Abstract
目的
探讨肝素/碱化利多卡因(利多卡因与碳酸氢钠混合)协同膀胱内灌注联合水扩张及经尿道电灼治疗女性间质性膀胱炎(interstitial cystitis, IC)的有效性和安全性。
方法
选择2012年1月至2020年12月于中国医科大学附属第一医院泌尿外科就诊,符合美国泌尿外科协会指南的诊断标准,新诊断为IC的女性患者的病例资料进行回顾性分析,诊断时对可疑病变进行膀胱镜检查和活检。所有患者均接受持续12个月的2%(质量分数)利多卡因10 mL + 5%(质量分数)碳酸氢钠5 mL +肝素25 000 IU的膀胱内灌注治疗,根据患者的意愿,选择接受或不接受水扩张及经尿道电灼治疗,将患者分为水扩张和经尿道电灼(hydrodistension and transurethral fulguration, HD/TF)组和非HD/TF组,记录患者治疗前和治疗后1、6、12个月O’Leary-Sant间质性膀胱炎症状指标评分(interstitial cystitis patient symptom index scores, ICSI)、问题指标评分(interstitial cystitis patient problem index scores, ICPI)、耻骨上疼痛视觉模拟评分(visual analog scale, VAS)、功能性膀胱容量(functional bladder capacity,FBC)等。
结果
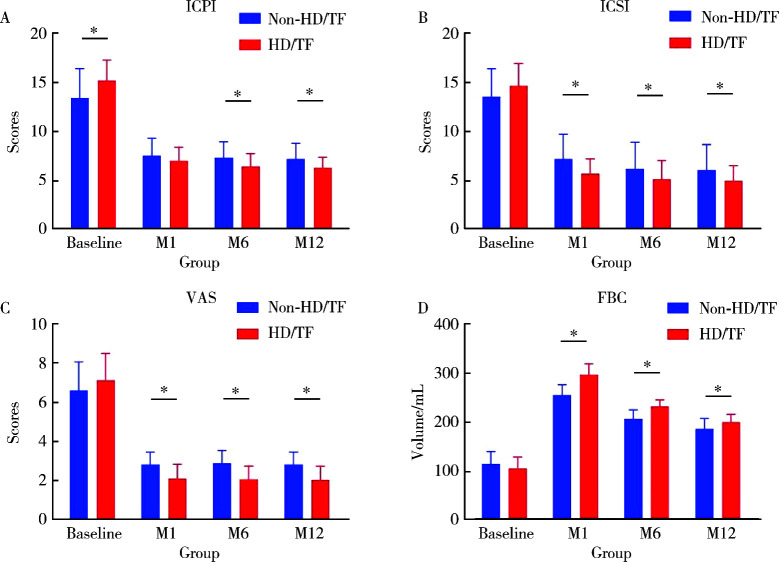
共收集到患者79例,其中有4例(5.1%)患者因病理诊断为癌或治疗失败而行膀胱切除术被剔除,其余患者均在治疗后1、6、12个月成功随访。重复测量方差分析显示:治疗后ICPI、ICSI和VAS较治疗前均显著降低(P < 0.05),FBC显著增加(P < 0.05)。治疗后1、6、12个月随访期间FBC持续下降,差异有统计学意义(P < 0.05);在治疗后1个月和6个月随访时ICSI持续降低,差异有统计学意义(P < 0.05),而治疗后的6个月与治疗后12个月的ICSI差异无统计学意义(P>0.05)。HD/TF组在治疗后1个月和6个月随访时ICPI持续降低,差异有统计学意义(P < 0.05),而治疗后6个月与12个月的ICPI差异无统计学意义(P>0.05)。治疗后1、6、12个月其余各项指标之间比较,差异均无统计学意义(P>0.05)。与非HD/TF组相比,HD/TF组的ICPI、ICSI、VAS和FBC改善更早,且VAS和FBC的变化更显著(P < 0.05)。
结论
肝素/碱化利多卡因协同膀胱内灌注加水扩张和经尿道电灼治疗IC是一种有效的治疗选择。膀胱内肝素/碱化利多卡因协同灌注可能成为首选治疗方法,可显著减轻患者和医保系统的经济负担,如果患者能接受,可考虑经尿道电灼联合水扩张的治疗方法。
Keywords: 间质性膀胱炎, 膀胱内灌注, 经尿道电灼, 水扩张
Abstract
Objective
To investigate the efficacy and safety of intravesical instillation of heparin/alkalized lidocaine (lidocaine mixed with sodium bicarbonate) combined with hydrodistension and transurethral fulguration in the treatment of female interstitial cystitis (IC).
Methods
Female patients who attended the Department of Urology at the First Hospital of China Medical University between January 2012 and December 2020 and met the diagnostic criteria proposed in the guidelines of the American Urological Association with a new diagnosis of IC were selected for retrospective analysis. Cystoscopy and biopsy of suspicious lesions were performed at the time of diagnosis. All the patients were treated with an intravesical instillation regimen of 2% lidocaine 10 mL + 5% sodium bicarbonate 5 mL + heparin 25 000 IU for a continuous period of 12 months, with or without water dilatation and transurethral electrocautery according to the patient's preference, categorized as hydrodistension and transurethral fulguration (HD/TF) group and non-HD/TF group. The patients were evaluated before and 1, 6, and 12 months after treatment for O'Leary-Sant interstitial cystitis patient symptom index scores (ICSI), interstitial cystitis patient problem index scores (ICPI), visual analog scale (VAS) of suprapubic pain, and functional bladder capacity (FBC) changes.
Results
A total of 79 patients were collected in this study. Four (5.1%) of these patients underwent cystectomy due to pathological diagnosis of cancer or treatment failure. The remaining patients were followed up 1, 6 and 12 months after treatment. Repeated-measures ANOVA showed a significant decrease in ICPI, ICSI and VAS and an increase in FBC after treatment compared with before treatment (P < 0.05). FBC continued to decrease during the 1, 6 and 12 months' post-treatment follow-ups, with statistically significant differences; ICSI continued to decrease during the 1 and 6 months post-treatment follow-ups, with statistically significant differences, while the difference between ICSI at 6 months post-treatment and at 12 months' post-treatment was not statistically significant. In the HD/TF group, ICPI continued to decrease in the follow-up from 1 and 6 months after treatment, and the difference was statistically significant, while the difference between ICPI 6 months after treatment and 12 months after treatment was not statistically significant. There was no statistically significant difference between the remaining indicators 1, 6 and 12 months after treatment. ICPI, ICSI, VAS and FBC improved earlier and the changes in VAS and FBC were more significant in the HD/TF group compared with the non-HD/TF group (P < 0.05).
Conclusion
Heparin/alkalized lidocaine combination of intravesical instillation with hydrodistension and transurethral fulguration for IC is an effective treatment option. Heparin/alkalized lidocaine combination of intravesical instillation may be the first choice of treatment, which can significantly reduce the economic burden of patients and medical insurance system. If patients can accept it, transurethral fulguration with hydrodistension may be considered.
Keywords: Interstitial cystitis, Intravesical instillation, Transurethral fulguration, Hydrodistension
间质性膀胱炎(interstitial cystitis, IC)是一种使人生活质量下降的慢性膀胱/盆腔疼痛疾病,其特征是尿频和不明原因的尿急,膀胱充盈时下腹或会阴区出现反复发作或持续存在的疼痛。据估计,美国6.5%的女性有与IC诊断相关的症状[1]。IC严重影响生活质量,且难以治愈,缓解症状是所有治疗方案的首要目标[2]。由于对IC的病因尚知之甚少,治疗方法多样,但治疗效果均不明确,很难达到完全治愈,且极易复发,故临床治疗多以缓解症状为主。一线的口服药物治疗,如阿米替林[3-4]、羟嗪[5]、戊聚糖多硫酸盐[6]和环孢素[7-9],已在安慰剂对照研究中被证明只能改善患者30%~68%的症状;然而,一些患者在治疗过程中会出现口干、体质量增加、过敏、胃肠功能紊乱和疼痛等不可耐受的毒副作用。
IC长期治疗给患者和社会带来了沉重的经济负担。在我国,肝素/碱化利多卡因(利多卡因与碳酸氢钠混合)联合治疗的费用约为透明质酸的1/90。本研究回顾性分析水扩张和经尿道电灼(hydrodistension and transurethral fulguration, HD/TF)加上肝素/碱化利多卡因协同膀胱灌注治疗IC的疗效,以确定其是否能以更低的费用成本和更少的侵入性获得快速、持久的症状缓解。
1. 资料与方法
1.1. 研究对象
2012年1月至2020年12月新诊断的79例女性患者均符合美国泌尿外科协会指南的IC诊断标准[1],包括: (1)合并与膀胱充盈/排空、尿频、夜尿、尿急相关的疼痛; (2)局部麻醉下膀胱镜检查可见Hunner病变或膀胱黏膜小球状出血; (3)排除其他有类似症状的疾病(如感染、膀胱过度活动症或恶性肿瘤)[10]。所有患者均进行详细的评估,包括病史、体格检查、O’Leary-Sant间质性膀胱炎症状指标评分(interstitial cystitis patient symptom index scores, ICSI)、问题指标评分(interstitial cystitis patient problem index scores, ICPI)、耻骨上疼痛视觉模拟评分(visual analog scale, VAS)和3 d排尿日记。所有患者均进行了尿液分析、尿培养、超声、CT和膀胱镜检查以排除其他疾病。
本回顾性研究遵循《赫尔辛基宣言》伦理标准,研究开始前已经中国医科大学附属第一医院伦理委员会审查批准(授权号: AF-SOP-07-1.1-01)。
所有患者被分成两组,在接诊患者后均建议行HD/TF治疗,根据患者意愿要求入组,其中同意行经尿道电灼+水扩张治疗的患者纳入HD/TF组,拒绝行有创治疗的患者纳入非HD/TF组。HD/TF治疗方式为首先在全身麻醉下用生理盐水行水扩张治疗,在这一过程可以见到Hunner病变(图 1)[7, 11]。水扩张的标准为膀胱在80 cmH2O(1 cmH2O=98 Pa)的压力下保持充盈3 min, 镜下会出现Hunner病灶瀑布状出血或膀胱黏膜弥漫性出血。对Hunner病灶及可疑病灶行电切活检取病理,其后对所有可疑病灶进行电灼治疗。所有患者均接受2%(质量分数)利多卡因10 mL + 5%(质量分数)碳酸氢钠5 mL +肝素25 000 IU的膀胱内灌注治疗,灌注液膀胱内留置60 min,每周1~2次,共8周,其后每月1~2次维持,治疗共进行1年。
图 1.
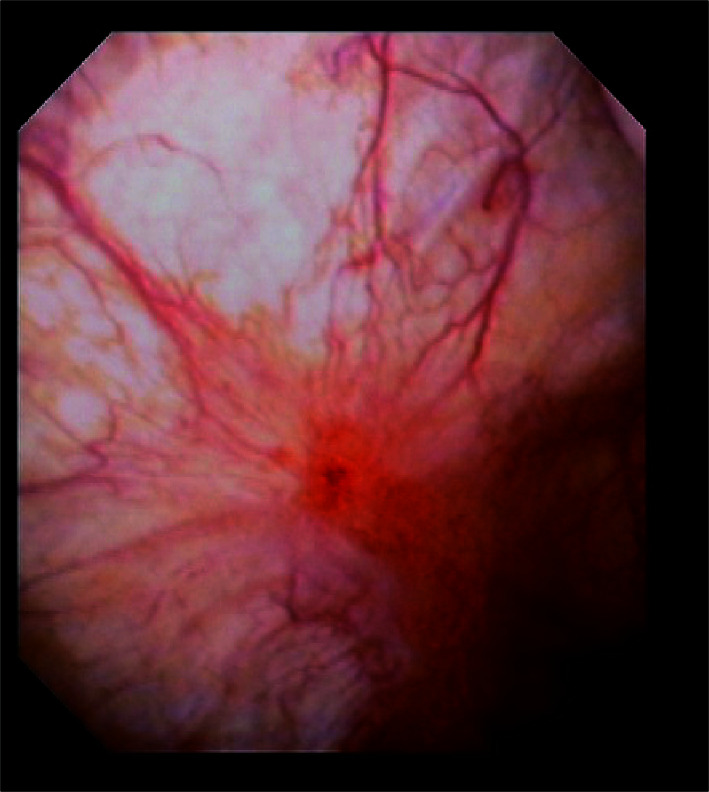
膀胱镜下典型的Hunner病变
Typical Hunner lesion on cystoscopy
1.2. 结局指标
采用ICSI、ICPI和VAS评分评价症状严重程度。以3 d排尿日记中记录功能性膀胱容量(functional bladder capacity,FBC)为平均容量。所有患者从治疗开始即接受调查,并分别在治疗后1、6、12个月进行了随访记录,以治疗前1周的各项指标为基线,对症状恶化的患者进行膀胱镜检查。
1.3. 统计学分析
采用SAS 9.4软件进行统计分析,Shapiro-Wilk检验结果显示各指标均符合正态分布,因此采用均数±标准差对各指标进行描述。研究中各结局指标涉及3次重复测量,因此,当数据满足方差齐性时,采用重复测量数据方差分析比较各指标(ICPI、ICSI、VAS、FBC)的组内差异和组间差异,当不符合Mauchly球形检验时,以Greenhouse-Geisser检验结果为准,且在比较不同时间各指标的组间差异时采用bonferroni法对α进行校正。若数据方差不齐,采用广义线性模型(generalize linear model, GLM)进行分析,以结局指标作为因变量,将时间及时间与自变量的相乘交互作用作为自变量纳入模型进行分析,所有检验均采用双侧检验,以P < 0.05为差异有统计学意义。
2. 结果
本研究79例患者中4例(5.1%,4/79)患者在随访期间因膀胱癌(2例)或治疗失败(2例)行膀胱切除术而被排除,最终共75例患者纳入分析,中位年龄60岁(35~82岁)。根据是否行HD/TF治疗将患者分为2组,其中50例(66.7%,50/75)患者纳入HD/TF组,25例(33.3%,25/75)患者纳入非HD/TF组。
治疗前患者基线水平ICPI为14.6±2.6,ICSI为14.2±2.5,VAS为6.9±1.4,FBC为(106.3±24.6) mL。4例行膀胱切除术的患者中,3例术后疼痛完全缓解,1例患者术后仍有不同程度的下腹痛。
两组患者在治疗前各指标的平均水平见表 1,其中ICSI、VAS、FBC基线水平在两组间差异无统计学意义,而HD/TF组的ICPI高于非HD/TF组(15.2 vs. 13.4),差异有统计学意义(P < 0.01)。
表 1.
非HD/TF组和HD/TF组的基线水平
Baseline of the Non-HD/TF and HD/TF groups
| Items | Group | Data | P value |
| ICSI, interstitial cystitis patient symptom index scores; ICPI, interstitial cystitis patient problem index scores; VAS, visual analog scale; FBC, functional bladder capacity; HD/TF, hydrodistension and transure-thral fulgurattion. | |||
| ICPI | Non-HD/TF | 13.4±3.0 | < 0.01 |
| HD/TF | 15.2±2.1 | ||
| ICSI | Non-HD/TF | 13.5±2.8 | 0.08 |
| HD/TF | 14.6±2.3 | ||
| VAS | Non-HD/TF | 6.6±1.5 | 0.14 |
| HD/TF | 7.1±1.4 | ||
| FBC | Non-HD/TF | 112.8±25.3 | 0.11 |
| HD/TF | 103.1±23.9 | ||
经分析发现,治疗方式和随访时间在对ICPI、ICSI、VAS、FBC的影响上均存在交互效应,所以研究进一步分析了随访时间和治疗方式的简单效应。
对治疗前后各项指标进行比较,ICPI在非HD/TF组中4次评价结果分别为13.4±3.0、7.5±1.8、7.3±1.7、7.2±1.6,而在HD/TF组中4次评价结果分别为15.2±2.1、7.0±1.4、6.4±1.4、6.3±1.1,不论在哪组中,治疗后较治疗前的ICPI均有降低,差异有统计学意义。ICSI在非HD/TF组中4次评价结果分别为13.5±2.8、7.2±2.5、6.2±2.7、6.1±2.6,而在HD/TF组中4次评价结果分别为14.6±2.3、5.7±1.5、5.1±1.9、5.0±1.6,不论在哪组中,治疗后较治疗前的ICSI均有降低,差异有统计学意义。VAS在非HD/TF组中4次评价结果分别为6.6±1.5、2.8±0.6、2.9±0.7、2.8±0.6,而在HD/TF组中4次评价结果分别为7.1±1.4、2.1±0.7、2.1±0.7、2.0±0.7,不论在哪组中,治疗后较治疗前的VAS均有降低,差异有统计学意义。FBC在非HD/TF组中4次评价结果分别为(112.8±25.3) mL、(253.6±21.4) mL、(204.8±18.7) mL、(184±22.0) mL,而在HD/TF组中4次评价结果分别为(103.1±23.9) mL、(294.8±22.7) mL、(230.4±13.5) mL、(198.0±16.3) mL,不论在哪组中,治疗后较治疗前的FBC均有升高,差异有统计学意义。治疗后1、6、12个月随访FBC持续下降,差异有统计学意义。在治疗后1个月和6个月随访时ICSI持续降低,差异有统计学意义,而治疗后的6个月与治疗后12个月的ICSI差异无统计学意义。HD/TF组在治疗后1个月和6个月随访时ICPI持续降低,差异有统计学意义,而治疗后的6个月与治疗后12个月的ICPI差异无统计学意义。治疗后1、6、12个月其余各项指标之间差异无统计学意义。
治疗后1、6和12个月HD/TF组的ICSI和VAS评分均低于非HD/TF组,FBC高于非HD/TF组,而HD/TF组ICPI在治疗后6个月和12个月低于非HD/TF组(图 2),说明与非HD/TF组相比,HD/TF组的ICPI、ICSI、VAS和FBC改善更早,且VAS和FBC的变化更显著(P < 0.05)。
图 2.
治疗前后ICPI、ICSI、VAS和FBC的变化趋势
Trends of ICPI, ICSI, VAS and FBC before and after treatment
与膀胱内灌注和水扩张相关的不良事件见表 2, 未发生与手术相关的膀胱破裂或大出血等严重不良事件。
表 2.
膀胱内灌注和水扩张的不良事件
Adverse events of the intravesical instillation and hydrodistension with transurethral fulguration
| Adverse events | Intravesical instillation, n(%) | Hydrodistension with transurethral fulguration, n(%) |
| Number of patients | 75 | 50 |
| Vomiting | 0 | 1 (2.0) |
| Fatigue | 3 (4.0) | 8 (16.0) |
| Headache | 1 (1.3) | 0 |
| Dizziness | 1 (1.3) | 0 |
| Anxiety | 2 (2.7) | 0 |
| Abdominal pain | 3 (4.0) | 5 (10.0) |
| Dysuria | 0 | 0 |
| Urethral pain | 25 (33.3) | 11 (22.0) |
| Pyrexia | 0 | 0 |
| Hematuria | 13 (17.3) | 23 (46.0) |
3. 讨论
IC的主要症状包括严重的疼痛和储尿症状,原因是膀胱容量减少。由于病因不明,目前尚无标准的病因治疗方法。IC治疗原则是提高生活质量,最佳管理应包括多种行为方式干预,生理和心理治疗。目前IC的治疗主要包括保守治疗、口服治疗、膀胱灌注治疗、膀胱镜检查和大手术5种方案[10]。
在过去的十年中,一些研究聚焦于IC的致病机制。有人提出,膀胱黏膜的糖胺聚糖(glycosami-noglycan,GAG)层缺陷会导致尿频、尿急、夜尿症和疼痛等症状[1]。因此,补充GAG层可以减轻神经源性炎症,防止上皮下肥大细胞活化,缓解IC症状。有几种药物已被证明具有与GAG相似的作用方式。已有研究关注IC患者膀胱内药物灌注的疗效,这些药物包括肝素[12-14]、透明质酸[12, 15-16]、硫酸软骨素[12, 17]和戊聚糖多硫酸盐[6, 12],已在临床广泛应用,并显示出良好的治疗效果。然而,这些药物的疗效不能长期维持,通常会逐渐减弱。目前国内只有肝素和透明质酸被批准用于IC患者的膀胱灌注,肝素比透明质酸更易获得,价格也更低,仅约为后者的1/180。此外,碱化利多卡因(利多卡因与碳酸氢钠混合)膀胱灌注对IC患者也有良好的疗效[12, 16, 18]。与肝素的作用机制不同,膀胱内注射利多卡因(一种局部麻醉剂)可降低膀胱感觉神经末梢的兴奋性,从而有助于缓解与IC相关的疼痛强度和频率,在碱性环境下,利多卡因可以更顺利地通过细胞膜,这意味着碱化利多卡因对控制IC引起的症状具有治疗潜力。
HD/TF通常被用于IC患者的诊断和治疗[1, 10],水扩张后1个月的疗效约为71%[19-20]。此外,膀胱内注射A型肉毒素[15, 21]或骶神经调节[22-23]被认为是其他治疗失败后的一种选择,但因具有侵入性强、费用高和并发症多等特点,所以不能被广泛应用。
本研究的治疗方案包括肝素和碱化利多卡因膀胱内灌注,以及经尿道膀胱病灶水扩张和电灼术。肝素是一种硫酸化多糖,是糖胺聚糖类似物,被认为能增强天然膀胱黏膜的保护作用,但是由于肝素灌注的延迟效应,碱化利多卡因被引入以下调膀胱的感觉神经阈值,肝素有助于膀胱上皮的修复,利多卡因似乎加速了感觉神经阈值下调的过程,在本研究中,经膀胱灌注肝素和碱化利多卡因,观察到ICPI/ICSI在1个月时显著下降,并在1年内保持不变。即使在重度IC患者中,此疗法也有可能更快、更明显地缓解症状。透明质酸是GAG的另一种类似物,其作用与肝素相似。在我国,肝素/碱化利多卡因的费用约为透明质酸的1/90。考虑到肝素/碱化利多卡因膀胱灌注治疗费用较低、作用持久且疗效满意,有望成为膀胱灌注治疗的首选药物,并可显著减轻患者和医保体系的经济负担。
膀胱镜检查对于区分IC与类似症状的疾病(如膀胱癌)很有价值,如发现典型或可疑征象,如Hunner病变、肿瘤或炎症性病变,应行经尿道电灼及病理检查。水扩张也有治疗作用,虽然水扩张的治疗机制尚不清楚,但在动物实验中,它已被证明对黏膜下神经丛造成损伤,被认为可以减轻疼痛和减少疼痛频率[24-26]。此外,HD/TF技术可对IC症状产生积极协同作用[7, 27-28]。本研究HD/TF组VAS评分显著降低,FBC评分显著升高,提示HD/TF能增强膀胱灌注的效果,并能立即缓解症状,考虑到症状可能立即消退,FBC改善,推荐HD/TF治疗。此外,虽然扩张的FBC在随访期间逐渐下降,但仍大于基线,提示持续的行为治疗在IC治疗中的重要性。
本研究未观察到与治疗方案相关的严重不良事件,最常见的不良反应为尿道疼痛和轻度血尿,无需任何干预即可恢复,这些结果表明,对于IC患者,本研究的治疗方案是一种安全的治疗选择。
组织活检对IC患者至关重要,据报道,IC患者随后被诊断为膀胱癌的可能性是正常人的2.95倍[29]。因此,在诊断IC患者时,经尿道活检是必要的,以检测恶性肿瘤,特别是原位癌。在本研究中,2例患者主诉症状复发,随后因膀胱镜和活检证实为肌层浸润性膀胱癌而接受了膀胱切除术。因此,IC患者在随访过程中应考虑到膀胱癌的风险,特别是症状恶化或复发的患者,应定期行膀胱镜检查。
此外,有研究提出,对于经其他治疗未能达到长期症状控制的IC患者,可以行膀胱切除术,膀胱切除术可能是难治性IC的唯一合理替代方案。在本研究中,2例患者接受了膀胱切除术,其中1例患者症状消失,另1例患者因持续下腹部疼痛仍不满意,因此,IC患者在选择膀胱切除术时应谨慎,患者应在术前充分了解膀胱切除术的风险获益比,以提高治疗满意度。
综上所述,IC的治疗方案包括肝素/碱化利多卡因膀胱灌注加经尿道电灼水扩张,可使症状得到相对长期的缓解。考虑到症状/疼痛的快速改善和较大的FBC,建议在治疗开始时进行水扩张和经尿道电灼。肝素/碱化利多卡因具有较好的疗效和较低的费用,可作为膀胱灌注治疗的首选药物,并可明显减轻患者的经济和医保负担。由于膀胱恶性肿瘤的发病率较高,应在随访期间进行膀胱镜检查,尤其是在症状加重或复发时。膀胱切除术可谨慎作为最终治疗手段。本研究为单中心的观察性研究,且样本量相对较小,干预效果评估的效能较低,未来需开展设计良好的临床随机对照试验加以验证。
References
- 1.Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment. J Urol. 2015;193(5):1545–1553. doi: 10.1016/j.juro.2015.01.086. [Hanno PM, Erickson D, Moldwin R, et al. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: AUA guideline amendment[J]. J Urol, 2015, 193(5): 1545-1553.] [DOI] [PubMed] [Google Scholar]
- 2.Engeler DS, Baranowski AP, Dinis-Oliveira P, et al. The 2013 EAU guidelines on chronic pelvic pain: Is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development. Eur Urol. 2013;64(3):431–439. doi: 10.1016/j.eururo.2013.04.035. [Engeler DS, Baranowski AP, Dinis-Oliveira P, et al. The 2013 EAU guidelines on chronic pelvic pain: Is management of chronic pelvic pain a habit, a philosophy, or a science? 10 years of development[J]. Eur Urol, 2013, 64(3): 431-439.] [DOI] [PubMed] [Google Scholar]
- 3.Generali JA, Cada DJ. Amitriptyline: Interstitial cystitis (painful bladder syndrome) Hosp Pharm. 2014;49(9):809–810. doi: 10.1310/hpj4909-809. [Generali JA, Cada DJ. Amitriptyline: Interstitial cystitis (painful bladder syndrome)[J]. Hosp Pharm, 2014, 49(9): 809-810.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, Fang Z, Ding Q, et al. Effect of amitriptyline in treatment interstitial cystitis or bladder pain syndrome according to two criteria: Does ESSIC criteria change the response rate. Neurourol Urodyn. 2014;33(3):341–344. doi: 10.1002/nau.22407. [Sun Y, Fang Z, Ding Q, et al. Effect of amitriptyline in treatment interstitial cystitis or bladder pain syndrome according to two criteria: Does ESSIC criteria change the response rate[J]. Neurourol Urodyn, 2014, 33(3): 341-344.] [DOI] [PubMed] [Google Scholar]
- 5.Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis. J Urol. 2003;170(3):810–815. doi: 10.1097/01.ju.0000083020.06212.3d. [Sant GR, Propert KJ, Hanno PM, et al. A pilot clinical trial of oral pentosan polysulfate and oral hydroxyzine in patients with interstitial cystitis[J]. J Urol, 2003, 170(3): 810-815.] [DOI] [PubMed] [Google Scholar]
- 6.Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: Insights from a randomized, double-blind, placebo controlled study. J Urol. 2015;193(3):857–862. doi: 10.1016/j.juro.2014.09.036. [Nickel JC, Herschorn S, Whitmore KE, et al. Pentosan polysulfate sodium for treatment of interstitial cystitis/bladder pain syndrome: Insights from a randomized, double-blind, placebo controlled study[J]. J Urol, 2015, 193(3): 857-862.] [DOI] [PubMed] [Google Scholar]
- 7.Vollstedt A, Tennyson L, Turner K, et al. Evidence for early cyclosporine treatment for hunner lesion interstitial cystitis. Female Pelvic Med Reconstr Surg. 2022;28(1):e1–e5. doi: 10.1097/SPV.0000000000001108. [Vollstedt A, Tennyson L, Turner K, et al. Evidence for early cyclosporine treatment for hunner lesion interstitial cystitis[J]. Female Pelvic Med Reconstr Surg, 2022, 28(1): e1-e5.] [DOI] [PubMed] [Google Scholar]
- 8.Ogawa T, Ishizuka O, Ueda T, et al. Pharmacological management of interstitial cystitis /bladder pain syndrome and the role cyclosporine and other immunomodulating drugs play. Expert Rev Clin Pharmacol. 2018;11(5):495–505. doi: 10.1080/17512433.2018.1457435. [Ogawa T, Ishizuka O, Ueda T, et al. Pharmacological management of interstitial cystitis /bladder pain syndrome and the role cyclosporine and other immunomodulating drugs play[J]. Expert Rev Clin Pharmacol, 2018, 11(5): 495-505.] [DOI] [PubMed] [Google Scholar]
- 9.Crescenze IM, Tucky B, Li J, et al. Efficacy, side effects, and monitoring of oral cyclosporine in interstitial cystitis-bladder pain syndrome. Urology. 2017;107:49–54. doi: 10.1016/j.urology.2017.05.016. [Crescenze IM, Tucky B, Li J, et al. Efficacy, side effects, and monitoring of oral cyclosporine in interstitial cystitis-bladder pain syndrome[J]. Urology, 2017, 107: 49-54.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malde S, Palmisani S, Al-Kaisy A, et al. Guideline of guidelines: Bladder pain syndrome. BJU Int. 2018;122(5):729–743. doi: 10.1111/bju.14399. [Malde S, Palmisani S, Al-Kaisy A, et al. Guideline of guidelines: Bladder pain syndrome[J]. BJU Int, 2018, 122(5): 729-743.] [DOI] [PubMed] [Google Scholar]
- 11.Nickel JC, Ehrlich GD, Krol JE, et al. The bacterial microbiota of Hunner lesion interstitial cystitis/bladder pain syndrome. BJU Int. 2022;129(1):104–112. doi: 10.1111/bju.15519. [Nickel JC, Ehrlich GD, Krol JE, et al. The bacterial microbiota of Hunner lesion interstitial cystitis/bladder pain syndrome[J]. BJU Int, 2022, 129(1): 104-112.] [DOI] [PubMed] [Google Scholar]
- 12.Meng E, Hsu YC, Chuang YC. Advances in intravesical therapy for bladder pain syndrome (BPS)/interstitial cystitis (IC) Low Urin Tract Symptoms. 2018;10(1):3–11. doi: 10.1111/luts.12214. [Meng E, Hsu YC, Chuang YC. Advances in intravesical therapy for bladder pain syndrome (BPS)/interstitial cystitis (IC)[J]. Low Urin Tract Symptoms, 2018, 10(1): 3-11.] [DOI] [PubMed] [Google Scholar]
- 13.Lim YN, Dwyer P, Murray C, et al. Long-term outcomes of intravesical dimethyl sulfoxide/heparin/hydrocortisone therapy for interstitial cystitis/bladder pain syndrome. Int Urogynecol J. 2017;28(7):1085–1089. doi: 10.1007/s00192-016-3232-0. [Lim YN, Dwyer P, Murray C, et al. Long-term outcomes of intravesical dimethyl sulfoxide/heparin/hydrocortisone therapy for interstitial cystitis/bladder pain syndrome[J]. Int Urogynecol J, 2017, 28(7): 1085-1089.] [DOI] [PubMed] [Google Scholar]
- 14.Generali JA, Cada DJ. Intravesical heparin: Interstitial cystitis (painful bladder syndrome) Hosp Pharm. 2013;48(10):822–824. doi: 10.1310/hpj4810-822. [Generali JA, Cada DJ. Intravesical heparin: Interstitial cystitis (painful bladder syndrome)[J]. Hosp Pharm, 2013, 48(10): 822-824.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li B, Leng Q, Li C, et al. Comparison of intravesical instillation of hyaluronic acid with intradetrusor botulinum toxin A injection or cystoscopic hydrodistention for ketamine-associated cystitis. J Int Med Res. 2020;48(11):300060520973100. doi: 10.1177/0300060520973100. [Li B, Leng Q, Li C, et al. Comparison of intravesical instillation of hyaluronic acid with intradetrusor botulinum toxin A injection or cystoscopic hydrodistention for ketamine-associated cystitis[J]. J Int Med Res, 2020, 48(11): 300060520973100.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lv YS, Zhou HL, Mao HP, et al. Intravesical hyaluronic acid and alkalinized lidocaine for the treatment of severe painful bladder syndrome/interstitial cystitis. Int Urogynecol J. 2012;23(12):1715–1720. doi: 10.1007/s00192-012-1802-3. [Lv YS, Zhou HL, Mao HP, et al. Intravesical hyaluronic acid and alkalinized lidocaine for the treatment of severe painful bladder syndrome/interstitial cystitis[J]. Int Urogynecol J, 2012, 23(12): 1715-1720.] [DOI] [PubMed] [Google Scholar]
- 17.Downey A, Hennessy DB, Curry D, et al. Intravesical chondroitin sulphate for interstitial cystitis/painful bladder syndrome. Ulster Med J. 2015;84(3):161–163. [Downey A, Hennessy DB, Curry D, et al. Intravesical chondroitin sulphate for interstitial cystitis/painful bladder syndrome[J]. Ulster Med J, 2015, 84(3): 161-163.] [PMC free article] [PubMed] [Google Scholar]
- 18.Nickel JC, Jain P, Shore N, et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: Safety and efficacy of a new drug delivery device. Sci Transl Med. 2012;4(143):143ra100. doi: 10.1126/scitranslmed.3003804. [Nickel JC, Jain P, Shore N, et al. Continuous intravesical lidocaine treatment for interstitial cystitis/bladder pain syndrome: Safety and efficacy of a new drug delivery device[J]. Sci Transl Med, 2012, 4(143): 143ra100.] [DOI] [PubMed] [Google Scholar]
- 19.Niimi A, Nomiya A, Yamada Y, et al. Hydrodistension with or without fulguration of hunner lesions for interstitial cystitis: Long-term outcomes and prognostic predictors. Neurourol Urodyn. 2016;35(8):965–969. doi: 10.1002/nau.22837. [Niimi A, Nomiya A, Yamada Y, et al. Hydrodistension with or without fulguration of hunner lesions for interstitial cystitis: Long-term outcomes and prognostic predictors[J]. Neurourol Urodyn, 2016, 35(8): 965-969.] [DOI] [PubMed] [Google Scholar]
- 20.Ham BK, Kim JH, Oh MM, et al. Effects of combination treatment of intravesical resiniferatoxin instillation and hydrodistention in patients with refractory painful bladder syndrome/interstitial cystitis: A pilot study. Int Neurourol J. 2012;16(1):41–46. doi: 10.5213/inj.2012.16.1.41. [Ham BK, Kim JH, Oh MM, et al. Effects of combination treatment of intravesical resiniferatoxin instillation and hydrodistention in patients with refractory painful bladder syndrome/interstitial cystitis: A pilot study[J]. Int Neurourol J, 2012, 16(1): 41-46.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Evans RJ, Overholt T, Colaco M, et al. Injection location does not impact botulinum toxin A efficacy in interstitial cystitis/bladder pain syndrome patients. Can J Urol. 2020;27(1):10125–10129. [Evans RJ, Overholt T, Colaco M, et al. Injection location does not impact botulinum toxin A efficacy in interstitial cystitis/bladder pain syndrome patients[J]. Can J Urol, 2020, 27(1): 10125-10129.] [PubMed] [Google Scholar]
- 22.Rahnamai MS, Marcelissen T, Apostolidis A, et al. The efficacy of botulinum toxin A and sacral neuromodulation in the management of interstitial cystitis (IC)/bladder pain syndrome (BPS), what do we know? ICI-RS 2017 think thank, Bristol. Neurourol Urodyn. 2018;37(Suppl 4):S99–S107. doi: 10.1002/nau.23493. [Rahnamai MS, Marcelissen T, Apostolidis A, et al. The efficacy of botulinum toxin A and sacral neuromodulation in the management of interstitial cystitis (IC)/bladder pain syndrome (BPS), what do we know? ICI-RS 2017 think thank, Bristol[J]. Neurourol Urodyn, 2018, 37(Suppl 4): S99-S107.] [DOI] [PubMed] [Google Scholar]
- 23.Peters KM, Jayabalan N, Bui D, et al. Effect of sacral neuro-modulation on outcome measures and urine chemokines in interstitial cystitis/painful bladder syndrome patients. Low Urin Tract Symptoms. 2015;7(2):77–83. doi: 10.1111/luts.12054. [Peters KM, Jayabalan N, Bui D, et al. Effect of sacral neuro-modulation on outcome measures and urine chemokines in interstitial cystitis/painful bladder syndrome patients[J]. Low Urin Tract Symptoms, 2015, 7(2): 77-83.] [DOI] [PubMed] [Google Scholar]
- 24.Xu R, Schachar J, Evans RJ, et al. Hydrodistention does not alter bladder gene expression profiles in patients with non-Hunner lesion interstitial cystitis/bladder pain syndrome. Neurourol Urodyn. 2021;40(5):1126–1132. doi: 10.1002/nau.24680. [Xu R, Schachar J, Evans RJ, et al. Hydrodistention does not alter bladder gene expression profiles in patients with non-Hunner lesion interstitial cystitis/bladder pain syndrome[J]. Neurourol Urodyn, 2021, 40(5): 1126-1132.] [DOI] [PubMed] [Google Scholar]
- 25.Walker SJ, Plair A, Hemal K, et al. Bladder hydrodistention does not result in a significant change in bladder capacity for interstitial cystitis/bladder pain syndrome patients. Urology. 2019;132:81–86. doi: 10.1016/j.urology.2019.06.031. [Walker SJ, Plair A, Hemal K, et al. Bladder hydrodistention does not result in a significant change in bladder capacity for interstitial cystitis/bladder pain syndrome patients[J]. Urology, 2019, 132: 81-86.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang MC, Hsieh CH, Chang WC, et al. Assessment of treatment outcomes of interstitial cystitis with hydrodistention and bladder training by O'Leary-Sant interstitial cystitis symptom and problem indices. Taiwan J Obstet Gynecol. 2018;57(5):718–721. doi: 10.1016/j.tjog.2018.08.019. [Huang MC, Hsieh CH, Chang WC, et al. Assessment of treatment outcomes of interstitial cystitis with hydrodistention and bladder training by O'Leary-Sant interstitial cystitis symptom and problem indices[J]. Taiwan J Obstet Gynecol, 2018, 57(5): 718-721.] [DOI] [PubMed] [Google Scholar]
- 27.Yu WR, Jhang JF, Ho HC, et al. Cystoscopic hydrodistention characteristics provide clinical and long-term prognostic features of interstitial cystitis after treatment. Sci Rep. 2021;11(1):455. doi: 10.1038/s41598-020-80252-x. [Yu WR, Jhang JF, Ho HC, et al. Cystoscopic hydrodistention characteristics provide clinical and long-term prognostic features of interstitial cystitis after treatment[J]. Sci Rep, 2021, 11(1): 455.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee SW, Kim WB, Lee KW, et al. Long-term outcomes of ulcerative interstitial cystitis after complete transurethral resection with therapeutic hydrodistention. Int Urol Nephrol. 2021;53(2):219–227. doi: 10.1007/s11255-020-02637-1. [Lee SW, Kim WB, Lee KW, et al. Long-term outcomes of ulcerative interstitial cystitis after complete transurethral resection with therapeutic hydrodistention[J]. Int Urol Nephrol, 2021, 53(2): 219-227.] [DOI] [PubMed] [Google Scholar]
- 29.Keller J, Chiou HY, Lin HC. Increased risk of bladder cancer following diagnosis with bladder pain syndrome/interstitial cystitis. Neurourol Urodyn. 2013;32(1):58–62. doi: 10.1002/nau.22283. [Keller J, Chiou HY, Lin HC. Increased risk of bladder cancer following diagnosis with bladder pain syndrome/interstitial cystitis[J]. Neurourol Urodyn, 2013, 32(1): 58-62.] [DOI] [PubMed] [Google Scholar]