Abstract
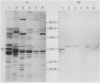
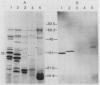
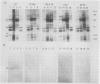
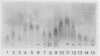
Photosystem II particles of Chlamydomonas reinhardtii contain three extrinsic polypeptides of 29, 20, and 16 kilodaltons, whose functions are incompletely defined. We prepared a monospecific polyclonal antibody against the 29 kilodalton protein and determined that it also specifically recognizes a protein of approximately 33 kilodaltons in thylakoid membrane fractions of several vascular plants, eukaryotic algae, and a cyanobacterium. The cross-reacting 33 kilodalton protein of pea was removed from inverted thylakoid vesicles by CaCl2 washes demonstrating the structural relationship between the Chlamydomonas polypeptide and the largest subunit of the water oxidation complex of vascular plants. Functional identity of the Chlamydomonas polypeptide was confirmed by antibody inhibition of O2 evolution in inverted pea vesicles. In contrast to wild-type cells, only low levels of the 29 kilodalton polypeptide are recovered with purified thylakoid membranes of the mutants examined. However, we show that the mature form of the 29 kilodalton polypeptide accumulates to wild-type levels in whole cell extracts of photosystem II deficient mutants and a water oxidation mutant of Chlamydomonas. Impaired membrane assembly has no effect on the maturation or stability of this component of the multi-subunit water oxidation complex.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blake M. S., Johnston K. H., Russell-Jones G. J., Gotschlich E. C. A rapid, sensitive method for detection of alkaline phosphatase-conjugated anti-antibody on Western blots. Anal Biochem. 1984 Jan;136(1):175–179. doi: 10.1016/0003-2697(84)90320-8. [DOI] [PubMed] [Google Scholar]
- Callahan F. E., Cheniae G. M. Studies on the photoactivation of the water-oxidizing enzyme : I. Processes limiting photoactivation in hydroxylamine-extracted leaf segments. Plant Physiol. 1985 Nov;79(3):777–786. doi: 10.1104/pp.79.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Bennoun P. Thylakoid membrane polypeptides of Chlamydomonas reinhardtii: wild-type and mutant strains deficient in photosystem II reaction center. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2175–2179. doi: 10.1073/pnas.72.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chua N. H., Gillham N. W. The sites of synthesis of the principal thylakoid membrane polypeptides in Chlamydomonas reinhardtii. J Cell Biol. 1977 Aug;74(2):441–452. doi: 10.1083/jcb.74.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman D. S., Levine R. P. Cytochrome f and plastocyanin: their sequence in the photosynthetic electron transport chain of Chlamydomonas reinhardi. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1665–1669. doi: 10.1073/pnas.54.6.1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchins J. P., Hind G. Concentration and function of membrane-bound cytochromes in cyanobacterial heterocysts. Plant Physiol. 1984 Oct;76(2):456–460. doi: 10.1104/pp.76.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nakatani H. Y. Photosynthetic oxygen evolution does not require the participation of polypeptides of 16 and 24 kilodaltons. Biochem Biophys Res Commun. 1984 Apr 16;120(1):299–304. doi: 10.1016/0006-291x(84)91448-7. [DOI] [PubMed] [Google Scholar]
- Plumley F. G., Kirchman D. L., Hodson R. E., Schmidt G. W. Ribulose Bisphosphate Carboxylase from Three Chlorophyll c-Containing Algae : Physical and Immunological Characterizations. Plant Physiol. 1986 Mar;80(3):685–691. doi: 10.1104/pp.80.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plumley F. G., Schmidt G. W. Rocket and crossed immunoelectrophoresis of proteins solubilized with sodium dodecyl sulfate. Anal Biochem. 1983 Oct 1;134(1):86–95. doi: 10.1016/0003-2697(83)90267-1. [DOI] [PubMed] [Google Scholar]
- Schmidt G. W., Mishkind M. L. Rapid degradation of unassembled ribulose 1,5-bisphosphate carboxylase small subunits in chloroplasts. Proc Natl Acad Sci U S A. 1983 May;80(9):2632–2636. doi: 10.1073/pnas.80.9.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talian J. C., Olmsted J. B., Goldman R. D. A rapid procedure for preparing fluorescein-labeled specific antibodies from whole antiserum: its use in analyzing cytoskeletal architecture. J Cell Biol. 1983 Oct;97(4):1277–1282. doi: 10.1083/jcb.97.4.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yocum C. F., Yerkes C. T., Blankenship R. E., Sharp R. R., Babcock G. T. Stoichiometry, inhibitor sensitivity, and organization of manganese associated with photosynthetic oxygen evolution. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7507–7511. doi: 10.1073/pnas.78.12.7507. [DOI] [PMC free article] [PubMed] [Google Scholar]