Abstract
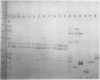
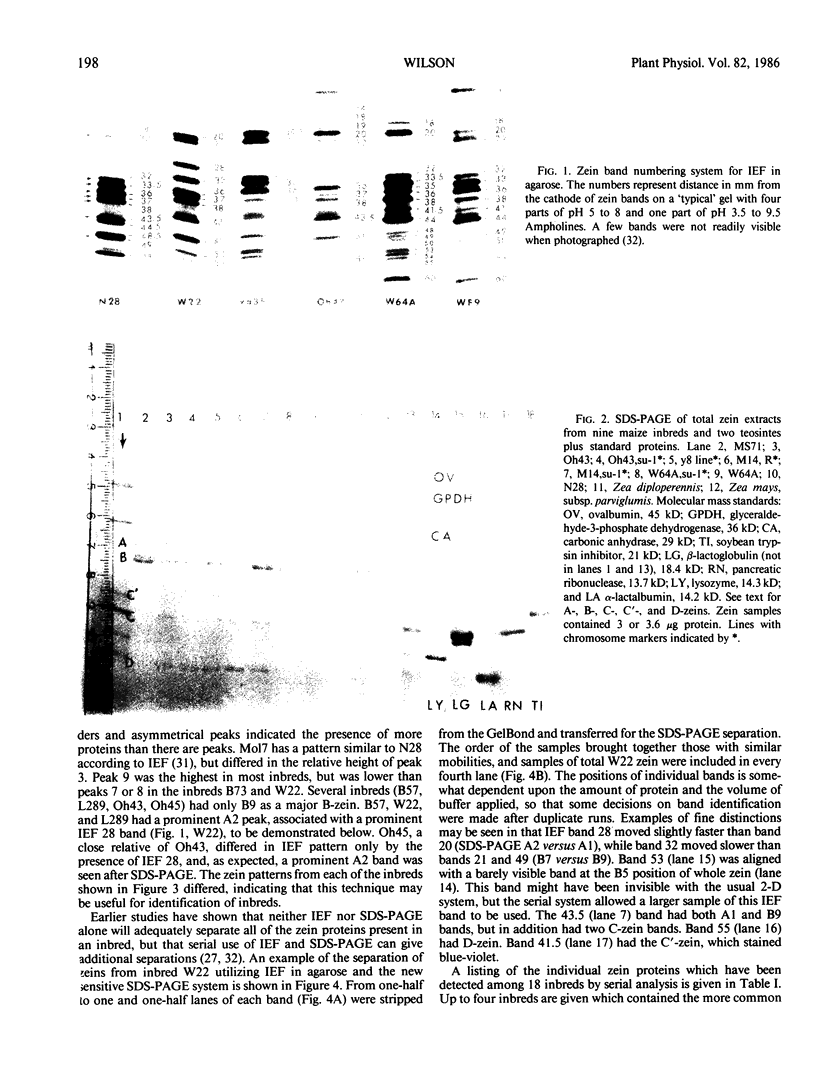
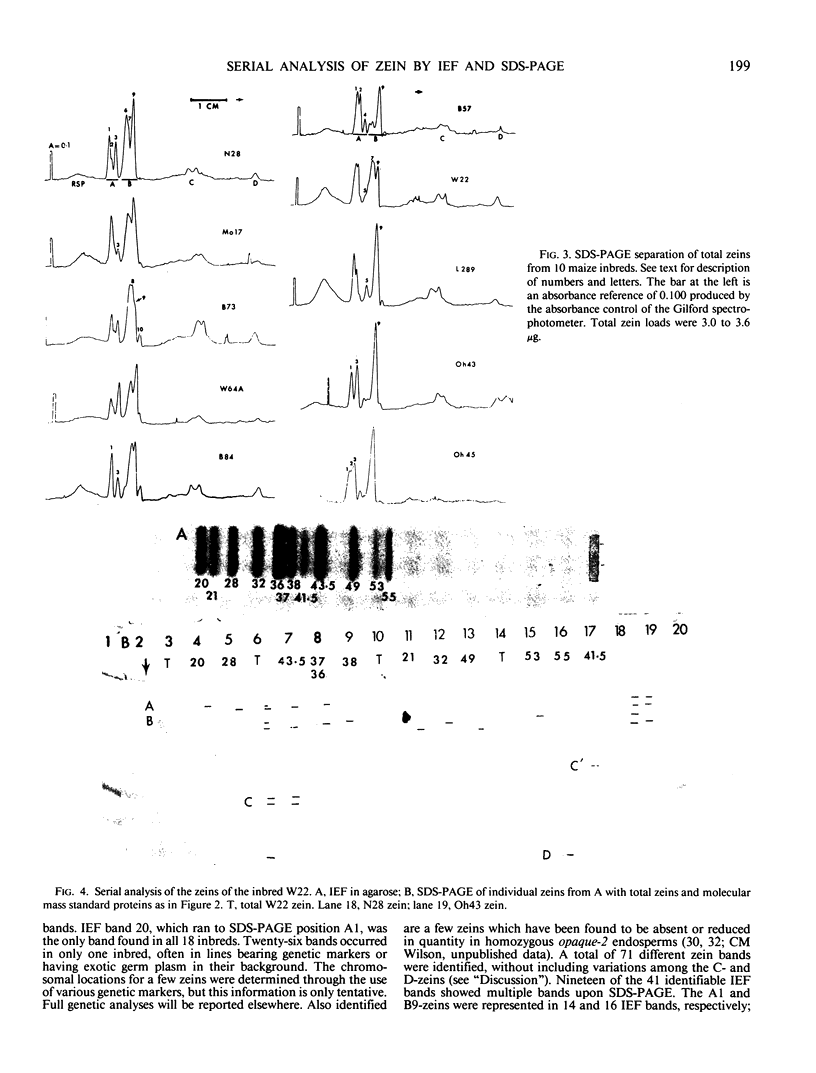
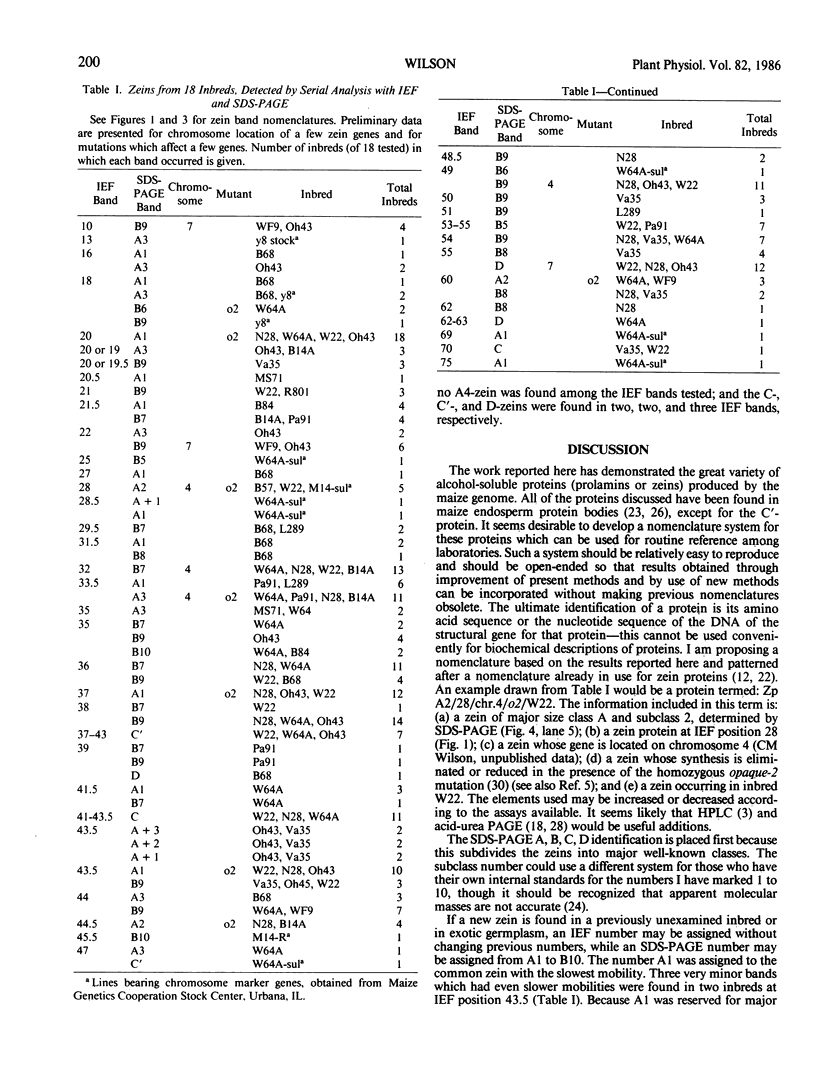
Zein, the major storage protein of maize (Zea mays L.) endosperm, was extracted from a number of inbreds with alcohol plus a reducing agent. Isoelectric focusing (IEF) separated total zeins into 41 components, while sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) separated total zeins into about 15 components. Each procedure gave characteristic patterns of zein bands for a number of maize inbreds. IEF and SDS-PAGE were used serially so that each band separated by IEF could be assayed as an individual SDS-PAGE sample. Some IEF bands revealed only a single band after SDS-PAGE, while others revealed two or more bands. A nomenclature system is presented which integrates the two separation systems with information about chromosome locations of zein genes, maize mutations which affect zein synthesis, and inbred sources for different zeins. SDS-PAGE of zein gives apparent molecular masses which vary widely according to the standards used and the properties of the gels, therefore an artificial nomenclature for identifying zein bands after SDS-PAGE is presented. The new nomenclature provides a flexible system which is useful and can be conveniently used in different laboratories.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L., Anderson N. Some perspectives on two-dimensional protein mapping. Clin Chem. 1984 Dec;30(12 Pt 1):1898–1905. [PubMed] [Google Scholar]
- Burr F. A., Burr B. Three mutations in Zea mays affecting zein accumulation: a comparison of zein polypeptides, in vitro synthesis and processing, mRNA levels, and genomic organization. J Cell Biol. 1982 Jul;94(1):201–206. doi: 10.1083/jcb.94.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landry J., Moureaux T. Distribution and amino acid composition of protein groups located in different histological parts of maize grain. J Agric Food Chem. 1980 Nov-Dec;28(6):1186–1191. doi: 10.1021/jf60232a042. [DOI] [PubMed] [Google Scholar]
- Melcher U., Fraij B. Methionine-rich protein fraction by cryoprecipitation from extracts of corn meal. J Agric Food Chem. 1980 Nov-Dec;28(6):1334–1336. doi: 10.1021/jf60232a038. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr, Glossmann H. Molecular weight determination of membrane protein and glycoprotein subunits by discontinuous gel electrophoresis in dodecyl sulfate. Methods Enzymol. 1974;32:92–102. doi: 10.1016/0076-6879(74)32012-5. [DOI] [PubMed] [Google Scholar]
- Read S. M., Northcote D. H. Minimization of variation in the response to different proteins of the Coomassie blue G dye-binding assay for protein. Anal Biochem. 1981 Sep 1;116(1):53–64. doi: 10.1016/0003-2697(81)90321-3. [DOI] [PubMed] [Google Scholar]
- Viotti A., Cairo G., Vitale A., Sala E. Each zein gene class can produce polypeptides of different sizes. EMBO J. 1985 May;4(5):1103–1110. doi: 10.1002/j.1460-2075.1985.tb03746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viotti A., Sala E., Marotta R., Alberi P., Balducci C., Soave C. Genes and mRNAs coding for zein polypeptides in Zea mays. Eur J Biochem. 1979 Dec;102(1):211–222. doi: 10.1111/j.1432-1033.1979.tb06282.x. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Mapping of zein polypeptides after isoelectric focusing on agarose gels. Biochem Genet. 1985 Feb;23(1-2):115–124. doi: 10.1007/BF00499117. [DOI] [PubMed] [Google Scholar]
- Wilson C. M. Staining of proteins on gels: comparisons of dyes and procedures. Methods Enzymol. 1983;91:236–247. doi: 10.1016/s0076-6879(83)91020-0. [DOI] [PubMed] [Google Scholar]
- de Jong W. W., Zweers A., Cohen L. H. Influence of single amino acid substitutions on electrophoretic mobility of sodium dodecyl sulfate-protein complexes. Biochem Biophys Res Commun. 1978 May 30;82(2):532–539. doi: 10.1016/0006-291x(78)90907-5. [DOI] [PubMed] [Google Scholar]