Abstract
Background
Managing acute pain is a common challenge in the emergency department (ED). Though widely used in perioperative settings, ED‐based ultrasound‐guided nerve blocks (UGNBs) have been slow to gain traction. Here, we develop a low‐cost, low‐fidelity, simulation‐based training curriculum in UGNBs for emergency physicians to improve procedural competence and confidence.
Methods
In this pre‐/postintervention study, ED physicians were enrolled to participate in a 2‐h, in‐person simulation training session composed of a didactic session followed by rotation through stations using handmade pork‐based UGNB models. Learner confidence with performing and supervising UGNBs as well as knowledge and procedural‐based competence were assessed pre‐ and posttraining via electronic survey quizzes. One‐way repeated‐measures ANOVAs and pairwise comparisons were conducted. The numbers of nerve blocks performed clinically in the department pre‐ and postintervention were compared.
Results
In total, 36 participants enrolled in training sessions, eight participants completed surveys at all three data collection time points. Of enrolled participants, 56% were trainees, 39% were faculty, 56% were female, and 53% self‐identified as White. Knowledge and competency scores increased immediately postintervention (mean ± SD t0 score 66.9 ± 8.9 vs. t1 score 90.4 ± 11.7; p < 0.001), and decreased 3 months postintervention but remained elevated above baseline (t2 scores 77.2 ± 11.5, compared to t0; p = 0.03). Self‐reported confidence in performing UGNBs increased posttraining (t0 5.0 ± 2.3 compared to t1 score 7.1 ± 1.5; p = 0.002) but decreased to baseline levels 3 months postintervention (t2 = 6.0 ± 1.9, compared to t0; p = 0.30).
Conclusions
A low‐cost, low‐fidelity simulation curriculum can improve ED provider procedural‐based competence and confidence in performing UGNBs in the short term, with a trend toward sustained improvement in knowledge and confidence. Curriculum adjustments to achieve sustained improvement in confidence performing and supervising UGNBs long term are key to increased ED‐based UGNB use.
Keywords: models, nerve block, POCUS, procedure, regional anesthesia, simulation, ultrasound
INTRODUCTION
Controlling acute pain is a cornerstone of Emergency Medicine practice and care. 1 In perioperative settings, ultrasound‐guided nerve blocks (UGNBs) are a targeted pain management strategy that is associated with decreased opioid use, reduced hospital length‐of‐stay, and increased patient satisfaction compared to traditional analgesic regimens. 2 , 3 , 4 Emerging evidence suggests that UGNBs are an effective analgesic approach for an impressive array of acute pain indications commonly encountered in the Emergency Department (ED) such as laceration repairs, fractures, dislocations, abscess incision and drainage, acute‐on‐chronic low back pain, shingles, or even acute pancreatitis. 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16
Despite the potential benefits of UGNBs for ED patients in acute pain, there has been a relatively slow rate of ED‐based adoption. Recent surveys demonstrate that only a small proportion of EDs offer training and credentialing pathways to their providers for UGNBs, with a minimal number of clinicians having received prior training. 17 Thus, when ED providers integrate UGNBs into clinical care, they have highly variable training, practice patterns, efficacy, and incidence of adverse events.
Performing UGNBs requires skills in ultrasound image acquisition and interpretation to identify relevant sonoanatomy and precise motor skills to guide the needle to the target. These motor skills are complex and require practice to achieve mastery. Medical simulation using models is a promising approach for training providers in point‐of‐care ultrasound (POCUS) and allows for safe skill development without jeopardizing patient safety. 18 , 19 Commercial simulation models for UGNB training and education are available; however, their cost can be prohibitive for widespread use, and they are limited in variety. 20 Low‐fidelity, cost‐effective UGNB models have been proposed; however, their impact on education and training outcomes long term has not been explored. 21 , 22 , 23
Here we investigate the impact of a novel, cost‐effective, low‐fidelity UGNB educational intervention on UGNBs performed in an academic emergency center. For our curriculum we developed simulation models that can be adapted to nearly any nerve block performed in the ED and are easily scalable for training in nearly any educational setting. Finally, we explore trainee comfort and competency with UGNBs as a result of our intervention.
METHODS
Study design and setting
This was a prospective, pilot pre‐/postintervention study aimed at assessing the impact of a brief simulation‐based curriculum on UGNB performance, competency, and self‐reported provider procedural comfort. The training took place in a simulation center of an urban, tertiary care academic medical center, which has an ED that serves 65,000 patients annually. All curriculum development and study proceedings were approved by the local institutional review board.
Participant recruitment and baseline knowledge and confidence assessment
Participants were recruited to participate via email to the faculty and resident email listservs. The study included emergency medicine faculty and/or residents at the host institution who were actively involved in clinical duties in the ED. After consenting to participate, all participants received a pretraining survey (t0) asking them to self‐report demographic data as well as their perceived comfort, confidence, and competency with both performing and supervising UGNBs, measured via 1–10 Likert scale as well as 17 multiple‐choice questions assessing knowledge‐based competency of UGNB techniques, utility, safety, POCUS image interpretation, and anatomy identification.
UGNB simulation curriculum
Participants completed a 2‐h, in‐person UGNB simulation‐based training session. The objectives of the training session were to improve (a) knowledge‐based competency of UGNB indications/contraindications, conceptual techniques, and safety including safe anesthetic dosing and possible complications; (b) POCUS image interpretation and anatomical landmark identification on human models; (c) needle visualization and manipulation on simulation models; and (d) the practice of hydrolocation and hydrodissection to accurately determine needle tip placement and to inject regional anesthesia between the identified fascial planes.
Training sessions included a 20‐min didactic session on UGNB indications, contraindications, conceptual techniques, and safety. Following the didactic session, participants were divided into small groups of three to five participants and rotated through each of four learning stations each equipped to teach one UGNB used for a common ED indication: (a) fascia iliaca block, (b) transgluteal sciatic nerve block, (c) serratus anterior block, and (d) interscalene nerve block. These blocks were selected due to their high‐yield applications in emergency medicine, and collectively they allow for comprehensive mastery of UGNBs of the upper extremities, lower extremities, and trunk as well as in targeting both nerve and plane block techniques.
Each station was attended by an educator who was a fellowship‐trained expert in emergency POCUS and who had advanced training in performing UGNBs. At each station learners participated in POCUS scanning on a live model to identify sonographic landmarks for the given block as well as hands‐on practice for mastering ultrasound‐guided needle visualization and control, hydrodissection, and anesthetic injection in low‐cost, low‐fidelity homemade reusable pork models.
UGNB low‐fidelity pork simulation models
Dedicated pork models were hand‐built using pork loin and household goods to mimic the anatomy of each of the four featured nerve blocks. Pork meat represented surrounding muscle, bundled yarn soaked in ultrasound gel imbedded in the meat was used to represent nerves, cuts in the meat were made and reapproximated using meat glue and a layer of tissue paper to represent fascial layers, wooden dowels imbedded in the meat were used to represent bones, and rubber tubing filled with water was used to represent blood vessels (Figure 1). Reusable pork models were hand‐made in advance of the training sessions and stored in a commercial freezer at −18°C for up to 1 week prior to use. Each model cost approximately $12 USD to produce, with the four models taking approximately 1.5–2 h to create.
FIGURE 1.
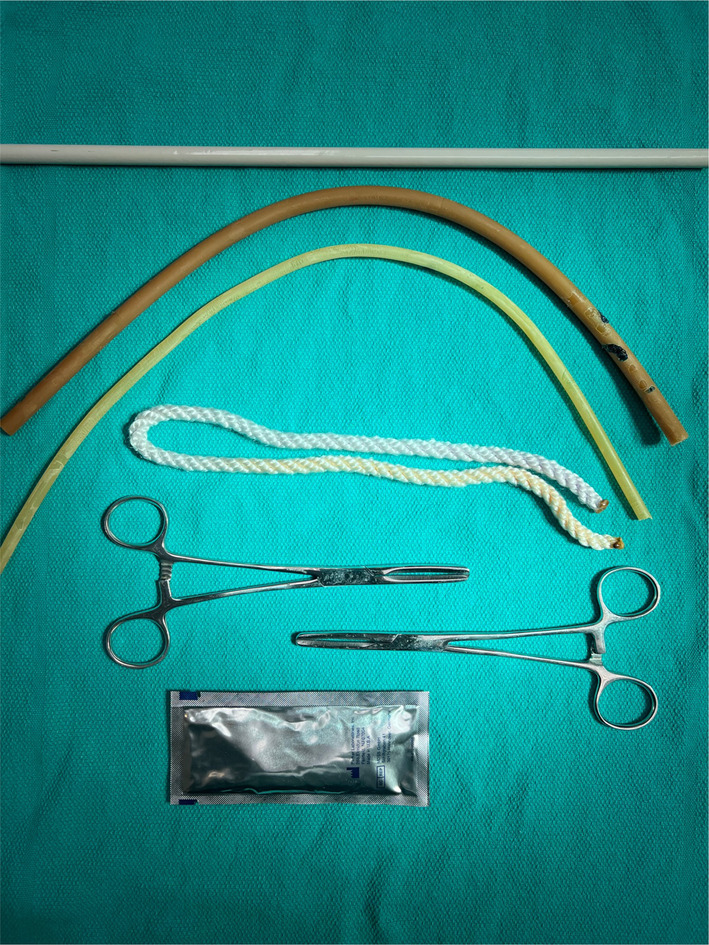
Materials used to make low‐fidelity pork‐based ultrasound‐guided regional anesthesia models.
Fascia iliaca compartment block
In the fascia iliaca compartment block, local anesthesia is deposited deep to the fascia iliaca to anesthetize several nerves in the hip and upper thigh including the femoral nerve. This model was created by making one horizontal cut in the superficial layer of the pork to approximate the fascia lata, another oblique cut for the fascia iliaca. Deep to the fascia iliaca cut, a yarn bundle was placed next to two sets of fluid‐filled tubing to create the femoral nerve alongside the femoral artery and vein (Figure 2A).
FIGURE 2.
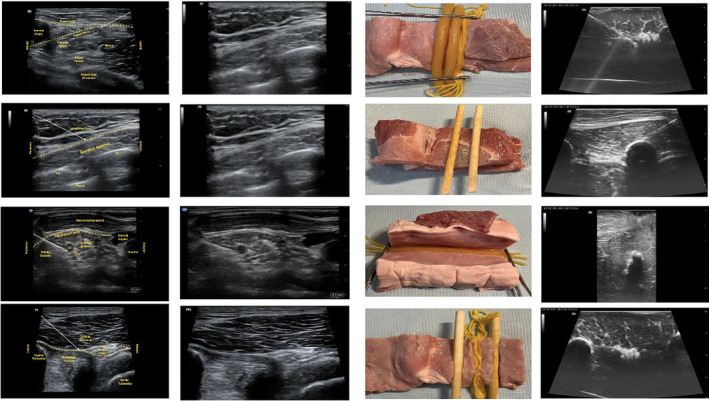
Images of targeted nerve block anatomy from a schematic, POCUS images from live patients, images of the pork models, and POCUS images from the pork models for (A) fascia iliaca compartment block, (B) serratus anterior plane block, (C) interscalene brachial plexus nerve block, and (D) transgluteal sciatic nerve block. POCUS, point‐of‐care ultrasound.
Serratus anterior plane block
In the serratus anterior plane block, local anesthetic is deposited in the fascial layer surrounding the serratus anterior muscle. As a plane block, there is no discrete nerve targeted. This model was created by making one horizontal cut in the superficial layer of the pork to approximate the border between the serratus anterior muscle and the latissimus dorsi muscle/superficial tissue. Another parallel horizontal cut was made to approximate the deep border of the serratus anterior muscle. Parallel wooden dowels were embedded in this layer to create ribs, and tissue paper was layered deep to the wooden dowels to represent the pleural line (Figure 2B).
Interscalene brachial plexus neve block (IBPNB)
Between the anterior and middle scalene muscles in the neck, the brachial plexus, which is targeted for this block, often has an appearance similar to clustered vessels on POCUS. Thus, this model was created by making an oblique cut in the pork to approximate the border between the anterior and middle scalene muscles. Three stacked sets of fluid‐filled tubing wrapped in tissue paper were embedded vertically along the cut surface (Figure 2C).
Transgluteal sciatic nerve block (TGSNB)
The sciatic nerve at the transgluteal level is identified deep to the gluteus maximus muscle between the bony landmarks of the greater trochanter and ischial tuberosity. This model was created by making a midline horizontal cut in the pork and embedding bundled yarn to represent the sciatic nerve between two wooden dowels. (Figure 2D).
Posttraining knowledge assessment and follow‐up
Immediately following the training session, study participants completed the same survey regarding their self‐reported confidence with both performing and supervising UGNBs. They also answered the same 17 knowledge‐based competency questions as part of the posttraining competency assessment (t1). The same survey was electronically sent out to all participants 3 months after the in‐person training session to evaluate the resilience of the intervention over time (t2).
UGNB performance in the ED pre‐ and posttraining
The electronic medical record at our institution was queried for UGNBs performed in the 3 months leading up to and the 3 months following the training session. UGNBs performed were captured via documented procedure notes as per standard operating practices at our facility.
Data analysis and statistical evaluation
Descriptive statistics were performed, and one‐way repeated‐measures ANOVA were conducted on our primary outcomes of provider self‐reported confidence/comfort in performing UGNBs and expertise as well as UGNB competence scores between the time points, after testing for normality and assumption of sphericity. Subsequent pairwise comparisons, adjusted for multiple comparisons, were performed. The number and type of nerve blocks performed pre‐ and posttraining sessions were also compared. All analysis was performed in IBM SPSS Statistics program version 27.
RESULTS
Participant demographics
A total of 36 participants completed the precourse survey and attended the in‐person training session. Of these, 20 participants (56%) were resident physicians, 14 (39%) were attending physicians, and two (5%) did not disclose their training level. Overall, 56% of participants were female and 53% of participants self‐identified as White.
UGNB knowledge‐based competency
While all participants completed the preintervention survey (t0), only eight participants completed surveys at all three time points. Among these eight participants, significant differences in scores across all three time points were observed (F(2,14) = 20.16, p < 0.001; Figure 3). Knowledge‐based competency scores increased immediately postintervention (mean ± SD t0 score 66.9 ± 8.9 compared to t1 score 90.4 ± 11.7; p < 0.001). Competency scores 3 months postintervention remained elevated above baseline t0 scores (t2 scores: 77.2 ± 11.5; p = 0.031). Competency scores at 3 months postintervention trended down compared to immediately postintervention; however, scores at 3 months remain statistically higher than at baseline (p = 0.010).
FIGURE 3.
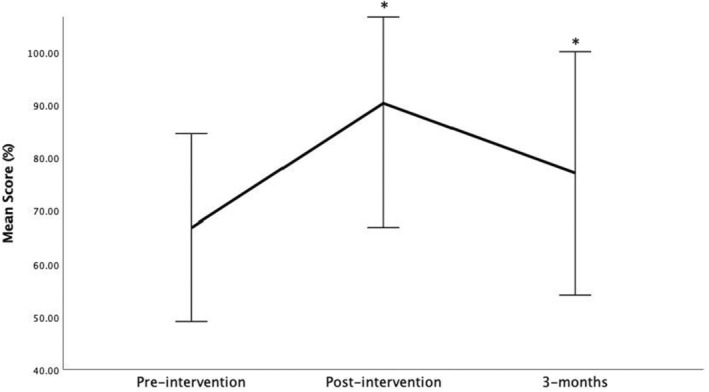
Ultrasound‐guided nerve block competency scores preintervention (t0), immediately postintervention (t1), and 3 months postintervention.
Self‐reported confidence performing and supervising UGNBs
Among the eight participants completing all time point surveys, self‐reported confidence in performing UGNBs differed (F(2,14) = 4.33, p = 0.034). Reported confidence increased immediately after training compared to baseline (mean ± SD t0 score 5.0 ± 2.3 compared to t1 score 7.1 ± 1.5; p = 0.002). By 3 months postintervention reported confidence/comfort had decreased to baseline levels (t2 6.0 ± 1.9 compared to t0; p = 0.30; Figure 4). There were no differences in self‐reported confidence in supervising UGNBs pre‐ or postintervention at any time points (mean ± SD t0 confidence 4.5 ± 2.4, t1 5.9 ± 2.1, t2 5.5 ± 1.8; p = 0.18).
FIGURE 4.
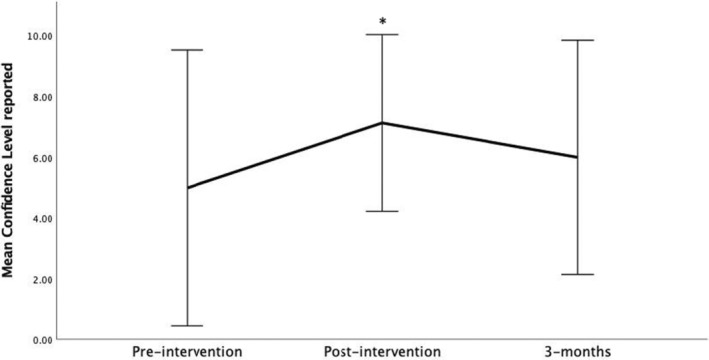
Self‐reported confidence/comfort with performing ultrasound‐guided nerve blocks preintervention (t0), immediately postintervention (t1), and 3 months postintervention.
UGNBs performed in the department pre‐ and postintervention
There were no significant differences in the number of UGNBs performed in the ED in the 3 months preintervention compared to the 3 months postintervention. Totals were small pre‐ and postintervention, with zero fascial iliaca blocks performed preintervention, and one block being performed postintervention. One TGSNB was performed clinically preintervention and three TGSNBs performed postintervention. There was one IBPNB performed preintervention, with no IBPNB performed in the 3 months postintervention.
DISCUSSION
Here we demonstrate that a brief, simulation‐based intervention using a combination of didactic and low‐fidelity meat‐based models can result in sustained knowledge‐based UGNB competency. Interestingly, this did not translate into improved provider confidence in either performing or supervising UGNBs long term and did not result in a greater volume of UGNBs being performed in the ED postintervention. These findings are likely related as we predict achieving increased volume of UGNBs is directly linked to provider confidence in performing these procedures, and in the case of attending physicians, the confidence in supervising these procedures. To improve the volume of UGNBs performed we likely need to incorporate elements into our training curriculum, which will improve provider confidence in addition to provider knowledge. Alternatively, offering longitudinal quality assurance review and feedback to providers could also result in sustained confidence and thus potentially UGNB volume over time.
In this work, competency was assessed via knowledge‐based surveys. While knowledge base is an important element of competency, competency in performing POCUS‐guided procedures including UGNBs is complex and involves a host of skills: mastery of probe positioning for optimal image, fine motor skills for needle control and guidance, and precision in anesthetic delivery. Assessing the overall effect of our educational intervention on UGNB competency will require future metrics such as performing objective structured clinical examinations with expert observation; quality assurance review of saved UGNB clips to evaluate image acquisition, needle manipulation, and anesthetic placement; and prospective evaluation of both efficacy and complications associated with UGNBs performed by our learners. These evaluations were out of the scope of this pilot study but will ideally be the focus of future work.
Model‐based simulation training has demonstrated efficacy in UGNB education. 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 While high‐fidelity commercial models for training UGNBs are available, costs of these models can be prohibitive, and they often do not allow for hydrodissection or anesthetic injection to confirm appropriate needle tip placement. Homemade UGNB training models are inexpensive compared to their commercial counterparts and often favored for both learners and educators. As an alternative to commercial models, cadaveric‐based UGNB training has also been described. 30 However, this resource is frequently unavailable outside of institutions without onsite medical schools, and cadavers cannot tolerate repeat injection. While this approach offers a relatively realistic training experience, the limited availability of cadaver labs makes this approach not scalable.
Low‐fidelity simulation models for UGNB training made of materials such as gelatin, meat products, or tofu have been previously described. 21 , 22 , 28 Generally, these models are well received by learners and are helpful in improving short‐term procedural competency. 23 Meat‐based models have even been shown to score higher on subjective “realism” of ultrasound image appearance and “feel” of needling assessments compared to commercial models. 29 While prior studies have described construction of meat‐based models for UGNB training, these studies have not assessed the effect of training with these models long term. Consistent with the existing literature, our meat‐model based curriculum demonstrates overall favorable educational outcomes in the short term, and additionally suggests there may also be longer term benefits to this training approach. Our models are cost‐effective and portable, can be constructed in nearly any setting, and can be stored for future use. Taken together, our work further supports that an UGNB training curriculum involving low‐fidelity meat‐based models may be an effective educational approach.
LIMITATIONS
While our work demonstrates promising results, the number of participants who provided data across all time points was quite small. This was sufficient for our pilot study; however, in subsequent work we should recruit a larger sample size and offer incentives for study completion posttraining session. As our participants represented only roughly 20% of ED providers at our institution, our limited sample size may also have impacted the likelihood of seeing an effect on overall UGNB volume performed in our ED posttraining. Additionally, this study was implemented immediately after the COVID‐19 pandemic, and unprecedented patient numbers, with high levels of boarding and increases in hallway care, may also have negatively impacted our providers from performing these procedures both given lack of space and capacity to step away from seeing new patients to perform these procedures. Developing a longitudinal training within the ED itself in addition to expanding our educational intervention across as many ED providers as is possible (and ideally across all providers) will be crucial to assessing the impact on UGNBs performed in the ED.
Our confidence evaluations were based on self‐report by providers. While this is a fairly common practice in educational intervention studies, additional more objective measures of intervention efficacy should be included in subsequent work. Similarly, competence assessments were knowledge‐based on surveys only. As described, in the future it will be important to assess competency in other modalities of UGNBs as well. Adjusting our assessment metrics in the future will strengthen our understanding of the potential value of our educational intervention.
Participants were voluntarily recruited for this work. This self‐selection may have introduced bias in participant investment in learning UGNBs. Finally, while our models are easy to recreate, cost‐effective, and portable, in terms of generalizability of our meat‐based models, using pork‐based models may not be culturally appropriate in some communities. While we suspect using an alternative product such as chicken or perhaps tofu may also have had a good educational effect, this will need to be explored further.
CONCLUSIONS
In this pilot study, our curriculum using homemade, low‐fidelity meat‐based simulation models demonstrated improved knowledge‐based competency. However, provider self‐reported confidence in performing ultrasound‐guided nerve blocks and the volume of ultrasound‐guided nerve blocks in our ED did not change. These models allow for scalable ultrasound‐guided nerve block training opportunities that may otherwise not be available. Further work is needed to assess additional competency metrics and clinical outcomes following this training.
AUTHOR CONTRIBUTIONS
Carrie D. Walsh, Andrew J. Goldsmith, and Nicole M. Duggan generated the study concept and design. Carrie D. Walsh, Joseph Stegeman, Andrew J. Eyre, Munaa Dashti, Andrew J. Goldsmith, and Nicole M. Duggan collected the data and ultrasound images. Irene W. Y. Ma, Carrie D. Walsh, and Nicole M. Duggan analyzed and interpereted the results and generated the figures and tables. Carrie D. Walsh, Andrew J. Goldsmith, and Nicole M. Duggan drafted the manusucript. Roger D. Dias, Andrew J. Eyre, Munaa Dashti, and Arun Nagdev provided critical expertise and revision of the manuscript for important intellectual content. Nicole M. Duggan and Andrew J. Goldsmith acquired funding for this work.
CONFLICT OF INTEREST STATEMENT
RDD has received funding from the National Institutes of Health, National Science Foundation, Department of Defense, and the National Aeronautics and Space Agency for investigator‐initiated research. AN is employed by Exo as the senior director for clinical education. AJG has received contract funding from Visby Medical for industry‐initiated research and has also received funding personally from Exo and Philips for consulting. NMD had received contract funding from Visby Medical for industry‐initiated research. The other authors declare no conflicts of interest.
Walsh CD, Ma IWY, Eyre AJ, et al. Implementing ultrasound‐guided nerve blocks in the emergency department: A low‐cost, low‐fidelity training approach. AEM Educ Train. 2023;7:e10912. doi: 10.1002/aet2.10912
Andrew J. Goldsmith and Nicole M. Duggan contributed equally toward senior authorship of this work.
Presented at the American College of Emergency Physicians Scientific Assembly San Francisco, CA, October 2022.
Supervising Editor: Jason Wagner
Funding informationThis work was supported by the Massachusetts College of Emergency Physicians Resident Research Award.
REFERENCES
- 1. Motov S, Strayer R, Hayes B, et al. The treatment of acute pain in the emergency department: a white paper position Statement prepared for the American Academy of emergency medicine. J Emerg Med. 2018;54(5):731‐736. doi: 10.1016/j.jemermed.2018.01.020 [DOI] [PubMed] [Google Scholar]
- 2. Gao Y, Tan H, Sun R, Zhu J. Fascia iliaca compartment block reduces pain and opioid consumption after total hip arthroplasty: a systematic review and meta‐analysis. Int J Surg. 2019;65:70‐79. doi: 10.1016/j.ijsu.2019.03.014 [DOI] [PubMed] [Google Scholar]
- 3. Kim Y‐M, Kang C, Joo Y‐B, Lee SH. The role of ultrasound‐guided single‐shot femoral and sciatic nerve blocks on pain management after total knee arthroplasty. Knee. 2019;26(4):881‐888. doi: 10.1016/j.knee.2019.05.002 [DOI] [PubMed] [Google Scholar]
- 4. Park MH, Kim JA, Ahn HJ, Yang MK, Son HJ, Seong BG. A randomized trial of serratus anterior plane block for analgesia after thorascopic surgery. Anaesthesia. 2018;73(10):1260‐1264. doi: 10.1111/anae.14424 [DOI] [PubMed] [Google Scholar]
- 5. Jaffe TA, Shokoohi H, Liteplo A, Goldsmith A. A novel application of ultrasound‐guided Interscalene anesthesia for proximal humeral fractures. J Emerg Med. 2020;59(2):265‐269. doi: 10.1016/j.jemermed.2020.05.013 [DOI] [PubMed] [Google Scholar]
- 6. Goldsmith AJ, Liteplo AS, Shokoohi H. Ultrasound‐guided serratus anterior plane block for intractable herpes zoster pain in the emergency department. J Emerg Med. 2020;S0736‐4679(20):30392‐30399. doi: 10.1016/j.jemergmed.2020.04.053 [DOI] [PubMed] [Google Scholar]
- 7. Goldsmith AJ, Liteplo A, Hayes BD, Duggan NM, Huang C, Shokoohi H. Ultrasound‐guided transgluteal sciatic nerve analgesia for refractory back pain in the ED: a case series. Am J Emerg Med. 2020;38(9):1792‐1795. doi: 10.1016/j.ajem.2020.06.001 [DOI] [PubMed] [Google Scholar]
- 8. Luftig J, Mantuani D, Herring AA, Dixon B, Clattenburg E, Nagdev A. Successful emergency pain control for posterior rib fractures with ultrasound‐guided erector spinae place block. Am J Emerg Med. 2018;36(8):1391‐1396. doi: 10.1016/j.ajem.2017.12.060 [DOI] [PubMed] [Google Scholar]
- 9. Lyons C, Herring AA. Ultrasound‐guided axillary nerve block for ED incision and drainage of deltoid abscess. Am J Emerg Med. 2017;35(7):1032.e3‐1032.e7. doi: 10.1016/j.ajem.2017.01.064 [DOI] [PubMed] [Google Scholar]
- 10. Mori T, Hagiwara Y. Ultrasound‐guided popliteal sciatic nerve block for an ankle laceration in a pediatric emergency department. Pediatr Emerg Care. 2017;33(12):803‐805. doi: 10.1097/PEC.0000000000001334 [DOI] [PubMed] [Google Scholar]
- 11. Mori T, Nomura O, Ihara T. Ultrasound‐guided peripheral forearm nerve block for digit fractures in a pediatric emergency department. Am J Emerg Med. 2019;37(3):489‐493. doi: 10.1016/j.ajem.2018.11.033 [DOI] [PubMed] [Google Scholar]
- 12. Nagel EM, Gantioque R, Taira T. Utilizing ultrasound‐guided femoral nerve blocks and fascia Iliaca compartment blocks for proximal femur fractures in the emergency department. Adv Emerg Nurs J. 2019;41(2):135‐144. doi: 10.1097/TME.0000000000000242 [DOI] [PubMed] [Google Scholar]
- 13. Ritcey B, Pageau P, Woo MY, Perry JJ. Regional nerve blocks for hip and femoral neck fractures in the emergency department: a systematic review. CJEM. 2016;18(1):37‐47. doi: 10.1017/cem.2015.75 [DOI] [PubMed] [Google Scholar]
- 14. Reavley P, Montgomery AA, Smith JE, et al. Randomised trial of the fascia iliaca block versus the ‘3‐in‐1’ block for femoral neck fractures in the emergency department. Emerg Med J. 2015;32(9):685‐689. doi: 10.1136/emermed-2013-203407 [DOI] [PubMed] [Google Scholar]
- 15. Flores S, Herring AA. Ultrasound‐guided greater auricular nerve block cor emergency department ear laceration and abscess drainage. J Emerg Med. 2016;50(4):651‐655. doi: 10.1016/j.jemermed.2015.10.003 [DOI] [PubMed] [Google Scholar]
- 16. Mantuani D, Luftig PAJ, Herring A, Mian MN. Successful emergency pain control for acute pancreatitis with ultrasound guided erector spinae plane blocks. Am J Emerg Med. 2020;38(6):1298.e5‐1298.e7. doi: 10.1016/j.ajem.2020.02.005 [DOI] [PubMed] [Google Scholar]
- 17. Amini R, Kartchner JZ, Nagdev A, Adhikari S. Ultrasound‐guided nerve blocks in emergency medicine practice. J Ultrasound Med. 2016;35(4):731‐736. doi: 10.7863/ultra.15.05095 [DOI] [PubMed] [Google Scholar]
- 18. Chiem AT, Soucy Z, Dinh VA, et al. Integration of ultrasound in undergraduate medical education at the California medical schools: a discussion of common challenges and strategies from the UMeCali experience. J Ultrasound Med. 2016;35(2):221‐233. doi: 10.7863/ultra.15.05006 [DOI] [PubMed] [Google Scholar]
- 19. Amini R, Stolz LA, Gross A, et al. Theme‐based teaching of point‐of‐care ultrasound in undergraduate medical education. Intern Emerg Med. 2015;10(5):613‐618. doi: 10.1007/s11739-015-1222-8 [DOI] [PubMed] [Google Scholar]
- 20. Lahham S, Smith T, Baker J, et al. Procedural simulation: medical student preference and value of three task trainers for ultrasound guided regional anesthesia. World J Emerg Med. 2017;8(4):287‐291. doi: 10.5847/wjem.j.1920-8642.2017.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Naraghi L, Lin J, Odashima K, Buttar S, Haines L, Dickman E. Ultrasound‐guided regional anesthesia simulation: use of meat glue in inexpensive and realistic nerve block models. BMC Med Educ. 2019;19(1):145. doi: 10.1186/s12909-019-1591-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sparks S, Evans D, Byars D. A low cost, high fidelity nerve block model. Crit Ultrasound J. 2014;6(1):12. doi: 10.1186/s13089-014-0012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Situ‐LaCasse EH, Amini R, Bain V, et al. Performance of ultrasound‐guided peripheral nerve blocks by medical students after one‐day training session. Cureus. 2019;11(1):e3911. doi: 10.7759/cureus.3911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chen XX, Trivedi V, AlSaflan AA, et al. Ultrasound‐guided regional anesthesia simulation training: a systematic review. Reg Anesth Pain Med. 2017;42(6):741‐750. doi: 10.1097/AAP.0000000000000639 [DOI] [PubMed] [Google Scholar]
- 25. Ahmed OMA, Azher I, Gallagher AG, Breslin DS, O'Donnell BD, Shorten GD. Deliberate practice using validated metrics improves skill acquisition in performance of ultrasound‐guided peripheral nerve block in a simulated setting. J Clin Anesth. 2018;48:22‐27. doi: 10.1016/j.jclinane.2018.04.015 [DOI] [PubMed] [Google Scholar]
- 26. Kim YH. Ultrasound phantoms to protect patients from novices. Korean J Pain. 2016;29(2):73‐77. doi: 10.3344/kjp.2016.29.2.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hocking G, Hebard S, Mitchell CH. A review of the benefits and pitfalls of phantoms in ultrasound‐guided regional anesthesia. Reg Anesth Pain Med. 2011;36(2):162‐170. doi: 10.1097/aap.0b013e31820d4207 [DOI] [PubMed] [Google Scholar]
- 28. Farjad Sultan S, Shorten G, Iohom G. Simulators for training in ultrasound guided procedures. Med Ultrason. 2013;15(2):125‐131. doi: 10.11152/mu.2013.2066.152.sfs1gs2 [DOI] [PubMed] [Google Scholar]
- 29. Samuel J, Kerr E, Young D, Watson M, Raj D. The use of joints of meat as phantoms for ultrasound‐guided needling skills: a prospective blinded study. Ultrasound J. 2022;14(1):14. doi: 10.1186/s13089-022-00263-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Barrington MJ, Wong DM, Slater B, Ivanusic JJ, Ovens M. Ultrasound‐guided regional anesthesia: how much practice do novices require before achieving competency in ultrasound needle visualization using a cadaver model. Reg Anesth Pain Med. 2012;37(3):334‐339. doi: 10.1097/AAP.0b013e3182475fba [DOI] [PubMed] [Google Scholar]
- 31. McLeod G, McKendrick M, Taylor A, et al. Validity and reliability of metrics for translation of regional anaesthesia performance from cadavers to patients. Br J Anaesth. 2019;123(3):368‐377. doi: 10.1016/j.bja.2019.04.060 [DOI] [PubMed] [Google Scholar]
- 32. Wiesmann T, Bornträger A, Neff M, Wulf H, Steinfeldt T. Needle visibility in different tissue models for ultrasound‐guided regional anaesthesia. Acta Anaesthesiol Scand. 2012;56(9):1152‐1155. doi: 10.1111/j.1399-6576.2012.02758.x [DOI] [PubMed] [Google Scholar]
- 33. Güven Aytaç B, Ünal Ş, Aytaç İ. A randomized, controlled simulation study comparing single and double operator ultrasound‐guided regional nerve block techniques using a gelatine‐based home‐made phantom. Medicine (Baltimore). 2022;101(35):e30368. doi: 10.1097/MD.0000000000030370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rueda Rojas VP, Meléndez Flórez HJ, Orozco GE. Analysis of previous training with simulated models on the success rate of ultrasound‐guided supraclavicular block. Prospective cohort study. Rev Esp Anestesiol Reanim (Engl Ed). 2019;66(5):241‐249. doi: 10.1016/j.redar.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 35. Niazi AU, Haldipur N, Prasad AG, Chan VW. Ultrasound‐guided regional anesthesia performance in the early learning period: effect of simulation training. Reg Anesth Pain Med. 2012;37(1):51‐54. doi: 10.1097/AAP.0b013e31823dc340 [DOI] [PubMed] [Google Scholar]


