Abstract
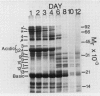
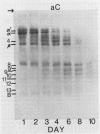
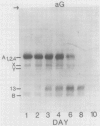
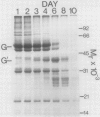
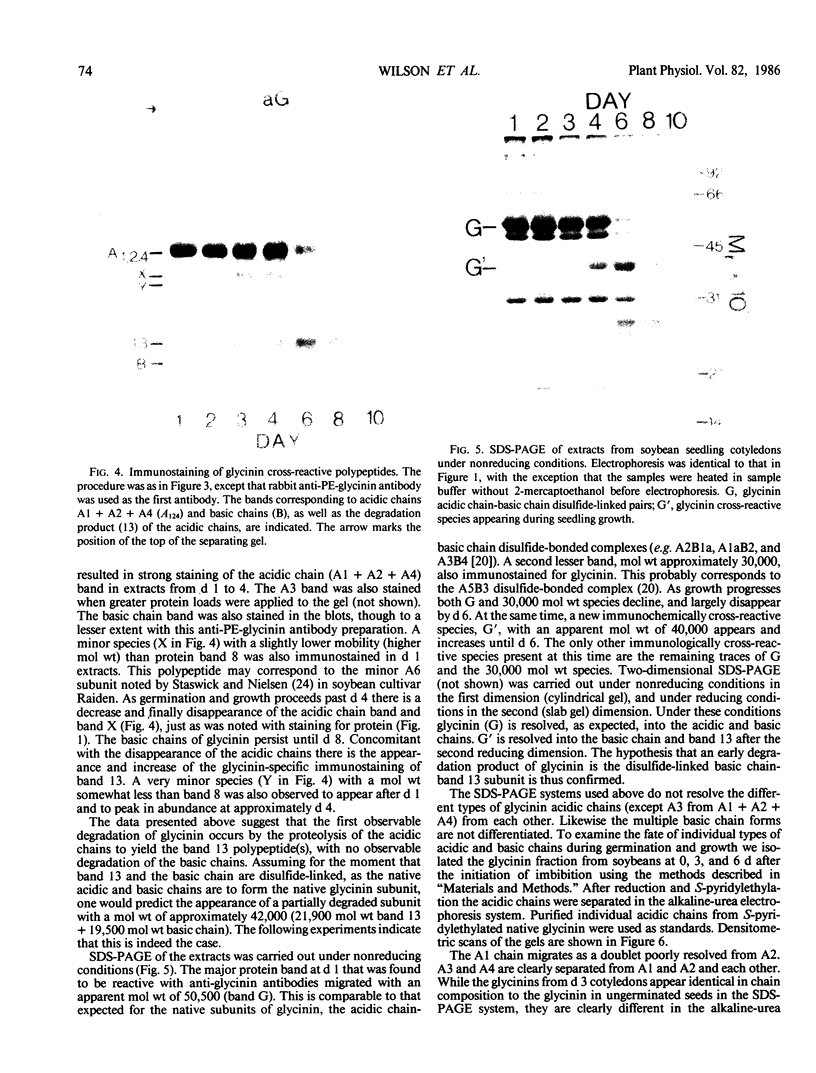
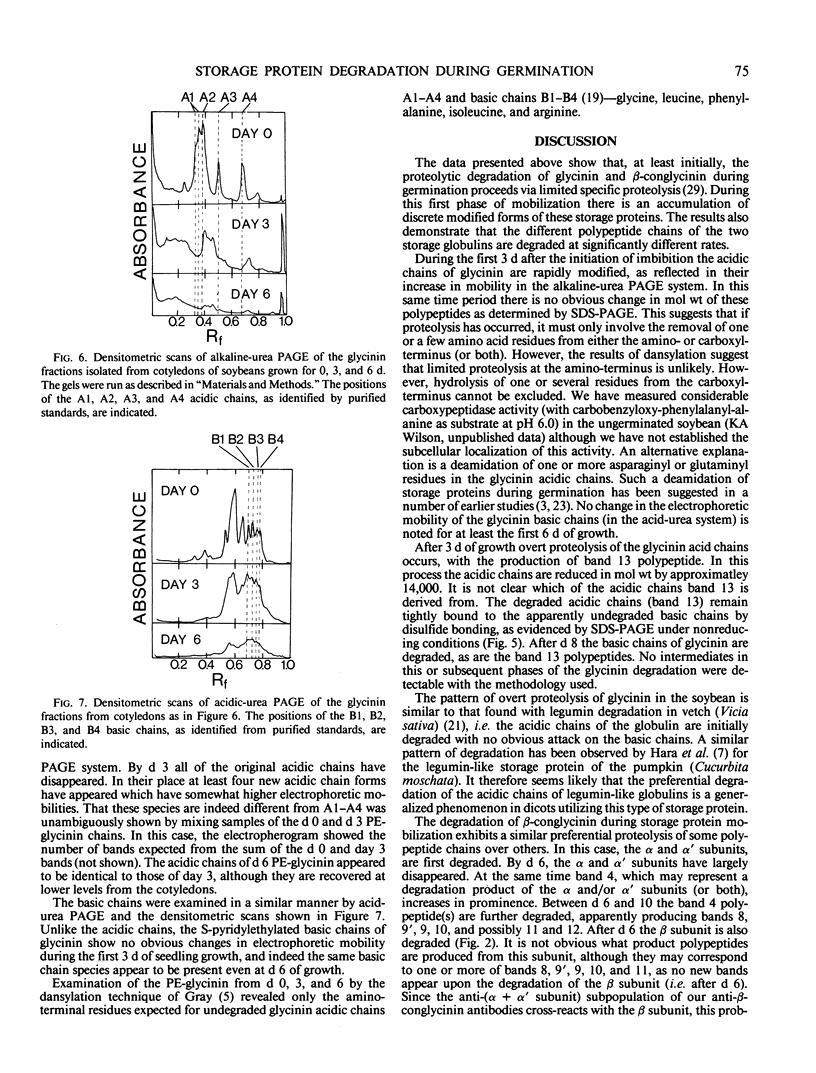
The degradation of the major seed storage globulins of the soybean (Glycine max [L.] Merrill) was examined during the first 12 days of germination and seedling growth. The appearance of glycinin and β-conglycinin degradation products was detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis of cotyledon extracts followed by electroblotting to nitrocellulose and immunostaining using glycinin and β-conglycinin specific antibodies. The three subunits of β-conglycinin were preferentially metabolized. Of the three subunits of β-conglycinin, the larger α and α′ subunits are rapidly degraded, generating new β-conglycinin cross-reactive polypeptides of 51,200 molecular weight soon after imbibition of the seed. After 6 days of growth the β-subunit is also hydrolyzed. At least six polypeptides, ranging from 33,100 to 24,000 molecular weight, appear as apparent degradation products of β-conglycinin. The metabolism of the glycinin acidic chains begins early in growth. The glycinin acidic chains present at day 3 have already been altered from the native form in the ungerminated seed, as evidenced by their higher mobility in an alkaline-urea polyacrylamide gel electrophoresis system. However, no change in the molecular weight of these chains is detectable by sodium dodecyl sulfate-polyarylamide gel electrophoresis. Examination of the glycinin polypeptide amino-termini by dansylation suggests that this initial modification of the acidic chains involves limited proteolysis at the carboxyl-termini, deamidation, or both. After 3 days of growth the acidic chains are rapidly hydrolyzed to a smaller (21,900 molecular weight) form. The basic polypeptides of glycinin appear to be unaltered during the first 8 days of growth, but are rapidly degraded thereafter to unidentified products. All of the original glycinin basic chains have been destroyed by day 10 of growth.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bond H. M., Bowles D. J. Characterization of soybean endopeptidase activity using exogenous and endogenous substrates. Plant Physiol. 1983 Jun;72(2):345–350. doi: 10.1104/pp.72.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catsimpoolas N., Campbell T. G., Meyer E. W. Immunochemical study of changes in reserve proteins of germinating soybean seeds. Plant Physiol. 1968 May;43(5):799–805. doi: 10.1104/pp.43.5.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harboe N., Ingild A. Immunization, isolation of immunoglobulins, estimation of antibody titre. Scand J Immunol Suppl. 1973;1:161–164. doi: 10.1111/j.1365-3083.1973.tb03798.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Moreira M. A., Hermodson M. A., Larkins B. A., Nielsen N. C. Partial characterization of the acidic and basic polypeptides of glycinin. J Biol Chem. 1979 Oct 10;254(19):9921–9926. [PubMed] [Google Scholar]
- Shutov A. D., Do N. L., Vaintraub I. A. Ochistka i chastichnaia kharakteristika proteazy B iz prorastaiushchikh semian viki. Biokhimiia. 1982 May;47(5):814–821. [PubMed] [Google Scholar]
- Staswick P. E., Nielsen N. C. Characterization of a soybean cultivar lacking certain glycinin subunits. Arch Biochem Biophys. 1983 May;223(1):1–8. doi: 10.1016/0003-9861(83)90565-9. [DOI] [PubMed] [Google Scholar]
- Tan-Wilson A. L., Rightmire B. R., Wilson K. A. Different Rates of Metabolism of Soybean Proteinase Inhibitors during Germination. Plant Physiol. 1982 Aug;70(2):493–497. doi: 10.1104/pp.70.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanh V. H., Shibasaki K. Beta-conglycinin from soybean proteins. Isolation and immunological and physicochemical properties of the monomeric forms. Biochim Biophys Acta. 1977 Feb 22;490(2):370–384. doi: 10.1016/0005-2795(77)90012-5. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]