Abstract
We evaluated the pharmacodynamic activities of fluconazole and amphotericin B given alone and in combination against Candida albicans by using an in vitro model of bloodstream infection that simulates human serum pharmacokinetic parameters for these antifungals. Fluconazole was administered as a bolus into the model to simulate regimens of 200 mg every 24 h (q24h) and 400 mg q24h. Amphotericin B was administered at doses producing the peak concentration (2.4 μg/ml) observed with a regimen of 1 mg/kg of body weight q24h. A combination regimen of fluconazole (400 mg q24h) and amphotericin B (1 mg/kg q24h) administered simultaneously and as a staggered regimen (amphotericin B bolus given 8 h after fluconazole bolus) was also simulated in the model to characterize possible antagonism between these agents. Fluconazole alone and amphotericin B alone demonstrated fungistatic (<99.9% reduction in numbers of CFU per milliliter from the starting inoculum) and fungicidal (>99.9% reduction) activity, respectively. When fluconazole and amphotericin B were administered simultaneously, fungicidal activity similar to that observed with amphotericin B alone was observed. Staggered administration of fluconazole and amphotericin B, however, resulted in a substantial reduction of the fungicidal activity of amphotericin B, producing fungistatic activity similar to that observed with noncombination fluconazole regimens. These results demonstrate the usefulness of this model for comparing the in vitro pharmacodynamic characteristics of different antifungal regimens and support the theory of azole-polyene antagonism. The effects of this antagonism on the in vivo activity and clinical usefulness of combination antifungal therapy, however, remain to be determined.
Antimicrobial resistance is a well-documented source of increasing cost, morbidity, and mortality (1, 4, 15). One method clinicians have employed to stem the development of bacterial resistance is simultaneous administration of antibiotics with distinct mechanisms of action to enhance the efficacy of drug therapy and decrease the likelihood of resistance. Additionally, using relatively low dosages of antibiotics with synergistic activity but independent toxicity profiles can minimize drug toxicities. Few antibiotic combinations, however, produce synergistic activity in vivo, and some antibiotic combinations demonstrate some degree of antagonism (10). Testing of synergistic combinations is frequently performed by in vitro methodologies, such as checkerboard titrations, agar dilutions, and time-kill studies, which do not account for the decline of drug concentrations that occurs in vivo after a dose is administered. Furthermore, the ratio of drugs administered in a combination may change in vivo, a characteristic that can considerably influence the activities of some combination regimens (7). By developing an in vitro pharmacodynamic model of infection that simulates human serum pharmacokinetic parameters, several researchers have overcome the inherent drawbacks of unrealistic drug pharmacokinetics present in many in vitro testing methodologies and have efficiently identified key pharmacodynamic characteristics for antibacterial regimens (6, 7, 14).
As fungi have become the fourth most common cause of nosocomial bloodstream infections in U.S. hospitals and the clinical problem of antifungal resistance has become more prevalent (16, 23), the organizers of several clinical trials have advocated the use of combination antifungal therapy to enhance the efficacy and decrease the toxicity of antifungal therapy (5, 30). Because fluconazole exerts its antifungal activity via the inhibition of ergosterol synthesis whereas amphotericin B primarily binds to ergosterol to exert its antifungal activity, the simultaneous administration of both antifungals would theoretically be antagonistic (19). Data generated from various in vitro and in vivo studies, however, have produced conflicting interpretations of polyene-azole antagonism. The objectives of this study were to examine the effectiveness of fluconazole and amphotericin B alone and in combination by using an in vitro dynamic mycotic (Candida albicans) infection model and to characterize the pharmacodynamics of amphotericin B-fluconazole combination regimens.
MATERIALS AND METHODS
Fungal isolates.
Two isolates of C. albicans were tested, an American Type Culture Collection (ATCC) reference strain (ATCC 90028) and a clinical isolate (OY31.5) obtained from the Special Microbiology Laboratory, Department of Pathology, University of Iowa College of Medicine (Iowa City).
Antifungal agents.
A 2-mg/ml fluconazole solution for injection was obtained from Pfizer-Roerig Pharmaceuticals (New York, N.Y.), and amphotericin B powder for injection was obtained from Apothecon Laboratories (Princeton, N.J.).
MIC determination.
MICs were determined by the microdilution technique with an inoculum of 2.5 × 103 CFU/ml by the methodology for broth dilution antifungal susceptibility testing of yeast adopted by the National Committee for Clinical Laboratory Standards (NCCLS) (M27-A) (20). Two ATCC strains, Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258, served as quality control isolates. The growth medium for the MIC determination was RPMI 1640 (Sigma Chemicals, St. Louis, Mo.) buffered to a pH of 7.0 with 0.165 M morpholinepropanesulfonic acid (MOPS). All microdilution trays were incubated for 48 h at 35°C in a dark, moist chamber. The MIC of amphotericin B was defined as the concentration resulting in complete inhibition of visible growth. The MIC of fluconazole was determined as the lowest concentration that resulted in 80% visible reduction of fungal growth compared to the control.
In vitro infection model.
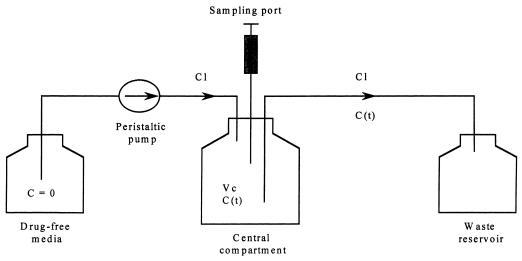
A one-compartment in vitro infection model capable of simulating human serum pharmacokinetic parameters for fluconazole and amphotericin B in the presence of viable yeast was constructed (Fig. 1) (6, 14). The central glass compartment of the model (4,000 ml) contained a magnetic stir bar for continuous mixing and sample ports sealed by rubber septa to allow for aseptic sampling from the central compartment. Sterile, drug-free RPMI 1640 was pumped into the central compartment of the model via a peristaltic pump (Masterflex 7524) at a fixed rate to simulate the 24-h half-lives of fluconazole and amphotericin B (8, 13). After the desired flow rate was established, a yeast suspension was prepared from 24-h culture plate and standardized to 0.5 McFarland turbidity standard (106 CFU/ml). Twenty milliliters of the standardized yeast suspension was then introduced into the central compartment to yield a starting inoculum of approximately 104 CFU/ml.
FIG. 1.
Schematic representation of a one-compartment in vitro infection model. Abbreviations: C, concentration of drug; Cl, flow rate; Vc, volume of the central compartment; C (t), concentration of drug in the central compartment.
Preliminary in vitro time-kill studies with fluconazole and amphotericin B performed in our laboratories demonstrated antagonism when amphotericin B was administered a minimum of 8 h after the introduction of fluconazole (31). Based on these results, a total of six intravenous antifungal regimens were simulated in the in vitro model. These included (i) the control (no drug), (ii) 200 mg of fluconazole administered every 24 h (q24h), (iii) 400 mg of fluconazole q24h, (iv) a simulation of 1 mg of amphotericin B/kg of body weight q24h, (v) 400 mg of fluconazole q24h and 1 mg of amphotericin B/kg simultaneously q24h, and (vi) 8-h staggered administration of fluconazole (400 mg q24h) followed by amphotericin B (1 mg/kg q24h). Because previous time-kill studies with amphotericin B indicated that few viable fungi remain in the in vitro system within 5 h after the administration of an amphotericin B bolus, we chose not to simulate a combination regimen of fluconazole administered 8 h after the amphotericin B bolus. All antifungals were administered into the central compartment as a rapid bolus to achieve target steady-state pharmacokinetic parameters (see Table 1). Throughout the experiment (48 h), the central chamber of the model was maintained at 37°C. Each experiment was performed in duplicate.
TABLE 1.
Target and actual pharmacokinetic valuesa
| Regimen | Cmax (μg/ml) | Cmin (μg/ml) | t1/2 (h)b |
|---|---|---|---|
| Fluconazole, 200 mg q24h | 10 (9.25) | 5 (3.8) | 24 (22) |
| Fluconazole, 400 mg q24h | 20 (19.4) | 10 (7.8) | 24 (21) |
| Amphotericin B, 1 mg/kg q24h | 2.4 (2.4) | 1.2 (0.5) | 24 (9.4) |
Actual values are shown in parentheses.
t1/2, half-life.
Antifungal assays.
Antifungal concentrations were determined by high-performance liquid chromatography (HPLC) by the slightly modified methodology of Ng et al. for analysis of antifungal concentrations in human serum (21). The HPLC system consisted of a Waters 717 Plus autosampler (Millipore Corp.), a Waters 501 HPLC pump, a Nova-Pak C18 column (8 by 100 mm; beads, 4 μm in diameter), a Waters 486 tunable multiwavelength detector, and a CR3A Chromatopac integrator (Shimadzu). The monitor wavelength was set at 260 nm for fluconazole and 405 nm for amphotericin B. Samples were diluted in the mobile phase (30:70 [vol/vol] methanol-ammonium acetate; pH 5.0) and analyzed by direct injection of 100 μl into the HPLC system. Mebendazole and antipyrine were used as the internal standards for samples that contained amphotericin B and fluconazole, respectively.
Pharmacokinetic analysis.
Samples (500 μl each) were acquired from the central compartment at 0, 2, 4, 6, 12, 24, 36, and 48 h for determination of antifungal concentrations and stored at −70°C until analysis. The peak concentration (Cmax), trough concentration (Cmin), and half-life for each antifungal regimen were calculated from the concentration-time plot by using a noncompartmental model for intravenous bolus administration (WinNonLin Software; Scientific Consulting, Inc., Cary, N.C.).
Pharmacodynamic analysis.
Factors not addressed by the NCCLS procedures for susceptibility testing, such as antifungal carryover, inocula, and agitation, were previously studied to develop a sampling methodology that minimizes the influence of these variables (17). This sampling methodology allows for a lower limit of quantitation of 50 CFU/ml without antifungal carryover. A 500-μl sample was removed from the model prior to the injection of the antifungal into the model (T = 0) and at predetermined time points following the addition of the antifungal. Samples were then diluted in sterile water, plated (at 30 μl/plate on potato dextrose agar, and incubated for 24 h at 35°C for colony count determination. Data from duplicate runs were then averaged and plotted as log10 CFU per milliliter for each time point. Data were analyzed to characterize fungistatic (<99.9% reduction in the number of CFU from the starting inoculum) and fungicidal (>99.9% reduction) activities by methods proposed by Pearson et al. (22).
RESULTS
Antifungal susceptibility testing.
The median MICs of fluconazole for ATCC 90028 and OY31.5 were 0.5 and 0.25 μg/ml, respectively. Both C. albicans isolates were considered susceptible to fluconazole based on NCCLS criteria (20). The median MICs of amphotericin B for the two isolates were 1 μg/ml.
Antifungal assay.
The limits of detection were 0.49 μg/ml for amphotericin B and 0.6 μg/ml for fluconazole. The results were reproducible for both fluconazole and amphotericin B, with median coefficients of variation of 3.64 and 3.14%, respectively.
Pharmacokinetic analysis.
The pharmacokinetic parameters measured in the model are shown in Table 1. Although the Cmax, Cmin, and half-life were relatively close to the target pharmacokinetic values, the elimination of amphotericin B from the model was more rapid than expected.
Pharmacodynamic analysis.
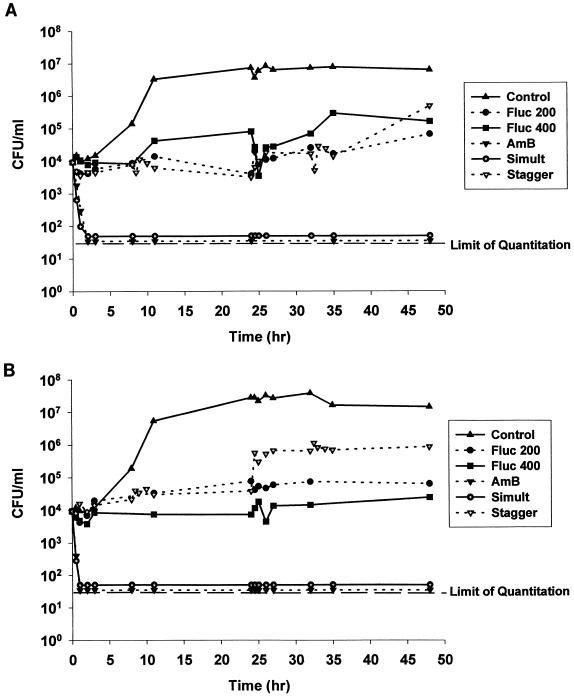
The numbers of CFU per milliliter were plotted versus time for the activities of fluconazole and amphotericin B alone and of the two in combination against C. albicans ATCC 90028 (Fig. 2A) and OY31.5 (Fig. 2B). Nearly a 3-log10-unit increase in CFU per milliliter was demonstrated for both isolates compared with the control regimen (no drug), thus demonstrating the viability of the selected C. albicans isolates in the model. Fluconazole administration into the model for both isolates resulted in fungistatic activity that was concentration independent. Doubling of the fluconazole dose did not increase the rate or extent of activity against either isolate. The administration of amphotericin B resulted in rapid fungicidal activity against both isolates that reduced the fungal count to below the lower limit of quantitation in the model within 5 h. When fluconazole and amphotericin B were administered simultaneously, fungicidal activity similar to that obtained with amphotericin B alone was observed. When a fluconazole bolus was administered 8 h prior to the amphotericin B bolus, the rapidly fungicidal activities of amphotericin B against both isolates were greatly reduced, to the extent that the fungistatic activities were similar to those observed with fluconazole alone. This antagonistic effect persisted throughout the experiment.
FIG. 2.
Time-kill plots of fluconazole (Fluc) and amphotericin B (AmB) regimens tested against C. albicans ATCC 90028 (A) and OY31.5 (B) in the in vitro mycosis infection model. Simult, simultaneous administration; stagger, staggered administration. See the text for details.
DISCUSSION
The optimization of systemic drug concentrations for the treatment of serious infections has been recognized as a critical factor in the success of anti-infection therapy (9, 24). We have developed an in vitro mycosis infection model for C. albicans that overcomes the inherent drawback of unrealistic drug concentration-time profiles presented by most in vitro testing methodologies. Our model simulates serum antifungal concentrations that occur in humans and allows the pharmacodynamic comparison of antifungal regimens. The time-kill data characteristics for each of the antifungal regimens simulated in this model also correlate well with previously reported antifungal time-kill data (13, 18, 24). Although the measured elimination rate for amphotericin B was higher than originally predicted, the rapidly fungicidal activity of this drug would likely be unaffected if its half-life was closer to the target pharmacokinetic values. Bannatyne and Cheung reported a propensity towards degradation of frozen amphotericin B samples (3). Therefore, we cannot rule out the possibility that some of the samples may have degraded prior to analysis.
With the importance of pathogenic fungi in clinical medicine growing and the introduction of new azole antifungals imminent, the clinical consequences of polyene-azole antagonism are becoming more considerable. Theoretically, the depletion of ergosterol in the cell membrane of fungi by fluconazole would antagonize the binding, and consequent antifungal activity, of amphotericin B. Using our model, we were able to demonstrate substantial antagonism of amphotericin B fungicidal activity following preexposure of the isolates to fluconazole. Other static in vitro studies of polyene-azole combination regimens have revealed antagonism for Candida and Aspergillus spp., while the results from in vivo studies of animal models of mycoses have been mixed. Many of these studies have been reviewed elsewhere (29). It is important to note, however, that the majority of studies that failed to detect polyene-azole antagonism utilized simultaneous administration of the azole antifungal and amphotericin B. As demonstrated in our model and reported by others (2, 28), the rapidly fungicidal activity of amphotericin B may preempt the detection or development of fluconazole-induced antagonism. These results suggest not only that the sequence of antifungal administration may be important for the development of polyene-azole antagonism but also that traditional methods for screening antifungal antagonism which rely on static concentrations of antifungal agents introduced simultaneously (e.g., checkerboard dilution) may be inappropriate for detecting polyene-azole antagonism.
Other studies that have employed regimens in which an azole antifungal agent was administered prior to amphotericin B have demonstrated substantial antagonism of amphotericin B activity. Schmitt et al. investigated the efficacy of combination ketoconazole-amphotericin B activity using a rat pulmonary-aspergillus model (26). When effective doses of amphotericin B (resulting in 100% survival at 30 days) were administered concomitantly with ketoconazole, the therapeutic effect of amphotericin B was completely annulled (mortality equal to that of the control). George and colleagues studied the potential for synergy of amphotericin B, fluconazole, and flucytosine against Aspergillus fumigatus using an immunosuppressed rabbit model (11). The cumulative 9-day mortality was greater for the rabbits that received fluconazole prophylaxis before amphotericin B therapy than for the rabbits that received amphotericin B alone. The authors, however, did not describe the fluconazole-amphotericin B combination as antagonistic. Schaffer and Böhler reported the failure of amphotericin B to halt the progression of aspergillosis in a renal transplant patient who was previously treated with itraconazole (25). Subsequent in vivo studies utilizing the A. fumigatus isolate recovered from the patient in a neutropenic mouse model demonstrated a significant difference in mortality between mice who received amphotericin B alone and mice who received itraconazole prior to amphotericin B therapy (40 versus 100% mortality, respectively; P < 0.0007). More recently, Sugar et al. examined the effects of high-dose and low-dose amphotericin B-fluconazole combination regimens against candidiasis in a murine model (27). Although the authors concluded that combination therapy was not antagonistic in vivo, the survival rate at 30 days for some groups of mice given concomitant high-dose fluconazole-amphotericin B therapy was nearly half that for mice that were given amphotericin B alone.
Clearly, much more information is needed before the question of amphotericin B-azole antagonism can be answered. A limitation of our study was that we tested only two isolates in the model. It is possible that antagonism between azoles and amphotericin B is both drug and fungus specific, and the nature of polyene-azole interaction in molds may be distinctly different from that in yeast (29). Ghannoum and colleagues noted that, unlike in C. albicans, the principle sterol component in Cryptococcus neoformans is not ergosterol (12). This may partially explain why amphotericin B-azole combinations have been described as having sequential activity (not synergy) against C. neoformans (2). Against this pathogen, azole antifungals may act as consolidation therapy subsequent to the rapid reduction in tissue fungal burden produced by amphotericin B. It may also be possible that less-appreciated mechanisms of azole antifungal activity (e.g., perturbation of membrane-bound enzymatic activity) enhance amphotericin B activity against some pathogens (13). Hopefully, future pharmacodynamic analysis, animal studies, and experience in clinical trials will provide a rational framework for the inevitable use of combination antifungal therapy.
ACKNOWLEDGMENTS
This study was supported by a grant from Pfizer Pharmaceuticals Inc.
We thank Joseph Miller and Lawrence Fleckenstein (University of Iowa, College of Pharmacy) for performing the antifungal concentration analysis. We also thank Shawn Messer and others at the Special Microbiology Laboratory (Department of Pathology, University of Iowa College of Medicine, Iowa City) for their technical assistance.
REFERENCES
- 1.Acar, J. F. 1997. Consequences of bacterial resistance to antibiotics in medical practice. Clin. Infect. Dis. 24(Suppl. 1):S17–S18. [DOI] [PubMed]
- 2.Atkinson B A, Bocanegra R, Colombo A L, Graybill J R. Treatment of disseminated Torulopsis glabrata infection with DO870 and amphotericin B. Antimicrob Agents Chemother. 1994;38:1604–1607. doi: 10.1128/aac.38.7.1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bannatyne R M, Cheung R. Discrepant results of amphotericin B assays on fresh versus frozen serum samples. Antimicrob Agents Chemother. 1977;12:550. doi: 10.1128/aac.12.4.550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck-Saugué C M, Jarvis W R the National Nosocomial Infections Surveillance System. Secular trends in nosocomial primary bloodstream infections in the United States 1980–1990. J Infect Dis. 1993;167:1247–1251. doi: 10.1093/infdis/167.5.1247. [DOI] [PubMed] [Google Scholar]
- 5.Bennett J E, Dismukes W E, Duma R J, Medoff G, Sande M A, Gallis H, Leonard J, Fields B T, Bradshaw M, Haywood H, McGee Z A, Cate T R, Cobbs C G, Warner J F, Alling D W. A comparison of amphotericin B alone and combined with flucytosine in the treatment of cryptococcal meningitis. N Engl J Med. 1979;301:126–131. doi: 10.1056/NEJM197907193010303. [DOI] [PubMed] [Google Scholar]
- 6.Blaser J, Zinner S H. In-vitro models for the study of antibiotic activities. Prog Drug Res. 1987;31:349–381. doi: 10.1007/978-3-0348-9289-6_11. [DOI] [PubMed] [Google Scholar]
- 7.Blaser, J. 1985. In-vitro model for the simultaneous simulation of the serum kinetics of two drugs with different half-lives. J. Antimicrob. Chemother. 15(Suppl. A):125–130. [DOI] [PubMed]
- 8.Daneshmend T K, Warnock D W. Clinical pharmacokinetics of systemic antifungal drugs. Clin Pharmacokinet. 1983;8:17–42. doi: 10.2165/00003088-198308010-00002. [DOI] [PubMed] [Google Scholar]
- 9.Ebert S C, Craig W A. Pharmacodynamic properties of antibiotics: application to drug monitoring and dosage regimen design. Infect Control Hosp Epidemiol. 1990;6:319–326. doi: 10.1086/646178. [DOI] [PubMed] [Google Scholar]
- 10.Eliopoulos G M. Synergism and antagonism. Infect Dis Clin N Am. 1989;3:399–405. [PubMed] [Google Scholar]
- 11.George D, Kordick D, Miniter P, Patterson T F, Andriole V T. Combination therapy in experimental invasive aspergillosis. Clin Infect Dis. 1993;168:692–698. doi: 10.1093/infdis/168.3.692. [DOI] [PubMed] [Google Scholar]
- 12.Ghannoum M A, Spellberg B J, Ibrahim A S, Ritchie J A, Currie B, Spitzer E D, Edwards J E, Jr, Casadevall A. Sterol composition of Cryptococcus neoformans in the presence and absence of fluconazole. Antimicrob Agents Chemother. 1994;38:2029–2033. doi: 10.1128/aac.38.9.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grant S M, Clissold S P. Fluconazole: a review of its pharmacodynamic and pharmacokinetic properties, and therapeutic potential in superficial and systemic mycosis. Drugs. 1990;39:877–916. doi: 10.2165/00003495-199039060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Grasson S, Meinardi G, de Carneri I, Tamassia V. New in vitro model to study the effect of antibiotic concentration and rate of elimination on antibacterial activity. Antimicrob Agents Chemother. 1978;13:570–576. doi: 10.1128/aac.13.4.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmberg S D, Solomon S L, Blake P A. Health and economic impact of antimicrobial resistance. Rev Infect Dis. 1987;9:1065–1078. doi: 10.1093/clinids/9.6.1065. [DOI] [PubMed] [Google Scholar]
- 16.Jones R N, Marshall S A, Pfaller M A, Wilke W W, Hollis R J, Erwin M E, Edmond M B, Wenzel R P the SCOPE Hospital Study Group. Nosocomial enterococcal bloodstream infections in the SCOPE program: antimicrobial resistance, species occurrence, molecular testing results, and laboratory testing accuracy. Diagn Microbiol Infect Dis. 1997;29:95–102. doi: 10.1016/s0732-8893(97)00115-6. [DOI] [PubMed] [Google Scholar]
- 17.Klepser M E, Ernst E J, Ernst M E, Lewis R E, Pfaller M A. Abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Antifungal time-kill curves, influence of test conditions on results, abstr. D-144; p. 109. [Google Scholar]
- 18.Klepser M E, Wolfe E J, Jones R N, Nightingale C H, Pfaller M A. Antifungal pharmacodynamic characteristics of fluconazole and amphotericin B tested against Candida albicans. Antimicrob Agents Chemother. 1997;41:1392–1395. doi: 10.1128/aac.41.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martin E, Maier F, Bhakdi S. Antagonistic effects of fluconazole and 5-fluorocytosine on candidacidal action of amphotericin B in human serum. Antimicrob Agents Chemother. 1994;38:1331–1338. doi: 10.1128/aac.38.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 21.Ng T K, Chan R C, Adeyemi-Doro F A, Cheung S W, Cheng A F. Rapid high performance liquid chromatographic assay for antifungal agents in human sera. J Antimicrob Chemother. 1996;37:465–472. doi: 10.1093/jac/37.3.465. [DOI] [PubMed] [Google Scholar]
- 22.Pearson R D, Steigbigel R T, Davis H T, Chapman S W. Method for reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980;18:699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pfaller, M. A. 1996. Nosocomial candidiasis: emerging species, reservoirs, and modes of transmission. Clin. Infect. Dis. 22(Suppl. 2):S89–S94. [DOI] [PubMed]
- 24.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vivo-in vitro correlation for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 25.Schaffer A, Böhler A. Amphotericin B refractory aspergillosis after itraconazole: evidence for significant antagonism. Mycoses. 1993;36:421–424. doi: 10.1111/j.1439-0507.1993.tb00732.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt H J, Bernard E M, Edwards F, Armstrong D. Combination therapy in a model of pulmonary aspergillosis. Mycoses. 1991;34:281–285. doi: 10.1111/j.1439-0507.1991.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 27.Sugar A M, Hitchcock C A, Troke P F, Picard M. Combination therapy of murine invasive candidiasis with fluconazole and amphotericin B. Antimicrob Agents Chemother. 1995;39:598–601. doi: 10.1128/AAC.39.3.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugar A M, Salibian M, Goldani L Z. Saperconazole therapy of murine disseminated candidiasis: efficacy and interactions with amphotericin B. Antimicrob Agents Chemother. 1994;38:371–373. doi: 10.1128/aac.38.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sugar A M. Use of amphotericin B with azole antifungal drugs: what are we doing? Antimicrob Agents Chemother. 1995;39:1907–1912. doi: 10.1128/aac.39.9.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Van Der Horst C M, Saag M S, Cloud G A, Hamill R J, Graybill J R, Sobel J D, Johnson P C, Tuazon C U, Kerkering T, Moskovitz B L, Powderly W G, Dismukes W E. Treatment of cryptococcal meningitis associated with the acquired immunodeficiency syndrome. N Engl J Med. 1997;337:15–21. doi: 10.1056/NEJM199707033370103. [DOI] [PubMed] [Google Scholar]
- 31.Wolfe E J, Klepser M E, Pfaller M A. Pharmacotherapy 17:189. (Abstract 39.) 1997. Antifungal dynamics of amphotericin B and fluconazole in combination against Candida albicans, effect of exposure time. [Google Scholar]