Abstract
This study aimed to conduct a survival analysis of thoracic esophageal squamous cell carcinoma (ESCC) patients treated with radical chemoradiotherapy and identify prognostic variables from among the hematological and radiation parameters. Cases of patients with ESCC receiving definitive chemoradiotherapy at Jiangsu Cancer Hospital between January 2018 and September 2020 were screened. A Cox proportional hazards model was used to assess the effect of hematological and radiation parameters on the overall survival (OS). The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the absolute neutrophil count (ANC) by the absolute lymphocyte count (ALC) in the week prior to radical chemoradiotherapy. Variables associated with radiation were gathered based on dose-volume histograms (DVH). X-tile software was used to determine the optimal cutoff values for pretreatment NLR and posttreatment ALC nadir. Associations between lymphopenia and dose-volume parameters were analyzed using multivariate logistic regression. The study included 104 ESCC patients. The median follow-up of surviving patients was 45.0 months (interquartile range: 40.2-52.2), with 1- and 3-year OS rates of 88.0% and 62.7%, respectively. Multivariate Cox regression analysis demonstrated a significant survival benefit in patients with low baseline NLR (≤ 2.2), high ALC nadir (> 0.24*109/L), and desirable radiation parameters for the heart and thoracic vertebrae. Increased dose-volume parameters of the heart, lungs, and thoracic vertebrae were correlated with a high probability of radiation-induced lymphopenia (RIL) risk (P < 0.05). Baseline NLR and RIL are significantly related to survival outcomes in ESCC patients. Optimization of radiation parameters of cardiopulmonary and thoracic vertebrae can be effective in the prevention of RIL.
Keywords: Hematological and radiation parameters, esophageal squamous cell carcinoma, prognosis, radical chemoradiotherapy
Introduction
An estimated 604,000 new cases and 544,000 fatalities of esophageal cancer (EC) had been recorded worldwide in 2020 [1], and approximately 320,000 new cases of EC were identified in China, which made it the fourth leading cause of cancer death in this country [2]. Squamous cell carcinoma was present in 90% of these cases in China [3]. Although concurrent chemoradiotherapy has somewhat improved the local control rate and survival rates of inoperable locally advanced esophageal squamous cell carcinoma (ESCC) to a certain extent [4,5], the prognosis of some patients is still not very satisfactory. Therefore, studies exploring the correlation between biological indicators and tumor prognosis have attracted extensive attention from scholars.
In recent years, the important role of immune and inflammatory responses in the tumor microenvironment has been gradually discovered. The systemic inflammatory response promotes vascular proliferation, DNA damage, and tumor invasion through the upregulation of cytokines [6]. Among many inflammatory indicators, studies have confirmed the significant correlation between the neutrophil-to-lymphocyte ratio (NLR) and malignancy, which can provide important information for prognosis [7-9]. The absolute neutrophil count and absolute lymphocyte count (ALC) reflect the tumor-induced inflammatory response and the level of antitumor immunity, respectively. Thus, the NLR can be used to determine whether the two variables are balanced. Radiation can suppress host immunity by killing immune cells, particularly cytotoxic T lymphocytes [10]. Radiation-induced lymphopenia (RIL) is a common hematologic toxicity because peripheral lymphocytes are known to be the most radiosensitive cells. The ALC nadir has been proven to be correlated with poor survival in a wide variety of malignancies, such as glioblastoma and cervical and non-small cell lung cancers [11-13]. This study aimed to explore the effect of peripheral hematologic indicators and radiation parameters on the overall survival (OS) of ESCC patients receiving radical chemoradiotherapy and determine the relationship between lymphopenia and radiation parameters.
Patients and methods
Patient selection
We retrospectively analyzed the medical records of 104 patients who underwent radical chemoradiotherapy at Cancer Hospital affiliated of Nanjing Medical University from January 2018 to September 2020. The specific inclusion criteria were: (1) 18 to 75 years of age; (2) histologically confirmed esophageal squamous cell carcinoma; (3) clinical stage II to IVA (American Joint Committee on Cancer, 8th edition); (4) the primary esophageal focus was limited to the thoracic segment; (5) Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2; (6) completed radical intensity-modulated radiotherapy radiotherapy (IMRT) no less than 50 Gy; (7) no serious hematopoietic, cardiac, pulmonary, hepatic or renal dysfunction; and adequate bone marrow function; (8) had retrievable full blood counts and radiation parameters (cardiac, lung and whole body dose). Primary exclusion criteria included patients who underwent surgery or lacked complete information on complete blood counts and dose-volume histogram (DVH) or had an immature follow-up period.
Definitive concurrent chemoradiotherapy
Radiotherapy was conducted using a Varian linear accelerator. All 104 cases were treated with involved-field radiotherapy (IFI) using the IMRT technique. Target volumes were defined in accordance with the International Commission on Radiation Units (ICRU) and Measurements Report #62. The gross tumor volume (GTV) was defined as including the primary tumor and all metastatic lymph nodes (the short diameter of the lymph node local in the tracheoesophageal sulcus ≥ 5 mm, in the mediastinum ≥ 1 cm, and biopsy-confirmed metastatic lymph nodes). The clinical target volume (CTV) included a 3-cm cephalad and caudad margin beyond GTV (without giving prophylactic irradiation). The planning target volume (PTV), defined by a 1-cm margin around CTV, was established. The field next to the spinal cord could be slightly adjusted to avoid excessive exposure. Dose limitations for the critical organs were as follows: (1) the V20 (percentage of the total lung volume receiving over 20 Gy) of lungs was ≤ 25%; (2) the V30 of the heart was ≤ 40%; (3) the V30 of the liver was ≤ 30%; and (4) the maximum spinal cord dose was ≤ 45 Gy.
Concurrent chemotherapy was conducted on the first day of radiotherapy: paclitaxel (175 mg/m2), continuous intravenous drip for 3 hours, day 1; cisplatin (25 mg/m2), i.v.drip., days 1 to 3. Cycles were duplicated every 4 weeks, for 2 courses altogether.
Dosimetric analysis
Critical organs were outlined on each axial section of the simulated CT scan. For example, for the heart, the superior side starts at the level of the inferior border of the pulmonary artery and extends down through the midline to the apical part of the heart. DVH of the organs at risk (OARs) were subsequently generated using the treatment planning system.
Data collection
The following clinical characteristics were obtained: age, gender, tumor site, tumor length, stage, ECOG performance status, etc. ALC and ANC were recorded within one week before definitive chemoradiotherapy (dCRT). The nadir of ALC was the lowest, appearing within two months after the dCRT started. The NLR was calculated by dividing the ANC by the ALC. The following radiotherapy-related variables were assessed based on the DVH: mean heart dose (MHD), mean lung dose (MLD), mean thoracic vertebrae dose (MTVD), V30 of heart, V20 of lung and V20 of thoracic vertebrae.
Statistical analysis
Categorical variables were descriptively analyzed by frequency and proportion. Median and inter-quartile range (IQR) were used to summarize continuous variables. X-tile 3.6.1 software (Yale University, New Haven, CT, USA) was used to determine the best critical value of pretreatment NLR and ALC nadir. On this basis, the receiver operating characteristics (ROC) curve was used to determine the cut-off points for radiation parameters with ALC nadir as the state variable. Survival curves were plotted using the Kaplan-Meier method, with log-rank tests used to compare OS in subgroups, and hazard ratios (HR) were estimated using Cox regression models. SPSS 26.0 was used for data analysis.
Results
Baseline characteristics
Of the 104 thoracic ESCC patients enrolled in our study, the majority were male (70.2%). Table 1 lists the baseline characteristics of the patients included in this work. The median age of all subjects was 65 years (range: 45-76). For the majority of patients, the tumor was in clinical stage III (49.0%), followed by those in clinical stage IVa (31.7%), and only 20 subjects had clinical stage II tumors (19.2%). The median tumor length was 5 cm (range: 1-8), and the tumor of 53 cases was located in the upper thoracic segment. The overwhelming of patients (93.3%) received radiotherapy with a prescribed dose of more than 60 Gy.
Table 1.
Baseline characteristics
| Characteristics | n (%) |
|---|---|
| Age (years) | |
| Median (range) | 65 (45-76) |
| Sex | |
| Male | 73 (70.2%) |
| Female | 31 (29.8%) |
| ECOG performance score | |
| 0-1 | 97 (93.3%) |
| 2 | 7 (6.7%) |
| Clinical stage (AJCC, 8th) | |
| II | 20 (19.2%) |
| III | 51 (49.0%) |
| IVa | 33 (31.7%) |
| Tumor length (cm) | |
| < 5 | 50 (48.1%) |
| ≥ 5 | 54 (51.9%) |
| Tumor location | |
| Upper (< 25 cm) | 53 (51.0%) |
| Middle (25-30 cm) | 33 (31.7%) |
| Lower (> 30 cm) | 15 (14.4%) |
| Multiple primary | 3 (2.9%) |
| Prescribed RT dose (Gy) | |
| < 60 | 7 (6.7%) |
| > 60 | 97 (93.3%) |
AJCC, American Joint Committee on Cancer.
OS and optimal cutoff values
As of March 1, 2023, the median follow-up for surviving patients was 45.0 months (IQR, 40.2-52.2, 95% confidence interval (CI) [30.7-37.0]). In the entire patient cohort, the median OS was not reached, with the 1- and 3-year OS rates being 88.0% and 62.7%, respectively. The median progression-free survival (PFS) was 39.2 months, with the 1-year and 3-year PFS rates of 77.8% and 51.5%, respectively (Supplementary Figure 1).
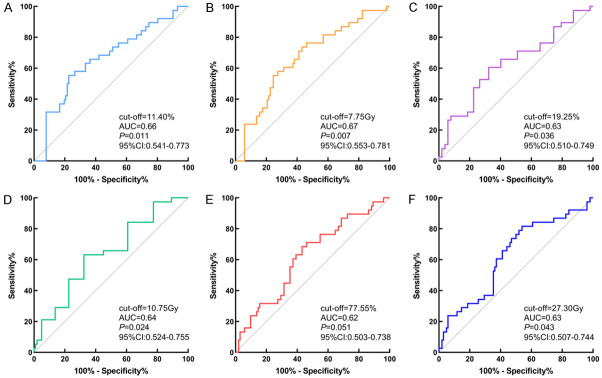
To dichotomize the hematologic indicators, we determined the optimal cutoff values of 2.2 for pretreatment NLR, 0.24*109/L for ALC nadir, and 91.3% for the maximum reduction in ALC pre- and post-treatment using the X-tile 3.6.1 software (Yale University, New Haven, CT, USA). The critical value of ALC nadir was subsequently used as a state variable in the ROC curve analysis to identify the cutoff values of the relevant radiation parameters (Figure 1). Consequently, the predictors of avoiding an ALC nadir below 0.24*109/L were as follows: heart V30 < 11.4%, MHD < 7.75 Gy, lung V20 < 19.25%, MLD < 10.75 Gy, thoracic vertebrae V20 < 77.55%, and MTVD < 27.30 Gy.
Figure 1.
ROC curve analysis to identify the cut-off values of the relevant radiation parameters. (A) Heart V30, (B) MHD, (C) Lung V20, (D) MLD, (E) Throcic vertebrae V20, (F) MTVD.
Predictive significance of cutoff values
Concerning Figures 2 and 3, the Kaplan-Meier curves for OS revealed that patients in the low baseline NLR group, high ALC nadir group, group with a greater proportion of lymphocyte count decline, and desirable radiation parameters for cardiopulmonary and thoracic vertebrae had longer OS. Table 2 summarizes the analysis of the factors associated with OS. Univariate analysis based on the Cox regression model indicated that ECOG score, tumor length, ALC nadir, and radiation parameters of the cardiopulmonary and thoracic vertebrae were associated with OS. The results of the multivariate analysis (Figure 4) showed that the independent indicators of OS include ECOG score (HR=0.30, 95% CI=0.10-0.85, P=0.024), baseline NLR (HR=0.33, 95% CI=0.15-0.77, P=0.010), degree of ALC decline (HR=0.36, 95% CI=0.15-0.89, P=0.026), heart V30 (HR=0.06, 95% CI=0.00-0.98, P=0.048), MHD (HR=0.04, 95% CI=0.00-0.39, P=0.007), thoracic vertebrae V20 (HR=0.28, 95% CI=0.09-0.83, P=0.022), and cycles of chemotherapy (HR=0.24, 95% CI=0.09-0.62, P=0.003).
Figure 2.
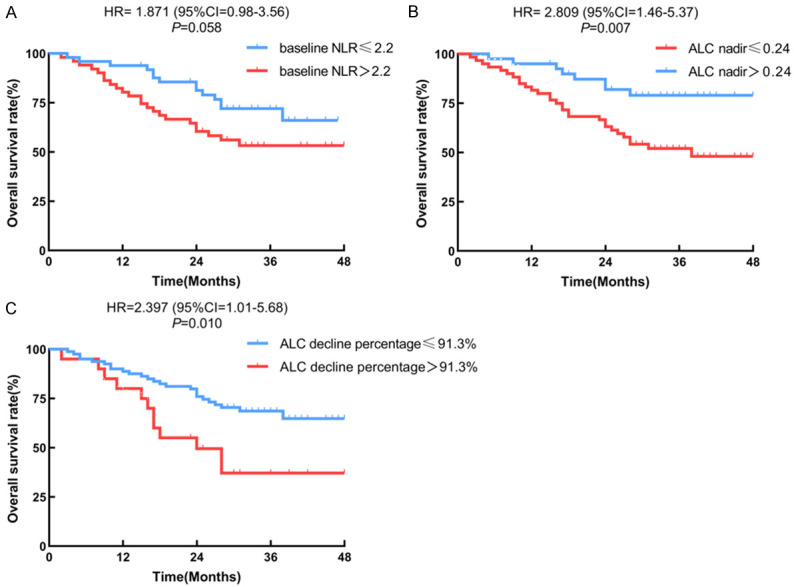
Kaplan-Meier curves of overall survival for subgroup populations (hematological parameters). (A) Baseline NLR, (B) ALC Nadir, (C) ALC decline percentage.
Figure 3.
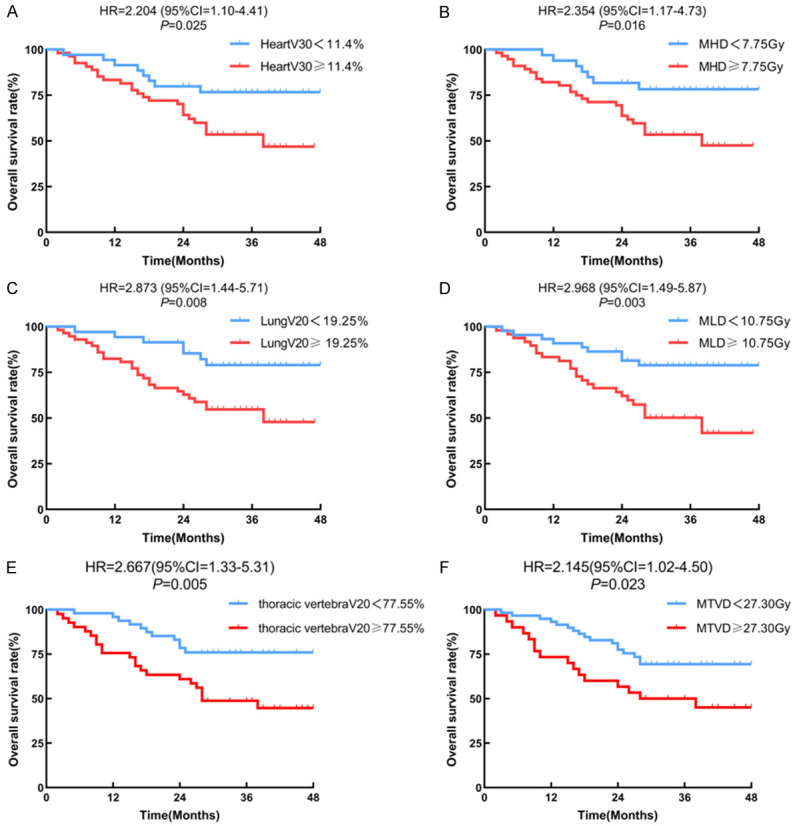
Kaplan-Meier curves of overall survival for subgroup populations (radiation parameters). (A) Heart V30, (B) MHD, (C) Lung V20, (D) MLD, (E) Throcic vertebrae V20, (F) MTVD.
Table 2.
Univariate Cox regression analysis of factors associated with OS
| Baseline characteristics | x2 value | P value | HR (95% CI) |
|---|---|---|---|
| Age, years (< 65 vs. ≥ 65) | 0.818 | 0.366 | 0.730 (0.369-1.445) |
| Sex (male vs. female) | 0.179 | 0.672 | 1.180 (0.548-2.538) |
| ECOG score (0-1 vs. 2) | 10.465 | 0.001 | 0.229 (0.094-0.559) |
| Tumor site (middle vs. non-middle) | 1.889 | 0.169 | 1.623 (0.813-3.240) |
| Clinical stage (II vs. III-IVa) | 1.642 | 0.200 | 0.504 (0.177-1.437) |
| Tumor length, cm (< 5 vs. ≥ 5) | 6.858 | 0.009 | 0.358 (0.166-0.772) |
| Baseline NLR (≤ 2.2 vs. > 2.2) | 3.189 | 0.074 | 0.529 (0.263-1.064) |
| ALC nadir, *109/L (≤ 0.24 vs. > 0.24) | 5.935 | 0.015 | 2.692 (1.214-5.970) |
| ALC decline percentage (≤ 91.3 vs. > 91.3) | 2.767 | 0.091 | 0.531 (0.252-1.119) |
| Heart V30, % (< 11.4 vs. ≥ 11.4) | 4.610 | 0.032 | 0.417 (0.188-0.927) |
| MHD, Gy (< 7.75 vs. ≥ 7.75) | 5.281 | 0.022 | 0.375 (0.163-0.866) |
| Lung V20, % (< 19.25 vs. ≥ 19.25) | 5.116 | 0.024 | 0.381 (0.165-0.879) |
| MLD, Gy (< 10.75 vs. ≥ 10.75) | 6.715 | 0.010 | 0.362 (0.168-0.781) |
| Thoracic vertebrae V20, % (< 77.55 vs. ≥ 77.55) | 7.097 | 0.008 | 0.373 (0.181-0.771) |
| MTVD, Gy (< 27.30 vs. ≥ 27.30) | 4.833 | 0.028 | 0.464 (0.234-0.920) |
| Chemotherapy cycles (1-2 vs. 3-4) | 3.001 | 0.083 | 2.098 (0.907-4.854) |
Figure 4.
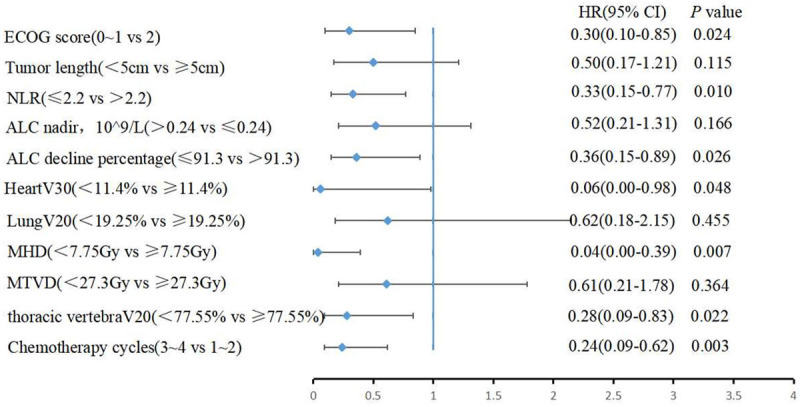
Multivariate Cox regression analysis of OS-related factors.
Multiple logistic regression analysis of lymphopenia
The prevalence of grades 1-2, 3, and 4 lymphopenia reached 9%, 45%, and 46%, respectively, in the whole patient cohort. To further attain more insights into the effect of relevant radiation parameters on lymphopenia, a multiple logistic regression analysis was performed. The results are presented in Table 3, which shows that where increased cardiopulmonary and thoracic vertebrae exposure doses are risk factors for the development of lymphopenia (given the proven multilinear relationship between the relevant independent variables, all radiation parameters were excluded in the analysis to avoid the role of interfering factors).
Table 3.
Multivariate logistic regression of the risk factors related to lymphopenia
| Variables | Multiple Regression | |
|---|---|---|
|
| ||
| RR (95% CI) | P | |
| MHD | 2.321 (1.163-4.629) | 0.017 |
| Lung V20 | 2.266 (1.130-4.545) | 0.021 |
| MTVD | 2.722 (1.378-5.378) | 0.004 |
Correlation of lymphopenia with acute radiation side effects
In this study, the incidences of grade 2 and above radiation skin reaction, radiation esophagitis, and radiation pneumonia in patients during radiotherapy were 5.8%, 49.0%, and 25.0%, respectively. Based on the results of the ROC analysis, the cutoff of RIL was somewhat predictive of grade 2 and above radiation pneumonitis, but the overall predictive value was faint (Supplementary Figure 2).
Discussion
With the development of radiotherapy technology and the continuous optimization of chemotherapy regimens, dCRT has become essential in inoperable locally advanced ESCC. However, some patients still require further improvement in their prognosis. This study investigated the impact of peripheral blood indicators during radiotherapy, including baseline NLR, ALC nadir, and degree of lymphopenia, on the OS of ESCC patients. We also examined the correlation between RIL and radiation parameters to provide a reference to better optimize radiotherapy planning to mitigate radiation-induced immunosuppression in the future.
Systemic inflammatory responses play an important role in tumorigenesis, progression, and metastasis [6]. NLR, a marker of the systemic inflammatory response, is straightforward, easy to obtain, and valuable in determining the treatment outcomes and predicting the prognosis of cancer patients. Patients in this retrospective study with lower NLR status had a more significant survival benefit compared to those with high baseline NLR values. Furthermore, it was established by the multivariate Cox regression analysis that baseline NLR is one of the independent prognostic factors for patients with ESCC.
A recent study on radiotherapy for esophageal cancer reported the presence of lymphopenia in 15.4% of patients before radiotherapy [14]. The value was 7.7% in our study, and thus, patients with concomitant lymphopenia before treatment had a significantly worse survival than those without lymphopenia (P=0.070). The reasons for the malignant tumors accompanying lymphopenia before antitumor therapy are currently ambiguous and may be related to either the direct induction of T lymphocyte apoptosis by malignancy via the FAS/FASL pathway or the increased levels of infiltrating lymphocytes from the peripheral blood circulation into the malignant tissues due to a collective immune response caused by cancer progression, which results in peripheral lymphopenia. Notably, the nutritional status of the organism itself should not be ignored.
Lymphocytes are the most radiosensitive hematopoietic cells, and they are typically depleted by radiation at a 50% lethal dose of 1 Gy to 2 Gy [15]. Treatment-related hemocytopenia and immunosuppression in thoracic malignancies usually predict a poor survival outcome. Our findings also revealed that patients with a low ALC nadir and heavy lymphopenia during radiotherapy are generally accompanied by an unsatisfactory prognosis. Furthermore, with the development of immunotherapy, radiation-induced hematological toxicity may have a more profound influence. Although most previous studies on radiation-related hematological toxicity focused on myelosuppression, radiotherapy can produce complex and long-lasting changes to the systemic immune system. For instance, the activity of lymphocytes in patients with Hodgkin’s lymphoma continues to be affected even though their counts recover to normals after radiotherapy [16,17]. In addition to immunosuppressive effects, immunostimulatory roles have also been documented for radiotherapy. Radiotherapy exerts a strong and substantial antitumor effects by boosting the immunogenicity of cancer cells [18]. Furthermore, the radiation-induced modulation of the tumor microenvironment can promote the recruitment of immune cells to the tumor and enhance the tumor cell detection and eradication by immune cells [19]. Preclinical studies have demonstrated that programmed death-ligand 1 expression is upregulated in the tumor microenvironment after radiotherapy [20,21]. Therefore, with the increased development of immunotherapy, eliminating the immunosuppressive effects of radiotherapy is essential to achieving the optimal combined antitumor effect [22].
Thoracic malignancies that are encompassed in the radiation portal, such as esophageal, lung, and left-sided breast cancers, frequently receive radiation close to the heart. Previous studies have highlighted the detrimental consequences of lung or heart radiation dose-volume in lung cancer radiotherapy [23]. Given that thoracic vertebrae radiation dose is also a potential driver of bone marrow suppression, this study attempted to explore the correlation between the occurrence of lymphopenia during radiotherapy and cardiopulmonary and thoracic vertebrae parameters. According to with our research findings, the high radiation parameters of the cardiopulmonary and thoracic vertebrae will result in severe lymphopenia. Thus, the prevention of severe RIL by continuous optimization of the radiotherapy plan may be one of the future directions to improving the prognosis of ESCC. A randomized phase III clinical trial (RTOG 0617) also revealed the potential of radiation to act as a risk factor for reducing immune function [24].
The range of radiotherapy targets for EC, including cervical, mediastinal, and upper abdominal lymph nodes, is broad and may contain multiple OARs. In clinical practice, the radiation target volume for oesophageal cancer, particularly whether in elective nodal irradiation (ENI) or involved field irradiation (IFI), is still the subject of ongoing debate. However, previous studies have confirmed that ENI did not alter the failure patterns and survival outcomes of locally advanced esophageal cancer patients treated with radical radiotherapy [25-28]. IFI has a smaller radiation target volume than ENI, and in our retrospective study that included all patients with IFI, the incidence of grade 4 lymphocytopenia was 46%, which is lower than that in previous research on concurrent radiotherapy [23]. All things considered, IFI may be a better option for reducing the incidence of RIL than ENI.
The study encountered several limitations. First, as a single-center retrospective research, selection bias was observed. Second, given that the included patients all received dCRT, exploring whether the presence or absence of concurrent chemotherapy during radiotherapy had a significant effect on the incidence of RIL in patients was impossible. Finally, as a result of sample size limitations, we did not include additional radiation parameters for analysis. Other than that, in our study, the dosimetric parameters of the lungs reflected the statistically significant differences in the univariate analysis only, but not in the multivariate Cox regression. We believe that this finding can be attributed to: (1) when the variables of the heart and lungs were simultaneously entered into the Cox regression, an interaction occurred between them, which interfered with the outcome; and/or (2) the relative contribution of radiation received by the lungs to the outcome was likely to be relatively small compared with that of the lung in ESCC patients receiving radical radiotherapy.
Conclusion
Our study showed that baseline NLR and RIL are significantly associated with survival outcomes in ESCC patients. The ALC nadir during radiotherapy can be predicted using the radiation parameters of the cardiopulmonary and thoracic vertebrae, and safeguarding the immune system by continuously improving the radiotherapy plans may be a significant future path to enhancing the prognosis of the EC population.
Acknowledgements
This study was supported by National Natural Science Foundation of China project (82273162), Wu Jieping Medical Foundation project (320.6750.19194-60), and Jiangsu Province “six talent peaks” innovative talent team project (TD-SWYY-007).
All patients signed informed consents prior to enrollment.
Disclosure of conflict of interest
None.
Abbreviations
- ESCC
esophageal squamous cell carcinoma
- OS
on overall survival
- NLR
neutrophil-to-lymphocyte ratio
- ANC
absolute neutrophil count
- ALC
absolute lymphocyte count
- LC
lymphocyte count
- DVH
dose-volume histograms
- RIL
radiation-induced lymphopenia
- MLD
mean lung dose
- NSCLC
non-small cell lung cancer
- AJCC
American Joint Committee on Cancer
- ECOG
Eastern Cooperative Oncology Group
- IMRT
intensity-modulated radiotherapy radiotherapy
- IFI
involved-field radiotherapy
- ICRU
International Commission on Radiation Units
- GTV
gross tumor volume
- CTV
clinical target volume
- PTV
planning target volume
- dCRT
definitive chemoradiotherapy
- MHD
mean heart dose
- IQR
inter-quartile range
- ROC
receiver operating characteristics
- HR
hazard ratios
- PFS
progression-free survival
- OARs
organs at risk
- ENI
elective nodal irradiation
Supporting Information
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Cao W, Chen HD, Yu YW, Li N, Chen WQ. Changing profiles of cancer burden worldwide and in China: a secondary analysis of the global cancer statistics 2020. Chin Med J (Engl) 2021;134:783–791. doi: 10.1097/CM9.0000000000001474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uhlenhopp DJ, Then EO, Sunkara T, Gaduputi V. Epidemiology of esophageal cancer: update in global trends, etiology and risk factors. Clin J Gastroenterol. 2020;13:1010–1021. doi: 10.1007/s12328-020-01237-x. [DOI] [PubMed] [Google Scholar]
- 4.Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr, Al-Sarraf M, Byhardt R, Russell AH, Beitler JJ, Spencer S, Asbell SO, Graham MV, Leichman LL. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281:1623–7. doi: 10.1001/jama.281.17.1623. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Ye J, Zhu Z, Zhao W, Zhou J, Wu C, Tang H, Fan M, Li L, Lin Q, Xia Y, Li Y, Li J, Jia H, Lu S, Zhang Z, Zhao K. Comparing paclitaxel plus fluorouracil versus cisplatin plus fluorouracil in chemoradiotherapy for locally advanced esophageal squamous cell cancer: a randomized, multicenter, phase III clinical trial. J. Clin. Oncol. 2019;37:1695–1703. doi: 10.1200/JCO.18.02122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferrucci PF, Gandini S, Battaglia A, Alfieri S, Di Giacomo AM, Giannarelli D, Cappellini GC, De Galitiis F, Marchetti P, Amato G, Lazzeri A, Pala L, Cocorocchio E, Martinoli C. Baseline neutrophil-to-lymphocyte ratio is associated with outcome of ipilimumab-treated metastatic melanoma patients. Br J Cancer. 2015;112:1904–10. doi: 10.1038/bjc.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo EJ, Park JC, Kim EH, Park CH, Shim CN, Lee HJ, Chung HS, Lee H, Shin SK, Lee SK, Lee CG, Lee YC. Prognostic value of neutrophil-to-lymphocyte ratio in patients treated with concurrent chemoradiotherapy for locally advanced oesophageal cancer. Dig Liver Dis. 2014;46:846–53. doi: 10.1016/j.dld.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Li KJ, Xia XF, Su M, Zhang H, Chen WH, Zou CL. Predictive value of lymphocyte-to-monocyte ratio (LMR) and neutrophil-to-lymphocyte ratio (NLR) in patients with oesophageal cancer undergoing concurrent chemoradiotherapy. BMC Cancer. 2019;19:1004. doi: 10.1186/s12885-019-6157-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cox S, Hurt C, Grenader T, Mukherjee S, Bridgewater J, Crosby T. The prognostic value of derived neutrophil to lymphocyte ratio in oesophageal cancer treated with definitive chemoradiotherapy. Radiother Oncol. 2017;125:154–159. doi: 10.1016/j.radonc.2017.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contreras JA, Lin AJ, Weiner A, Speirs C, Samson P, Mullen D, Campian J, Bradley J, Roach M, Robinson C. Cardiac dose is associated with immunosuppression and poor survival in locally advanced non-small cell lung cancer. Radiother Oncol. 2018;128:498–504. doi: 10.1016/j.radonc.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 11.Mendez JS, Govindan A, Leong J, Gao F, Huang J, Campian JL. Association between treatment-related lymphopenia and overall survival in elderly patients with newly diagnosed glioblastoma. J Neurooncol. 2016;127:329–335. doi: 10.1007/s11060-015-2037-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu ES, Oduyebo T, Cobb LP, Cholakian D, Kong X, Fader AN, Levinson KL, Tanner EJ 3rd, Stone RL, Piotrowski A, Grossman S, Roche KL. Lymphopenia and its association with survival in patients with locally advanced cervical cancer. Gynecol Oncol. 2016;140:76–82. doi: 10.1016/j.ygyno.2015.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang C, Liao Z, Gomez D, Levy L, Zhuang Y, Gebremichael RA, Hong DS, Komaki R, Welsh JW. Lymphopenia association with gross tumor volume and lung V5 and its effects on non-small cell lung cancer patient outcomes. Int J Radiat Oncol Biol Phys. 2014;89:1084–1091. doi: 10.1016/j.ijrobp.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Zhou XL, Zhu WG, Zhu ZJ, Wang WW, Deng X, Tao WJ, Ji FZ, Tong YS. Lymphopenia in esophageal squamous cell carcinoma: relationship to malnutrition, various disease parameters, and response to concurrent chemoradiotherapy. Oncologist. 2019;24:e677–e686. doi: 10.1634/theoncologist.2018-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yovino S, Grossman SA. Severity, etiology and possible consequences of treatment-related lymphopenia in patients with newly diagnosed high-grade gliomas. CNS Oncol. 2012;1:149–54. doi: 10.2217/cns.12.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuks Z, Strober S, Bobrove AM, Sasazuki T, McMichael A, Kaplan HS. Long term effects of radiation of T and B lymphocytes in peripheral blood of patients with Hodgkin’s disease. J Clin Invest. 1976;58:803–14. doi: 10.1172/JCI108532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoppe RT, Fuks ZY, Strober S, Kaplan HS. The long term effects of radiation of T and B lymphocytes in the peripheral blood after regional irradiation. Cancer. 1977;40:2071–8. doi: 10.1002/1097-0142(197711)40:5<2071::aid-cncr2820400513>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Deng W, Li N, Neri S, Sharma A, Jiang W, Lin SH. Combining immunotherapy and radiotherapy for cancer treatment: current challenges and future directions. Front Pharmacol. 2018;9:185. doi: 10.3389/fphar.2018.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liao XY, Liu CY, He JF, Wang LS, Zhang T. Combination of checkpoint inhibitors with radiotherapy in esophageal squamous cell carcinoma treatment: a novel strategy. Oncol Lett. 2019;18:5011–21. doi: 10.3892/ol.2019.10893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, Fu YX. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen MF, Chen PT, Chen WC, Lu MS, Lin PY, Lee KD. The role of PD-L1 in the radiation response and prognosis for esophageal squamous cell carcinoma related to IL-6 and T-cell immunosuppression. Oncotarget. 2016;7:7913–24. doi: 10.18632/oncotarget.6861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schaue D, Ratikan JA, Iwamoto KS, McBride WH. Maximizing tumor immunity with fractionated radiation. Int J Radiat Oncol Biol Phys. 2012;83:1306–10. doi: 10.1016/j.ijrobp.2011.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davuluri R, Jiang W, Fang P, Xu C, Komaki R, Gomez DR, Welsh J, Cox JD, Crane CH, Hsu CC, Lin SH. Lymphocyte nadir and esophageal cancer survival outcomes after chemoradiation therapy. Int J Radiat Oncol Biol Phys. 2017;99:128–135. doi: 10.1016/j.ijrobp.2017.05.037. [DOI] [PubMed] [Google Scholar]
- 24.Thor M, Deasy JO, Hu C, Gore E, Bar-Ad V, Robinson C, Wheatley M, Oh JH, Bogart J, Garces YI, Kavadi VS, Narayan S, Iyengar P, Witt JS, Welsh JW, Koprowski CD, Larner JM, Xiao Y, Bradley J. Modeling the impact of cardiopulmonary irradiation on overall survival in NRG oncology trial RTOG 0617. Clin Cancer Res. 2020;26:4643–4650. doi: 10.1158/1078-0432.CCR-19-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao KL, Ma JB, Liu G, Wu KL, Shi XH, Jiang GL. Three-dimensional conformal radiation therapy for esophageal squamous cell carcinoma: is elective nodal irradiation necessary. Int J Radiat Oncol Biol Phys. 2010;76:446–51. doi: 10.1016/j.ijrobp.2009.02.078. [DOI] [PubMed] [Google Scholar]
- 26.Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, Nakagawa K. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study. Radiat Oncol. 2015;10:171. doi: 10.1186/s13014-015-0482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashita H, Okuma K, Wakui R, Kobayashi-Shibata S, Ohtomo K, Nakagawa K. Details of recurrence sites after elective nodal irradiation (ENI) using 3D-conformal radiotherapy (3D-CRT) combined with chemotherapy for thoracic esophageal squamous cell carcinoma-a retrospective analysis. Radiother Oncol. 2011;98:255–60. doi: 10.1016/j.radonc.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 28.Cheng YJ, Jing SW, Zhu LL, Wang J, Wang L, Liu Q, Yang CR, Wang Y, Cao F, Jiao WP, Wu YJ. Comparison of elective nodal irradiation and involved-field irradiation in esophageal squamous cell carcinoma: a meta-analysis. J Radiat Res. 2018;59:604–15. doi: 10.1093/jrr/rry055. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.