Abstract
目的
探讨HER-2相关基因(the human epidermal growth factor receptor-2-related genes, HRGs)与膀胱癌生存预后的相关性,并基于HRGs构建一种膀胱癌患者生存预后的预测模型。
方法
从癌症基因组图谱(the cancer genome atlas, TCGA)中下载膀胱肿瘤组织mRNA测序数据和临床数据,通过与分子签名数据库(the molecular signatures database, MSigDB)中HER-2相关的基因联合分析鉴定膀胱癌中的HRGs。利用单因素和多因素Cox回归分析进一步明确与膀胱癌生存相关的HRGs(P < 0.05),并构建HRGs风险模型(HRGs risk score model, HRSM),根据风险评分取中位数将膀胱癌患者分成高风险组和低风险组。利用R语言对高风险和低风险组的患者进行生存分析,并对HRGs与临床特征的相关性进行分析。利用多因素Cox回归分析,验证影响膀胱癌患者预后的独立因素。计算HRSM的受试者工作特征曲线(receiver operating characteristic curve, ROC)下的面积(area under the curve, AUC),并构建诺模图(nomogram)对膀胱癌患者进行生存预测。利用TIMER数据库对HRSM和患者免疫细胞浸润相关性进行分析。
结果
共鉴定到13个与患者生存相关的HRGs。通过多因素Cox回归分析,筛选出5个基因(BTC、CDC37、EGF、PTPRR和EREG)构建HRSM,高风险组的膀胱癌患者5年生存率明显低于低风险组患者。通过临床相关性分析发现,PTPRR的高表达与肿瘤分级、分期呈显著负相关,而EREG的高表达与肿瘤分级、分期呈正相关;EGF表达量的增加和患者的高级别有相关性,而CDC37的高表达却呈现出了相反的结果;BTC的表达与临床特征无显著相关性。通过对HRSM与免疫细胞的相关性分析发现,风险评分与树突状细胞、CD8+T细胞、CD4+T细胞、中性粒细胞和巨噬细胞的浸润呈正相关。
结论
HRGs对膀胱癌患者的预后有重要作用,可能作为新的预测性生物标志物和治疗的潜在靶点。
Keywords: 膀胱癌, HER-2相关基因, 风险模型, 预后, 免疫细胞浸润
Abstract
Objective
To investigate the correlation between the human epidermal growth factor receptor-2-related genes (HRGs) and survival prognosis of bladder cancer and to construct a predictive model for survival prognosis of bladder cancer patients based on HRGs.
Methods
HRGs in bladder cancer were found by downloading bladder tumor tissue mRNA sequencing data and clinical data from the cancer genome atlas (TCGA), downloading HER-2 related genes from the molecular signatures database (MsigDB), and crossing the two databases. Further identifying HRGs associated with bladder cancer survival (P < 0.05) by using single and multi-factor Cox regression analysis and constructing HRGs risk score model (HRSM), the bladder cancer patients were categorized into high-risk and low-risk groups accor-ding to the median risk score. Survival analysis of the patients in high- and low-risk groups was conducted using R language and correlation of HRGs with clinical characteristics. A multi-factor Cox regression analysis was used to verify the independent factors affecting the prognosis of the patients with bladder cancer. The area under the curve (AUC) of the receiver operating characteristic curve (ROC) of HRSM was calculated, and a nomogram was constructed for survival prediction of the bladder cancer patients. Analysis of HRSM and patient immune cell infiltration correlation was made using the TIMER database.
Results
A total of 13 HRGs associated with patient survival were identified in this study. Five genes (BTC, CDC37, EGF, PTPRR and EREG) were selected for HRSM by multi-factor Cox regression analysis. The 5-year survival rate of the bladder cancer patients in the high-risk group was significantly lower than that of the patients in the low-risk group. High expression of PTPRR was found to be significantly and negatively correlated with tumor grade and stage by clinical correlation analysis, while EREG was found to be the opposite; Increased expression of EGF was associated with high grade, however, the high expression ofCDC37showed the opposite result. And no significant correlation was found between BTC expression and clinical features. Correlation analysis of HRSM with immune cells revealed a positive correlation between risk score and infiltration of dendritic cells, CD8+T cells, CD4+T cells, neutrophils and macrophages.
Conclusion
HRGs have an important role in the prognosis of bladder cancer patients and may serve as new predictive biomarkers and potential targets for treatment.
Keywords: Bladder cancer, HER-2 related genes, Risk model, Prognosis, Immune cell infiltration
膀胱癌是全世界范围内最常见的十大癌症之一, 死亡率排名第13位[1]。尿路上皮癌(urothelial carcinoma, UC) 是迄今为止最常见的膀胱癌组织学类型,占膀胱癌的90% 以上。根据肿瘤侵犯的程度,膀胱癌可分为非肌层浸润性膀胱癌(non-muscle-invasive bladder cancer, NMIBC)和肌层浸润性膀胱癌(muscle-invasive bladder cancer, MIBC),MIBC占膀胱癌的30%左右[2]。尽管有可用的治疗方法,MIBC的复发率、进展率和死亡率仍然很高,严重影响了我们的生活[3]。MIBC患者行根治性膀胱切除术后,约半数最终会因转移或局部复发而死亡[4]。当MIBC发生转移后,尽管进行积极治疗,5年生存率也不到50%[5],因此,提高患者的总体生存率和降低复发率仍然是临床面临的主要挑战。随着分子水平研究的深入和基因检测技术的进步,确定新的有效的治疗靶点、构建预后模型判断膀胱癌患者的预后、进一步了解膀胱癌的发病机制、制定更有效的综合治疗策略是当前研究的重要方向。
人类上皮生长因子受体2(the human epidermal growth factor receptor-2, HER-2)被确定为转移性UC靶向治疗的潜在候选基因。HER-2是一种由ERBB2原癌基因编码的蛋白质,位于17号染色体的长臂上(17q12),属于上皮生长因子受体家族,其家族包括HER-1(EGFR、ERBB1)、HER-2(ERBB2)、HER-3(ERBB3)和HER-4(ERBB4)。这些蛋白是酪氨酸激酶型受体,通过激活下游信号通路参与细胞增殖、血管形成等,如丝裂原活化蛋白激酶通路(the mitogen-activated protein kinase, MAPK)和磷脂酰肌醇3激酶通路(the phosphatidylinositol 3-kinase, PI3K/Akt)。与其他受体不同的是,HER-2的胞外区缺乏配体结合活性,其信号功能是通过与其他受体结合形成异二聚体实现[6]。HER-2基因的过度表达在乳腺癌和胃癌中是很好的特征,它展示出负面的预后效应,可能促使癌细胞获得更强的致癌力和侵袭性[7],基因扩增是HER-2在这些肿瘤中过表达的主要机制[8-9]。尿路上皮癌是HER-2过度表达比例第三高的癌症,在6%~17%的样本中有HER-2基因的突变或扩增,有时共存[10]。有研究证实HER-2的过度表达与膀胱UC的恶性程度有关,可作为预测复发的独立预后因素[11]。另外,随着免疫治疗时代的到来,一些研究表明HER-2阳性的肿瘤浸润淋巴细胞水平通常高于HER-2阴性的肿瘤,这意味着HER-2阳性疾病可能更具免疫原性[12-13],免疫治疗可能是一种不错的选择。但近年来,一些HER-2的靶向药物(如曲妥珠单抗、拉帕替尼等)治疗UC的研究结果并不理想[14-15],耐药可能是其中原因之一。目前,针对HER-2的抗体偶联药物(antibody drug conjugate, ADC)治疗在晚期膀胱UC患者中显示出较好的疗效和安全性,但是仍有不少患者无法从中受益[16-17],因此,寻找新的预后标志物和治疗靶点是晚期膀胱UC治疗的重要研究方向。
本研究中,我们筛选了与膀胱癌生存预后相关的HER-2相关基因(HER-2 related genes, HRGs),并构建HRGs风险模型(HRGs risk score model, HRSM),分析其对膀胱癌患者预后的预测价值。目前已有基于铁死亡相关基因、凋亡相关基因和膜蛋白等构建的膀胱癌预后模型,相较于这些研究者提出的模型,我们的模型有着更加精准的预测预后的能力。基于HRGs建立的模型,可以更加准确地指导临床上ADC等药物的使用,从而减少患者的医疗负担,改善患者预后。
1. 资料与方法
1.1. 数据下载和数据处理
我们从癌症基因组图谱(the cancer genome atlas, TCGA;https://portal.gdc.cancer.gov/)中下载并整理了414个膀胱癌样本的mRNA数据和临床信息,中位随访时间为17.6个月,排除生存时间小于30 d的患者,因为这些患者可能会死于出血和感染性疾病。从分子签名数据库(the molecular signatures database, MSigDB)中提取HER-2相关的基因,这些数据更新于2022年12月12日。用Perl软件(http://www.perl.org/)将mRNA序列和临床数据整理合并到矩阵文件中,利用Ensembl数据库(http://asia.ensembl.org/index.html)将基因的Ensembl ID转换成基因官方名称。
1.2. 生存相关的HRGs提取及HRSM构建
利用单因素Cox回归分析寻找生存相关的HRGs(P < 0.05),随后利用多因素Cox回归分析进行HRSM的构建,并根据HRGs表达量将患者分成高风险组和低风险组(P < 0.05)。通过R语言survival包对高风险和低风险组患者的总生存期(overall survival, OS)进行分析。
1.3. HRSM与临床相关性分析
为进一步探究HRSM和临床指标的相关性,我们采用多因素Cox回归分析,验证影响膀胱癌患者预后的独立因素。利用R语言ggpubr包对HRGs和临床分期进行相关性分析。利用survival受试者工作特征曲线(receiver operating characteristic curve, ROC)包计算HRSM的曲线下面积(area under the curve, AUC)。利用R语言rms包构建诺模图(nomogram)去预测膀胱癌患者的生存率并绘制列线图的校准曲线。利用TIMER数据库对HRSM和患者免疫细胞浸润相关性进行分析。
1.4. 统计学分析
所有数据均通过R软件(4.0.2)进行,数据分析使用到了R语言的limma包、survival包、survivalROC包、ggplot2包、ggpubr包和rms包。两组差异比较采用t检验,两组以上组间差异比较采用单因素方差分析处理, P < 0.05为差异具有统计学意义。
2. 结果
2.1. HRGs基因的获取
从TCGA数据库下载并整理了膀胱癌患者的转录组RNA测序数据和临床数据(表 1),在MSigDB数据库中下载了HER-2相关的基因,随后通过两个数据库取交集找到了膀胱癌中的HRGs。
表 1.
TCGA数据库中膀胱癌患者的一般临床特征
General clinical characteristics of patients with bladder cancer in the TCGA database
| Characteristics | Value, n(%) |
| Some patients were not counted in the table due to missing data. TCGA, the cancer genome atlas. | |
| Age/year | |
| ≤65 | 141 (37.7) |
| >65 | 233 (62.3) |
| Gender | |
| Female | 96 (25.7) |
| Male | 278 (74.3) |
| Grade | |
| High | 354 (94.7) |
| Low | 20 (5.3) |
| Clinical stage | |
| Ⅰ | 2 (0.5) |
| Ⅱ | 105 (28.1) |
| Ⅲ | 138 (36.9) |
| Ⅳ | 129 (34.5) |
| T stage | |
| T0 | 1 (0.3) |
| T1 | 3 (0.8) |
| T2 | 118 (31.6) |
| T3 | 194 (51.9) |
| T4 | 58 (15.5) |
| N stage | |
| N0 | 222 (59.4) |
| N1-3 | 126 (33.7) |
| Nx | 26 (7.0) |
| M stage | |
| M0 | 178 (47.6) |
| M1 | 8 (2.1) |
| Mx | 188 (50.3) |
2.2. 生存相关的HRGs提取和HRSM构建
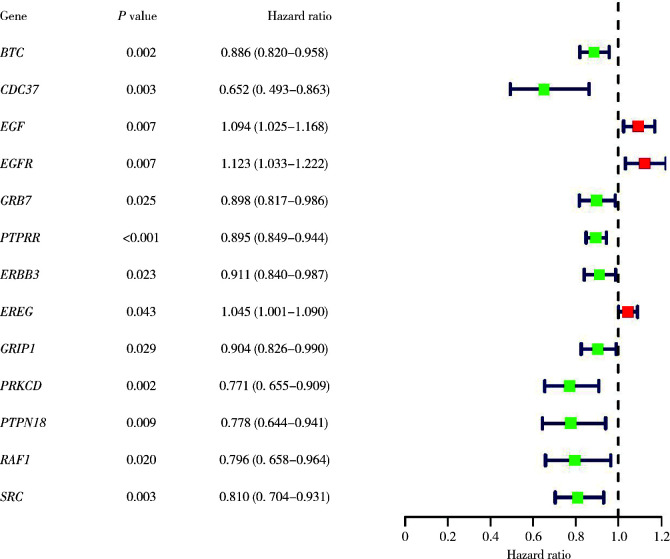
利用单因素Cox回归分析对HRGs和膀胱癌患者的预后情况进行分析,共找到了13个与患者生存相关的HRGs(图 1)。随后,利用多因素Cox回归分析对生存相关的HRGs进行筛选并构建HRSM(P < 0.05),公式如下:BTC的表达量×(-0.11)+CDC37的表达量×(-0.43)+EGF的表达量×0.11+PTPRR的表达量×(-0.07)+EREG的表达量×0.05。根据风险评分取中位数,将414例患者分为了高风险组和低风险组(图 2A),发现随着风险评分的增长,患者的死亡率有着显著的上升,并且EGF和ERGE的表达量也会上调(图 2B、C)。生存曲线结果也提示高风险组的膀胱癌患者有着更差的5年生存率(图 2D)。
图 1.
13个与患者生存相关的HRGs的单因素Cox回归分析
Single-factor Cox regression analysis of 13 HRGs associated with patient survival
图 2.

构建HRSM进行生存分析
Construction of HRSM for survival analysis
2.3. HRSM的临床相关性
为了证明HRSM可以作为独立预后因素对膀胱癌患者的预后进行评估,我们进行了多因素Cox回归分析,发现只有性别和风险评分可以作为膀胱癌患者的独立预后指标(图 3)。随后对HRSM、膀胱癌分级和临床分期进行分析,发现PTPRR的高表达与肿瘤分级、分期呈显著负相关,而EREG的高表达与肿瘤分级、分期呈正相关;EGF的表达量增加和患者的高级别有着相关性,而CDC37的高表达却呈现出了相反的结果;BTC的表达与临床特征无显著相关性(图 4)。我们利用ROC曲线对HRSM的准确性进行验证,发现HRSM、年龄、性别、分级、分期、T分期、M分期和N分期的AUC值分别是0.765、0.571、0.442、0.445、0.669、0.644、0.521和0.646(图 5),此结果说明HRSM相较于其他临床指标能更准确地预测膀胱癌患者的预后。
图 3.
风险评分的多因素Cox回归分析
Multi-factor Cox regression analysis of risk scores
图 4.
HRGs与临床的相关性
Correlation between HRGs and clinical
图 5.
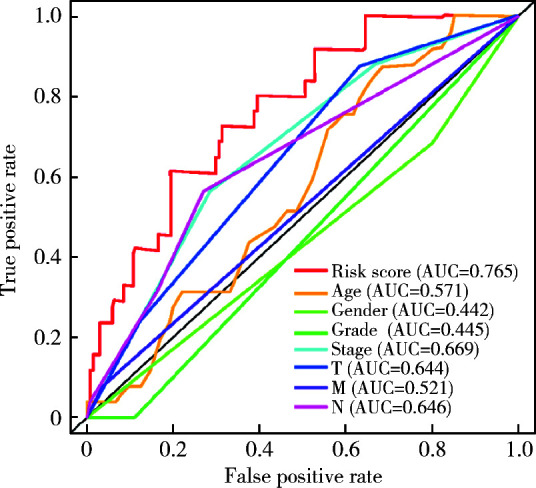
HRSM和一般临床特征的ROC曲线
ROC curves for HRSM and general clinical features
2.4. HRSM在临床中的潜在应用
我们构建了诺模图,该图可根据患者HRGs表达量对其1、3和5年的生存进行预测(图 6)。同时也绘制了校准曲线评估列线图模型的性能,发现该诺模图具有较好的预测能力(图 7)。随着膀胱癌免疫治疗的普及,ADC+免疫检查点抑制剂的联合治疗在局部晚期或转移性尿路上皮癌中的使用越来越多,免疫细胞的浸润情况也是临床治疗要考虑的一个问题,所以对HRSM与免疫细胞的相关性进行了分析,发现HRSM与树突状细胞、CD8+T细胞、CD4+T细胞、中性粒细胞和巨噬细胞都有着一定的相关性(图 8)。
图 6.
基于HRGs构建膀胱癌患者的预后诺模图
Construction of a prognostic nomogram for bladder cancer patients based on HRGs
图 7.

预后诺模图的校准曲线
Calibration curves for prognostic nomograms
图 8.
风险评分与免疫细胞浸润的关系图
Graph of the relationship between risk score and immune cell infiltration
3. 讨论
膀胱癌的高发生率、高复发率和高病死率严重破坏了人们的健康生活,寻找有效的预测预后的标志物和治疗靶点是当前研究的关键。在我们的研究中,共筛选了5个HRGs(BTC、CDC37、EGF、PTPRR和EREG), 并构建了HRSM。通过风险评分将患者分为了低风险组和高风险组,并对其进行预后评估、生存分析及与免疫细胞相关性的分析。
BTC蛋白是表皮生长因子受体(epidermal growth factor receptor, EGFR)的配体,除了EGFR,BTC蛋白还可以结合和激活ERBB4同源二聚体和所有可能的ERBB异源二聚体组合,包括高度致癌的ERBB2/3二聚体[18-19]。受体激活后,BTC蛋白可以激活大量的细胞内信号通路,包括p38MAPK、PI3K/AKT和c-Jun N-末端激酶(c-Jun N-terminal kinase, JNK),从而参与各种细胞反应,包括细胞增殖、血管生成和抗凋亡[20],这与多种癌症的发病机制有关。
根据以往的研究,已经在几种类型的恶性肿瘤中检测到BTC的过度表达[21-22],并且BTC的上调被证明与乳腺癌的不良预后有关[23]。在我们的研究中,BTC在低风险组的表达高于高风险组,可能与膀胱癌患者更好的预后有关。CDC37基因是在对酿酒酵母细胞分裂周期基因的筛选中发现的,但它似乎主要作为一种辅助伴侣蛋白,通过增强蛋白激酶的稳定性和活性来发挥功能[24]。热休克蛋白90(heat shock proteins 90, HSP90)是一种分子伴侣,它与一系列辅助伴侣一起发挥作用,引导一系列信号蛋白的稳定和激活,包括致癌蛋白、转录因子和激素受体。CDC37蛋白被认为是这种多聚体伴侣机制的关键组成部分,在与信号转导、增殖和生存相关的一大类蛋白激酶的成熟和稳定过程中发挥着不可或缺的作用[14, 25]。蛋白激酶在癌症中的频繁突变和过度表达是肿瘤发生的关键,研究表明CDC37在间变性大细胞淋巴瘤、急性髓母细胞白血病、多发性骨髓瘤和肝细胞癌等癌症中有高表达[26],而HER-2是目前已知的CDC37调节的激酶中的一种[27],因此,在这些蛋白激酶参与的肿瘤中存在大量潜在的HSP90/CDC37靶点,比如已有研究发现抑制剂FW-04-806通过抑制HSP90/CDC37相互作用,导致HSP90/CDC37/HER-2复合体解离,从而使HER-2降解,尤其对HER-2过表达的乳腺癌细胞具有良好的抗肿瘤活性[28]。
本研究发现,CDC37表达量的增加和膀胱癌的更低级别有相关性,且在低风险组的表达明显高于高风险组,所以该基因的表达增加也可能预示膀胱癌患者更好的生存结局。EGF最早是半个多世纪前在小鼠体内发现的,并确定了其一级结构[29]。EGF蛋白被认为是第一组表皮生长因子家族的原型,该家族还包括TGF-α蛋白、HB-EGF蛋白、双调蛋白、BTC蛋白、EREG蛋白和表观基因蛋白[30]。在功能上,这些生长因子与EGFR结合,激活其固有的酪氨酸激酶活性,并耦合控制细胞增殖、分化、生存或运动的下游信号通路。在一项研究中,用EGF处理的各种癌细胞显示出EGFR过度表达、脱磷和黏着斑激酶表达不足,同时也有癌细胞侵袭、迁移和转移能力的增加。同样,当用EGF处理乳腺癌细胞时,发现细胞的迁移和耐药性增加[31],这说明EGF的表达可能与肿瘤的进展有关。我们的研究也发现,EGF基因的高表达和膀胱癌的更高级别有关,并且在高风险组高表达,在低风险组低表达,所以它可能在膀胱癌进展方面起到一定的作用。PTPRR基因位于12号染色体上,该基因编码的蛋白质是蛋白质酪氨酸磷酸酶家族的成员,通过使酪氨酸去磷酸化在细胞信号传递和细胞功能调节中发挥重要作用[32]。有研究在结直肠癌中报道了PTPRR,揭示了它的肿瘤抑制作用[33]。随后,该基因的肿瘤抑制作用在其他几种癌症中也逐渐被证实,如卵巢癌、宫颈癌和前列腺癌[34-36]。在本研究中,我们发现PTPRR的高表达和更高级别、更高临床分期和更高的T分期呈负相关,与先前研究证实的PTPRR的肿瘤抑制作用一致。EREG基因位于人类染色体4q13.3上,与EGF家族其他成员有24%~50%的序列相似性[37],所以EREG也调控细胞增殖、侵袭、转移、血管生成和抗凋亡等过程,在肿瘤的发生发展中起着重要的作用。根据多项研究表明,EREG在几种类型的肿瘤中均有过表达,而在对应的正常组织中表达水平极低[38-40]。
我们的研究也表明EREG在膀胱癌患者高风险组中的表达水平明显高于低风险组,与上述研究结果相似,这说明EREG可能起着促癌基因的作用,并且与膀胱癌患者的不良预后有关。
综上所述,在这项研究中,我们筛选出5个HRGs构建HRSM,并明确其与膀胱癌患者生存预后及临床病理特征的相关性。同时,我们发现HRSM与免疫细胞浸润有着显著的相关性,这为预测膀胱癌患者靶向HER-2治疗的预后提供了新的标志物,同时为进一步探索HER-2在膀胱癌发生发展中的作用机制及其与免疫抑制剂的相互作用提供了理论基础。
References
- 1.Aneoni S, Ferlay J, Soerjomatarm I, et al. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71(1):96–108. doi: 10.1016/j.eururo.2016.06.010. [Aneoni S, Ferlay J, Soerjomatarm I, et al. Bladder cancer incidence and mortality: A global overview and recent trends [J]. Eur Urol, 2017, 71(1): 96-108.] [DOI] [PubMed] [Google Scholar]
- 2.Nielsen ME, Smith AB, Merer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the Uni-ted States: 1988 to 2006. Cancer. 2014;120(1):86–95. doi: 10.1002/cncr.28397. [Nielsen ME, Smith AB, Merer AM, et al. Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the Uni-ted States: 1988 to 2006 [J]. Cancer, 2014, 120(1): 86-95.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer. Cancer Immunol Immunother. 2020;69(1):3–14. doi: 10.1007/s00262-019-02443-4. [Crispen PL, Kusmartsev S. Mechanisms of immune evasion in bladder cancer [J]. Cancer Immunol Immunother, 2020, 69(1): 3-14.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goossens-Laan CA, Leliveld AM, Verhoeven RH, et al. Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer. Int J Cancer. 2014;135(4):905–912. doi: 10.1002/ijc.28716. [Goossens-Laan CA, Leliveld AM, Verhoeven RH, et al. Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer [J]. Int J Cancer, 2014, 135(4): 905-912.] [DOI] [PubMed] [Google Scholar]
- 5.Redondo-Gonzalez E, de Castro LN, Moreno-Sierra J, et al. Bladder carcinoma data with clinical risk factors and molecular mar-kers: A cluster analysis. Biomed Res Int. 2015;2015:168682. doi: 10.1155/2015/168682. [Redondo-Gonzalez E, de Castro LN, Moreno-Sierra J, et al. Bladder carcinoma data with clinical risk factors and molecular mar-kers: A cluster analysis [J]. Biomed Res Int, 2015, 2015: 168682.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moasser MM. The oncogene HER2 : Its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469–6487. doi: 10.1038/sj.onc.1210477. [Moasser MM. The oncogene HER2 : Its signaling and transforming functions and its role in human cancer pathogenesis [J]. Oncogene, 2007, 26(45): 6469-6487.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome. J Clin Oncol. 2011;29(22):3030–3036. doi: 10.1200/JCO.2010.33.6313. [Begnami MD, Fukuda E, Fregnani JH, et al. Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome [J]. J Clin Oncol, 2011, 29(22): 3030-3036.] [DOI] [PubMed] [Google Scholar]
- 8.Bang YJ, van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. doi: 10.1016/S0140-6736(10)61121-X. [Bang YJ, van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial [J]. Lancet, 2010, 376(9742): 687-697.] [DOI] [PubMed] [Google Scholar]
- 9.Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Patho-logists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Patho-logists clinical practice guideline update [J]. J Clin Oncol, 2013, 31(31): 3997-4013.] [DOI] [PubMed] [Google Scholar]
- 10.Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer. J Clin Oncol. 2013;31(25):3133–3140. doi: 10.1200/JCO.2012.46.5740. [Iyer G, Al-Ahmadie H, Schultz N, et al. Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer [J]. J Clin Oncol, 2013, 31(25): 3133-3140.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai X, He W, Yin H, et al. Prognostic significance of HER2 status evaluation using immunohistochemistry in patients with urothelial carcinoma of the bladder: A retrospective single-center experience. Exp Ther Med. 2022;24(5):704. doi: 10.3892/etm.2022.11640. [Bai X, He W, Yin H, et al. Prognostic significance of HER2 status evaluation using immunohistochemistry in patients with urothelial carcinoma of the bladder: A retrospective single-center experience [J]. Exp Ther Med, 2022, 24(5): 704.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase Ⅲ randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J Clin Oncol. 2013;31(7):860–867. doi: 10.1200/JCO.2011.41.0902. [Loi S, Sirtaine N, Piette F, et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase Ⅲ randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98 [J]. J Clin Oncol, 2013, 31(7): 860-867.] [DOI] [PubMed] [Google Scholar]
- 13.Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review. JAMA Oncol. 2016;2(10):1354–1360. doi: 10.1001/jamaoncol.2016.1061. [Stanton SE, Adams S, Disis ML. Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review [J]. JAMA Oncol, 2016, 2(10): 1354-1360.] [DOI] [PubMed] [Google Scholar]
- 14.Jia Y, Kodumudi KN, Ramamoorthi G, et al. Th1 cytokine interferon gamma improves response in HER2 breast cancer by modulating the ubiquitin proteasomal pathway. Mol Ther. 2021;29(4):1541–1556. doi: 10.1016/j.ymthe.2020.12.037. [Jia Y, Kodumudi KN, Ramamoorthi G, et al. Th1 cytokine interferon gamma improves response in HER2 breast cancer by modulating the ubiquitin proteasomal pathway [J]. Mol Ther, 2021, 29(4): 1541-1556.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wülfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115(13):2881–2890. doi: 10.1002/cncr.24337. [Wülfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma [J]. Cancer, 2009, 115(13): 2881-2890.] [DOI] [PubMed] [Google Scholar]
- 16.Sheng X, Yan X, Wang L, et al. Open-label, multicenter, phase Ⅱ study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res. 2021;27(1):43–51. doi: 10.1158/1078-0432.CCR-20-2488. [Sheng X, Yan X, Wang L, et al. Open-label, multicenter, phase Ⅱ study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma [J]. Clin Cancer Res, 2021, 27(1): 43-51.] [DOI] [PubMed] [Google Scholar]
- 17.Sheng X, Zhou AP, Yao X, et al. A phase Ⅱ study of RC48-ADC in HER2-positive patients with locally advanced or metastatic urothelial carcinoma. J Clin Oncol. 2019;37(Suppl 15):4509. [Sheng X, Zhou AP, Yao X, et al. A phase Ⅱ study of RC48-ADC in HER2-positive patients with locally advanced or metastatic urothelial carcinoma [J]. J Clin Oncol, 2019, 37(Suppl 15): 4509.] [Google Scholar]
- 18.Rush JS, Peterson JL, Ceresa BP. Betacellulin (BTC) biases the EGFR to dimerize with ErbB3. Mol Pharmacol. 2018;94(6):1382–1390. doi: 10.1124/mol.118.113399. [Rush JS, Peterson JL, Ceresa BP. Betacellulin (BTC) biases the EGFR to dimerize with ErbB3 [J]. Mol Pharmacol, 2018, 94(6): 1382-1390.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shen T, Yang T, Yao M, et al. BTC as a novel biomarker contri-buting to EMT via the PI3K-AKT pathway in OSCC. Front Genet. 2022;13:875617. doi: 10.3389/fgene.2022.875617. [Shen T, Yang T, Yao M, et al. BTC as a novel biomarker contri-buting to EMT via the PI3K-AKT pathway in OSCC [J]. Front Genet, 2022, 13: 875617.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dahlhoff M, Wolf E, Schneider MR. The ABC of BTC: Structural properties and biological roles of betacellulin. Semin Cell Dev Biol. 2014;28:42–48. doi: 10.1016/j.semcdb.2014.01.002. [Dahlhoff M, Wolf E, Schneider MR. The ABC of BTC: Structural properties and biological roles of betacellulin [J]. Semin Cell Dev Biol, 2014, 28: 42-48.] [DOI] [PubMed] [Google Scholar]
- 21.Olsen DA, Kjaer IM, Brandslund I. Development of a three-plex single molecule immunoassay enabling measurement of the EGFR ligands amphiregulin, betacellulin and transforming growth factor alpha simultaneously in human serum samples. J Immunol Methods. 2018;459:63–69. doi: 10.1016/j.jim.2018.05.002. [Olsen DA, Kjaer IM, Brandslund I. Development of a three-plex single molecule immunoassay enabling measurement of the EGFR ligands amphiregulin, betacellulin and transforming growth factor alpha simultaneously in human serum samples [J]. J Immunol Methods, 2018, 459: 63-69.] [DOI] [PubMed] [Google Scholar]
- 22.Lee YS, Song GJ, Jun HS. Betacellulin-induced alpha-cell proli-feration is mediated by ErbB3 and ErbB4, and may contribute to beta-cell regeneration. Front Cell Dev Biol. 2020;8:605110. doi: 10.3389/fcell.2020.605110. [Lee YS, Song GJ, Jun HS. Betacellulin-induced alpha-cell proli-feration is mediated by ErbB3 and ErbB4, and may contribute to beta-cell regeneration [J]. Front Cell Dev Biol, 2020, 8: 605110.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen DA, Bechmann T, ϕstergaard B, et al. Increased concentrations of growth factors and activation of the EGFR system in breast cancer. Clin Chem Lab Med. 2012;50(10):1809–1818. doi: 10.1515/cclm-2011-0823. [Olsen DA, Bechmann T, ϕstergaard B, et al. Increased concentrations of growth factors and activation of the EGFR system in breast cancer [J]. Clin Chem Lab Med, 2012, 50(10): 1809-1818.] [DOI] [PubMed] [Google Scholar]
- 24.Pearl LH. Hsp90 and Cdc37: A chaperone cancer conspiracy. Curr Opin Genet Dev. 2005;15(1):55–61. doi: 10.1016/j.gde.2004.12.011. [Pearl LH. Hsp90 and Cdc37: A chaperone cancer conspiracy [J]. Curr Opin Genet Dev, 2005, 15(1): 55-61.] [DOI] [PubMed] [Google Scholar]
- 25.Serwetnyk MA, Blagg BSJ. The disruption of protein-protein interactions with co-chaperones and client substrates as a strategy towards Hsp90 inhibition. Acta Pharm Sin B. 2021;11(6):1446–1468. doi: 10.1016/j.apsb.2020.11.015. [Serwetnyk MA, Blagg BSJ. The disruption of protein-protein interactions with co-chaperones and client substrates as a strategy towards Hsp90 inhibition [J]. Acta Pharm Sin B, 2021, 11(6): 1446-1468.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gray PJ, J r., Prince T, Cheng J, et al. Targeting the oncogene and kinome chaperone CDC37. Nat Rev Cancer. 2008;8(7):491–495. doi: 10.1038/nrc2420. [Gray PJ, Jr., Prince T, Cheng J, et al. Targeting the oncogene and kinome chaperone CDC37 [J]. Nat Rev Cancer, 2008, 8(7): 491-495.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells. J Biol Chem. 2005;280(10):8875–8883. doi: 10.1074/jbc.M410882200. [Ghatak S, Misra S, Toole BP. Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells [J]. J Biol Chem, 2005, 280(10): 8875-8883.] [DOI] [PubMed] [Google Scholar]
- 28.Huang W, Ye M, Zhang LR, et al. FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation. Mol Cancer. 2014;13:150. doi: 10.1186/1476-4598-13-150. [Huang W, Ye M, Zhang LR, et al. FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation [J]. Mol Cancer, 2014, 13: 150.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Esparís-Ogando A, Montero JC, Arribas J, et al. Targeting the EGF/HER ligand-receptor system in cancer. Curr Pharm Des. 2016;22(39):5887–5898. doi: 10.2174/1381612822666160715132233. [Esparís-Ogando A, Montero JC, Arribas J, et al. Targeting the EGF/HER ligand-receptor system in cancer [J]. Curr Pharm Des, 2016, 22(39): 5887-5898.] [DOI] [PubMed] [Google Scholar]
- 30.Wang Z. ErbB receptors and cancer. Methods Mol Biol. 2017;1652:3–35. doi: 10.1007/978-1-4939-7219-7_1. [Wang Z. ErbB receptors and cancer [J]. Methods Mol Biol, 2017, 1652: 3-35.] [DOI] [PubMed] [Google Scholar]
- 31.Garousi S, Jahanbakhsh-Godehkahriz S, Esfahani K, et al. Meta-analysis of EGF-stimulated normal and cancer cell lines to discover EGF-associated oncogenic signaling pathways and prognostic biomarkers. Iran J Biotechnol. 2022;20(3):e3245. doi: 10.30498/ijb.2022.323464.3245. [Garousi S, Jahanbakhsh-Godehkahriz S, Esfahani K, et al. Meta-analysis of EGF-stimulated normal and cancer cell lines to discover EGF-associated oncogenic signaling pathways and prognostic biomarkers [J]. Iran J Biotechnol, 2022, 20(3): e3245.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laczmanska I, Sasiadek MM. Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer. Acta Biochim Pol. 2011;58(4):467–470. [Laczmanska I, Sasiadek MM. Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer [J]. Acta Biochim Pol, 2011, 58(4): 467-470.] [PubMed] [Google Scholar]
- 33.Menigatti M, Cattaneo E, Sabates-Bellver J, et al. The protein tyrosine phosphatase receptor type R gene is an early and frequent target of silencing in human colorectal tumorigenesis. Mol Cancer. 2009;8:124. doi: 10.1186/1476-4598-8-124. [Menigatti M, Cattaneo E, Sabates-Bellver J, et al. The protein tyrosine phosphatase receptor type R gene is an early and frequent target of silencing in human colorectal tumorigenesis [J]. Mol Cancer, 2009, 8: 124.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y, Cao J, Liu W, et al. Protein tyrosine phosphatase receptor type R (PTPRR) antagonizes the wnt signaling pathway in ovarian cancer by dephosphorylating and inactivating β-catenin. J Biol Chem. 2019;294(48):18306–18323. doi: 10.1074/jbc.RA119.010348. [Wang Y, Cao J, Liu W, et al. Protein tyrosine phosphatase receptor type R (PTPRR) antagonizes the wnt signaling pathway in ovarian cancer by dephosphorylating and inactivating β-catenin [J]. J Biol Chem, 2019, 294(48): 18306-18323.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su PH, Lin YW, Huang RL, et al. Epigenetic silencing of PTPRR activates MAPK signaling, promotes metastasis and serves as a biomarker of invasive cervical cancer. Oncogene. 2013;32(1):15–26. doi: 10.1038/onc.2012.29. [Su PH, Lin YW, Huang RL, et al. Epigenetic silencing of PTPRR activates MAPK signaling, promotes metastasis and serves as a biomarker of invasive cervical cancer [J]. Oncogene, 2013, 32(1): 15-26.] [DOI] [PubMed] [Google Scholar]
- 36.Munkley J, Lafferty NP, Kalna G, et al. Androgen-regulation of the protein tyrosine phosphatase PTPRR activates ERK1/2 signalling in prostate cancer cells. BMC Cancer. 2015;15:9. doi: 10.1186/s12885-015-1012-8. [Munkley J, Lafferty NP, Kalna G, et al. Androgen-regulation of the protein tyrosine phosphatase PTPRR activates ERK1/2 signalling in prostate cancer cells [J]. BMC Cancer, 2015, 15: 9.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng WL, Feng PH, Lee KY, et al. The role of EREG/EGFR pathway in tumor progression. Int J Mol Sci. 2021;22(23):12828. doi: 10.3390/ijms222312828. [Cheng WL, Feng PH, Lee KY, et al. The role of EREG/EGFR pathway in tumor progression [J]. Int J Mol Sci, 2021, 22(23): 12828.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang L, Nan F, Yang L, et al. Differentially expressed EREG and SPP1 are independent prognostic markers in cervical squamous cell carcinoma. J Obstet Gynaecol Res. 2022;48(7):1848–1858. doi: 10.1111/jog.15265. [Zhang L, Nan F, Yang L, et al. Differentially expressed EREG and SPP1 are independent prognostic markers in cervical squamous cell carcinoma [J]. J Obstet Gynaecol Res, 2022, 48(7): 1848-1858.] [DOI] [PubMed] [Google Scholar]
- 39.Liu S, Wang Y, Han Y, et al. EREG-driven oncogenesis of head and neck squamous cell carcinoma exhibits higher sensitivity to erlotinib therapy. Theranostics. 2020;10(23):10589–10605. doi: 10.7150/thno.47176. [Liu S, Wang Y, Han Y, et al. EREG-driven oncogenesis of head and neck squamous cell carcinoma exhibits higher sensitivity to erlotinib therapy [J]. Theranostics, 2020, 10(23): 10589-10605.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xia Q, Zhou Y, Yong H, et al. Elevated epiregulin expression predicts poor prognosis in gastric cancer. Pathol Res Pract. 2019;215(5):873–879. doi: 10.1016/j.prp.2019.01.030. [Xia Q, Zhou Y, Yong H, et al. Elevated epiregulin expression predicts poor prognosis in gastric cancer [J]. Pathol Res Pract, 2019, 215(5): 873-879.] [DOI] [PubMed] [Google Scholar]