Abstract
We evaluate National Cancer Institute (NCI) funding distribution to the most common cancers, considering their respective public health burdens, and explore associations between funding and racial and ethnic burden of disease. The NCI’s Surveillance, Epidemiology and End Results, US Cancer Statistics database, and Funding Statistics were used to calculate funding-to-lethality (FTL) scores. Breast and prostate cancer had the first (179.65) and second (128.90) highest FTL scores, and esophagus and stomach cancer ranked 18th (2.12) and 19th (1.78). We evaluated whether there were differences between the FTL and cancer incidence and/or mortality within individual racial and ethnic groups. NCI funding correlated highly with cancers afflicting a higher proportion of non-Hispanic White individuals (Spearman correlation coefficient = 0.84; P < .001). Correlation was stronger for incidence than mortality. These data reveal that funding across cancer sites is not concordant with lethality and that cancers with high incidence among racial and ethnic minorities receive lower funding.
In 2016, the White House launched the Cancer Moonshot initiative to accelerate cancer research with a crucial cross-cutting feature of mitigating cancer disparities (1). Prior studies have shown inequitable research funding for certain cancers, accounting for incidence and/or lethality (eg, gynecologic cancers), but these studies were limited as they 1) did not focus solely on federal (ie, National Cancer Institute [NCI]) funding; 2) evaluated funding data prior to the launch of the Cancer Moonshot; and/or 3) did not evaluate whether funding disparities were associated with racial and ethnic disparities in cancer incidence and/or mortality (2-4). Here, we evaluate disparities in NCI research funding for the most common cancers considering their respective public health burdens (incidence rate, mortality rate, and person-years of life lost) and explore associations between funding and racial and ethnic burden of disease. Previous efforts to illuminate funding distribution have evaluated these 3 metrics separately, but we use a previously validated measure, lethality, which incorporates the 3 metrics above and provides a composite objective measure for burden of disease (2).
We obtained data from the NCI’s Surveillance, Epidemiology and End Results (SEER) database, US Cancer Statistics database, and Funding Statistics between 2014 and 2018 (5-7). For each year, we identified overall (and by race and ethnicity) incidence rate and mortality rate per 100 000 persons for the 19 most common cancer sites, as well as NCI funding for each cancer. We calculated ratios for funding to incidence, funding to mortality, mortality to incidence, and lethality scores (mortality to incidence ratios * person-years of life lost per death). Funding-to-lethality (FTL) scores (NCI funding divided by lethality) were identified by cancer site and represent a previously validated measure to identify funding disparities (2). Correlation between FTL and incidence and mortality rates by race and ethnicity were assessed with Spearman correlation coefficients. In doing so, we sought to evaluate whether there were differences between the FTL and cancer incidence and/or mortality within individual racial and ethnic groups.
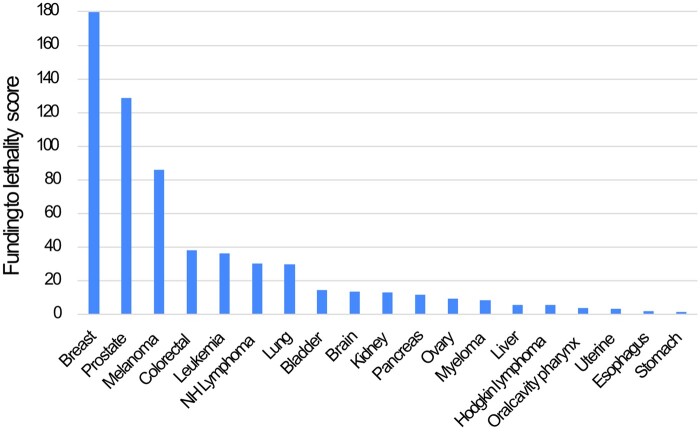
Cancers with the highest average annual funding were breast ($542.2 million) and lung cancer ($292.9 million), and stomach cancer ($13.2 million) and Hodgkin lymphoma ($13.7 million) had the lowest average annual funding (Supplementary Figure 1, available online). There was a more than 100-fold difference in the FTL score of the highest (breast: 179.65) and lowest (stomach: 1.78) funded cancers, accounting for lethality (Figure 1). In fact, breast cancer had a FTL score that was more than 10 times higher than 12 other cancers. Although there was a slight increase in funding over the years 2014-2018, the disparities across the cancers included in the analysis were unchanged over time (Supplementary Figure 2, A and B, available online).
Figure 1.
Bar graph depicting funding-to-lethality ratios by cancer site. NH = non-Hodgkin.
Overall, there was a stronger correlation between FTL scores and race and ethnicity–specific cancer incidence, rather than mortality (Table 1). Although there was a strong correlation between a cancer’s incidence among non-Hispanic White individuals and its FTL score (ρ = 0.84), this correlation was only moderate to weak for other racial and ethnic groups (ρ = 0.25-0.57). Similarly, although the correlation was moderate to strong between a cancer’s mortality rate among non-Hispanic White individuals and its FTL score (ρ = 0.69), this correlation was weak for all other racial and ethnic groups (ρ = 0.33-0.44). These correlation coefficients suggest that funding is strongly correlated with a cancer’s incidence among non-Hispanic White individuals.
Table 1.
Correlation between cancer-specific funding-to-lethality ratios and cancer incidence and mortality stratified by race and ethnicitya
| Race | Funding to lethality: incidence | Funding to lethality: mortality |
|---|---|---|
| Hispanic | 0.52 | 0.41 |
| Non-Hispanic American Indian Alaska Native | 0.57 | 0.37 |
| Non-Hispanic Asian and Pacific Islander | 0.25 | 0.33 |
| Non-Hispanic Black | 0.45 | 0.44 |
| Non-Hispanic White | 0.84 | 0.69 |
These data represent Spearman rank correlation coefficients assessing the correlation between funding-to-lethality ratio for a given cancer (ie, x axis on a scatter plot) plotted against incidence (or mortality) for a given cancer (ie, y axis on a scatter plot), stratified by race and ethnicity. For example, there was a strong correlation between the funding-to-lethality ratio and cancer incidence among non-Hispanic White individuals suggesting that funding relative to lethality was increased as the incidence of the disease increased among non-Hispanic White patients. However, this correlation was weak to moderate among non-Hispanic Black individuals, suggesting limited correlation between the incidence of the cancer among non-Hispanic Black patients and the funding relative to lethality.
Despite initiatives to bolster cancer research funding and to mitigate disparities in cancer outcomes, there are marked disparities in federally funded cancer research that do not correlate with lethality. The federal government (NCI, Department of Defense) is a major source of cancer research funding, followed by private sector sources (charity foundations and pharmaceutical industry) (8). For pancreatic cancer in 2018, for example, NCI contributed $122.4 million, Department of Defense contributed $6 million, and the Pancreatic Cancer Action Network (a private charity) contributed $8.5 million (7,9). Accordingly, these data raise concerns that funding for different cancer sites is not concordant with disease burden. Importantly, cancers that disproportionately afflict non-Hispanic White individuals (breast cancer, leukemia, and lymphoma) receive more funding than those cancers with high incidence rates among racial and ethnic minorities (stomach, uterine, and liver cancers). This finding is supported by a 2022 study that demonstrated that NCI and nonprofit funding increased proportionately as incidence increased for White patients, whereas cancers with higher incidence in the ethnic minority populations were relatively underfunded (10).
Cancer funding allocation is complex and multifaceted. Prior studies have sought to understand funding distribution, as we note above. A 2019 study demonstrated that cancers with stigmatized behaviors (smoking, alcohol, drug use) are underfunded (2). Fundraising campaigns and advertising for well-funded cancers such as the Susan G. Komen Foundation and the Leukemia and Lymphoma Society should be praised for their success, as effective patient advocacy has increased public awareness and helped secure billions of dollars in cancer research funding. Yet, it is crucial we continue to critically examine disparities in funding and allocate resources to mitigate these disparities, especially where underrepresented groups are disproportionately impacted.
Our paper identifies discrepancies in funding by demographic groups and highlights the need to ensure that federal funds are equitably distributed. This is especially important given the discrepancies in cancer outcomes for minorities, particularly in the more underfunded cancers. For example, Black Americans, Hispanics, and Asians and Pacific Islanders are 2-3 times more likely to die from stomach cancer than non‐Hispanic White individuals (11). Similarly, although White and Black women are diagnosed with uterine cancer at similar rates, Black women are twice as likely to die from it compared with White women (6). Some may argue that the discrepancy in funding in absolute terms between highly funded cancers like breast cancer and lower-funded cancers like stomach cancer is warranted given overall differences in incidence or mortality. However, even in absolute terms there were marked disparities. For example, in 2018, estimated deaths for breast cancer was nearly 4 times that of stomach cancer, yet breast cancer received approximately 50 times more funding (Supplementary Figure 1, available online) (6). Concerted efforts are required to align funding allocation and requests for applications that account for disease lethality and burden on historically disadvantaged groups.
There are potential limitations to this study. First, this study only included NCI in the primary analysis as the source of research funding and did not include other sources of federal (Department of Defense Cancer Research Program) or private (nonprofit organization) funding because of the variability of how funds are allocated to cancer sites among funding sources. Second, the SEER registry does not provide disaggregated data on Asian and Pacific Islander subgroups, which is problematic because these are heterogenous groups—Asia consists of more than 40 countries, the Pacific Islands are grouped by 3 subregions, and there are myriad genetic, environmental, and sociodemographic differences between them (12). Within the groups, there are marked differences in cancer incidence and outcomes, and aggregating the data can mask important cancer disparities (13). Third, racial and ethnic disparities in incidence and outcomes exist among subtypes of certain cancers (eg, triple-negative breast cancer), yet we were not able to include this in our analysis as such data were not made available by SEER. Fourth, we could not separate funding based on research type (ie, basic vs clinical), which may limit our ability to delineate funding allocation focusing on racial and ethnic minorities (for example, those specifically investigating disparities or genetic predispositions). Lastly, we refer to cancer lethality as a surrogate for a cancer’s public health or disease burden, yet it is important to recognize that disease burden is subject to varying interpretations including treatment burden that accompanies the burden of cancer survivorship (eg, taking medications, maintaining medical appointments, managing acute and/or chronic complications from treatment or disease).
Access to health care and discrimination and bias in the health-care system are well-documented drivers of cancer disparities. To dismantle decades of structural racism, a top-down, policy approach to equitably distribute cancer research funding across racial and ethnic groups is paramount. Funding should be prioritized for cancers that disproportionately impact minorities to mitigate disparities and reduce our cancer burden. Additionally, cancer lethality may be a more appealing means of justifying increased research funding, as this measure can reflect the true burden and efficacy of cancer control programs.
Supplementary Material
Acknowledgements
The funder had no role in the study design; data collection, analysis, or interpretation; writing of the manuscript or decision to submit it for publication.
Contributor Information
Shida Haghighat, Division of Gastroenterology and Hepatology, University of Miami Miller School of Medicine, Miami, FL, USA.
Chunsu Jiang, Division of Gastroenterology and Hepatology, University of Miami Miller School of Medicine, Miami, FL, USA.
Wael El-Rifai, Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL, USA; Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
Alexander Zaika, Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL, USA; Department of Surgery, University of Miami Miller School of Medicine, Miami, FL, USA.
David S Goldberg, Division of Gastroenterology and Hepatology, University of Miami Miller School of Medicine, Miami, FL, USA; Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL, USA.
Shria Kumar, Division of Gastroenterology and Hepatology, University of Miami Miller School of Medicine, Miami, FL, USA; Sylvester Comprehensive Cancer Center, University of Miami Health System, Miami, FL, USA.
Data availability
The data used for this study are publicly available data from SEER and United States Cancer Statistics. The underlying data for funding can be shared upon request. The SEER data require an investigator sign a data use agreement so cannot be shared by the investigators but the process to obtain the data can be shared upon request.
Author contributions
Shida Haghighat, MD, MPH (Data curation; Formal analysis; Methodology; Visualization; Writing – original draft; Writing – review & editing), Chunsu Jiang, MD (Data curation; Formal analysis), Wael El-Rifai, MD, PhD (Writing – review & editing), Alexander Zaika, PhD (Writing – review & editing), David S. Goldberg, MD, MSCE (Conceptualization; Supervision; Writing – review & editing), and Shria Kumar, MD, MSCE (Conceptualization; Formal analysis; Supervision; Writing – review & editing).
Funding
Shida Haghighat, MD, MPH, is supported by an NIH training grant T32 DK 116678-05.
Conflicts of interest
The authors declare no personal, professional, or financial conflicts of interest.
References
- 1.National Cancer Institute. Cancer Moonshot. https://www.cancer.gov/research/key-initiatives/moonshot-cancer-initiative. Accessed November 4, 2022.
- 2. Spencer RJ, Rice LW, Ye C, Woo K, Uppal S.. Disparities in the allocation of research funding to gynecologic cancers by funding to lethality scores. Gynecol Oncol. 2019;152(1):106-111. doi: 10.1016/j.ygyno.2018.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kamath SD, Kircher SM, Benson AB.. Comparison of cancer burden and nonprofit organization funding reveals disparities in funding across cancer types. J Natl Compr Cancer Netw. 2019;17(7):849-854. doi: 10.6004/jnccn.2018.7280. [DOI] [PubMed] [Google Scholar]
- 4. Hall BR, Cannon A, Atri P, et al. A comparative analysis of survival and funding discrepancies in cancers with high mortality. Ann Surg. 2020;271(2):296-302. doi: 10.1097/SLA.0000000000003042. [DOI] [PubMed] [Google Scholar]
- 5. National Cancer Institute, DCCPS, Surveillance Research Program. Surveillance, Epidemiology, and End Results (SEER) Program Research Data (2000–2018). 2020. www.seer.cancer.gov. Accessed November 4, 2022.
- 6.National Program of Cancer Registries and Surveillance, Epidemiology, and End Results Program SEERStat Database: NPCR and SEER Incidence–US Cancer Statistics Public Use Research Database, 2021 sub (2001–2019). National Cancer Institute, US Department of Health and Human Services, Centers for Disease Control and Prevention. 2022. www.cdc.gov/cancer/uscs/public-use. Accessed November 4, 2022.
- 7.National Cancer Institute. Research funding statistics for FY 2014 - 2018 cancer type. https://fundedresearch.cancer.gov/nciportfolio/search/funded?fy=PUB2014&type=site. Accessed October 3, 2022.
- 8. Trasta A. Where does public funding for cancer research go: allocation of research funding for cancer and COPD is not always proportional to disease burden. EMBO Rep. 2018;19(3):e45859. doi: 10.15252/embr.201845859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reynolds K. Big win: Congress Creates dedicated $6 million pancreatic cancer research program. Pancreatic Cancer Action Network; 2019. https://pancan.org/news/big-win-congress-creates-dedicated-6-million-pancreatic-cancer-research-program/. Accessed May 14, 2023.
- 10. Kamath SD, Chen Y. Disparities in NCI and nonprofit organization funding and effect on cancers with high incidence rates among Black patients and mortality rates. Poster presented at 2022 American Society of Clinical Oncology Annual Meeting; June 3-7, 2022; Chicago, IL.
- 11. Laszkowska M, Tramontano AC, Kim J, et al. Racial and ethnic disparities in mortality from gastric and esophageal adenocarcinoma. Cancer Med. 2020;9(15):5678-5686. doi: 10.1002/cam4.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bhakta S. Data disaggregation: the case of Asian and Pacific Islander data and the role of health sciences librarians. J Med Libr Assoc. 2022;110(1):133-138. doi: 10.5195/jmla.2022.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Medina HN, Callahan KE, Morris CR, Thompson CA, Siweya A, Pinheiro PS.. Cancer mortality disparities among Asian American and native Hawaiian/Pacific Islander populations in California. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1387-1396. doi: 10.1158/1055-9965.EPI-20-1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used for this study are publicly available data from SEER and United States Cancer Statistics. The underlying data for funding can be shared upon request. The SEER data require an investigator sign a data use agreement so cannot be shared by the investigators but the process to obtain the data can be shared upon request.