Abstract
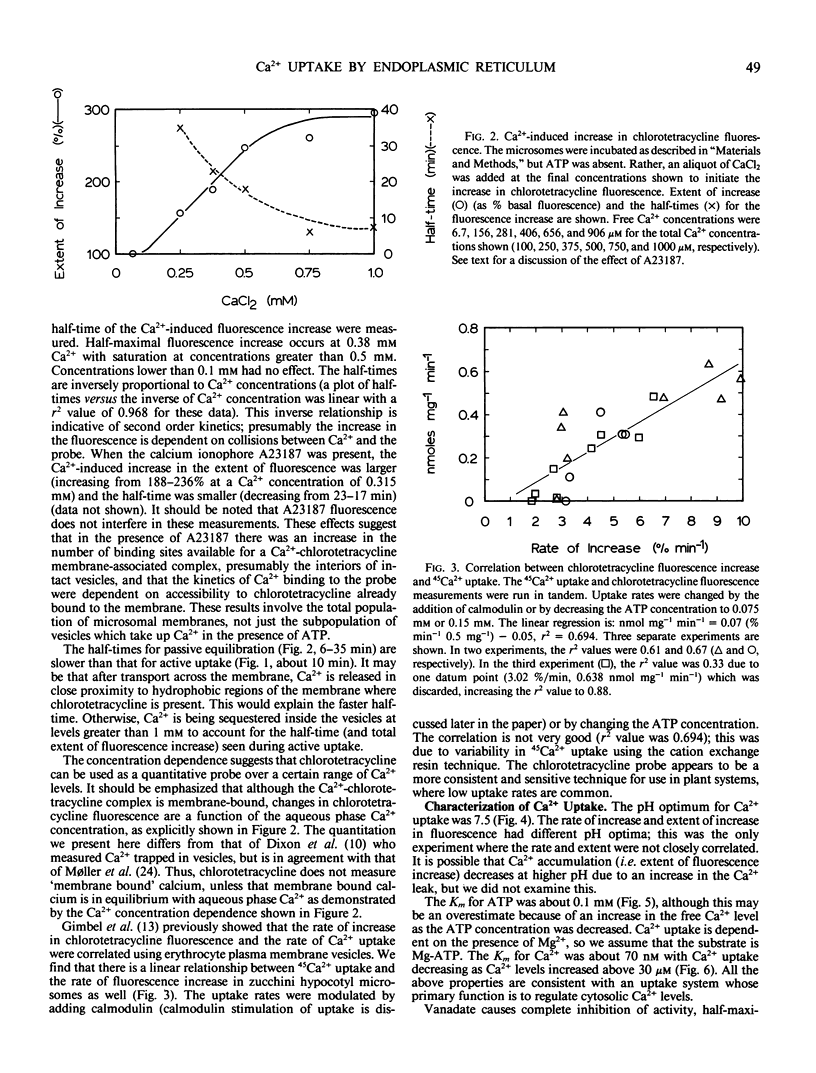
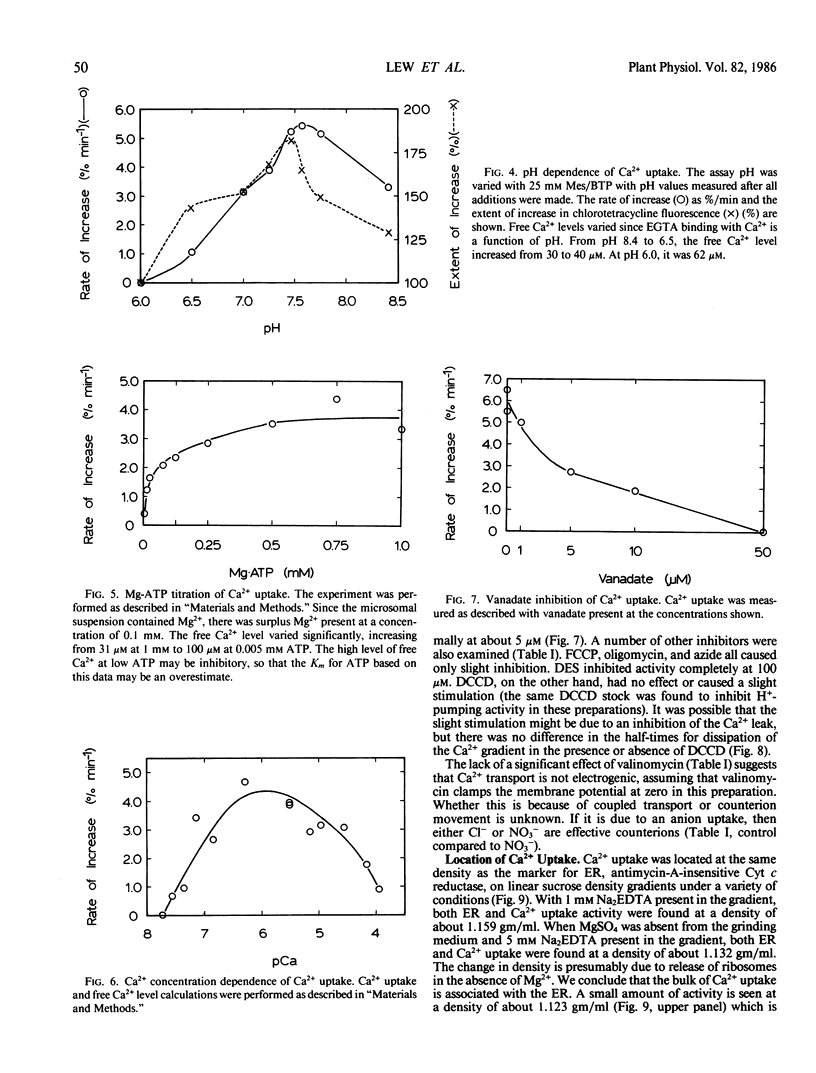
Ca2+ uptake into microsomal vesicles was measured using the fluorescent probe chlorotetracycline. The Ca2+ uptake was ATP-dependent and did not occur in the presence of the calcium ionophore A23187. There was a linear relationship between the rate of ATP-dependent fluorescence increase using chlorotetracycline and ATP-dependent 45Ca2+ uptake, indicating that chlorotetracycline can be used as a quantitative probe for Ca2+ uptake. The fluorescent probe allows measurements to be made in real time, and avoids the use of radioisotopes. Ca2+ transport was associated with endoplasmic reticulum on linear gradients when the endoplasmic reticulum was in either rough or smooth form. The Ca2+ uptake had a pH optimum of 7.5, a Km for ATP of 0.1 millimolar, a Km for Ca2+ of about 70 nanomolar, and was stimulated 2-fold by calmodulin. Vanadate inhibited uptake completely at a concentration of 50 micromolar, half-maximally at 5 micromolar. Carbonyl cyanide 4-(trifluoromethoxy)-phenyl-hydrazone, oligomycin, azide, and nitrate caused only slight inhibition. Dicyclohexylcarbodiimide (DCCD) stimulated slightly at concentrations as high as 400 micromolar. The hormones gibberellic acid, indoleacetic acid, and abscisic acid at 10 micromolar had no significant effect. Myo-inositol 1,4,5-trisphosphate did not cause release of Ca2+ after uptake. The properties of the enzyme suggest that it has a functional role in regulating cytosolic Ca2+ levels. Based on the lack of an effect by hormones, it may not act as a mediator of second messenger roles of Ca2+. The inhibition by vanadate and slight stimulation by DCCD may be useful as a `signature' for this endoplasmic reticulum Ca2+ uptake system.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Buckhout T. J. Characterization of Ca Transport in Purified Endoplasmic Reticulum Membrane Vesicles from Lepidium sativum L. Roots. Plant Physiol. 1984 Dec;76(4):962–967. doi: 10.1104/pp.76.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dieter P., Marmé D. Calmodulin activation of plant microsomal Ca uptake. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7311–7314. doi: 10.1073/pnas.77.12.7311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon D., Brandt N., Haynes D. H. Chlorotetracycline fluorescence is a quantitative measure of the free internal Ca2+ concentration achieved by active transport. In situ calibration and application to bovine cardiac sarcolemmal vesicles. J Biol Chem. 1984 Nov 25;259(22):13737–13741. [PubMed] [Google Scholar]
- Dupont F. M., Bennett A. B., Spanswick R. M. Localization of a proton-translocating ATPase on sucrose gradients. Plant Physiol. 1982 Oct;70(4):1115–1119. doi: 10.1104/pp.70.4.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasko O. D., Knowles A. F., Shertzer H. G., Suolinna E. M., Racker E. The use of ion-exchange resins for studying ion transport in biological systems. Anal Biochem. 1976 May 7;72:57–65. doi: 10.1016/0003-2697(76)90506-6. [DOI] [PubMed] [Google Scholar]
- Gimble J. M., Gustin M., Goodman D. B., Rasmussen H. Studies on the Ca2+ transport mechanism of human erythrocyte inside-out plasma membrane vesicles. V. Chlortetracycline fluorescence. Biochim Biophys Acta. 1982 Mar 8;685(3):253–259. doi: 10.1016/0005-2736(82)90065-7. [DOI] [PubMed] [Google Scholar]
- Gross J., Marmé D. ATP-dependent Ca uptake into plant membrane vesicles. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1232–1236. doi: 10.1073/pnas.75.3.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges T. K., Leonard R. T. Purification of a plasma membrane-bound adenosine triphosphatase from plant roots. Methods Enzymol. 1974;32:392–406. doi: 10.1016/0076-6879(74)32039-3. [DOI] [PubMed] [Google Scholar]
- Kauss H. Volume regulation in poterioochromonas: involvement of calmodulin in the ca-stimulated activation of isofloridoside-phosphate synthase. Plant Physiol. 1983 Jan;71(1):169–172. doi: 10.1104/pp.71.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubowicz B. D., Vanderhoef L. N., Hanson J. B. ATP-Dependent Calcium Transport in Plasmalemma Preparations from Soybean Hypocotyls : EFFECT OF HORMONE TREATMENTS. Plant Physiol. 1982 Jan;69(1):187–191. doi: 10.1104/pp.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTZEHL H., CALDWELL P. C., RUEEGG J. C. THE DEPENDENCE OF CONTRACTION AND RELAXATION OF MUSCLE FIBRES FROM THE CRAB MAIA SQUINADO ON THE INTERNAL CONCENTRATION OF FREE CALCIUM IONS. Biochim Biophys Acta. 1964 May 25;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Pick U., Racker E. Inhibition of the (Ca2+)ATPase from sarcoplasmic reticulum by dicyclohexylcarbodiimide: evidence for location of the Ca2+ binding site in a hydrophobic region. Biochemistry. 1979 Jan 9;18(1):108–113. doi: 10.1021/bi00568a017. [DOI] [PubMed] [Google Scholar]
- Ranjeva R., Refeno G., Boudet A. M., Marmé D. Activation of plant quinate:NAD 3-oxidoreductase by Ca and calmodulin. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5222–5224. doi: 10.1073/pnas.80.17.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium- and calmodulin-regulated phosphorylation of soluble and membrane proteins from corn coleoptiles. Plant Physiol. 1984 Oct;76(2):359–365. doi: 10.1104/pp.76.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]


