Abstract
Introduction
Epidemiological modeling is widely used to offer insights into the COVID-19 pandemic situation in Asia. We reviewed published computational (mathematical/simulation) models conducted in Asia that assessed impacts of pharmacological and non-pharmacological interventions against COVID-19 and their implications for vaccination strategy.
Methods
A search of the PubMed database for peer-reviewed, published, and accessible articles in English was performed up to November 2022 to capture studies in Asian populations based on computational modeling of outcomes in the COVID-19 pandemic. Extracted data included model type (mechanistic compartmental/agent-based, statistical, both), intervention type (pharmacological, non-pharmacological), and procedures for parameterizing age. Findings are summarized with descriptive statistics and discussed in terms of the evolving COVID-19 situation.
Results
The literature search identified 378 results, of which 59 met criteria for data extraction. China, Japan, and South Korea accounted for approximately half of studies, with fewer from South and South-East Asia. Mechanistic models were most common, either compartmental (61.0%), agent-based (1.7%), or combination (18.6%) models. Statistical modeling was applied less frequently (11.9%). Pharmacological interventions were examined in 59.3% of studies, and most considered vaccination, except one study of an antiviral treatment. Non-pharmacological interventions were also considered in 84.7% of studies. Infection, hospitalization, and mortality were outcomes in 91.5%, 30.5%, and 30.5% of studies, respectively. Approximately a third of studies accounted for age, including 10 that also examined mortality. Four of these studies emphasized benefits in terms of mortality from prioritizing older adults for vaccination under conditions of a limited supply; however, one study noted potential benefits to infection rates from early vaccination of younger adults. Few studies (5.1%) considered the impact of vaccination among children.
Conclusion
Early in the COVID-19 pandemic, non-pharmacological interventions helped to mitigate the health burden of COVID-19; however, modeling indicates that high population coverage of effective vaccines will complement and reduce reliance on such interventions. Thus, increasing and maintaining immunity levels in populations through regular booster shots, particularly among at-risk and vulnerable groups, including older adults, might help to protect public health. Future modeling efforts should consider new vaccines and alternative therapies alongside an evolving virus in populations with varied vaccination histories.
Keywords: COVID-19, vaccination, epidemiological modeling, Asia, intervention
1. Introduction
The SARS-CoV-2, which causes COVID-19, rapidly spread from the first reported symptoms in Wuhan, China, in December 2019 through to the declaration of an International Public Health Emergency by the World Health Organization in January 2020 (1, 2). The nature of the virus has since changed through its evolution into new variants of concern, with differing potentials for infection, severe disease course, and negation of immunity from vaccination or prior infection (3, 4). The human response to the pandemic has also developed from the implementation of non-pharmacological countermeasures through to pharmacological and vaccination strategies, which have mitigated the impact of the pandemic with varying degrees of effectiveness (5, 6).
In the early pandemic, non-pharmacological interventions were implemented across many regions to ease the immediate disease burden of COVID-19 and prevent the overwhelming of public health systems. Although lockdowns and school/workplace closures were effective in reducing transmission and patient healthcare costs, the economic disruption, declining mental health, and social discontent caused by these interventions raised concerns (7–9). The development and wider rollout of effective vaccines has further changed the dynamics of the situation (10–13). In the context of limited vaccine supply, the WHO recommended administration strategies that aimed to reduce mortality from COVID-19 infection (14). Individuals at risk of infection and/or severe disease were targeted, namely, front-line healthcare workers and older adults, the latter group being at particular risk of morbidity and mortality from COVID-19, compared with younger age groups (6, 15, 16). As supplies have stabilized, campaigns to vaccinate the wider public have faced challenges in the form of hesitancy among certain populations to receive initial doses of the vaccine and/or subsequent boosters (17, 18). Additionally, from 2022, the progression from a prevalent Delta to Omicron variant has shifted the burden of disease to younger people, including pediatric patients in Asia (19–21).
Ongoing high infection rates, an evolving virus, vaccine fatigue, and the relaxation of pandemic countermeasures suggest that COVID-19 will pose ongoing challenges to public health with potential differential effects across age groups (22, 23). Owing to large and diverse populations with different cultural practices and healthcare systems, the development of the COVID-19 situation in Asian settings is likely to pose unique questions for healthcare professionals and policymakers in the region. The dynamics of vaccination against SARS-CoV-2 are complicated by age-dependent factors, type of vaccines used and population vaccine coverage, changing levels of infection and the prevailing strains of concern, and the relaxation of non-pharmaceutical interventions, hence, necessitating the use of mathematical modeling studies in Asia.
Throughout the crisis, epidemiological models have been widely developed and used by policymakers to estimate the impact of interventions on projected disease burden and demands on public healthcare systems (24, 25). Mitigation strategies, including the mandating of mask-wearing, social distancing, restrictions on movement and gatherings, through to pharmaceutical and vaccination strategies, have been implemented to control the spread of virus with varying degrees of effectiveness (5, 6). Although models cannot exactly predict key factors such as the basic reproduction number (R0), these tools have been applied both globally and in Asia to guide potential care needs in terms of stratifying risk, directing limited resources, and planning for future outbreaks (26, 27). Models of increasing complexity have also been developed to account for the dynamics of transmission among different age groups in the context of different vaccination statuses and waning vaccine effectiveness over time or against emerging variants (28).
Mechanistic models are formulated to mimic the nature of spreading diseases, allowing the simulation of the complex dynamics and nonlinear feedback of COVID-19 transmission within a population (29). An example is the Susceptible-Infected-Exposed-Removed (SEIR) compartmental model, which considers interactions among cohorts of a population, categorized according to disease status. Individuals within a compartment are not differentiated from one another but can flow from one compartment to another at rates defined by parameter inputs. This approach can be effective for examining disease dynamics at a macro level. As a type of mechanistic model, individual-level models treat members of a population as unique agents; this may better capture phenomena such as super-spreader events. Statistically derived models (for example, distribution fitting and regression-type analysis) can also be applied to accurately characterize data from a small number of parameters and are suitable for short-term forecasts that are accurate, repeatable, and sensitive to momentary changes in a system (30). These tools include regression using least-squares or Bayesian estimations. These features make statistical models valuable for producing up-to-date estimates of COVID-19’s underlying nature as a disease (e.g., the basic reproduction rate) and the demographic patterns of the population (e.g., how long people self-isolate). Although statistical models can be highly accurate, they treat the situation as a black box and do not mimic the underlying nature of the disease or its consequences. Thus, these models are not well suited for long-term projections or for exploring hypothetical scenarios.
This review aims to examine published COVID-19 epidemiological modeling studies conducted in Asian settings and assess the models used and their findings regarding the impact of vaccination and non-pharmacological measures on COVID-19 infections, hospitalization, mortality, and policies. We discuss the implications of modeling studies in Asia in the context of the latest data on COVID-19 vaccines, the emergence of SARS-CoV-2 variants, and regional vaccination policies.
2. Methods
2.1. Literature search
For the purposes of this study, eligible reports examined the infection, mortality, hospitalization, vaccination, and/or policy outcomes from computational models for COVID-19 set in Asia. We followed the PRISMA guidelines in conducting systematic elements of this review. A literature search in the PubMed database for peer-reviewed, published, and accessible articles in English, available from the start of the COVID-19 pandemic, was conducted on 9 November 2022. Search terms focused on keywords identified from PICO (populations, intervention, comparison, outcome) elements and included terms common among modeling studies (e.g., Susceptible-Infected-Recovered, simulation, agent-based), types of outcomes (e.g., hospitalization, infection, and mortality) and the target interventions (e.g., vaccination) (See Supplementary Table S1 for full search terms). The search was filtered for articles that had available full text, and the results were exported to Microsoft Excel for initial screening based on the titles and abstracts. Articles that did not include an epidemiological model (e.g., laboratory and clinical studies) and/or were not concerned with COVID-19 (e.g., other diseases examined within a COVID-19 setting) were excluded.
Screened articles were further categorized based on the abstract and display items to identify a smaller set of articles that fulfilled any of the following conditions: (i) the article mentions collaborations with, or recommendations to, policy-makers; or (ii) the article considers the effects size of pharmacological and/or non-pharmacological interventions on any of the outcomes of interest (infection, mortality, hospitalization, and/or vaccination).
2.2. Data extraction
One research assistant performed data extraction for model classification, location, time, and scale, interventions applied, consideration of age groups, and impacts of interventions on rates of infection, hospitalization/severe disease, mortality, and/or policy. Another research assistant confirmed the extracted data.
Non-pharmacological interventions were categorized as “reducing contact” (measures that reduce transmission from infected individuals, such as lockdowns, school/workplace closure, and social distancing), “border control” (preventing infected individuals from entering the population; for instance, airport quarantine and border closure), “contact tracing” (testing/quarantining of people having close contact with infected individuals), “hygiene” (e.g., hand washing, ventilation), “PPE” (e.g., wearing of masks, gloves), and “other.” In categorizing models employed in reports, we differentiated between “mechanistic” (i.e., any model that applied a priori mathematical equations describing physical systems, with or without stochastic elements) and “statistical models” (e.g., models based on regression or Bayesian analysis, or machine learning of data sets), and classified combinations as “both.” We further categorized mechanistic models as compartmental (cohort), agent-based (individual) or combination models (combining elements from both types).
3. Results
3.1. Study settings
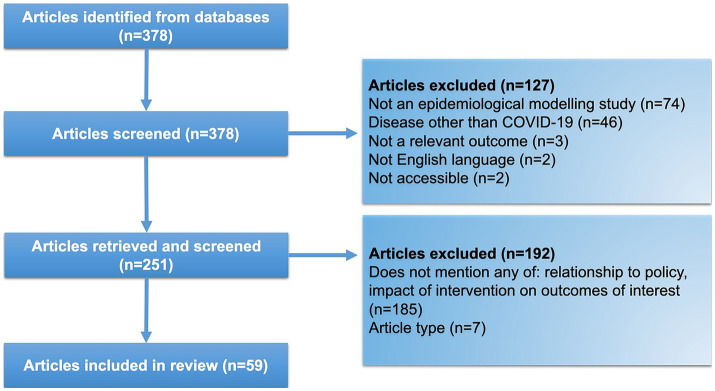
The literature search returned 378 results, which, after exclusions for irrelevance (n = 127) and ineligibility (n = 192), resulted in a set of 59 reports selected for data extraction (Figure 1; Table 1). Most reports were from East Asia (China, 21/59 [3 of which were set in Hong Kong], 35.6%; Japan, 7/59, 11.9%; South Korea 6/59, 10.2%), followed by South Asia (India, 5/59, 8.5%; Bangladesh, 3/59, 5.1%; Sri Lanka, 1/59, 1.7%) and South-East Asia (Malaysia, 3/59, 5.1%; the Philippines, 2/59, 3.4%; Thailand, 1/59, 1.7%; Vietnam; 1/59, 1.7%). Multiple countries were considered in 6.8% (4/59) of reports, and 5.1% (3/59) presented modeling results that examined specific Asian regions as part of a global setting. The majority of reports (59.3%, 35/59) applied a modeling start date in 2020, with fewer studies starting in 2021 (33.9%, 20/59) and 2022 (3.4%, 2/59).
Figure 1.
Literature search flow diagram.
Table 1.
Key characteristics of included reports (N = 59 studies).
| Author Year (citation) | Locality | Intervention | Model type | Age groups | Outcomes | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Nonpharmacological | Pharmacological | Vaccination efficacy parametersa | Infection | Hospitalization and severe disease | Mortality | |||||
| Baniasad 2021 (31) | Iran, Turkey, Saudi Arabia, United Arab Emirates, India, Russia, Philippines, South Korea | Contact tracing, reducing contact | Vaccination (unspecified) | Unspecified | Both | Combination | N/A | X | X | X |
| Cai 2022 (32) | China | Reducing contact | Vaccination (Sinovac/CoronaVac) | 51.8%, as a variable | Mechanistic | Compartmental | 14 age groups | X | X | X |
| Ferguson 2022 (33) | Bangladesh | Reducing contact, PPE | N/A | N/A | Mechanistic | Compartmental | N/A | X | X | X |
| Jung 2020 (34) | North Korea | Reducing contact | Vaccination (unspecified) | 50–80% in reducing susceptibility 80–95% reducing hospitalization |
Mechanistic | Compartmental | N/A | X | X | |
| Leung 2021 (35) | Global, Hong Kong, China | Border control | Vaccination (unspecified) | Weighted average of available vaccines | Mechanistic | Compartmental | Age as variable | X | X | X |
| Qian 2022 (36) | China | Contact tracing, reducing contact | Vaccination (unspecified) | Unspecified | Mechanistic | Compartmental | N/A | X | X | X |
| Suphanchaimat 2021 (37) | Thailand | N/A | Vaccination (unspecified) | 50% against infection | Mechanistic | Compartmental | N/A | X | X | X |
| Cai 2022 (38) | China | N/A | Vaccination (unspecified) | Variable | Mechanistic | Compartmental | 16 age groups | X | X | |
| Ediriweera 2020 (39) | Sri Lanka | Reducing contact | N/A | Weighted average of available vaccines | Mechanistic | Compartmental | N/A | X | X | |
| Kong 2022 (40) | Japan, China | Contact tracing, border control, reducing contact | Vaccination (unspecified) | Unspecified | Mechanistic | Compartmental | N/A | X | X | |
| Rajput 2021 (41) | India | Contact tracing, reducing contact | Vaccination (unspecified) | 80%, variable | Mechanistic | Compartmental | N/A | X | X | |
| Shah 2022 (42) | India | Reducing contact | N/A | N/A | Mechanistic | Combination | N/A | X | X | |
| Shankaranarayanan 2022 (43) | India | Reducing contact | Vaccination (unspecified) | Statistical | N/A | N/A | X | X | ||
| De Lara-Tuprio 2022 (44) | The Philippines | Contact tracing, reducing contact, hygiene, PPE | N/A | N/A | Mechanistic | Compartmental | N/A | X | ||
| Dong 2022 (45) | China | Reducing contact | N/A | N/A | Mechanistic | Agent-based | Age as variable | X | ||
| Foy 2021 (46) | India | Reducing contact | Vaccination (unspecified) | 0–100% | Mechanistic | Compartmental | 7 age groups | X | X | |
| Fu 2022 (47) | China | N/A | Vaccination (CoronaVac/BBIBP-CorV) | Unspecified | Statistical | N/A | 3 age groups | X | X | |
| Gaudou 2020 (48) | Vietnam | Reducing contact, PPE | N/A | N/A | Mechanistic | Combination | Age as variable | X | X | |
| Ko 2021 (49) | South Korea | Border control, reducing contact | Vaccination (unspecified) | Variable | Mechanistic | Compartmental | 4 age groups | X | X | |
| Ko 2021 (50) | South Korea | Reducing contact | Vaccination (unspecified) | 84% | Both | Compartmental | 5 age groups | X | X | |
| Lin 2021 (51) | China | N/A | Antivirals | N/A | Mechanistic | Compartmental | 4 age groups | X | X | |
| Omae 2021 (52) | Japan | N/A | BNT162b2 | Decreased infection by 60 and 92% for 1st and 2nd doses | Mechanistic | Compartmental | N/A | X | X | |
| Yufeng 2022 (53) | China | Reducing contact | N/A | N/A | Mechanistic | Compartmental | N/A | X | X | |
| Zhang 2022 (54) | Pakistan, Bangladesh | Reducing contact | Vaccination (unspecified) | Unspecified | Mechanistic | Compartmental | N/A | X | X | |
| Zhao 2021 (55) | China | N/A | Vaccination (unspecified) | Unspecified | Mechanistic | Compartmental | 4 age groups | X | X | |
| Akamatsu 2021 (56) | Japan | Reducing contact | N/A | N/A | Mechanistic | Compartmental | Age as variable | X | X | |
| Alsayed (57) | Malaysia | Reducing contact | N/A | N/A | Mechanistic | Combination | N/A | X | ||
| Chen 2021 (58) | China, Singapore | Border control | N/A | N/A | Mechanistic | Compartmental | N/A | X | ||
| Estadilla 2021 (59) | The Philippines | Contact tracing, reducing contact | Vaccination (unspecified) | Weighted average of available vaccines | Mechanistic | Compartmental | N/A | X | ||
| Hassan 2020 (60) | Bangladesh | Reducing contact | N/A | N/A | Mechanistic | Combination | N/A | X | ||
| Herng 2022 (61) | Malaysia | Reducing contact | N/A | N/A | Both | Combination | X | |||
| Hirata 2022 (62) | Japan | Reducing contact | Vaccination (Pfizer/BioNTech) | Statistical | N/A | N/A | X | |||
| Islam 2021 (63) | Bangladesh | Border control, reducing contact | N/A | N/A | Statistical | N/A | N/A | X | ||
| Kobayashi 2022 (64) | Japan | Reducing contact | Vaccination (unspecified) | Estimated at population level | Mechanistic | Compartmental | Age as variable | X | ||
| Kong 2021 (65) | Global | Border control, reducing contact | N/A | N/A | Mechanistic | Compartmental | N/A | X | ||
| Libotte 2020 (66) | China | N/A | Vaccination (unspecified) | Variable | Mechanistic | Compartmental | N/A | X | ||
| Liu 2022 (67) | China | Contact tracing, border control, reducing contact, hygiene, PPE | N/A | N/A | Both | Compartmental | N/A | X | ||
| Liu 2022 (68) | China | Reducing contact, PPE | Vaccination (unspecified) | Parameterized for susceptibility and infectiousness | Mechanistic | Compartmental | N/A | X | ||
| Lym 2022 (69) | South Korea | Reducing contact | N/A | N/A | Statistical | N/A | 2 age groups | X | ||
| Mandal 2020 (70) | India | Border control, reducing contact | N/A | N/A | Mechanistic | Compartmental | N/A | X | ||
| Min 2021 (71) | South Korea | Reducing contact | AstraZeneca, Moderna, Janssen, Pfizer, COVAX facility | 52–94% | Mechanistic | Compartmental | 3 age groups | X | ||
| Salman 2021 (72) | Malaysia | Reducing contact, education | N/A | N/A | Mechanistic | Combination | N/A | X | ||
| Seok 2022 (73) | South Korea | Contact tracing, reducing contact, Other | Vaccination (unspecified) | 48.1–96.1% | Mechanistic | Compartmental | 9 age groups | X | ||
| Shen 2022 (74) | China | Reducing contact | Vaccination (unspecified) | Unspecified | Mechanistic | Combination | 3 age groups | X | ||
| Wu 2022 (75) | China | Reducing contact | Vaccination (unspecified) | 30% | Mechanistic | Statistical | N/A | X | ||
| Xing 2021 (76) | Global | Reducing contact | N/A | N/A | Statistical | N/A | N/A | X | ||
| Yasuda 2022 (77) | Japan | N/A | Vaccination (unspecified) | Mechanistic | Compartmental | 2 age groups | X | |||
| Yin 2021 (78) | China | Contact tracing, PPE | N/A | N/A | Mechanistic | Combination | Age as a variable | X | ||
| Yu 2021 (79) | China, Hong Kong | Contact tracing, reducing contact | Vaccination (unspecified) | 30, 50, 70% | Mechanistic | Compartmental | N/A | X | ||
| Zhang 2021 (80) | China, Hong Kong | Border control, contact tracing, reducing contact | Vaccination (unspecified) | 0–80% | Mechanistic | Combination | 4 age groups | X | ||
| Zhao 2021 (81) | China | Education | Vaccination (unspecified) | 100% | Mechanistic | Compartmental | N/A | X | ||
| Zhao 2021 (82) | South East Asia | Contact tracing, reducing contact, PPE | Vaccination (unspecified) | 70% | Mechanistic | Compartmental | N/A | X | ||
| Zhou 2020 (83) | China | Reducing contact | N/A | N/A | Mechanistic | Compartmental | N/A | X | ||
| Zhu 2021 (84) | Japan | Border control, reducing contact | Vaccination (unspecified) | 78.1% | Both | Compartmental | N/A | X | ||
| Zou 2022 (85) | China | Reducing contact | Vaccination (unspecified) | 50–90% | Mechanistic | Compartmental | N/A | X | ||
| Hou 2021 (86) | China | Other | N/A | N/A | Mechanistic | Combination | N/A | X | ||
| Jung 2022 (87) | South Korea | Reducing contact, PPE | N/A | N/A | Mechanistic | Compartmental | N/A | X | X | |
| Li 2020 (88) | China | N/A | N/A | N/A | Statistical | N/A | Age as variable | X | ||
| Sunohara 2021 (89) | Japan | Reducing contact | Vaccination (unspecified) | 100% against transmission | Mechanistic | Compartmental | 3 age groups | X | ||
Referring to infection unless otherwise specified.
3.2. Modeling approaches
Mechanistic models dominated the reports (79.7.0%, 47/59), with fewer statistical (11.9%, 7/59), and combinations of both approaches (8.5%, 5/59) (Table 1). Among the mechanistic models, the majority were based on a compartmental model (61.0%, 36/59). For example, Jung et al. (34) used a susceptible-infected-recovered (SIR) model with time-dependent parameters determined by machine learning. Only one study (1.7%) focused on an agent-based model constructed from statistical data on buildings and the local population to model individual interactions (45); however, a number of studies integrated combination analysis models (18.6%, 11/59), including a study by Shen et al. which compared results from separate SEIR and agent-based models (74).
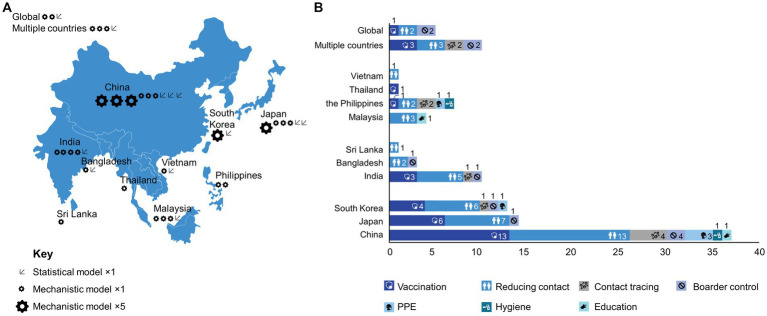
These proportions were generally similar across the geographic regions (Figure 2A). More than a third of reports accounted for the effects of age in their modeling (37.3%, 22/59), either as an analytical parameter or by separation of specific age groups. For example, Sunohara et al. (89) applied a SEIR model, stratifying the population into three age groups: young (15–49 years), middle (50–64 years), and old (>64 years), ignoring children (0–14 years). A large proportion of studies were prospective (44.1%, 26/59) or included a component of prospective analysis (28.8%, 17/59); completely retrospective studies were less common (27.1%, 16/59).
Figure 2.
Geographical distribution of reports by (A) use of statistical and mechanistic model; (B) intervention category.
3.3. Interventions
Pharmacological and non-pharmacological interventions were considered in 59.3% (35/59) and 84.7% (50/59) of reports, respectively (not mutually exclusive). Vaccination was the most common pharmacological intervention, considered in 57.6% (34/59) of studies. Notably, vaccination was a factor considered in more than half of the reports from East Asian settings (China, 13/21, 61.9%; Japan, 6/7, 85.7%; South Korea, 4/6, 66.7%), as well as India (3/5, 60.0%) and Thailand (1/1, 100%) (Figure 2B). However, models that incorporated vaccination were either absent or less common than models that incorporated non-pharmacological interventions in many regions of South Asia (Bangladesh, 0/3, 0%; Sri Lanka, 0/1, 0%) and South-East Asia (Malaysia, 0/3, 0%; the Philippines, 1/2, 50.0%; Vietnam; 0/1, 0%). Among studies concerning vaccination, 25.4% (15/59) also considered the influence of age in their models; however, vaccination was explicitly extended to pediatric patients (≥3 years old) in only three studies (32, 38, 55) and adolescents in one study (≥15 years old) (89). In other studies, vaccines were considered to be administered only to the adult population (typically ≥20 years old).
The type of vaccine was generally unspecified or maintained as a ‘hypothetical’ vaccine within the model with effectiveness as a variable. Where vaccine effectiveness was an input parameter in models, a wide range of values for effectiveness in reducing infection were typically used. Several models also parameterized the effectiveness of vaccination for reducing infectiousness and risk of mortality, as well as vaccination type, number of doses, and time from vaccination. Two studies from China specified the use of vaccines based on the inactivated virus, and two studies from Japan specified the use of mRNA vaccines. A study from South Korea compared a variety of different mRNA vaccines and a viral vector vaccine. Traditional Chinese medicine was also considered in one report and antiviral medication in two reports.
A large proportion of the reports that considered non-pharmacological interventions examined the effects of reducing contact (76.3%, 45/59). These kinds of interventions ranged from strict lockdowns on individual movement in regions that report infections (32) to studies that parameterized changes in individual mobility at public transport facilities and spaces (62). Contact tracing (20.3%, 12/59); border control (16.9%, 10/59); and the use of PPE (13.6%, 8/59), i.e., masks and face shields, were also relatively common targets. The effects of hygiene (3.4%, 2/59) and education (3.4%, 2/59) were less commonly measured. For several lower-income regions (i.e., Vietnam, Malaysia, Sri Lanka, Bangladesh), modeling targeted only non-pharmacological interventions (Figure 2B).
3.4. Outcomes
3.4.1. Infection
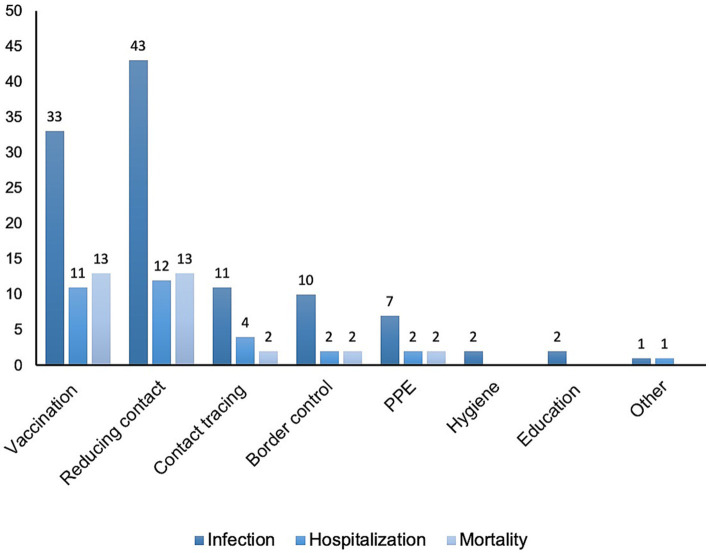
Symptomatic infection was a target outcome of almost all models (91.5%, 54/59) (Table 1; Figure 3). Among the 57.6% (34/59) of reports that considered the influence of vaccination, almost all (97.1%, 33/34) examined infection as an outcome. Of the 85.2% (46/54) of reports concerning non-pharmacological impacts on infection (Figure 3), those categorized as reducing contact were most common (93.5%, 43/46), followed by contact tracing (23.9%, 11/46), border control (21.7%, 10/46), and PPE (15.2%, 7/46). Fewer reports considered the effects of hygiene (4.3%, 2/46) or education (4.3%, 2/46) on infection.
Figure 3.
Outcome categories explored in models targeting specific interventions.
Among models that considered infection, 35.2% (19/54) of reports accounted for the influence of age as a factor in their models, including 14 reports of vaccinated populations. With respect to recommendations for prioritizing vaccines to different age groups during the acute stage of the pandemic, only one study in Japan noted potential benefits to infection rates by vaccinating younger people first (89), and two South Korean reports specifically recommended prioritizing older adults first (49, 50). Other reports from China proposed targets for overall population coverage necessary to flatten case numbers. Two studies highlighted the importance of expanding vaccination programs to include young people (32, 55).
3.4.2. Hospitalization and severe disease
Incidences of severe cases of COVID-19 that required medical intervention and/or hospitalization were considered in 30.5% (18/59) of studies (Table 1; Figure 3). Vaccination was considered in 61.1% (11/18) of these studies. A third of reports (33.3%, 6/18) stratified this outcome by age, in which two studies noted that targeted vaccination of older adults or children would decrease hospitalization (32, 38). Additionally, 77.8% (14/18) of reports considered non-pharmacological countermeasures, including reducing contact (66.7%, 12/18), contact tracing (22.2%, 4/18), and border control (11.1%, 2/18).
3.4.3. Mortality
Among the included reports, 30.5% (18/59) considered mortality as a modeling outcome (Table 1; Figure 3); of these, 72.2% (13/18) examined vaccination. Age was a factor in 55.6% (10/18) of reports, and 22.2% (4/18) explicitly noted that vaccination schedules prioritizing older adults during the acute phase of the pandemic would likely reduce overall mortality most effectively (46, 49, 50, 55). One Japanese report suggested that a vaccination strategy that prioritized younger generations might offer benefits in terms of overall mortality (89). However, the stringency of and compliance with non-pharmacological countermeasures was acknowledged to modulate the effectiveness of age-based strategies. Models that incorporated non-pharmacological countermeasures were a focus of 77.8% (14/18) of reports; these countermeasures included contact reduction (72.2%, 13/18), contact tracing (11.1%, 2/18), and border control (11.1%, 2/18).
4. Discussion
We examined modeling studies applied to the COVID-19 pandemic in Asia to identify patterns that might guide decision-making on appropriate and timely countermeasures for the ongoing endemic situation and management of future potential SARS-CoV-2 variants of interest. Although, global studies have raised concerns that modeling studies may not give accurate long-term forecasts of numbers of COVID-19 patients, hospitalizations, and deaths, the scenarios generated by such models are still useful for guiding decision-making and resource prioritization (90–92). Across many regions, modeling studies have also played roles in messaging to the public in order to explain and justify otherwise undesirable interventions (93). Our review showed that data-driven modeling studies play a critical role in the understanding of disease and various preventive policies in Asia.
We identified more reports from East Asian nations, particularly dominated by reports from China. However, the strong tendency to use mechanistic modeling was generally reflected across all regions. Despite the considerable differences in public health infrastructure and substantial variations in population densities, income levels, and sociocultural aspects (82), the common use of mechanistic cohort models across regions suggests that this approach is universally attractive for understanding general disease dynamics and the effectiveness of different interventions (94). This choice may also reflect the intuitive nature of this type of model and the ability to easily develop tools suitable for use by policymakers. Other key advantages of mechanistic models include the insights into the disease that might be gained, particularly from validation of the modeling against real-world evidence. Hence, mechanistic disease-spread models are often used as a basis for cost-effectiveness analyses (95). Additionally, the broad range of symptoms that manifest in COVID-19 introduces considerable uncertainty into national reporting of disease statistics; thus, more sophisticated models (i.e., agent-based models) may not offer particular benefits in accuracy based on available input data.
Particularly early in the pandemic, prior to the wider availability of vaccines, models examined the influence of non-pharmacological interventions on disease-related outcomes. Models set in South and South-East Asian regions were particularly focused on non-pharmacological interventions rather than vaccination, which may reflect the timing of this literature search and the slower vaccine approval, acquisition, and administration in these regions. The majority of models examined here looked at reducing contact through restrictions of movement and gatherings. This focus may reflect the economic disruption caused by these interventions. Although such measures are acknowledged to be effective in reducing disease transmission, their impact is difficult to assess in terms of financial burden and sustainability and compliance; thus, it remains challenging to gauge the accuracy of modeling applied to these measures (96). Few studies here included explicit cost-effectiveness assessments for managing COVID-19 with non-pharmacological interventions or vaccination strategies (47, 88, 89). Whereas vaccination is considered to be cost-effective in terms of treatment costs, strictly enforced countermeasures can pose considerable socio-economic and mental health burdens on populations, which are not fully considered in any of the models identified here (97, 98).
Following the introduction of vaccines from late 2020, reports more often incorporated the effects of vaccination into models. Although information on the specific vaccines used was often limited, actual administration patterns may be inferred from local approval schedules and government procurement policies. Whereas China has almost exclusively used inactivated vaccines (e.g., CoronaVac, Sinovac Biotech), Japan and South Korea granted early approval for mRNA vaccines (e.g., Comirnaty, Pfizer–BioNTech, Pfizer Inc.; Spikevax, Moderna Inc), which have been widely administered. In India, a viral vector vaccine (Vaxzevria, Oxford-AstraZeneca) was used extensively; and across other South and South-East Asian countries, a variety of vaccine types have been deployed due to varying availability and access.
Early recommendations on vaccination strategies were broadly similar, regardless of the vaccination type and local situation, emphasizing the need for rapid distribution and high overall population coverage. Models examining vaccination identified here underline the importance of rapidly achieving 67–83% population coverage with effective vaccines to reduce infection, hospitalization, and/or mortality rates (99). Direct comparisons of modeling results with actual real-world data are complicated by various factors. The methods of diagnosing infection have developed from polymerase chain reaction (PCR) testing initially, through to antigen rapid testing later in the pandemic. Additionally, the implementation of home-based recovery programs in some regions may have underestimated the actual number of infections and hospital admissions (100). Nevertheless, predictions of vaccination outcomes from Asian models have been widely confirmed by lower rates of infection and hospitalization among vaccinated individuals and higher excess mortality among unvaccinated adults across these regions (101, 102).
Reports diverged in terms of specific recommendations for prioritizing vaccination of different age groups. In China and South Korea, where strict social measures were implemented alongside widespread use of inactivated virus or mRNA vaccines, respectively, the majority of models recommended priority vaccination of older adults, a groups that has being prioritized for vaccination since the beginning of the pandemic (103). This is consistent with results from early global modeling studies that also prioritized older adults (90, 104, 105).
In Japan, which implemented voluntary social distancing and predominantly administered mRNA vaccines, one study noted a potential for benefits in terms of indirectly reducing mortality by targeting younger age groups to reduce overall transmission, depending on the strength of the lockdown (89). The actual vaccination strategy implemented in Japan, however, prioritized older adults, which may have helped reduce the hospital burden but could have been suboptimal in reducing transmission, particularly in the context of weakly enforced social measures. However, attitudes towards COVID-19 suggested remarkable concern among the Japanese public, which contributed to good compliance with government advice to wear face masks, social distance, and accept vaccination/boosters (106). Globally, the attitudes of populations towards the disease, trust in policy makers, and enforcement of interventions are factors that may need to be accounted for in modeling studies (107).
The evolution of new viral strains may also influence vaccination strategies. The main variants of SARS-CoV-2 circulating in different regions have changed, from the original strain prevalent in 2020, to four main variants of concern – the Alpha, Beta, and Delta strains, common across Asia in 2021 – through to the Omicron variants from 2022 (3, 4, 108). Notably, the changes from the original variant to the Delta variant and then later to the Omicron variant were accompanied by increases in R0 values, from 2.79 to 5.08 to 8.20, respectively (109, 110). The recently emerged Omicron subvariants, such as XBB and XBF, appear to be key drivers of infection waves, although they appear to pose lower risks of mortality (111). The emergence of new variants presents challenges for modeling in terms of shifting profiles for infectiousness, severe disease and mortality, and breakthrough infection against different vaccines and vaccination statuses (i.e., number/type of vaccination and time since last booster) (112). For example, individuals with hybrid immunity (developed through both infection and vaccination) might experience greater and more sustained protection, which might allow for longer intervals between boosters (113).
The outcomes of many models have anticipated that sufficiently high vaccination coverage in a population might ‘break’ transmission, allowing for the relaxation of non-pharmacological interventions (71, 114). In reality, vaccinated populations in Asia have continued to fall short of the high proportions predicted to be necessary to confer ‘herd immunity’ despite large numbers having received initial vaccinations and boosters (115). The waning effectiveness of mRNA vaccination/booster shots over time (after approximately 20 weeks) – particularly among older adults, who typically have weakened immune systems – may increase rates of infection (116). The potential impact of immune imprinting also motivates further studies to understand the effects and limitations of boosters (117). Reduced effectiveness of vaccines against Omicron strains might also shift initial targets for vaccination coverage in a population upwards, obstructing the relaxation of non-pharmacological interventions (118). Ongoing viral transmission from viral shedding from convalescent individuals, in addition to cessation of mask usage, may contribute to future surges of the disease.
There is also a need to deeply understand the importance of using vaccines that confer broad protection in the Omicron era, such as bivalent vaccines (119). Recent real-world evidence on bivalent vaccines points to additional protection, primarily against hospitalization, among older adults with monovalent vaccines; however, there may also be some additional benefits against symptomatic disease in other age groups during Omicron subvariant circulation (120). The high-priority populations are defined as older adults, adults with significant comorbidities, children or adults with immunocompromising conditions, pregnant women, and frontline health workers.
We noted that few studies (32, 38, 55) examined here modeled the vaccination of pediatric populations in Asia. Modeling of the impact of vaccinating children (5–11 years) in Europe has been proposed as a route to the relaxation of restrictions in schools (121). Modeling studies have also suggested that extending vaccination to children may reduce hospitalization and mortality across all age groups in developing countries (122). The latest update from the WHO’s Strategic Advisory Group of Experts on Immunization (SAGE) on COVID-19 vaccination continues to prioritize the populations at greatest risk of mortality or severe disease to safeguard healthcare systems. As a low priority group, children are not routinely recommended for vaccination and boosters, but are instead determined by individual countries’ evaluation of the burden, cost, and effectiveness (123). Although data on the impact of COVID-19 among children are lacking overall, there appears to be greater risk of mortality and hospitalization in low and middle-income countries (124). Thus, there is a need for further studies focused on the local burden of COVID-19 disease among younger populations in Asia, where future modeling studies might also guide decision-making on the impact of vaccinations (121, 122, 125).
Moving into a period where more vaccines are approved and made accessible, the hesitancy of uptake is likely to pose an ongoing barrier to booster strategies and sustainable protection (126). Among children and their caregivers, common reasons for fear and hesitancy to receive a vaccine include assumptions that the vaccine has side effects and may not be safe (18). Adults may also be complacent that the COVID-19 situation is no longer severe, and/or a negative perception of the vaccine, government, and/or pharmaceutical company may discourage them from receiving initial vaccination and/or boosters (17, 18). General fatigue over the topics of COVID-19 and vaccines and a wider circulation of misinformation on vaccines may contribute to these attitudes (127). Some reports examined here accounted for vaccine hesitancy, but this factor will likely be more important in future COVID-19 models, where the majority of a population have been vaccinated but coverage falls short of requirements for herd immunity. Modeling may also need to account for the impacts of educational interventions to address gaps in knowledge and attitudes among hesitant groups. Therefore, from a public health perspective, it is crucial to understand the drivers of vaccine uptake and vaccine hesitancy; this might help to identify groups that might have lower than average uptake and plan accordingly. Such pockets of immunity gaps and high susceptibility in the population could result in small-scale outbreaks that reduce the effect of population immunity. We predict that relaxation of control measures might be associated with new waves of infection and associated deaths; however, these outcomes will be reduced by increased levels of vaccine-derived immunity as well as hybrid immunity from infections in vaccinated individuals in the population.
The evolving nature of the COVID-19 situation especially motivates a dynamic vaccine-development pipeline to deliver more effective options against new strains of SARS-CoV-2 (128, 129). Furthermore, in an endemic scenario, non-vaccine pharmacological interventions are likely to become important for the treatment of severe disease. Here, two studies were identified that considered the impact of antiviral treatments, which may reflect the regional timeline for approval of such medicines. The introduction of monoclonal antibodies may help to manage cases of severe COVID-19 and improve these outcomes (10).
There are several limitations to this study. First, the scope of the literature search, particularly the focus on journal articles published in the English language, may have omitted more recent pre-print and local-language documents. The specific search terms may also have introduced bias into the types of modeling studies captured here. Furthermore, the large diversity of regional situations, model designs, interventions, and outcome targets limit the ability to systematically extract relevant quantitative data from these reports. Additionally, few studies examined the influence of pharmacological interventions other than vaccines. As common limitations of all modeling studies, there is considerable uncertainty in many parameters such as population immunity, infectious rate, and contact and the level of health-related behaviors in study populations (130). Thus, modeling studies are subject to unreliable predictions on the impact on outcomes of interest.
5. Conclusion
Computational modeling is an important tool to address a limited understanding of SARS-CoV-2 epidemiology, and the quantitative estimates of the duration of protection from infection and vaccinations. Whereas non-pharmacological interventions have had an early role in managing health outcomes of the COVID-19 pandemic, modeling studies underline the importance of vaccines. High population coverage and vaccine effectiveness are key to mitigating the outcomes of the disease and supporting the relaxation of disruptive restrictions on movement. Compartmental mechanistic modeling offers an adequate approach to projecting disease outcomes across large populations; however, future models may need to account for complications from evolving variants and vaccination statuses/inclinations within populations, age group stratification, especially including pediatric populations. Deeper consideration of the socioeconomic burdens associated with strict non-pharmacological interventions may also be useful for policymakers and further underscore the cost-effectiveness and social benefits of vaccination programs. In the near future, increasing vaccine coverage, particularly among at-risk populations and through outreach to vaccine-adverse groups, may help to ease infection and severe disease. The rapidly evolving nature of the virus also motivates the development of new vaccines and alternative therapies. Future administration strategies may also be nuanced by competing needs to reduce overall transmissibility or severe disease burden in high-risk groups.
Author contributions
KT, JS, EO, JY, and MK: conceptualization and methodology. KT, JS, JY, MK, and CM: formal analysis and data curation. KT, JS, JY, MK, EO, CM, and HO: validation, writing, reviewing, and editing of manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
We thank Stephen Heap, Andrew Jackson and Magdalene Chu of MIMS Pte Ltd. for literature research and medical writing support provided in compliance with Good Publication Practice (GPP) 2022.
Funding Statement
The study received funding from Pfizer Inc. The funder had the following involvement with the study: study design, collection, analysis, interpretation of data, funding for medical writing support provided by Stephen Heap, Andrew Jackson and Magdalene Chu at MIMS Pte Ltd.
Conflict of interest
MK, CM, EO, JS, KT, and JY were employed by Pfizer Inc.
The remaining author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2023.1252719/full#supplementary-material
References
- 1.Habibzadeh P, Stoneman EK. The novel coronavirus: a Bird’s eye view. Int J Occup Environ Med. (2020) 11:65–71. doi: 10.15171/ijoem.2020.1921, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheng ZJ, Shan J. 2019 novel coronavirus: where we are and what we know. Infection. (2020) 48:155–63. doi: 10.1007/s15010-020-01401-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chinta S, Rodriguez-Guerra M, Shaban M, Pandey N, Jaquez-Duran M, Vittorio TJ. COVID-19 therapy and vaccination: a clinical narrative review. Drugs Context. (2023):12. doi: 10.7573/dic.2022-7-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolf JM, Wolf LM, Bello GL, Maccari JG, Nasi LA. Molecular evolution of SARS-CoV-2 from December 2019 to august 2022. J Med Virol. (2023) 95:e28366. doi: 10.1002/jmv.28366, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flaxman S, Mishra S, Gandy A, Unwin HJT, Mellan TA, Coupland H, et al. Estimating the effects of non-pharmaceutical interventions on COVID-19 in Europe. Nature. (2020) 584:257–61. doi: 10.1038/s41586-020-2405-7, PMID: [DOI] [PubMed] [Google Scholar]
- 6.CDC . Assessing risk factors for severe COVID-19 illness. (2022). Available at: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/assessing-risk-factors.html
- 7.Segarra-Blasco A, Teruel M, Cattaruzzo S. The economic reaction to non-pharmaceutical interventions during Covid-19. Econ Anal Policy. (2021) 72:592–608. doi: 10.1016/j.eap.2021.10.006, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toffolutti V, Plach S, Maksimovic T, Piccitto G, Mascherini M, Mencarini L, et al. The association between COVID-19 policy responses and mental well-being: evidence from 28 European countries. Soc Sci Med. (2022) 301:114906. doi: 10.1016/j.socscimed.2022.114906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou Y, Rahman MM, Khanam R, Taylor BR. Individual preferences, government policy, and COVID-19: a game-theoretic epidemiological analysis. Appl Math Model. (2023) 122:401–16. doi: 10.1016/j.apm.2023.06.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chary M, Barbuto AF, Izadmehr S, Tarsillo M, Fleischer E, Burns MM. COVID-19 therapeutics: use, mechanism of action, and toxicity (vaccines, monoclonal antibodies, and immunotherapeutics). J Med Toxicol. (2023) 19:205–18. doi: 10.1007/s13181-023-00931-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hillary VE, Ceasar SA. An update on COVID-19: SARS-CoV-2 variants, antiviral drugs, and vaccines. Heliyon. (2023) 9:e13952. doi: 10.1016/j.heliyon.2023.e13952, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oyebanji OA, Mylonakis E, Canaday DH. Vaccines for the prevention of coronavirus disease 2019 in older adults. Infect Dis Clin N Am. (2023) 37:27–45. doi: 10.1016/j.idc.2022.11.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO . COVID-19 vaccine tracker. (2021). Available at: https://covid19.trackvaccines.org/agency/who/
- 14.WHO . WHO SAGE roadmap for prioritizing uses of COVID-19 vaccines in the context of limited supply: An approach to inform planning and subsequent recommendations based on epidemiological setting and vaccine supply scenarios. Geneva: WHO; (2021). [Google Scholar]
- 15.Felsenstein S, Hedrich CM. SARS-CoV-2 infections in children and young people. Clin Immunol. (2020) 220:108588. doi: 10.1016/j.clim.2020.108588, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y, Mao B, Liang S, Yang JW, Lu HW, Chai YH, et al. Shanghai clinical treatment experts group for COVID-19. Association between age and clinical characteristics and outcomes of COVID-19. Eur Respir J. (2020) 55:2001112. doi: 10.1183/13993003.01112-2020, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hong Y, Xie F, An X, Lan X, Liu C, Yan L, et al. Evolution of public attitudes and opinions regarding COVID-19 vaccination during the vaccine campaign in China: year-long infodemiology study of Weibo posts. J Med Internet Res. (2023) 25:e42671. doi: 10.2196/42671, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaufishan Z, Usman M, Fishan Mumtaz K, Bilal R, Arshad A, Khan HM. Safety, effectiveness and hesitancy of COVID-19 vaccination in children: a cross-sectional study in Pakistan. Front Public Health. (2022) 10:1084017. doi: 10.3389/fpubh.2022.1084017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fergie J, Moran MM, Cane A, Pather S, Tureci Ō, Srivastava A. COVID-19 epidemiology, immunity, and vaccine development in children: a review. Vaccines (Basel). (2022) 10. doi: 10.3390/vaccines10122039, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi DS, Whitaker M, Marks KJ, Anglin O, Milucky J, Patel K, et al. Hospitalizations of children aged 5-11 years with laboratory-confirmed COVID-19 - COVID-NET, 14 states, March 2020-February 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:574–81. doi: 10.15585/mmwr.mm7116e1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choi SH, Choi JH, Lee JK, Eun BW, Song SH, Ahn B, et al. Clinical characteristics and outcomes of children with SARS-CoV-2 infection during the Delta and omicron variant-dominant periods in Korea. J Korean Med Sci. (2023) 38:e65. doi: 10.3346/jkms.2023.38.e65, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graham F. Daily briefing: no room for COVID complacency in 2023. Nature. (2022). doi: 10.1038/d41586-022-04588-2 [E-pub ahead of print]., PMID: [DOI] [PubMed] [Google Scholar]
- 23.Sarfraz A, Hasan Siddiqui S, Iqbal J, Ali SA, Hasan Z, Sarfraz Z, et al. COVID-19 age-dependent immunology and clinical outcomes: implications for vaccines. J Dev Orig Health Dis. (2022) 13:277–83. doi: 10.1017/S2040174421000398, PMID: [DOI] [PubMed] [Google Scholar]
- 24.Jewell NP, Lewnard JA, Jewell BL. Predictive mathematical models of the COVID-19 pandemic: underlying principles and value of projections. JAMA. (2020) 323:1893–4. doi: 10.1001/jama.2020.6585, PMID: [DOI] [PubMed] [Google Scholar]
- 25.Afzal A, Saleel CA, Bhattacharyya S, Satish N, Samuel OD, Badruddin IA. Merits and limitations of mathematical Modeling and computational simulations in mitigation of COVID-19 pandemic: a comprehensive review. Arch Comput Methods Eng. (2022) 29:1311–37. doi: 10.1007/s11831-021-09634-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pandey A, Sah P, Moghadas SM, Mandal S, Banerjee S, Hotez PJ, et al. Challenges facing COVID-19 vaccination in India: lessons from the initial vaccine rollout. J Glob Health. (2021) 11:03083. doi: 10.7189/jogh.11.03083, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nojiri S, Irie Y, Kanamori R, Naito T, Nishizaki Y. Mortality prediction of COVID-19 in hospitalized patients using the 2020 diagnosis procedure combination administrative database of Japan. Intern Med. (2023) 62:201–13. doi: 10.2169/internalmedicine.0086-22, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy J, Heath SM, Wang S, Ramkrishna D. Modeling COVID-19 transmission between age groups in the United States considering virus mutations, vaccinations, and reinfection. Sci Rep. (2022) 12:20098. doi: 10.1038/s41598-022-21559-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lessler J, Azman AS, Grabowski MK, Salje H, Rodriguez-Barraquer I. Trends in the mechanistic and dynamic modeling of infectious diseases. Curr Epidemiol Rep. (2016) 3:212–22. doi: 10.1007/s40471-016-0078-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sharif O, Hasan MZ, Rahman A. Determining an effective short term COVID-19 prediction model in ASEAN countries. Sci Rep. (2022) 12:5083. doi: 10.1038/s41598-022-08486-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Baniasad M, Mofrad MG, Bahmanabadi B, Jamshidi S. COVID-19 in Asia: transmission factors, re-opening policies, and vaccination simulation. Environ Res. (2021) 202:111657. doi: 10.1016/j.envres.2021.111657, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cai J, Deng X, Yang J, Sun K, Liu H, Chen Z, et al. Modeling transmission of SARS-CoV-2 omicron in China. Nat Med. (2022) 28:1468–75. doi: 10.1038/s41591-022-01855-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ferguson EA, Brum E, Chowdhury A, Chowdhury S, Kundegorski M, Mahmud AS, et al. Modelling how face masks and symptoms-based quarantine synergistically and cost-effectively reduce SARS-CoV-2 transmission in Bangladesh. Epidemics. (2022) 40:100592. doi: 10.1016/j.epidem.2022.100592, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jung SY, Jo H, Son H, Hwang HJ. Real-world implications of a rapidly responsive COVID-19 spread model with time-dependent parameters via deep learning: model development and validation. J Med Internet Res. (2020) 22:e19907. doi: 10.2196/19907, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Leung K, Wu JT, Leung GM. Effects of adjusting public health, travel, and social measures during the roll-out of COVID-19 vaccination: a modelling study. Lancet Public Health. (2021) 6:e674–82. doi: 10.1016/S2468-2667(21)00167-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Qian Y, Cao S, Zhao L, Yan Y, Huang J. Policy choices for Shanghai responding to challenges of Omicron. Front Public Health. (2022) 10:927387. doi: 10.3389/fpubh.2022.927387, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Suphanchaimat R, Tuangratananon T, Rajatanavin N, Phaiyarom M, Jaruwanno W, Uansri S. Prioritization of the target population for coronavirus disease 2019 (COVID-19) vaccination program in Thailand. Int J Environ Res Public Health. (2021) 18:10803. doi: 10.3390/ijerph182010803, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cai J, Yang J, Deng X, Peng C, Chen X, Wu Q, et al. Assessing the transition of COVID-19 burden towards the young population while vaccines are rolled out in China. Emerg Microbes Infect. (2022) 11:1205–14. doi: 10.1080/22221751.2022.2063073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ediriweera DS, de Silva NR, Malavige GN, de Silva HJ. An epidemiological model to aid decision-making for COVID-19 control in Sri Lanka. PLoS One. (2020) 15:e0238340. doi: 10.1371/journal.pone.0238340, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kong L, Duan M, Shi J, Hong J, Zhou X, Yang X, et al. Optimization of COVID-19 prevention and control measures during the Beijing 2022 winter Olympics: a model-based study. Infect Dis Poverty. (2022) 11:95. doi: 10.1186/s40249-022-01019-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rajput A, Sajid M, Tanvi SC, Aggarwal R. Optimal control strategies on COVID-19 infection to bolster the efficacy of vaccination in India. Sci Rep. (2021) 11:20124. doi: 10.1038/s41598-021-99088-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shah PV. Prediction of the peak, effect of intervention, and total infected by COVID-19 in India. Disaster Med Public Health Prep. (2022) 16:40–50. doi: 10.1017/dmp.2020.321, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shankaranarayanan A, Wei HC. Mathematical modeling of SARS-nCoV-2 virus in Tamil Nadu, South India. Math Biosci Eng. (2022) 19:11324–44. doi: 10.3934/mbe.2022527, PMID: [DOI] [PubMed] [Google Scholar]
- 44.de Lara-Tuprio E, Estadilla CDS, Macalalag JMR, Teng TR, Uyheng J, Espina KE, et al. Policy-driven mathematical modeling for COVID-19 pandemic response in the Philippines. Epidemics. (2022) 40:100599. doi: 10.1016/j.epidem.2022.100599, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dong T, Dong W, Xu Q. Agent simulation model of COVID-19 epidemic agent-based on GIS: a case study of Huangpu District, Shanghai. Int J Environ Res Public Health. (2022) 19:10242. doi: 10.3390/ijerph191610242, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foy BH, Wahl B, Mehta K, Shet A, Menon GI, Britto C. Comparing COVID-19 vaccine allocation strategies in India: a mathematical modelling study. Int J Infect Dis. (2021) 103:431–8. doi: 10.1016/j.ijid.2020.12.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fu Y, Zhao J, Wei X, Han P, Yang L, Ren T, et al. Effectiveness and cost-effectiveness of inactivated vaccine to address COVID-19 pandemic in China: evidence from randomized control trials and real-world studies. Front Public Health. (2022) 10:917732. doi: 10.3389/fpubh.2022.917732, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaudou B, Huynh NQ, Philippon D, Brugiere A, Chapuis K, Taillandier P, et al. COMOKIT: a modeling kit to understand, analyze, and compare the impacts of mitigation policies against the COVID-19 epidemic at the scale of a City. Front Public Health. (2020) 8:563247. doi: 10.3389/fpubh.2020.563247, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ko Y, Lee J, Kim Y, Kwon D, Jung E. COVID-19 vaccine priority strategy using a heterogenous transmission model based on maximum likelihood estimation in the Republic of Korea. Int J Environ Res Public Health. (2021) 18:6469. doi: 10.3390/ijerph18126469, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko Y, Lee J, Seo Y, Jung E. Risk of COVID-19 transmission in heterogeneous age groups and effective vaccination strategy in Korea: a mathematical modeling study. Epidemiol Health. (2021) 43:e2021059. doi: 10.4178/epih.e2021059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin SN, Rui J, Chen QP, Zhao B, Yu SS, Li ZY, et al. Effectiveness of potential antiviral treatments in COVID-19 transmission control: a modelling study. Infect Dis Poverty. (2021) 10:53. doi: 10.1186/s40249-021-00835-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Omae Y, Kakimoto Y, Sasaki M, Toyotani J, Hara K, Gon Y, et al. SIRVVD model-based verification of the effect of first and second doses of COVID-19/SARS-CoV-2 vaccination in Japan. Math Biosci Eng. (2022) 19:1026–40. doi: 10.3934/mbe.2022047, PMID: [DOI] [PubMed] [Google Scholar]
- 53.Yufeng Z, Huaxin P, Lanting L, Pei Z, Kaining W, Shengxing C, et al. Risk assessment and analysis of traditional Chinese medicine intervention in coronavirus disease. J Tradit Chin Med. (2022) 42:472–8. doi: 10.19852/j.cnki.jtcm.20220408.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang W, Huggins T, Zheng W, Liu S, Du Z, Zhu H, et al. Assessing the dynamic outcomes of containment strategies against COVID-19 under different public health governance structures: a comparison between Pakistan and Bangladesh. Int J Environ Res Public Health. (2022) 19:9239. doi: 10.3390/ijerph19159239, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhao ZY, Niu Y, Luo L, Hu QQ, Yang TL, Chu MJ, et al. The optimal vaccination strategy to control COVID-19: a modeling study in Wuhan City, China. Infect Dis Poverty. (2021) 10:140. doi: 10.1186/s40249-021-00922-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Akamatsu T, Nagae T, Osawa M, Satsukawa K, Sakai T, Mizutani D. Model-based analysis on social acceptability and feasibility of a focused protection strategy against the COVID-19 pandemic. Sci Rep. (2021) 11:2003. doi: 10.1038/s41598-021-81630-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Alsayed A, Sadir H, Kamil R, Sari H. Prediction of epidemic peak and infected cases for COVID-19 disease in Malaysia, 2020. Int J Environ Res Public Health. (2020) 17:4076. doi: 10.3390/ijerph17114076, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen T, Huang S, Li G, Zhang Y, Li Y, Zhu J, et al. An integrated framework for modelling quantitative effects of entry restrictions and travel quarantine on importation risk of COVID-19. J Biomed Inform. (2021) 118:103800. doi: 10.1016/j.jbi.2021.103800, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Estadilla CDS, Uyheng J, de Lara-Tuprio EP, Teng TR, Macalalag JMR, Estuar M. Impact of vaccine supplies and delays on optimal control of the COVID-19 pandemic: mapping interventions for the Philippines. Infect Dis Poverty. (2021) 10:107. doi: 10.1186/s40249-021-00886-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hassan R, Dosar AS, Mondol JK, Khan TH, Noman AA, Sayem MS, et al. Prediction of epidemics trend of COVID-19 in Bangladesh. Front Public Health. (2020) 8:559437. doi: 10.3389/fpubh.2020.559437, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Herng LC, Singh S, Sundram BM, Zamri A, Vei TC, Aris T, et al. The effects of super spreading events and movement control measures on the COVID-19 pandemic in Malaysia. Sci Rep. (2022) 12:2197. doi: 10.1038/s41598-022-06341-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hirata A, Kodera S, Diao Y, Rashed EA. Did the Tokyo Olympic games enhance the transmission of COVID-19? An interpretation with machine learning. Comput Biol Med. (2022) 146:105548. doi: 10.1016/j.compbiomed.2022.105548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Islam A, Sayeed MA, Rahman MK, Zamil S, Abedin J, Saha O, et al. Assessment of basic reproduction number (R(0)), spatial and temporal epidemiological determinants, and genetic characterization of SARS-CoV-2 in Bangladesh. Infect Genet Evol. (2021) 92:104884. doi: 10.1016/j.meegid.2021.104884, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kobayashi T, Nishiura H. Prioritizing COVID-19 vaccination. Part 2: real-time comparison between single-dose and double-dose in Japan. Math Biosci Eng. (2022) 19:7410–24. doi: 10.3934/mbe.2022350, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Kong L, Hu Y, Wang Q, Chen X, Yao T, Wang Y, et al. Could COVID-19 pandemic be stopped with joint efforts of travel restrictions and public health countermeasures? A modelling study. BMJ Open. (2021) 11:e046157. doi: 10.1136/bmjopen-2020-046157, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Libotte GB, Lobato FS, Platt GM, Silva Neto AJ. Determination of an optimal control strategy for vaccine administration in COVID-19 pandemic treatment. Comput Methods Prog Biomed. (2020) 196:105664. doi: 10.1016/j.cmpb.2020.105664, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu W, Guo Z, Abudunaibi B, Ouyang X, Wang D, Yang T, et al. Model-based evaluation of transmissibility and intervention measures for a COVID-19 outbreak in Xiamen City. China Front Public Health. (2022) 10:887146. doi: 10.3389/fpubh.2022.887146, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu J, Gao B, Bao HX, Shi Z. Isolating the net effect of multiple government interventions with an extended susceptible-exposed-infectious-recovered (SEIR) framework: empirical evidence from the second wave of COVID-19 pandemic in China. BMJ Open. (2022) 12:e060996. doi: 10.1136/bmjopen-2022-060996, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lym Y, Lym H, Kim K, Kim KJ. Spatiotemporal associations between local safety level index and COVID-19 infection risks across capital regions in South Korea. Int J Environ Res Public Health. (2022) 19:824. doi: 10.3390/ijerph19020824, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mandal S, Bhatnagar T, Arinaminpathy N, Agarwal A, Chowdhury A, Murhekar M, et al. Prudent public health intervention strategies to control the coronavirus disease 2019 transmission in India: a mathematical model-based approach. Indian J Med Res. (2020) 151:190–9. doi: 10.4103/ijmr.IJMR_504_20, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Min KD, Tak S. Dynamics of the COVID-19 epidemic in the post-vaccination period in Korea: a rapid assessment. Epidemiol Health. (2021) 43:e2021040. doi: 10.4178/epih.e2021040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Salman AM, Ahmed I, Mohd MH, Jamiluddin MS, Dheyab MA. Scenario analysis of COVID-19 transmission dynamics in Malaysia with the possibility of reinfection and limited medical resources scenarios. Comput Biol Med. (2021) 133:104372. doi: 10.1016/j.compbiomed.2021.104372, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Seok J, Lee Y, Choi JY, Choi JP, Seo H, Lee S, et al. Evaluation of intervention policies for the COVID-19 epidemic in the Seoul/Gyeonggi region through a model simulation. Yonsei Med J. (2022) 63:707–16. doi: 10.3349/ymj.2022.63.8.707, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shen S, Li W, Wei H, Zhao L, Ye R, Ma K, et al. A chess and card room-induced COVID-19 outbreak and its agent-based simulation in Yangzhou, China. Front Public Health. (2022) 10:915716. doi: 10.3389/fpubh.2022.915716, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wu JW, Jiao XK, Du XH, Jiao ZT, Liang ZR, Pang MF, et al. Assessment of the benefits of targeted interventions for pandemic control in China based on machine learning method and web service for COVID-19 policy simulation. Biomed Environ Sci. (2022) 35:412–8. doi: 10.3967/bes2022.057, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xing X, Xiong Y, Yang R, Wang R, Wang W, Kan H, et al. Predicting the effect of confinement on the COVID-19 spread using machine learning enriched with satellite air pollution observations. Proc Natl Acad Sci U S A. (2021) 118:e2109098118. doi: 10.1073/pnas.2109098118, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yasuda H, Ito F, Hanaki KI, Suzuki K. COVID-19 pandemic vaccination strategies of early 2021 based on behavioral differences between residents of Tokyo and Osaka, Japan. Arch Public Health. (2022) 80:180. doi: 10.1186/s13690-022-00933-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yin L, Zhang H, Li Y, Liu K, Chen T, Luo W, et al. A data driven agent-based model that recommends non-pharmaceutical interventions to suppress coronavirus disease 2019 resurgence in megacities. J R Soc Interface. (2021) 18:20210112. doi: 10.1098/rsif.2021.0112, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu Z, Zhu X, Liu X, Wei T, Yuan HY, Xu Y, et al. Reopening international boarders without quarantine: contact tracing integrated policy against COVID-19. Int J Environ Res Public Health. (2021) 18:7494. doi: 10.3390/ijerph18147494, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang N, Jack Chan PT, Jia W, Dung CH, Zhao P, Lei H, et al. Analysis of efficacy of intervention strategies for COVID-19 transmission: a case study of Hong Kong. Environ Int. (2021) 156:106723. doi: 10.1016/j.envint.2021.106723, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao X, Zhou Q, Wang A, Zhu F, Meng Z, Zuo C. The impact of awareness diffusion on the spread of COVID-19 based on a two-layer SEIR/V-UA epidemic model. J Med Virol. (2021) 93:4342–50. doi: 10.1002/jmv.26945, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhao Z, Li X, Liu F, Jin R, Ma C, Huang B, et al. Stringent nonpharmaceutical interventions are crucial for curbing COVID-19 transmission in the course of vaccination: a case study of South and Southeast Asian countries. Healthcare (Basel). (2021) 9:1292. doi: 10.3390/healthcare9101292, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou Y, Xu R, Hu D, Yue Y, Li Q, Xia J. Effects of human mobility restrictions on the spread of COVID-19 in Shenzhen, China: a modelling study using mobile phone data. Lancet Digit Health. (2020) 2:e417–24. doi: 10.1016/S2589-7500(20)30165-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhu W, Feng J, Li C, Wang H, Zhong Y, Zhou L, et al. COVID-19 risk assessment for the Tokyo Olympic games. Front Public Health. (2021) 9:730611. doi: 10.3389/fpubh.2021.730611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zou Y, Yang W, Lai J, Hou J, Lin W. Vaccination and quarantine effect on COVID-19 transmission dynamics incorporating Chinese-spring-festival travel rush: modeling and simulations. Bull Math Biol. (2022) 84:30. doi: 10.1007/s11538-021-00958-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hou C, Jiang H. Methodology of emergency medical logistics for multiple epidemic areas in public health emergency. PLoS One. (2021) 16:e0253978. doi: 10.1371/journal.pone.0253978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jung SM, Jung J. The possible impact of Nationwide vaccination on outcomes of the COVID-19 epidemic in North Korea: a modelling study. J Korean Med Sci. (2022) 37:e300. doi: 10.3346/jkms.2022.37.e300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Li XZ, Jin F, Zhang JG, Deng YF, Shu W, Qin JM, et al. Treatment of coronavirus disease 2019 in Shandong, China: a cost and affordability analysis. Infect Dis Poverty. (2020) 9:78. doi: 10.1186/s40249-020-00689-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sunohara S, Asakura T, Kimura T, Ozawa S, Oshima S, Yamauchi D, et al. Effective vaccine allocation strategies, balancing economy with infection control against COVID-19 in Japan. PLoS One. (2021) 16:e0257107. doi: 10.1371/journal.pone.0257107, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gerlee P, Joud A, Spreco A, Timpka T. Computational models predicting the early development of the COVID-19 pandemic in Sweden: systematic review, data synthesis, and secondary validation of accuracy. Sci Rep. (2022) 12:13256. doi: 10.1038/s41598-022-16159-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cramer EY, Ray EL, Lopez VK, Bracher J, Brennen A, Castro Rivadeneira AJ, et al. Evaluation of individual and ensemble probabilistic forecasts of COVID-19 mortality in the United States. Proc Natl Acad Sci U S A. (2022) 119:e2113561119. doi: 10.1073/pnas.2113561119, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Childs ML, Kain MP, Harris MJ, Kirk D, Couper L, Nova N, et al. The impact of long-term non-pharmaceutical interventions on COVID-19 epidemic dynamics and control: the value and limitations of early models. Proc Biol Sci. (1957) 2021:20210811. doi: 10.1098/rspb.2021.0811, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Imai N, Gaythorpe KAM, Abbott S, Bhatia S, van Elsland S, Prem K, et al. Adoption and impact of non-pharmaceutical interventions for COVID-19. Wellcome Open Res. (2020) 5:59. doi: 10.12688/wellcomeopenres.15808.1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ahmed DA, Ansari AR, Imran M, Dingle K, Bonsall MB. Mechanistic modelling of COVID-19 and the impact of lockdowns on a short-time scale. PLoS One. (2021) 16:e0258084. doi: 10.1371/journal.pone.0258084, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lessler J, Cummings DA. Mechanistic models of infectious disease and their impact on public health. Am J Epidemiol. (2016) 183:415–22. doi: 10.1093/aje/kww021, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Brauner JM, Mindermann S, Sharma M, Johnston D, Salvatier J, Gavenciak T, et al. Inferring the effectiveness of government interventions against COVID-19. Science. (2021) 371. doi: 10.1126/science.abd9338, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Reuge N, Jenkins R, Brossard M, Soobrayan B, Mizunoya S, Ackers J, et al. Education response to COVID 19 pandemic, a special issue proposed by UNICEF: editorial review. Int J Educ Dev. (2021) 87:102485. doi: 10.1016/j.ijedudev.2021.102485, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Peng W, Berry EM. Coping with the challenges of COVID-19 using the Sociotype framework: a rehearsal for the next pandemic. Rambam Maimonides Med J. (2021) 12:e0005. doi: 10.5041/RMMJ.10425, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Steele MK, Couture A, Reed C, Iuliano D, Whitaker M, Fast H, et al. Estimated number of COVID-19 infections, hospitalizations, and deaths prevented among vaccinated persons in the US, December 2020 to September 2021. JAMA Netw Open. (2022) 5:e2220385. doi: 10.1001/jamanetworkopen.2022.20385, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.WHO . Eight steps to help with safe home-based recovery for COVID-19. (2022). Available at: https://www.who.int/westernpacific/news-room/feature-stories/item/eight-steps-to-help-with-safe-home-based-recovery-for-covid-19
- 101.Smith DJ, Hakim AJ, Leung GM, Xu W, Schluter WW, Novak RT, et al. COVID-19 mortality and vaccine coverage - Hong Kong special administrative region, China, January 6, 2022-march 21, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:545–8. doi: 10.15585/mmwr.mm7115e1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wichaidit M, Nopsopon T, Sunan K, Phutrakool P, Ruchikachorn P, Wanvarie D, et al. Breakthrough infections, hospital admissions, and mortality after major COVID-19 vaccination profiles: a prospective cohort study. Lancet Reg Health Southeast Asia. (2023) 8:100106. doi: 10.1016/j.lansea.2022.100106, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.WHO . WHO SAGE roadmap on uses of COVID-19 vaccines in the context of OMICRON and substantial population immunity. Geneva: WHO; (2020). [Google Scholar]
- 104.Moore S, Hill EM, Dyson L, Tildesley MJ, Keeling MJ. Modelling optimal vaccination strategy for SARS-CoV-2 in the UK. PLoS Comput Biol. (2021) 17:e1008849. doi: 10.1371/journal.pcbi.1008849, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hogan AB, Winskill P, Watson OJ, Walker PGT, Whittaker C, Baguelin M, et al. Within-country age-based prioritisation, global allocation, and public health impact of a vaccine against SARS-CoV-2: a mathematical modelling analysis. Vaccine. (2021) 39:2995–3006. doi: 10.1016/j.vaccine.2021.04.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Innovation IoGH . COVID-19 global behaviours and attitudes. Spokane, WA: Innovation IoGH; (2022). [Google Scholar]
- 107.Chowdhury R, Heng K, Shawon MSR, Goh G, Okonofua D, Ochoa-Rosales C, et al. Global dynamic interventions strategies for COVID-19 collaborative group. Dynamic interventions to control COVID-19 pandemic: a multivariate prediction modelling study comparing 16 worldwide countries. Eur J Epidemiol. (2020) 35:389–99. doi: 10.1007/s10654-020-00649-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.CoVarients . Overview of variants in countries. Available at: https://covariants.org/per-country
- 109.Liu Y, Rocklöv J. The reproductive number of the Delta variant of SARS-CoV-2 is far higher compared to the ancestral SARS-CoV-2 virus. J Travel Med. (2021) 28:taab124. doi: 10.1093/jtm/taab124, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liu Y, Rocklöv J. The effective reproductive number of the Omicron variant of SARS-CoV-2 is several times relative to Delta. J Travel Med. (2022) 29. doi: 10.1093/jtm/taac037, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Chatterjee S, Bhattacharya M, Nag S, Dhama K, Chakraborty C. A detailed overview of SARS-CoV-2 Omicron: its sub-variants, mutations and pathophysiology, clinical characteristics, immunological landscape, immune escape, and therapies. Viruses. (2023) 15:167. doi: 10.3390/v15010167, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li T, Xiao Y. Optimal strategies for coordinating infection control and socio-economic activities. Math Comput Simul. (2023) 207:533–55. doi: 10.1016/j.matcom.2023.01.017, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bobrovitz N, Ware H, Ma X, Li Z, Hosseini R, Cao C, et al. Protective effectiveness of previous SARS-CoV-2 infection and hybrid immunity against the Omicron variant and severe disease: a systematic review and meta-regression. Lancet Infect Dis. (2023) 23:556–67. doi: 10.1016/S1473-3099(22)00801-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pan J, Zhu W, Tian J, Liu Z, Xu A, Yao Y, et al. Vaccination as an alternative to non-drug interventions to prevent local resurgence of COVID-19. Infect Dis Poverty. (2022) 11:36. doi: 10.1186/s40249-022-00960-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Y, Quigley A, Wang Q, MacIntyre CR. Non-pharmaceutical interventions during the roll out of covid-19 vaccines. BMJ. (2021) 375:n2314. doi: 10.1136/bmj.n2314, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Andrews N, Tessier E, Stowe J, Gower C, Kirsebom F, Simmons R, et al. Duration of protection against mild and severe disease by Covid-19 vaccines. N Engl J Med. (2022) 386:340–50. doi: 10.1056/NEJMoa2115481, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chemaitelly H, Ayoub HH, Tang P, Coyle P, Yassine HM, Al Thani AA, et al. Long-term COVID-19 booster effectiveness by infection history and clinical vulnerability and immune imprinting: a retrospective population-based cohort study. Lancet Infect Dis. (2023). doi: 10.1016/S1473-3099(23)00058-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chen B, Zhao Y, Jin Z, He D, Li H. Twice evasions of omicron variants explain the temporal patterns in six Asian and Oceanic countries. BMC Infect Dis. (2023) 23:25. doi: 10.1186/s12879-023-07984-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lin DY, Xu Y, Gu Y, Zeng D, Wheeler B, Young H, et al. Effectiveness of bivalent boosters against severe Omicron infection. N Engl J Med. (2023) 388:764–6. doi: 10.1056/NEJMc2215471, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Surie D, DeCuir J, Zhu Y, Gaglani M, Ginde AA, Douin DJ, et al. Early estimates of bivalent mRNA vaccine effectiveness in preventing COVID-19–associated hospitalization among immunocompetent adults aged ≥65 years — IVY network, 18 states, September 8–November 30, 2022. MMWR Morb Mortal Wkly Rep. (2022) 71:1625–30. doi: 10.15585/mmwr.mm715152e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Colosi E, Bassignana G, Contreras DA, Poirier C, Boelle PY, Cauchemez S, et al. Screening and vaccination against COVID-19 to minimise school closure: a modelling study. Lancet Infect Dis. (2022) 22:977–89. doi: 10.1016/S1473-3099(22)00138-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Muller GC, Ferreira LS, Mesias Campos FE, Borges ME, Berg de Almeida G, Poloni S, et al. Modeling the impact of child vaccination (5-11 y) on overall COVID-19 related hospitalizations and mortality in a context of Omicron variant predominance and different vaccination coverage paces in Brazil. Lancet Reg Health Am. (2023) 17:100396. doi: 10.1016/j.lana.2022.100396, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.WHO . SAGE updates COVID-19 vaccination guidance. Geneva: WHO; (2023). [Google Scholar]
- 124.Kitano T, Kitano M, Krueger C, Jamal H, Al Rawahi H, Lee-Krueger R, et al. The differential impact of pediatric COVID-19 between high-income countries and low- and middle-income countries: a systematic review of fatality and ICU admission in children worldwide. PLoS One. (2021) 16:e0246326. doi: 10.1371/journal.pone.0246326, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Link-Gelles R, Ciesla AA, Roper LE, Scobie HM, Ali AR, Miller JD, et al. Early estimates of bivalent mRNA booster dose vaccine effectiveness in preventing symptomatic SARS-CoV-2 infection attributable to Omicron BA.5- and XBB/XBB.1.5-related sublineages among immunocompetent adults - increasing community access to testing program, United States, December 2022-January 2023. MMWR Morb Mortal Wkly Rep. (2023) 72:119–24. doi: 10.15585/mmwr.mm7205e1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Andreas M, Iannizzi C, Bohndorf E, Monsef I, Piechotta V, Meerpohl JJ, et al. Interventions to increase COVID-19 vaccine uptake: a scoping review. Cochrane Database Syst Rev. (2022) 8:CD015270. doi: 10.1002/14651858.CD015270, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zimmerman T, Shiroma K, Fleischmann KR, Xie B, Jia C, Verma N, et al. Misinformation and COVID-19 vaccine hesitancy. Vaccine. (2023) 41:136–44. doi: 10.1016/j.vaccine.2022.11.014, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mirtaleb MS, Falak R, Heshmatnia J, Bakhshandeh B, Taheri RA, Soleimanjahi H, et al. An insight overview on COVID-19 mRNA vaccines: advantageous, pharmacology, mechanism of action, and prospective considerations. Int Immunopharmacol. (2023) 117:109934. doi: 10.1016/j.intimp.2023.109934, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Khoshnood S, Ghanavati R, Shirani M, Ghahramanpour H, Sholeh M, Shariati A, et al. Viral vector and nucleic acid vaccines against COVID-19: a narrative review. Front Microbiol. (2022) 13:984536. doi: 10.3389/fmicb.2022.984536, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Holmdahl I, Buckee C. Wrong but useful – what Covid-19 epidemiologic models can and cannot tell us. N Engl J Med. (2020) 383:303–5. doi: 10.1056/NEJMp2016822, PMID: [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.