Abstract
The pharmacokinetic parameters determining antibiotic efficacy are peak concentrations (Cmax), minimum (trough) concentrations (Cmin), and area under the concentration-time curve (AUC). There is general agreement about the importance of Cmax and AUC for aminoglycosides, but this is not so for maintenance of Cmin. With in vitro exposures modelling in vivo administration, Pseudomonas aeruginosa reference strain ATCC 27853 (MIC, 1 mg/liter) and a higher-MIC (relatively resistant) clinical isolate (MIC, 4 mg/liter) were used to explore bacteriostatic and bactericidal outcomes. With P. aeruginosa ATCC 27853, kill followed a complete bolus profile with a 30-min postdistribution peak (Cpeak30) of 10 mg/liter. The clinical isolate required a Cpeak30 bolus profile of 20 mg/liter for kill, and there was no difference between the efficacies of the bolus and infusion exposures. Bolus profiles that were truncated at 8.5 h and producing sublethal effects were then combined with a wide range of Cmins. With a Cpeak30 profile of 8 mg/liter, P. aeruginosa ATCC 27853 showed a graded bacteriostatic response until a Cmin of ≥0.8 mg/liter, when complete kill resulted. In contrast, bactericidal effects on the clinical isolate required a Cpeak30 profile of 18 mg/liter with a Cmin of ≥1.0 mg/liter. Therefore, Cmin also contributes to the bactericidal effect of tobramycin, with requirements showing minor variation with change in MIC. Dosing principles for relatively resistant (higher-MIC) organisms are suggested from the data. Relatively higher aminoglycoside doses via infusion regimens are likely to be needed to generate higher peak concentrations and higher AUC values necessary for bactericidal effect in resistant organisms. Maintenance of trough concentrations on the order of 1.0 mg/liter during the interdose interval will tend to guard against the possibility of inadequate peak and AUC exposures for kill.
With the capacity for concentration-time profiles to be customized for individual patients in routine clinical practice (4, 10, 11), it is of critical importance to know what pharmacokinetic parameter or parameters determine therapeutic and toxic outcomes.
Using reference strains of Escherichia coli (NCTC 10418) and Pseudomonas aeruginosa (ATCC 27853), we have carried out a series of studies which have established that aminoglycoside action can be separated from a direct, linear dependence on area under the concentration-time curve (AUC) (1, 5, 7, 12), that bactericidal outcomes are significantly related to peak concentrations (1, 3, 6, 12), and that there is a critical requirement for a maintained minimum (trough) concentration (Cmin) of aminoglycoside following “peak-only” exposure (7). The trough concentration required is itself a function of the peak profile and the level of the peak exposure (7).
The prior studies used reference bacterial strains with fixed values for the MIC. In the clinical setting, however, there are numerous isolates of gram-negative organisms which are recognized as relatively resistant to aminoglycosides as measured by laboratory determinations of the MIC. In this study, we aimed to determine what changes in the in vitro concentration-time profile are important in determining the responses of a clinical isolate for which the MIC of aminoglycoside was altered (higher). In our design, we had particular interest in the influence of the presence or absence of a maintained minimum (trough [Cmin]) concentration following in vitro exposures modelling clinical concentration-time profiles.
MATERIALS AND METHODS
A relatively resistant clinical isolate of P. aeruginosa (MIC, 4 mg/liter) was studied in parallel with a reference strain, P. aeruginosa ATCC 27853 (MIC, 1 mg/liter). The clinical isolate was characterized as a P. aeruginosa strain by standard criteria with the VITEK system (BioMerieux, Hazelwood, Mo.) and was lyophilized and stored in glycerol broth at −70°C until required. Each isolate was inoculated in brain heart infusion broth (Oxoid, Basingstoke, England) and incubated at 37°C for 45 h.
The in vitro antibiotic exposures initially involved modelling of clinical concentration-time bolus profiles (1), including 30-min postdistribution peaks (Cpeak30s) of up to 10 mg/liter for the reference strain.
Profiles of exposure for the clinical isolate included bolus and infusion profiles with Cpeak30s of up to 20 mg/liter.
The second series of studies involved bolus profiles truncated to 8.5 h for both strains, and then tobramycin was removed by centrifugation and the cell pellet was reconstituted with medium containing maintained tobramycin concentrations. For the reference strain, a Cpeak30 of 8 mg/liter was combined with maintained Cmins over the range 0 to 1.0 mg/liter (Table 1). The clinical isolate was studied with a bolus profile to 8.5 h, including a Cpeak30 of 18 mg/liter combined with reconstitution (Cmin) concentrations across the range 0 to 1.2 mg/liter (Table 2).
TABLE 1.
Summary of results for P. aeruginosa reference strain ATCC 27853a
| Tobramycin profile | Cmax (mg/liter) | AUC0–48.5 h (mg · h/liter) | Bacterial counts (log10 CFU/ml) atb:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 2.5 h | 10.5 h | 24.5 h | 48.5 h | |||
| Postdistribution peak (mg/liter) of complete bolus | ||||||||
| 0 (control) | 0 | 0 | 5.86 ± 0.53 | 5.00 ± 0.24 | 5.71 ± 0.44 | 7.83 ± 0.60 | 8.78 ± 0.52 | 9.16 ± 0.37 |
| 8 | 27.84 ± 0.74 | 35.90 ± 1.69 | 6.01 ± 0.19 | 2.22 ± 1.28* | 0.22 ± 0.53* | 0.22 ± 0.53* | 3.61 ± 1.00* | 8.40 ± 0.78 |
| 10 | 35.53 ± 5.06 | 42.29 ± 3.96 | 6.01 ± 0.16 | 0.73 ± 0.33† | NGD*c | NGD* | NGD† | NGD† |
| Postdistribution peak of 8 mg/liter to 8.5 h and then reconstituted to (mg/liter): | ||||||||
| Control (no antibiotic) | 0 | 0 | 5.89 ± 0.02 | 5.29 ± 0.11 | 5.64 ± 0.36 | 8.02 ± 0.02 | 9.08 ± 0.03 | 9.13 ± 0.02 |
| 0 | 27.31 ± 0.42 | 30.07 ± 0.32 | 5.89 ± 0.04 | 0.92 ± 0.72* | NGD* | NGD* | 6.99 ± 0.75 | 8.93 ± 0.04 |
| 0.2 | 26.42 ± 1.5 | 32.92 ± 0.38 | 5.88 ± 0.03 | 0.92 ± 0.72* | NGD* | NGD* | 6.97 ± 0.06 | 8.93 ± 0.03 |
| 0.4 | 26.99 ± 1.31 | 35.91 ± 0.94 | 5.89 ± 0.03 | 0.92 ± 0.72* | NGD* | NGD* | 6.14 ± 0.54* | 8.93 ± 0.05 |
| 0.6 | 27.66 ± 0.60 | 38.87 ± 0.67 | 5.89 ± 0.03 | 0.70 ± 0.77* | NGD* | NGD* | 3.88 ± 3.06‡ | 5.97 ± 4.62 |
| 0.8 | 27.39 ± 1.11 | 41.51 ± 0.56 | 5.89 ± 0.03 | 0.65 ± 0.71* | NGD* | NGD* | NGD‡ | NGD‡ |
| 1.0 | 27.46 ± 1.13 | 44.66 ± 0.54 | 5.90 ± 0.02 | 0.70 ± 0.77* | NGD* | NGD* | NGD‡ | NGD‡ |
For all groups, values are means ± standard deviations (n = 6).
*, significantly different from control (P < 0.001); †, significantly different from all other exposures (P < 0.001). ‡, Significantly different from other exposures, including control (P < 0.001).
NGD, no growth defect.
TABLE 2.
Summary of results for the clinical isolate of P. aeruginosaa
| Tobramycin profile | Cmax (mg/liter) | AUC0–48.5 h (mg · h/liter) | Bacterial counts (log10 CFU/ml) atb:
|
|||||
|---|---|---|---|---|---|---|---|---|
| 0 h | 0.5 h | 2.5 h | 10.5 h | 24.5 h | 48.5 h | |||
| Postdistribution peak (mg/liter) of complete bolus profile | ||||||||
| 0 (control) | 0 | 0 | 5.86 ± 0.06 | 5.32 ± 0.34 | 5.31 ± 0.71 | 8.27 ± 0.39 | 8.96 ± 0.12 | 9.05 ± 0.15 |
| 18 | 62.67 ± 1.59 | 80.36 ± 1.47 | 5.86 ± 0.08 | 3.11 ± 0.65* | 0.48 ± 0.75* | 0.21 ± 0.21* | 4.72 ± 0.15* | 8.96 ± 0.02 |
| 20 | 69.89 ± 0.36 | 89.81 ± 0.50 | 5.90 ± 0.03 | 2.97 ± 0.07* | NGD*c | NGD* | NGD† | NGD† |
| Postdistribution peak of 18 mg/liter to 8.5 h and then reconstituted to (mg/liter): | ||||||||
| Control (no antibiotic) | 0 | 0 | 5.87 ± 0.04 | 5.41 ± 0.28 | 5.56 ± 0.43 | 7.98 ± 0.07 | 9.02 ± 0.07 | 9.15 ± 0.6 |
| 0 | 63.33 ± 1.66 | 69.99 ± 1.41 | 5.90 ± 0.04 | 3.21 ± 0.32* | 0.70 ± 0.77* | NGD* | 6.15 ± 0.79* | 8.95 ± 0.03 |
| 0.2 | 61.23 ± 2.61 | 71.59 ± 1.46 | 5.90 ± 0.05 | 3.21 ± 0.23* | 0.65 ± 0.71* | NGD* | 6.14 ± 0.79* | 8.94 ± 0.02 |
| 0.4 | 62.38 ± 0.81 | 75.45 ± 0.34 | 5.89 ± 0.04 | 3.18 ± 0.27* | 0.43 ± 0.67* | NGD* | 5.69 ± 1.56* | 8.94 ± 0.02 |
| 0.6 | 62.30 ± 1.55 | 79.15 ± 0.77 | 5.92 ± 0.05 | 3.07 ± 0.43* | 0.43 ± 0.67* | NGD* | 5.08 ± 1.95* | 8.95 ± 0.03 |
| 0.8 | 62.38 ± 0.68 | 81.31 ± 0.82 | 5.89 ± 0.05 | 3.24 ± 0.25* | 0.43 ± 0.67* | NGD* | 2.05 ± 1.84‡ | 5.96 ± 4.62 |
| 1.0 | 62.80 ± 0.31 | 84.44 ± 0.33 | 5.88 ± 0.02 | 3.16 ± 0.29* | 0.22 ± 0.53* | NGD* | NGD‡ | NGD‡ |
| 1.2 | 63.05 ± 0.29 | 87.49 ± 0.61 | 5.89 ± 0.03 | 3.11 ± 0.23* | NGD* | NGD* | NGD‡ | NGD‡ |
For all groups, values are means ± standard deviations (n = 6).
*, significantly different from control (P < 0.001); †, significantly different from all other exposures (P < 0.001); ‡, significantly different from other exposures (P < 0.001).
NGD, no growth detected.
Six replicates for the clinical isolate and reference strain were run for all exposure profiles, including antibiotic-free controls. Tobramycin concentrations were measured at times of 0, 0.16, 0.33, 0.5, 2.5, 4.5, 6.5, 8.5, 10.5, 24.5, and 48.5 h, and assay concentrations were determined with an EMIT immunoassay (Solaris; Syva Laboratories, San Jose, Calif.). The threshold for detection was 0.6 mg/liter. Assay results were linear over the range 0.6 to 10 mg/liter (r2 = 0.99). The interrun coefficient of variation was 8.4 to 8.8% over the range 2.0 to 7.0 mg/liter (n = 6), and the intrarun coefficient of variation was 2.8% at 2.3 mg/liter, 5.8% at 5.5 mg/liter, and 6.0% at 6.3 mg/liter (n = 7).
Viable cell counts were measured at 0, 0.5, 2.5, 4.5, 6.5, 8.5, 10.5, 24.5, and 48.5 h.
Statistical testing involved repeated-measures analysis of variance with the Minitab program (Minitab, Inc., State College, Pa.).
RESULTS
Responses of Pseudomonas strain ATCC 27853 and a Pseudomonas clinical isolate to tobramycin concentration-time exposures modelling bolus in vivo dosing.
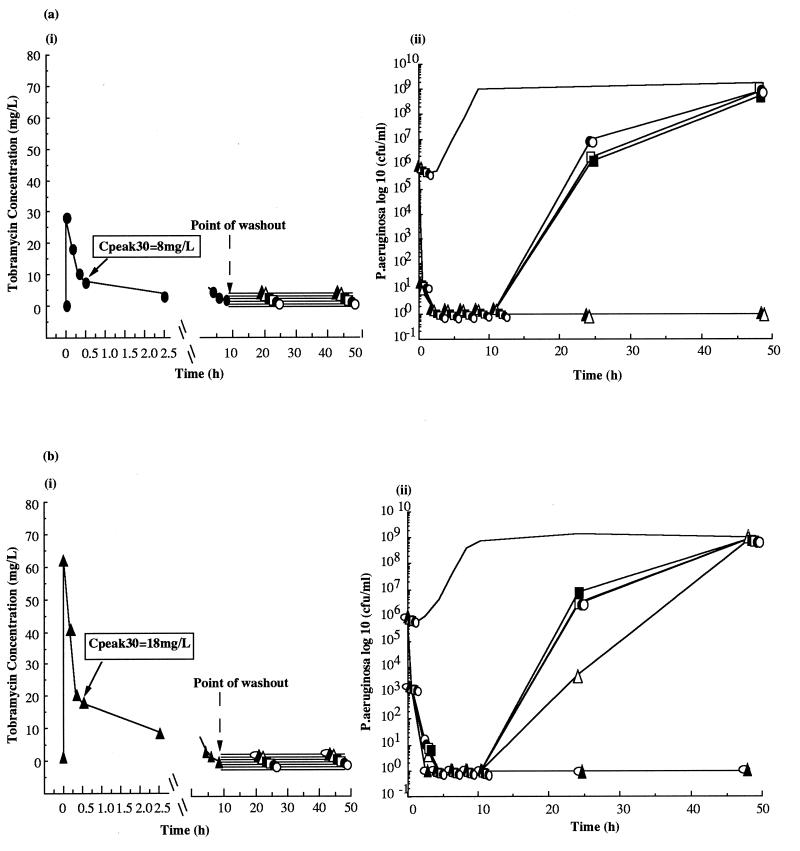
In studies with the reference strain, exposure to a complete (48.5-h) profile of tobramycin with a Cpeak30 of 10 mg/liter produced complete kill [Fig. 1a(i) and a(ii) and Table 1], while exposure to a profile with a Cpeak30 of 8 mg/liter caused substantial initial kill with recovery by 48.5 h [Fig. 1a(i) and a(ii) and Table 1].
In marked contrast, studies with the clinical isolate showed that bactericidal effect required a complete bolus profile with a Cpeak30 of 20 mg/liter [Fig. 1b(i) and b(ii) and Table 2]. Lower concentrations produced graded responses with recovery of cell growth by 48.5 h [Fig. 1b(i) and b(ii) and Table 2].
These results indicate that there is a requirement for a greater exposure to tobramycin for bactericidal effect in the clinical strain. A peak/MIC ratio of 5:1 for the clinical isolate compares to a 10:1 ratio for the reference strain. The respective AUC/MIC ratios were 22:1 and 42:1.
Responses of a Pseudomonas clinical isolate to tobramycin concentration-time exposures modelling infusion in vivo dosing.
Exposure to a complete (48.5-h) infusion profile of tobramycin with a Cpeak30 of 20 mg/liter produced complete kill (Table 2), while exposure to a profile with a Cpeak30 of 18 mg/liter caused substantial initial kill with recovery by 48.5 h (Table 2).
Responses of Pseudomonas strains to bolus profile exposure to 8.5 h with varied reconstitution to maintained concentrations of tobramycin.
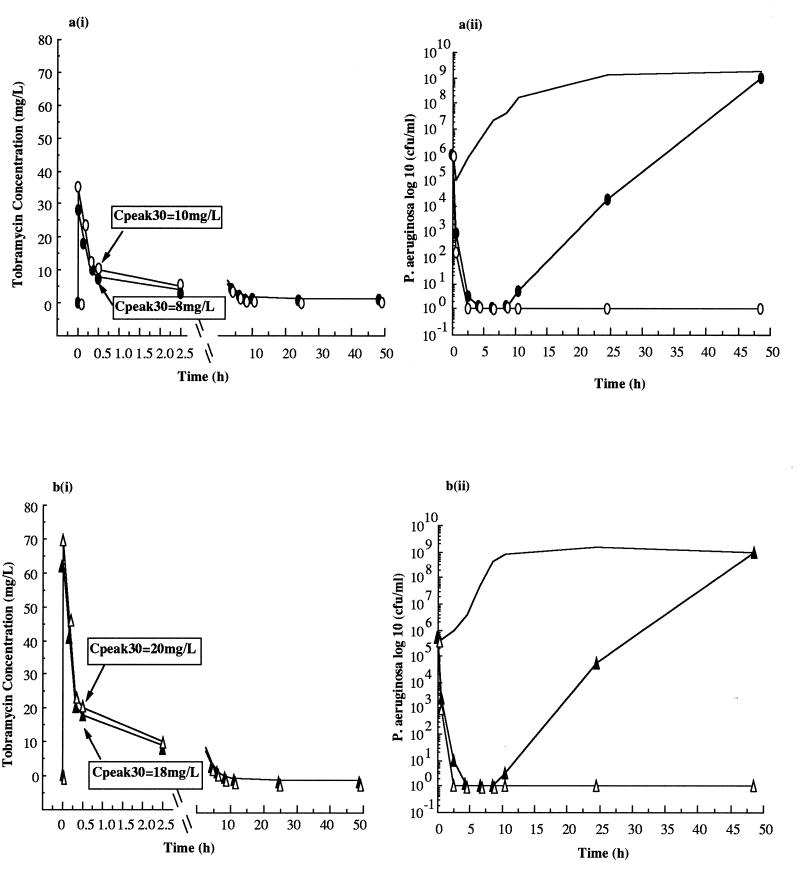
The responses of the reference strain to a bolus profile to 8.5 h incorporating a Cpeak30 of 8 mg/liter were significantly augmented by the reconstituted trough (Cmin) concentrations [Fig. 2a(i) and a(ii) and Table 1].
Reconstituted (trough [Cmin]) concentrations of tobramycin at or above 0.6 mg/liter produced significant differences in regrowth patterns, with bactericidal effects observed when Cmin was ≥0.8 mg/liter.
The responses of the clinical isolate to a bolus profile to 8.5 h incorporating a Cpeak30 of 18 mg/liter similarly showed that outcomes were strongly influenced by the reconstituted concentration [Fig. 2b(i) and b(ii) and Table 2]. The responses at various times after the beginning of exposure to tobramycin were clearly not simply related to AUC (Table 2). Reconstituted (trough [Cmin]) concentrations of tobramycin at or above 0.8 mg/liter produced significant differences in regrowth patterns, with complete bactericidal effects observed when Cmin was ≥1.0 mg/liter.
DISCUSSION
Studies of a higher-MIC, relatively resistant clinical isolate of P. aeruginosa with related changes in aminoglycoside sensitivity may have provided further insights into the necessary design of clinical exposures to aminoglycosides for therapeutic effect.
The clinical isolate required a markedly different exposure to tobramycin in vitro to produce complete kill compared to the relatively sensitive reference strain. A bolus profile with a high postdistributional peak of 20 mg/liter was required for the P. aeruginosa clinical isolate (MIC, 4 mg/liter) compared to the reference strain (MIC, 1 mg/liter), which required a bolus profile with a postdistributional peak of only 10 mg/liter. These results indicate that the peak/MIC ratio is not a constant (2, 9). Similarly, there is no common AUC/MIC ratio.
The question of whether there is dependence of bactericidal outcomes on AUC can be assessed from the profile exposure studies truncated at 8.5 h. These data indicate that the differences in outcome are most sensitive to the trough (Cmin) concentration rather than variation in AUC.
When the initial antibiotic exposure was a bolus clinical profile to 8.5 h, the subsequent required trough concentration for effect was a function of MIC (Fig. 2), without a common MIC/trough ratio for the two strains. Accordingly, there is an interaction between elements of the clinical profile to 8.5 h and the subsequent bactericidal effect; however, no simple general rule emerges.
FIG. 2.
Concentration-time profiles (i) under in vitro conditions following bolus dosing with tobramycin and bacterial colony counts (ii) for the P. aeruginosa reference strain ATCC 27853 (a) when exposed to a postdistribution peak concentration of 8 mg/liter to 8.5 h and then reconstituted to 0 (○), 0.2 (•), 0.4 (□), 0.6 (▪), 0.8 (▵), and 1.0 (▴) mg/liter and for a clinical isolate of P. aeruginosa (b) when exposed to a postdistribution peak concentration of 18 mg/liter to 8.5 h and then reconstituted to 0 (○), 0.2 (•), 0.4 (□), 0.6 (▪), 0.8 (▵), 1.0 (▴), and 1.2 (○) mg/liter. A bolus control (— [no antibiotic]) was also run in parallel for both isolates. For all studies, n = 6.
In vitro results must be translated cautiously into clinical practice; however, these findings can be considered for their potential clinical application, taking into account clinically determined efficacy and toxicity data. Clearly, the tobramycin peak/MIC ratio requirements documented here for the clinical isolate would take clinical bolus dosing into an exposure zone which is beyond the limit of our objective clinical safety knowledge for auditory and/or vestibular toxicity (3, 13). The observation that infusion exposures are equivalent to bolus exposures in the clinical isolate thus becomes important, because we have published detailed patient auditory data indicating the clinical safety of the infusion profiles modelled here (3).
The trough concentrations documented here as contributing to bactericidal outcomes are well within the conventional boundaries for aminoglycoside use (8); hence, the use of target levels of 1.0 mg/liter appears to be safe. There appears to be little variation in the trough (Cmin) requirement, even with a major change in the MIC; accordingly, this may represent an important element of antibiotic exposure in the clinical setting of likely exposure to resistant organisms. However, the recent report of lower ototoxic outcomes in patients with cystic fibrosis exposed to tobramycin trough levels of 0.5 mg/liter, versus trough levels of 0.8 mg/liter in the comparator group (13), suggests that further detailed studies of clinical toxicity associated with maintained trough concentrations are required.
While final clinical guidelines do not emerge from these studies, because they are limited to a single strain for which the MIC is altered (higher), certain principles emerge for consideration in the therapy of resistant organisms with aminoglycosides.
Given that bolus and infusion profiles are equally effective at higher exposures in the clinical isolate, relatively higher aminoglycoside doses delivered via infusion regimens are likely to be needed to generate higher peak concentrations and higher AUC values necessary for bactericidal effect in organisms with higher MIC requirements. Maintenance of trough concentrations of the order of 1.0 mg/liter during the interdose interval will tend to minimize the peak exposure requirement and will tend to guard against the possibility of inadequate peak exposures for kill. Close clinical monitoring for tinnitus, disturbance of balance, and auditory toxicity are indicated in all instances, especially if bolus dosing is used and higher concentrations are targeted.
FIG. 1.
Concentration-time profiles under in vitro conditions following bolus dosing with tobramycin (i) and bacterial colony counts (ii) for the P. aeruginosa reference strain ATCC 27853 when exposed to postdistribution peak concentrations of 8 mg/liter (•) and 10 mg/liter (○) (a) and for a clinical isolate of P. aeruginosa when exposed to postdistribution peak concentrations of 18 mg/liter (▴) and 20 mg/liter (▵) (b). A bolus control (— [no antibiotic]) was run in parallel for both isolates. For all studies, n = 6.
REFERENCES
- 1.Bastone E B, Li S C, Ioannides-Demos L L, Spicer W J, McLean A J. Kill kinetics and regrowth patterns of Escherichia coli exposed to gentamicin concentration-time profiles simulating in vivo bolus or infusion dosing. Antimicrob Agents Chemother. 1993;37:914–917. doi: 10.1128/aac.37.4.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg E J, Peddie B A, Chambers S T, et al. Comparison of gentamicin dosing regimens using an in-vitro model. J Antimicrob Chemother. 1992;29:427–433. doi: 10.1093/jac/29.4.427. [DOI] [PubMed] [Google Scholar]
- 3.Ioannides-Demos L L, Li S C, Bastone E B, Spelman D W, Hooper R, Cousins V C, McLean A J. Absence of toxicity in patients with malignant otitis externa following long-term treatment with high dosage tobramycin. J Antimicrob Chemother. 1994;34:267–274. doi: 10.1093/jac/34.2.267. [DOI] [PubMed] [Google Scholar]
- 4.Li S C, Ioannides-Demos L L, Spicer W J, Spelman D W, Tong N, McLean A J. Prospective audit of the impact of an aminoglycoside pharmacokinetic and consultative service on the utilization patterns and clinical toxicology of aminoglycosides in a general hospital. Med J Aust. 1992;157:308–311. [PubMed] [Google Scholar]
- 5.McLean A J, Ioannides-Demos L L, Li S C, Bastone E B, Spicer W J. Bactericidal effect of gentamicin peak concentration provides a rationale for administration of bolus doses. J Antimicrob Chemother. 1993;32:301–305. doi: 10.1093/jac/32.2.301. [DOI] [PubMed] [Google Scholar]
- 6.McLean A J, Liolios L, Bastone E B, Ioannides-Demos L L, Spicer W J, Christophidis N. Concentration-dependence of gentamicin post-bolus peak effect on Escherichia coli kill kinetics. J Antimicrob Chemother. 1995;35:444–446. doi: 10.1093/jac/35.3.444. [DOI] [PubMed] [Google Scholar]
- 7.McLean A J, Bastone E B, Ioannides-Demos L L, Spicer W J. Bactericidal effect of gentamicin trough concentration provides a rationale for administration of bolus doses and maintenance of trough levels. J Antimicrob Chemother. 1994;33:999–1004. doi: 10.1093/jac/33.5.999. [DOI] [PubMed] [Google Scholar]
- 8.McLean A J, Ioannides-Demos L L, Spicer W J, Christophidis N. Aminoglycoside dosing: one, two or three times a day? Med J Aust. 1996;164:39–42. doi: 10.5694/j.1326-5377.1996.tb94111.x. [DOI] [PubMed] [Google Scholar]
- 9.Moore R D, Lietman P S, Smith C R. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis. 1987;155:93–99. doi: 10.1093/infdis/155.1.93. [DOI] [PubMed] [Google Scholar]
- 10.Sawchuk R J, Zaske D E, Cipolle R J, et al. Kinetic model for gentamicin dosing with the use of individual patient parameters. Clin Pharmacol Ther. 1977;21:362–365. doi: 10.1002/cpt1977213362. [DOI] [PubMed] [Google Scholar]
- 11.Sveska K J, Roff B D, Solomon D K, Hoffman R P. Outcome of patients treated by an aminoglycoside pharmacokinetic dosing service. Am J Hosp Pharm. 1985;42:2472–2478. [PubMed] [Google Scholar]
- 12.Wood P J, Ioannides-Demos L L, Bastone E B, Spicer W J, McLean A J. Kill kinetics and regrowth patterns of Pseudomonas aeruginosa exposed to tobramycin concentration-time profiles simulating in vivo bolus or infusion dosing. Antimicrob Agents Chemother. 1996;40:1321–1324. doi: 10.1128/aac.40.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wood P J, Ioannides-Demos L L, Li S C, Williams T, Hickey B, Spicer W J, Hooper R E, McLean A J. Minimisation of aminoglycoside toxicity in patients with cystic fibrosis. Thorax. 1996;51:369–373. doi: 10.1136/thx.51.4.369. [DOI] [PMC free article] [PubMed] [Google Scholar]