (1 to 4 cells), moderate numbers of cells (5 to 30 cells), and many (>30 cells).
Cerebrospinal fluid was obtained on day 3 by percutaneous intracisternal puncture to detect possible meningeal involvement.
Pharmacokinetic studies.
Amoxicillin and cefuroxime were administered to healthy animals by the subcutaneous route at doses of 5 and 20 mg/kg. Groups of six animals were killed with CO2 and were exsanguined by intracardiac puncture at 0.25, 0.5, 1, and 2 h after drug administration. Amoxicillin and cefuroxime, concentrations were determined by microbiological assay with Micrococcus luteus ATCC 9341 and Bacillus subtilis 1904E, respectively. The concentration of antibiotic in the samples was derived from a standard solution prepared in pooled gerbil serum. The variability of the assay for individual samples was <10%. Pharmacokinetic analysis were performed by routine graphical methods (13).
Statistical analysis.
The number of ears with a positive count divided by the total number of ears was calculated to give the percentages of ears with positive counts for each group of animals. The percentage of treated animals with positive counts was compared with that for animals in the untreated control group, and the percentages between the groups were compared by Fisher’s exact test corrected for by the Bonferroni method, due to the multiple comparisons that were performed. The overall type I error was 5%, adjusted for the 10 pairwise treatment group comparisons. Bacterial counts in untreated and treated animals were expressed as the arithmetic mean log10 CFU per 20 μl of washing fluid; culture-negative samples were included in the calculation of means, assuming a value at the detection limit, and the results were analyzed by Kruskal-Wallis and Mann-Whitney tests, and the Bonferroni correction (α = 0.005) was used. These tests were also used to analyze the differences in the volume of fluid recovered from the ME as well as the relative weight loss.
The study was performed in accordance with prevailing regulations regarding the care and use of laboratory animals in the European Community (9).
RESULTS
In vitro studies.
The respective median MICs and MBCs for the test strain were 1 and 1 μg/ml and 4 and 4 μg/ml for amoxicillin and cefuroxime, respectively.
Therapeutic efficacy in experimental otitis.
After inoculation, unilateral or bilateral purulent otitis media was detected in 92.3% of untreated animals at day 3, with 88.5% of the ME specimens being culture positive. The mean bacterial concentration was 2.58 ± 1.67 log10 CFU per 20 μl of washing fluid. Intra- and extracellular pneumococci were seen in Gram-stained ME samples. From the first day of the experimental study the animals started to lose weight, with the loss being more marked on day 2 and then with a slow recovery afterward. Lethargy and otorrhea were observed in most animals on day 1, and the otoscopic examination, performed on day 3, showed marked inflammation with retrotympanic exudate. By day 7, 62.5% of the untreated animals had cleared the organism from their ME samples, and their behavior was normal, with almost complete weight recovery. Table 1 presents the comparative therapeutic results for control and treated animals. Both doses of amoxicillin and the high dose of cefuroxime significantly reduced the number of culture-positive ME specimens (P = 0.0001 for amoxicillin at 5 or 20 mg/kg and P = 0.0009 for cefuroxime at 20 mg/kg) compared with the results obtained for untreated animals. When the results obtained with different antibiotics and doses were compared, only amoxicillin at 5 mg/kg was found to be more efficacious than cefuroxime at 5 mg/kg (P = 0.0012). As far as the mean concentration of bacteria in the ME is concerned, amoxicillin (at both doses) and cefuroxime (at 5 mg/kg) significantly reduced the number of organisms compared with the numbers in the MEs of the untreated controls. No differences between antibiotics and doses were observed.
TABLE 1.
Outcome on day 3 of AOM caused by penicillin-resistant S. pneumoniae treated with amoxicillin and cefuroxime
| Group | No. of animals | No. (%) of animals with culture-positive ME samples
|
No. (%) of ME culture-positive samples | Bacterial counts (log10 CFUa (mean [SD]) | |
|---|---|---|---|---|---|
| Bilateral | Unilateral | ||||
| Untreated controls | 13 | 11 (84.6) | 1 (7.7) | 23 (88.5) | 2.58 (1.67) |
| Amoxicillin, 5 mg/kg | 12 | 1 (8.3) | 2 (16.6) | 4 (16.7)b | 0.74 (1.16)b |
| Amoxicillin, 20 mg/kg | 12 | 3 (25.0) | 0 (0.0) | 6 (25.0)b | 0.94 (1.31)b |
| Cefuroxime, 5 mg/kg | 13 | 6 (46.2) | 4 (30.8) | 16 (61.5) | 1.25 (1.11)b |
| Cefuroxime, 20 mg/kg | 12 | 4 (33.3) | 4 (33.3) | 12 (50.0)b | 1.88 (1.90) |
Values are the mean log10 CFU in 20 μl of washing fluid. Data for culture-negative samples were included in the calculation of means by assuming a value at the detection limit (0.30 log10 CFU).
Differences between values for treated animals versus those for untreated control animals were statistically significant.
By day 7 there were no significant differences in the numbers of positive cultures or in bacterial concentrations in ME samples between control and treated animals.
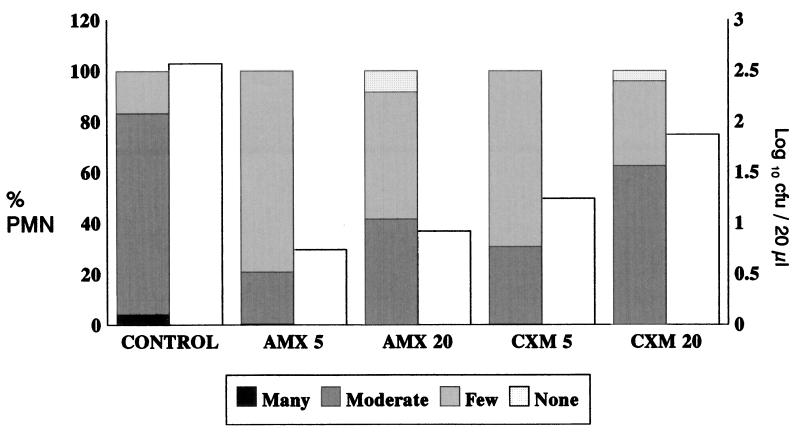
Figure 1 presents the results of an analysis of the level of inflammation in comparison with the number of microorganisms recovered. Higher inflammatory responses were found in ME samples with high bacterial counts, particularly in untreated animals, and lower inflammatory responses were found in those treated with 5 mg of amoxicillin per kg, from which the lowest numbers of organisms were recovered.
FIG. 1.
Inflammatory response on day 3 (evaluated by the presence of polymorphonuclear leukocytes [PMN]) and number of recovered microorganisms (in log10 CFU/20 μl) from washing fluid. Shaded bars, percent polymorphonuclear cells; open bars, bacterial counts (log10 CFU/20 μl). AMX, amoxicillin; CXM, cefuroxime; 5 and 20, doses of 5 and 20 mg/kg, respectively.
Analysis of the fluid volume recovered at day 3 after washing of the ME showed that all treated animals had higher volumes (range, 38.2 to 52.0 μl) than untreated controls (26.4 μl), but the difference was statistically significant only for animals treated with the higher dose of either antibiotic. By day 7 significant differences in volume recovered between treated and untreated animals were observed only for those treated with the low dose of cefuroxime; animals in that group had larger amounts of fluid compared with the amounts in the control animals (P < 0.005).
As far as the loss of body weight, as recorded on day 2, is concerned, all treated animals lost less weight than the untreated controls, but the differences were statistically significant only for those animals receiving any dose of amoxicillin (range, 4.9 to 5.9% versus 11.8% loss of initial body weight; P < 0.005). By day 7 most animals had not fully recovered their initial body weight, although significant increases with respect to the body weights on day 2 had occurred. There were no significant differences in body weights between treated and untreated animals or between any of the treated animal groups.
The cerebrospinal fluid of only three animals was positive for the inoculated microorganism (two in the group treated with amoxicillin and one in the group treated with cefuroxime, both at the low dose).
Pharmacokinetic and pharmacodynamic data.
Table 2 presents the results of the pharmacokinetic and pharmacodynamic analyses in relation to the inoculated organism. A bacteriological eradication rate of ≥75% was obtained only when the concentration at 15 min/MIC ratio was >10, when the area under the concentration-time curve/MIC ratio was >10, or when the levels in serum exceeded the MIC for the pathogen for >15% of the dosing interval, which were obtained only with amoxicillin.
TABLE 2.
Pharmacokinetic and pharmacodynamic data for amoxicillin and cefuroxime in serum in relation to S. pneumoniae susceptibility
| Antibiotic (dose [mg/kg]) | C15 (μg/ml)a | Half-life (min) | C15/MIC | Time above MIC (min [% dose interval]) | AUC/ MIC |
|---|---|---|---|---|---|
| Amoxicillin (5) | 12.2 ± 2.8 | 18.0 | 12.2 | 78 (16.25) | 10.5 |
| Amoxicillin (20) | 35.4 ± 10.0 | 20.7 | 35.4 | 108 (22.50) | 30.5 |
| Cefuroxime (5) | 10.1 ± 2.1 | 16.0 | 2.5 | 36 (7.50) | 2.8 |
| Cefuroxime (20) | 23.3 ± 8.8 | 17.8 | 5.8 | 55 (11.50) | 4.8 |
C15, means ± standard deviation concentrations in six animals measured 15 min after the administration of each antibiotic at each dose.
DISCUSSION
AOM is usually a self-limited infection with a favorable evolution even in the absence of antimicrobial treatment (4, 26). For these reasons, it is very difficult for clinical trials to show the superiority of one regimen over another, by which bacteriological efficacy should be always considered. On the other hand, animal models of AOM can provide both clinical and bacteriological parameters that can be used to compare the efficacies between antibiotics. Amoxicillin and cefuroxime are both commonly used for the treatment of respiratory tract infections, including AOM, usually with favorable clinical results (17, 22). However, the emergence of penicillin-resistant S. pneumoniae strains (1, 6, 16) has created a great deal of concern about the efficacies of such antibiotics because these strains usually show reduced susceptibilities to both antibiotics. We selected for the animal model one S. pneumoniae strain of a serotype that is usually involved in AOM in humans (16, 20) and that was also penicillin resistant, which is the case for up to 54% of pneumococcal strains involved in this pathology (6, 8). It is also common that the cefuroxime MICs for such strains are higher than those of amoxicillin (12). In addition, this strain was able to induce AOM in gerbils, as described by other investigators (3, 10).
Our experimental model showed that for animals treated with either amoxicillin or cefuroxime, the number of cultures of ME fluids that were positive was reduced and there was also a reduction in the number of microorganisms in treated animals on day 3 compared with the numbers in untreated animals. However, only amoxicillin at either dose was able to significantly reduce both the number of culture-positive samples and the bacterial concentration in ME samples versus those for untreated animals.
Comparison of efficacy between antibiotics showed that at the low dose amoxicillin was significantly more active than cefuroxime. The increase in the dose did not increase therapeutic efficacy. The beneficial effects of the antibiotics and the superiority of amoxicillin were also confirmed by the ponderal control of the animals, with those treated with amoxicillin losing the least body weight.
The lower efficacy of cefuroxime shown in our experimental model correlates with the recently published clinical and bacteriological failures of the drug in treating AOM caused by penicillin-resistant pneumococcal strains. Gehanno et al. (11) reported 93.5% clinical efficacy when cefuroxime axetil was used for the treatment of AOM caused by pneumococci, with MICs of this antibiotic being ≤0.5 μg/ml, while its efficacy fell to 76.3% for strains for which MICs were ≥1 μg/ml. Bacteriological failure rates of up to 50% were shown by Dagan et al. (8) for those pneumococcal strains for which cefuroxime MICs were ≥1 μg/l, while a bacteriological failure rate of only 9% was found for those strains for which cefuroxime MICs were ≤0.5 μg/ml (8).
With our animal model of AOM we also showed that after antimicrobial treatment most animals had higher amounts of ME liquid compared with the amounts in untreated control animals. This could be due, at least in part, to the fact that many untreated animals suffered tympanic perforation, which caused leaks of ME liquid, while in most animals treated with antibiotics, the membrane remained intact. The higher antibiotic doses were related to the presence of more polymorphonuclear cells and to higher volumes of ME liquid compared with those in animals treated with lower doses. This might be explained by the fact that beta-lactam antibiotics increase the level of inflammation in experimental pneumococcal infections (otitis and meningitis) because cell wall debris can induce such an inflammatory response (15, 25). This phenomenon could be related to the frequent complication observed after an AOM episode, namely, otitis media with effusion, which merits further investigation.
Pharmacokinetic parameters alone were not predictive of efficacy since similar concentrations in serum at 15 min and half-lives were obtained with the low doses of both antibiotics. On the other hand, the pharmacodynamic parameters were more predictive of efficacy, with amoxicillin being very efficacious and with the percentage of the dose interval during which the levels of amoxicillin in serum exceeded the MIC for the pathogen being at least two times the percentage obtained with cefuroxime. Nevertheless, these percentages for amoxicillin were lower than those previously suggested for bacteriological efficacy (7), which could be explained by the pharmacokinetics of antimicrobial agents in ME resulting in lower peak concentrations but higher trough concentrations than those in serum (7) and/or longer half-lives in the ME (2).
Although S. pneumoniae was considered cefuroxime sensitive when the MIC for the strain was ≤8 μg/ml (18), clinical experience has demonstrated that failure may occur when the MICs for the strains are ≥1 μg/ml, particularly in infections in which the antibiotic has difficulties in penetration, such as to the ME (8, 11). Our data also show that a pneumococcal strain for which the cefuroxime MIC was 4 μg/ml cannot be eradicated when cefuroxime is used for the treatment of AOM, even when it is administered at a high dose. While we were carrying out this investigation we became aware of the most recent proposition of the National Committee for Clinical Laboratory Standards changing the cefuroxime susceptibility breakpoint to ≤0.5 μg/ml (19); according to that breakpoint our strain should be considered nonsensitive, which would explain both the clinical and bacteriological failures. On the other hand, although the susceptibility of our strain to amoxicillin should be considered intermediate, the use of higher doses in animal models (2, 23) and for the treatment of pneumococcal pneumonia in humans (21) is usually associated with a favorable outcome.
ACKNOWLEDGMENTS
This work was supported by a grant from SmithKline Beecham Pharmaceuticals, Madrid, Spain. E.N. and G.G.-C. were aided by scholarships from the Fundación Conchita Rábago, Madrid, Spain.
REFERENCES
- 1.Applebaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Barry B, Muffat-Joly M, Gehanno P, Pocidalo J J. Effect of increased dosages of amoxicillin in the treatment of experimental middle ear otitis due to penicillin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1993;37:1599–1603. doi: 10.1128/aac.37.8.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barry B, Muffat-Joly M, Bauchet J, Faurisson F, Gehanno P, Pocidalo J J, Carbon C. Efficacy of single-dose ceftriaxone in experimental otitis media induced by penicillin- and cephalosporin-resistant Streptococcus pneumoniae. Antimicrob Agents Chemother. 1996;40:1977–1982. doi: 10.1128/aac.40.9.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bluestone C D. Otitis media in children: to treat or not to treat? N Engl J Med. 1982;306:1399–1404. doi: 10.1056/NEJM198206103062305. [DOI] [PubMed] [Google Scholar]
- 5.Bluestone, C. D., J. S. Stephenson, and L. M. Martin. 1992. Ten-year review of otitis media pathogens. Pediatr. Infect. Dis. J. 11(Suppl):7–11. [DOI] [PubMed]
- 6.Castillo F, García-Perea A, Baquero Artiago F. Bacteriology of acute otitis media in Spain: a prospective study based on tympanocentesis. Pediatr Infect Dis J. 1996;15:541–543. doi: 10.1097/00006454-199606000-00014. [DOI] [PubMed] [Google Scholar]
- 6a.Cenjor, C., C. Ponte, A. Parra, E. Nieto, G. García, M. J. Giménez, L. Aguilar, and F. Soriano. 1997. Comparative efficacy of amoxicillin (AMX) and cefuroxime (CXM) in experimental otitis media (OM) caused by a penicillin-resistant pneumococcus (PRPP). Clin. Microbiol. Infect. 3(Suppl. 2):328.
- 7.Craig W A, Andes D. Pharmacokinetics and pharmacodynamics of antibiotics in otitis media. Pediatr Infect Dis J. 1996;15:255–259. doi: 10.1097/00006454-199603000-00015. [DOI] [PubMed] [Google Scholar]
- 8.Dagan R, Abramson O, Leibovitz E, Lang R, Goshen S, Greenberg D, Yagupsky P, Leiberman A, Fliss D M. Impaired bacteriologic response to oral cephalosporins in acute otitis media caused by pneumococci with intermediate resistance to penicillin. Pediatr Infect Dis J. 1996;15:980–985. doi: 10.1097/00006454-199611000-00010. [DOI] [PubMed] [Google Scholar]
- 9.European Community. Journal officiel des communautés européennes, 18 Décembre. Report L358. Luxembourg: Office des Publications Officielles des Communautés Européennes; 1986. [Google Scholar]
- 10.Fulghum R S, Brinn J E, Smith A M, Daniel III H J, Loesche P J. Experimental otitis media in gerbils and chinchillas with Streptococcus pneumoniae, Haemophilus influenzae, and other aerobic and anaerobic bacteria. Infect Immun. 1982;36:802–810. doi: 10.1128/iai.36.2.802-810.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gehanno P, Lenoir G, Berche P. In vivo correlates for Streptococcus pneumoniae penicillin resistance in acute otitis media. Antimicrob Agents Chemother. 1995;39:271–272. doi: 10.1128/aac.39.1.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldstein, F. W., J. F. Acar, and the Alexander Project Collaborative Group. 1996. Antimicrobial resistance among lower respiratory tract isolates of Streptococcus pneumoniae: results of a 1992–93 western Europe and USA collaborative surveillance study. J. Antimicrob. Chemother. 38(Suppl. A):71–84. [DOI] [PubMed]
- 13.Greenblatt D J, Koch-Wesser J. Clinical pharmacokinetics. N Engl J Med. 1975;297:702–705. doi: 10.1056/NEJM197510022931406. [DOI] [PubMed] [Google Scholar]
- 14.Grenier, B. 1997. Decision-making in otitis media in children. Part I. Epidemiologic data and definitions for a reliable cost-effectiveness analysis. Clin. Microbiol. Infect. 3(Suppl. 3):63–68. [PubMed]
- 15.Kawana M, Kawana C, Giebink G S. Penicillin treatment accelerates middle ear inflammation in experimental pneumococcal otitis media. Infect Immun. 1992;60:1908–1912. doi: 10.1128/iai.60.5.1908-1912.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Latorre C, Muñoz C, Trujillo G, Juncosa T, Clarós P. Susceptibility of pneumococci isolated from middle ear effusions to antimicrobial agents commonly used in otitis media. J Antimicrob Chemother. 1994;33:186–187. doi: 10.1093/jac/33.1.186. [DOI] [PubMed] [Google Scholar]
- 17.McLinn S E, Moskal M, Goldfarb J, Bodor F, Aronovitz G, Schwartz R, Self P, Ossi M J. Comparison of cefuroxime axetil and amoxicillin-clavulanate suspensions in treatment of acute otitis media with effusion in children. Antimicrob Agents Chemother. 1994;38:315–318. doi: 10.1128/aac.38.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. Standards for antimicrobial susceptibility testing: methods for dilution antimicrobial susceptibility tests for bacteria that growth aerobically, 2nd ed. Approved standard M7-A2. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1990. [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. Performance standards for antimicrobial susceptibility testing. Sixth informed supplement. NCCLS document M100-S6. Vol. 15 1995. , no. 14. National Committee for Clinical Laboratory Standards, Villanova, Pa. [Google Scholar]
- 20.Nelson C T, Mason E O, Jr, Kaplan S L. Activity of oral antibiotics in middle ear and sinus infections caused by penicillin-resistant Streptococcus pneumoniae: implications for treatment. Pediatr Infect Dis J. 1994;13:585–589. doi: 10.1097/00006454-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Pallarés R, Liñares J, Vadillo M, Cabellos C, Manresa F, Viladrich P F, Martin R, Gudiol F. Resistance to penicillin and cephalosporin and mortality from severe pneumococcal pneumonia in Barcelona, Spain. N Engl J Med. 1995;333:474–480. doi: 10.1056/NEJM199508243330802. [DOI] [PubMed] [Google Scholar]
- 22.Pechère, J. C., J. Garau, and P. Gehanno. 1997. Current trends in the antibiotic therapy of uncomplicated acute otitis media in children: concluding remarks. Clin. Microbiol. Infect. 3(Suppl. 3):73–74. [PubMed]
- 23.Ponte C, Parra A, Nieto E, Soriano F. Development of experimental pneumonia by infection with penicillin-insensitive Streptococcus pneumoniae in guinea pigs and their treatment with amoxicillin, cefotaxime, and meropenem. Antimicrob Agents Chemother. 1996;40:2698–2702. doi: 10.1128/aac.40.12.2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teele D W, Klein J O, Rossner B the Greater Boston Otitis Media Study Group. Epidemiology of otitis media during the first seven years of life in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 25.Toumanen E, Hengsler B, Rich R, Bray M A, Zak O, Tomaz A. Nonsteroidal inflammatory agents in the therapy for experimental pneumococcal meningitis. J Infect Dis. 1987;155:985–990. doi: 10.1093/infdis/155.5.985. [DOI] [PubMed] [Google Scholar]
- 26.Van Buchem F L, Dunk J H M, Van’t Hof M A. Therapy of acute otitis media: myringotomy, antibiotics or neither? A double blind study in children. Lancet. 1981;ii:883–887. doi: 10.1016/s0140-6736(81)91388-x. [DOI] [PubMed] [Google Scholar]