Abstract
Serum urate is a risk factor for hypertension and gout. The DASH diet and losartan independently lower blood pressure (BP); however, their effects on serum urate are understudied. We performed a post‐hoc analysis of the DASH‐losartan trial, which randomized participants with hypertension in parallel fashion to the DASH diet or a standard American diet (control) and in crossover fashion to 4‐week losartan or placebo. Serum urate was measured at baseline and after each 4‐week period. Diets were designed to maintain weight constant. We examined the effects of DASH (vs control) and/or losartan (vs placebo) on serum urate, overall and among those with baseline serum urate ≥6 mg/dL, using generalized estimating equations. Of 55 participants (mean age 52 years, 58% women, 64% Black), mean (±SD) baseline ambulatory SBP/DBP was 146±12/91±9 and mean (±SD) serum urate was 5.2±1.2 mg/dL. The DASH diet did not significantly reduce urate levels overall (mean difference −0.05 mg/dL; 95%CI: −0.39, 0.28), but did decrease levels among participants with baseline hyperuricemia (−0.33 mg/dL; 95%CI: −0.87, 0.21; P‐interaction=0.007 across hyperuricemia groups). Losartan significantly decreased serum urate (−0.23 mg/dL; 95%CI: −0.40, −0.05) with greater effects on serum urate among adults <60 years old versus adults ≥60 years old (−0.33 mg/dL vs 0.16 mg/dL, P interaction = 0.003). In summary, the DASH diet significantly decreased serum urate among participants with higher urate at baseline, while losartan significantly reduced serum urate, especially among younger adults. Future research should examine the effects of these interventions in patients with hyperuricemia or gout.
Keywords: diet, losartan, trial, urate
1. INTRODUCTION
Gout and hyperuricemia commonly occur in persons with hypertension. Gout is a painful arthropathy affecting 7.7 million adults in the United States between 2007−2020. 1 Moreover, the prevalence of gout is rising, mirroring trends in hyperuricemia and cardiovascular risk factors among the US population. 2 , 3 Hypertension is a particularly common comorbidity, present among over 70% of adults with gout. 4 The co‐occurrence of hypertension and gout has driven interest in identifying lifestyle and pharmacologic strategies that might benefit both hypertension and hyperuricemia. However, prior dietary recommendations (e.g., a low purine diet) may worsen hypertension, while some antihypertensive agents (e.g., thiazide diuretics and beta blockers), may worsen hyperuricemia and gout. 5 , 6 , 7 , 8
The DASH (Dietary Approaches to Stop Hypertension) diet is a well‐established dietary intervention to lower blood pressure, and it also has efficacy in lowering serum urate, particularly among adults with hyperuricemia. 3 In addition, losartan has known uricosuric effects, but the magnitude of its effects on serum urate have not been clearly shown. 9 Furthermore, it is possible that both the DASH diet and losartan in combination could have even greater effects on serum urate, as prior work demonstrated that the DASH diet combined with losartan were additive in lowering blood pressure, an effect magnified in Black individuals. 10 However, the impact of both interventions on serum urate is unknown.
Using data from the DASH‐Losartan trial, 10 we examined the effects, alone and combined, of the DASH diet (vs. a control diet) or losartan (vs. placebo) on serum urate. The objective of this study was to determine whether the DASH diet and/or losartan lowered serum urate levels among individuals with normal or high baseline serum urate. We hypothesized a priori that the DASH diet and losartan would lower serum urate levels to a greater extent among participants with baseline hyperuricemia.
2. METHODS
DASH‐Losartan was an investigator‐initiated, multi‐center trial conducted in three clinical centers (Baltimore, MD; Boston, MA, and Durham, NC) between September 1994, and March 1996. 10 The study was designed to test the hypothesis that inhibition of the renin angiotensin system would mitigate the effects of higher renin levels, observed with the DASH diet, and thus enhance blood pressure reduction. The study's primary results were previously published, however, the urate data collected at the time of the trial had never been analyzed. The DASH‐losartan trial was an independent trial from the DASH and DASH‐sodium trials conducted at about the same time. The trial protocol was reviewed and approved by Institutional Review Boards at each center. All participants provided written informed consent before entering into the study. The current study was determined by the Institutional Review Board of Beth Israel Deaconess Medical Center to be exempt research.
2.1. Core design
The study was a randomized, placebo‐controlled trial that compared the effects of losartan versus placebo in the context of the DASH diet or a typical American diet on blood pressure. After screening, there was a run‐in phase that lasted 2 weeks. Then, participants were randomized 1:1 following a parallel arm design to one of two diets (a control diet or the DASH diet) for 8 weeks. Concurrently, within each diet, participants were randomized in crossover fashion to sequence of medication (losartan, then placebo, or placebo, then losartan) with each period lasting 4 weeks. While the assignment to losartan or placebo was blinded, the dietary assignment was not blinded. However, all lab assessments were blinded to assignment for both drug and dietary contrasts.
2.2. Participants
DASH‐Losartan enrolled adults aged 22 years and older with a mean systolic blood pressure (SBP) < 180 mm Hg and mean diastolic blood pressure (DBP) of 90−109 mm Hg during each of three screening visits. Participants treated with anti‐hypertensive medications underwent withdrawal of antihypertensive medications prior to screening. Adults with a prior history of poorly controlled hyperlipidemia, diabetes mellitus, cardiovascular disease (e.g., myocardial infarction, coronary artery bypass graft, angioplasty, symptomatic ischemic heart disease, stroke, or congestive heart failure), or renal insufficiency were excluded from the trial. Serum urate was not a factor in the inclusion/exclusion criterion. DASH‐Losartan also excluded adults with a BMI > 35 kg/m2, those requiring antihypertensive agents or insulin during the trial, and persons consuming more than 14 alcoholic drinks a week. 10 , 11
2.3. Dietary interventions
Participants received prepared foods for all meals and snacks during the feeding phase of the study. All participants started a two‐week run‐in period on the standard American diet (the control diet) to assess adherence with the feeding protocol. The control diet was designed with potassium, magnesium, and calcium levels at the 25th percentile of U.S. consumption, and was consequently low in fruit, vegetables, and dairy products. Meanwhile, the macronutrient profile and fiber content for the control diet were kept at average U.S. consumption levels. By comparison, the DASH diet was comparatively higher in fruits and vegetables, emphasizing low‐fat dairy products, whole grains, fish, nuts, and poultry, with decreased levels of cholesterol and total and saturated fats. Sodium content, ∼3 g of sodium per day, was the same in both the control and DASH diets.
All meals were prepared and provided in metabolic kitchens at the three clinical centers. One meal/day was required to be consumed on‐site during weekdays, while prepared meals were eaten off‐site during weekends. Caloric intake of the meals was adjusted to maintain a constant weight. Participants continued their typical baseline physical activities through the 10‐week study. Dietary compliance was assessed through participants’ daily attendance at weekday, on‐site meals (once a week), attendance at study visits for BP measurement, and self‐reported adherence.
2.4. Losartan
At the start of the 8‐week intervention period, participants were also randomly assigned to receive 50 mg/day of losartan or placebo for 4 weeks. Four weeks into the 8‐week period, participants were switched in crossover fashion from losartan to placebo and vice versa. 10 , 11 The half‐life of losartan is 2 h with active metabolites having a half‐life of 6−9 h. 12
2.5. Serum urate measurement
The primary outcome measurement for this post hoc study was serum urate concentration. Serum specimens were obtained following the 2‐week run‐in period, at the 4‐week crossover period, and at the end of the 8‐week study. Fasting samples were obtained by trained staff via phlebotomy performed after 30 min of sitting. Serum urate was measured via spectrophotometry in fresh specimens at the time of the original DASH‐losartan trial. 10 , 11 , 13
2.6. Other covariate measurements and definitions
Study covariates were ascertained via physical measurements, laboratory specimens, and questionnaires. Blood pressure during screening visits was measured with participants seated for 5 min using a random‐zero sphygmomanometer. Blood pressure measurements were obtained at the end of each of the 4‐week intervention periods with ambulatory blood pressure monitoring. High blood pressure at baseline was defined by a mean systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg. Body mass index (BMI) was derived from standardized measurements of height and weight.
2.7. Statistical analyses
Study population characteristics were described using means (SD) and proportions. The main outcome of interest in the present study was the change in serum urate concentration. The main contrasts in our study were (1) the DASH diet versus the control diet: a parallel contrast with two follow‐up measurements at 4 weeks and 8 weeks; (2) losartan versus placebo: a crossover contrast comparing serum urate after 4 weeks of losartan to serum urate after 4 weeks of placebo; and (3) DASH‐Losartan versus control‐placebo: a parallel contrast comparing serum urate after 4 weeks of DASH and losartan to serum urate after 4 weeks of the control diet and placebo. In a sensitivity analysis, we also compared DASH‐Placebo versus control‐losartan (a parallel contrast).
We compared serum urate, using generalized estimating equations with robust standard errors and an exchangeable covariance matrix to account for correlated measures. These models yield valid standard errors even if the correlation structure is not as specified. 14
We repeated the DASH versus control and losartan versus placebo contrasts in strata of age (<60 or ≥60 years), sex (men or women), race (non‐Black or Black), obesity (body mass index < 30 or ≥30 kg/m2), and baseline hyperuricemia (<6 or ≥6 mg/dL). We assessed for interactions across strata using three‐way interaction terms and Wald tests (for dietary comparisons) or two‐way interaction terms for the losartan versus placebo comparisons. In a sensitivity analysis, we further examined the effect of the interventions by baseline hyperuricemia among Black participants alone, given the greater synergy of the two interventions on BP reported elsewhere. 10
Analyses were performed with Stata version 15.1 (Stata Corporation, College Station, TX, USA). A two‐tailed P‐value < 0.05 was considered statistically significant without adjustment for multiple comparisons.
3. RESULTS
3.1. Baseline characteristics
Baseline characteristics for the 55 participants are shown in Table 1. The mean age of participants was 52 ± 9 years; 58% were women, and 64% were Black. Mean baseline ambulatory systolic blood pressure was 146 ± 12 mm Hg and ambulatory diastolic blood pressure was 91 ± 9 mmHg. Mean serum urate was 5.2 ± 1.2 mg/dL; 49% (27 of 55) had an elevated baseline serum urate > 6 mg/dL.
TABLE 1.
Baseline characteristics according to diet assignment, mean (SD) or %.
| Overall, N = 55 | Control, N = 28 | DASH, N = 27 | |
|---|---|---|---|
| Age, years * | 52.3 (9.3) | 52.0 (7.7) | 52.7 (10.9) |
| Age ≥60 years, % * | 20.8 | 14.8 | 26.9 |
| Female, % | 58.2 | 50.0 | 66.7 |
| Black race, % | 63.6 | 64.3 | 63.0 |
| Mean ambulatory systolic blood pressure, mm Hg | 145.6 (12.2) | 145.3 (12.9) | 145.9 (11.6) |
| Mean ambulatory diastolic blood pressure, mm Hg | 90.5 (8.7) | 90.9 (7.8) | 90.2 (9.7) |
| Body mass index, kg/m2 ** | 29.0 (4.6) | 29.6 (5.3) | 28.5 (3.6) |
| Body mass index ≥30 kg/m2 ** , % | 46.3 | 50.0 | 42.3 |
| Mean serum urate, mg/dL ** | 5.2 (1.2) | 5.4 (1.2) | 5.0 (1.2) |
| Serum urate ≥6 mg/dL, % ** | 27.8 | 33.3 | 22.2 |
Only 53 had age reported at baseline: 27 among the control group and 26 among the DASH group.
Only 54 contributed these measurements at baseline. For body mass index, only 26 had values on the DASH diet. For serum urate only 27 had serum urate measured at baseline on the control diet.
3.2. DASH diet comparison
The changes in serum urate on the DASH diet versus control diet are shown in Table 2 and Figure 1. The DASH diet did not reduce serum urate overall (mean difference −0.05; 95% CI: −0.39, 0.28). Among patients with hyperuricemia at baseline (mean serum urate of 6.7 mg/dL), the DASH versus control diet lowered serum urate with a mean difference of −0.38 mg/dL (95% CI: −0.92, 0.16), while among those without hyperuricemia (mean serum urate of 4.6 mg/dL), the mean difference was −0.01 (95% CI: −0.41, 0.38; P‐interaction = 0.007 across hyperuricemia groups).
TABLE 2.
Effects of dietary assignment on serum urate, overall and by baseline serum urate concentration.
| Baseline, mean (SE) | End of period, mean (SE) | Change from baseline, mean (95% CI) | DASH versus control, mean (95% CI) | P | |
|---|---|---|---|---|---|
| Overall, N = 55 (161 measurements) | |||||
| Control, N = 28 | 5.35 (0.22) | 5.09 (0.21) | −0.26 (−0.50, −0.02) | Referent | Referent |
| DASH, N = 27 | 5.00 (0.23) | 4.69 (0.18) | −0.31 (−0.55, −0.07) | −0.05 (−0.39, 0.28) | 0.77 |
| Baseline serum urate < 6 mg/dL, N = 39 (115 measurements) | |||||
| Control, N = 18 | 4.68 (0.17) | 4.54 (0.19) | −0.13 (−0.45, 0.18) | Referent | Referent |
| DASH, N = 21 | 4.53 (0.19) | 4.38 (0.17) | −0.15 (−0.38, 0.08) | −0.01 (−0.41, 0.38) | 0.95 |
| Baseline serum urate ≥6 mg/dL, N = 15 (44 measurements) | |||||
| Control, N = 9 | 6.74 (0.14) | 6.24 (0.23) | −0.50 (−0.78, −0.22) | Referent | Referent |
| DASH, N = 6 | 6.67 (0.28) | 5.78 (0.26) | −0.88 (−1.34, −0.42) | −0.38 (−0.92, 0.16) * | 0.16 |
Using a Wald test to assess for interaction yielded a P‐value of 0.0069.
FIGURE 1.
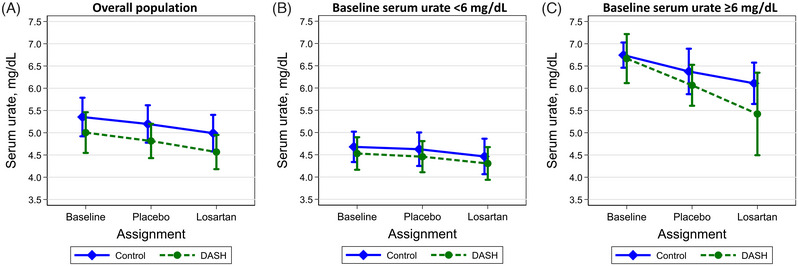
Mean (95% CI) serum urate concentrations by dietary assignment at baseline (diamond represents the control diet; circle represents the DASH diet), after the placebo period, and after the losartan period among participants with (A) the overall population, (B) a baseline serum urate < 6 mg/dL, or (C) a baseline serum urate ≥6 mg/dL.
3.3. Losartan comparison
Compared to placebo, losartan reduced serum urate by 0.23 mg/dL (95% CI: −0.40, −0.05) (Table 3). There was no evidence that the effects of losartan on serum urate differed by baseline hyperuricemia (P‐interaction = 0.31).
TABLE 3.
The effect of losartan on serum urate.
| Mean (SE) | Placebo or losartan versus baseline, mean (95% CI) | Losartan versus placebo, mean (95% CI) | P | |
|---|---|---|---|---|
| Overall, N = 55 (161 measurements) * | ||||
| Baseline | 5.2 (0.2) | Referent | – | – |
| Placebo | 5.0 (0.1) | −0.17 (−0.35, 0.00) | Referent | Referent |
| Losartan | 4.8 (0.1) | −0.40 (−0.60, −0.20) | −0.23 (−0.40, −0.05) | 0.011 |
| Baseline serum urate < 6 mg/dL, N = 39 (115 measurements) | ||||
| Baseline | 4.6 (0.1) | Referent | – | – |
| Placebo | 4.5 (0.1) | −0.06 (−0.26, 0.13) | Referent | Referent |
| Losartan | 4.4 (0.1) | −0.22 (−0.44, 0.00) | −0.16 (−0.33, 0.02) | 0.082 |
| Baseline serum urate ≥6 mg/dL, N = 15 (44 measurements) | ||||
| Baseline | 6.7 (0.1) | Referent | – | – |
| Placebo | 6.3 (0.2) | −0.46 (−0.80, −0.12) | Referent | Referent |
| Losartan | 5.9 (0.2) | −0.85 (−1.21, −0.50) | −0.39 (−0.84, 0.05) | 0.081 |
Note: 1 participant did not have a baseline serum urate measurement and thus was excluded from the stratified analysis.
Since the losartan and placebo comparison relied on end of period measurements all 55 were included in the overall comparison.
The P‐value for an interaction between diet and losartan was 0.72.
Test of interaction for losartan versus placebo was 0.31.
3.4. Combined effect
There was no evidence of a combined effect of DASH‐Losartan versus control‐placebo (Table 4). Compared to control‐placebo, DASH‐Losartan reduced serum urate by 0.28 mg/dL (95% CI: −0.65, 0.10). These effects differed by baseline hyperuricemia with a reduction of 0.90 (95% CI: −1.68, −0.12) among those with baseline hyperuricemia versus a reduction of 0.17 (95% CI: −0.56, 0.22) among those without baseline hyperuricemia (P‐interaction = 0.015). There were no significant differences between DASH‐placebo versus control‐losartan (Table ST1).
TABLE 4.
Combined effects of dietary assignment and losartan on serum urate.
| Baseline, mean (SE) | End of period, mean (SE) | Change from baseline, mean (95% CI) | DASH‐Losartan versus control‐placebo, mean (95% CI) | P | |
|---|---|---|---|---|---|
| Overall, N = 55 (107 measurements) | |||||
| Control‐placebo, N = 28 | 5.36 (0.22) | 5.19 (0.22) | −0.16 (−0.40, 0.07) | Referent | Referent |
| DASH‐Losartan, N = 27 | 5.00 (0.23) | 4.56 (0.20) | −0.44 (−0.73, −0.15) | −0.28 (−0.65, 0.10) | 0.15 |
| Baseline serum urate < 6 mg/dL, N = 39 (77 measurements) | |||||
| Control‐Placebo, N = 18 | 4.68 (0.17) | 4.62 (0.19) | −0.05 (−0.35, 0.24) | Referent | Referent |
| DASH‐Losartan, N = 21 | 4.53 (0.19) | 4.30 (0.19) | −0.22 (−0.48, 0.03) | −0.17 (−0.56, 0.22) | 0.39 |
| Baseline serum urate ≥6 mg/dL, N = 15 (29 measurements) | |||||
| Control‐Placebo, N = 9 | 6.74 (0.14) | 6.38 (0.26) | −0.37 (−0.76, 0.02) | Referent | Referent |
| DASH‐Losartan, N = 6 | 6.67 (0.28) | 5.40 (0.47) | −1.27 (−1.94, −0.59) | ‐0.90 (−1.68, −0.12) | 0.024 |
The P‐value for an interaction between diet and losartan overall was 0.72.
The P‐value for an interaction across strata of baseline serum urate using a Wald test was 0.015.
3.5. Additional subgroup analyses
There was no evidence that age, sex, Black race, or obesity modified the effects of the DASH diet versus the control diet on serum urate (Figures 2). In contrast, losartan had greater effects on serum urate among adults <60 years‐old versus adults ≥60 years‐old (−0.33 mg/dL vs. 0.16 mg/dL, P‐interaction = 0.003). With regards to the combined effect of DASH and losartan versus control and placebo, effects differed by participants without obesity versus participants with obesity (0.21 mg/dL vs. −0.84 mg/dL, P‐interaction = 0.03) (Figure SF1).
FIGURE 2.
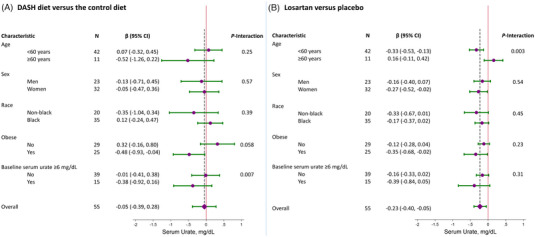
Effects of the DASH diet versus the control diet (A) or losartan versus placebo (B) on mean serum (95% CI) urate in strata of age, sex, race, obesity, and baseline hyperuricemia. Comparisons across strata were performed using interaction terms. The diamond represents the overall contrasts with the vertical dashed line representing the summary estimate.
There was no evidence of effect modification by Black race. However, the effects of the DASH diet versus the control diet among Black participants without baseline hyperuricemia was 0.17 mg/dL (95% CI: −0.25, 0.59) versus −0.39 mg/dL (95% CI: −0.76, −0.02) among Black participants with baseline hyperuricemia (P‐interaction < 0.001) (Table ST2). Greater reductions in serum urate were also observed among Black participants for the combined effect of DASH and losartan versus the control diet and placebo (P‐interaction = 0.15; Figure SF1). There was also no evidence of a difference in losartan versus placebo among Black adults across hyperuricemia status (Table ST3). While there was no effect from DASH and losartan on serum urate among all Black adults, a greater combined effect was noted among Black adults with hyperuricemia (P‐interaction = 0.04) (Table ST4).
4. DISCUSSION
In this randomized trial of 55 adults with mean DBP of 90−109 mm Hg and mean SBP < 180 mm Hg, the DASH diet did not reduce serum urate significantly when compared to a control diet. However, there were significant reductions in serum urate among participants with baseline hyperuricemia. In contrast, losartan reduced serum urate overall and this effect did not differ by baseline hyperuricemia; a significant effect of losartan on serum urate was observed among adults < 60 years old, not among those ≥60 years of age. Like the DASH diet, the combination of DASH and losartan did not reduce serum urate overall, but significantly reduced serum urate among adults with baseline hyperuricemia. These findings suggest that the DASH diet and losartan might lower serum urate among adults with hyperuricemia and hypertension.
Other studies have documented that the DASH diet reduces serum urate. 15 , 16 A DASH dietary pattern has been associated with a lower risk of gout in observational studies compared to a typical Western diet. 17 Moreover, in both DASH and DASH‐Sodium trials, the DASH diet lowered serum urate by 0.25 mg/dL (95% CI: −0.43, −0.08) and 0.35 mg/dL (95%CI: −0.65, −0.05) respectively, compared to the control diet. 3 , 13 In both of these trials, effects were greater among adults with baseline hyperuricemia. A similar trend was observed in a study of 123 African American adults with controlled hypertension 18 ; in this trial, there was a significant trend toward greater reductions in serum urate from DASH‐like groceries among adults with higher baseline urate levels. The present trial is consistent with these previous studies, showing that the DASH diet reduced serum urate among adults with baseline hyperuricemia.
Losartan has been considered a preferred antihypertensive agent among adults with gout, due to its uricosuric effects. 9 Losartan has been shown to prevent increases in serum urate levels. 19 , 20 A number of physiology studies show that losartan and its metabolites (e.g., E3174) do not inhibit xanthine oxidase activity, 9 , 21 but rather inhibit urate transporter 1 (URAT1)‐mediated renal tubule urate reabsorption, 9 , 22 which leads to decreased absorption in the proximal tubule of the kidney. 23 While several trials have examined the urate‐lowering effects of losartan alone or in combination with other agents, 24 , 25 , 26 the present study is unique in documenting the magnitude of the effect of losartan on serum urate in a placebo‐controlled trial. Our results were modest, consistent with a recent trial of high versus low dose losartan that reduced serum urate by 0.27 mg/dL among adults with heart failure, 27 and did not vary by baseline hyperuricemia. Compared to other studies of antihypertensive medications and serum urate, the magnitude of urate reduction observed was similar to the effects expected from starting or stopping other antihypertensive agents. 5 , 6 In the Treatment of Mild Hypertension Trial, compared to baseline, enalapril lowered urate by 0.31 mg/dL and amlodipine lowered urate by 0.48 mg/dL, while acebutolol (a beta blocker) increased urate by 0.33 mg/dL and chlorthalidone increased urate by 1.17 mg/dL. 28 Both beta blockers and chlorthalidone are associated with greater gout risk. 5 , 6 Our findings of modest effects of losartan on serum urate are informative for practitioners treating hypertension. Longer‐acting angiotensin receptor blockers are advantageous for hypertension management compared to losartan (a shorter‐acting agent). 29 Moreover, they have also been shown to inhibit urate uptake receptors, 30 but they have not consistently lowered serum urate compared to losartan 26 , 31 or placebo 32 in part due to agent‐specific differences in urate receptor inhibition. 33 However, our study suggests that depending on the degree of hypertension control, the modest reduction in urate from losartan may not be adequate to forego the benefits of a longer‐acting blood pressure agent.
The greatest reductions in serum urate were observed among participants less than 60 years old. Losartan has been shown to decrease blood pressure regardless of age, kidney function, or liver function. However, we found that the effects of losartan on serum urate differed by age. To our knowledge, the impact of age on the effects of losartan has not been reported in clinical studies; nevertheless, there is a body of mechanistic evidence, which supports such a relationship. Following oral administration, losartan is converted to its active metabolite, E3174, by the cytochrome P450 pathway in the liver. Both losartan and E3174 act on URAT‐1 in the kidney to induce uricosuria. With age, adults undergo both a decrease in glomerular filtration rate (GFR) at a rate of 6.3 mL/min/1.73 m2 per decade, 34 and the liver decreases in volume and blood flow, affecting cytochrome P450 activity. 35 We speculate that both reduced conversion of losartan to E3174 coupled with decreased glomerular filtration and thus access to URAT1, may contribute to losartan's reduced effectiveness in lowering serum urate among older adults in our study. However, these findings need replication particularly in a study with a larger number of older adults.
Among Black participants, we found greater urate‐lowering effects from DASH among adults with hyperuricemia. The greater BP lowering effects of the DASH diet among Black adults compared to White adults has been previously recorded, as has its trend towards decreasing serum urate levels. 10 , 36 , 37 However, our analyses were limited by small sample size.
Our study has limitations. First, our trial was relatively small, and the majority of participants had normal serum urate levels. Second, the study duration was short, limiting our ability to determine the longer‐term effects of these interventions on serum urate. This may be particularly relevant for the effects of the DASH diet on urate, which have been shown to achieve greater effects with longer durations. 38 Third, participants were not asked about a prior diagnosis of gout, and this was not an exclusion criterion or a study outcome. Given that serum urate is a poor correlate of gout flares, it is possible that DASH or losartan might prevent gout flares despite modest effects on serum urate. Fourth, our study did not examine other classes of antihypertensive agents that have been observed to modestly lower serum urate (e.g., calcium channel blockers). 5 Moreover, the trial only examined one dose of losartan. Whether effects might be greater at higher doses should be evaluated further. Fifth, the larger effects from DASH among adults with hyperuricemia could reflect regression to the mean and should be replicated in a study focused on adults with hyperuricemia. Sixth, our stratified analyses were exploratory and given the multiple comparisons could be susceptible to falsely significant findings. Moreover, small numbers limit our power to detect significant interactions across subgroups and should be re‐examined in a larger population. Lastly, the crossover design for losartan (vs. placebo) had increased statistical power compared to the parallel arm design for the DASH (vs. control) diet.
Strengths of this study include its core design, namely a randomized trial with tightly controlled dietary interventions, high follow‐up rates, and standardized data collection procedures. Moreover, the study enrolled a clinically relevant population of adults with hypertension, that is, a population at higher risk for gout, 2 and participants were diverse, that is, over half Black and over half women. Finally, measurement of serum urate occurred within six months of collection, minimizing the effects of sublimation and long‐term storage on urate concentrations.
In conclusion, the DASH diet reduced serum urate among adults with baseline hyperuricemia, while losartan reduced serum urate particularly among younger participants. These findings add to the growing evidence that the DASH diet and losartan lower serum urate. However, replication among adults with hyperuricemia and gout is needed.
CONFLICT OF INTEREST STATEMENT
The authors have no conflicts of interest to report.
Supporting information
Supporting Information
ACKNOWLEDGMENTS
We are indebted to the study participants for their sustained commitment to the DASH‐Losartan Trial. SPJ supported by a NIH/NHLBI K23HL135273 and NIH/NHLBI R21HL144876.
Castilla‐Ojo N, Turkson‐Ocran RA, Conlin PR, Appel LJ, Miller ER III, Juraschek SP. Effects of the DASH diet and losartan on serum urate among adults with hypertension: Results of a randomized trial. J Clin Hypertens. 2023;25:915–922. 10.1111/jch.14721
This trial was conducted before the clinicaltrial.gov registration requirement and thus has not be registered.
REFERENCES
- 1. Juraschek SP, Kovell LC, Miller ER, Gelber AC. Gout, urate‐lowering therapy, and uric acid levels among adults in the United States. Arthritis Care Res (Hoboken). 2015;67(4):588‐592. doi: 10.1002/acr.22469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Juraschek SP, Kovell LC, Miller ER, Gelber AC. Dose‐response association of uncontrolled blood pressure and cardiovascular disease risk factors with hyperuricemia and gout. PLoS One. 2013;8(2):e56546. doi: 10.1371/journal.pone.0056546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Juraschek SP, Yokose C, McCormick N, Miller ER, Appel LJ, Choi HK. Effects of dietary patterns on serum urate: results from a randomized trial of the effects of diet on hypertension. Arthritis Rheumatol. 2021;73(6):1014‐1020. doi: 10.1002/art.41614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhu Y, Pandya BJ, Choi HK. Comorbidities of gout and hyperuricemia in the US general population: nHANES 2007–2008. Am J Med. 2012;125(7):679‐687. doi: 10.1016/j.amjmed.2011.09.033 e1 [DOI] [PubMed] [Google Scholar]
- 5. Juraschek SP, Simpson LM, Davis BR, et al. The effects of antihypertensive class on gout in older adults: secondary analysis of the antihypertensive and lipid‐lowering treatment to prevent heart attack trial. J Hypertens. 2020;38(5):954‐960. doi: 10.1097/HJH.0000000000002359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Juraschek SP, Appel LJ, Miller ER. Metoprolol increases uric acid and risk of gout in african americans with chronic kidney disease attributed to hypertension. Am J Hypertens. 2017;30(9):871‐875. doi: 10.1093/ajh/hpx113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Neaton JD, Grimm RH, Prineas RJ, et al. Treatment of mild hypertension study final results. Treatment of mild hypertension study research group. JAMA. 1993;270(6):713‐724. [PubMed] [Google Scholar]
- 8. Yokose C, McCormick N, Choi HK. Dietary and lifestyle‐centered approach in gout care and prevention. Curr Rheumatol Rep. 2021;23(7):51. doi: 10.1007/s11926-021-01020-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hamada T, Ichida K, Hosoyamada M, et al. Uricosuric action of losartan via the inhibition of urate transporter 1 (URAT 1) in hypertensive patients. Am J Hypert. 2008;21(10):1157‐1162. doi: 10.1038/ajh.2008.245 [DOI] [PubMed] [Google Scholar]
- 10. Conlin PR, Erlinger TP, Bohannon A, et al. The DASH diet enhances the blood pressure response to losartan in hypertensive patients. Am J Hypertens. 2003;16:337‐342. doi: 10.1016/s0895-7061(03)00056-6 Pt 1 [DOI] [PubMed] [Google Scholar]
- 11. Erlinger TP, Conlin PR, Macko RF, et al. The impact of angiotensin II receptor blockade and the DASH diet on markers of endogenous fibrinolysis. J Hum Hypertens. 2002;16(6):391‐397. doi: 10.1038/sj.jhh.1001401 [DOI] [PubMed] [Google Scholar]
- 12. Ripley E, Hirsch A. Fifteen years of losartan: what have we learned about losartan that can benefit chronic kidney disease patients? Int J Nephrol Renovasc Dis. 2010;3:93‐98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Juraschek SP, McAdams‐Demarco M, Gelber AC, et al. Effects of lowering glycemic index of dietary carbohydrate on plasma uric acid levels: the omnicarb randomized clinical trial. Arthr Rheumatol (Hoboken, NJ). 2016;68(5):1281‐1289. doi: 10.1002/art.39527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. White H. A heteroskedasticity‐consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48(4):817‐838. [Google Scholar]
- 15. Juraschek SP, Miller ER, Wu B, et al. A randomized pilot study of DASH patterned groceries on serum urate in individuals with gout. Nutrients. 2021;13(2):538. doi: 10.3390/nu13020538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Belanger MJ, Wee CC, Mukamal KJ, et al. Effects of dietary macronutrients on serum urate: results from the OmniHeart trial. Am J Clin Nutr. 2021;113(6):1593‐1599. doi: 10.1093/ajcn/nqaa424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rai SK, Fung TT, Lu N, Keller SF, Curhan GC, Choi HK. The dietary approaches to stop hypertension (DASH) diet, Western diet, and risk of gout in men: prospective cohort study. BMJ. 2017;357:j1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Miller ER, Cooper LA, Carson KA, et al. A dietary intervention in urban African Americans: results of the “five plus nuts and beans” randomized trial. Am J Prev Med. 2016;50(1):87‐95. doi: 10.1016/j.amepre.2015.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Katsiki N, Tsioufis K, Ural D, Volpe M. Fifteen years of LIFE (Losartan intervention for endpoint reduction in hypertension)‐lessons learned for losartan: an ‘‘old dog playing good tricks. J Clin Hypertens (Greenwich). 2018;20(8):1153‐1159. doi: 10.1111/jch.13325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Høieggen A, Alderman MH, Kjeldsen SE, et al. The impact of serum uric acid on cardiovascular outcomes in the LIFE study. Kidney Int. 2004;65(3):1041‐1049. doi: 10.1111/j.1523-1755.2004.00484.x [DOI] [PubMed] [Google Scholar]
- 21. Enomoto A, Endou H. Roles of organic anion transporters (OATs) and a urate transporter (URAT1) in the pathophysiology of human disease. Clin Exp Nephrol. 2005;9(3):195‐205. doi: 10.1007/s10157-005-0368-5 [DOI] [PubMed] [Google Scholar]
- 22. Song D, Zhao X, Wang F, Wang G. A brief review of urate transporter 1 (URAT1) inhibitors for the treatment of hyperuricemia and gout: current therapeutic options and potential applications. Eur J Pharmacol. 2021;907:174291. doi: 10.1016/j.ejphar.2021.174291 [DOI] [PubMed] [Google Scholar]
- 23. BardinT. Fenofibrate and losartan. Ann Rheum Dis. 2003;62(6):497‐498. doi: 10.1136/ard.62.6.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Würzner G, Gerster JC, Chiolero A, et al. Comparative effects of losartan and irbesartan on serum uric acid in hypertensive patients with hyperuricaemia and gout. J Hypertens. 2001;19(10):1855‐1860. [DOI] [PubMed] [Google Scholar]
- 25. Manolis AJ, Grossman E, Jelakovic B, et al. Effects of losartan and candesartan monotherapy and losartan/hydrochlorothiazide combination therapy in patients with mild to moderate hypertension Losartan Trial Investigators. Clin Ther. 2000;22(10):1186‐1203. doi: 10.1016/s0149-2918(00)83062-3 [DOI] [PubMed] [Google Scholar]
- 26. Elliott WJ, Calhoun DA, DeLucca PT, Gazdick LP, Kerns DE, Zeldin RK. Losartan versus valsartan in the treatment of patients with mild to moderate essential hypertension: data from a multicenter, randomized, double‐blind, 12‐week trial. Clin Ther. 2001;23(8):1166‐1179. doi: 10.1016/s0149-2918(01)80099-0 [DOI] [PubMed] [Google Scholar]
- 27. Ferreira JP, Zannad F, Kiernan MS, Konstam MA. High‐ versus low‐dose losartan and uric acid: an analysis from HEAAL. J Cardiol. 2023;82(1):57‐61. doi: 10.1016/j.jjcc.2023.04.005 [DOI] [PubMed] [Google Scholar]
- 28. Elmer PJ, Grimm R, Laing B, et al. Lifestyle intervention: results of the treatment of mild hypertension study (TOMHS). Prev Med. 1995;24(4):378‐388. doi: 10.1006/pmed.1995.1062 [DOI] [PubMed] [Google Scholar]
- 29. Nishimura T, Hashimoto J, Ohkubo T, et al. Efficacy and duration of action of the four selective angiotensin II subtype 1 receptor blockers, losartan, candesartan, valsartan and telmisartan, in patients with essential hypertension determined by home blood pressure measurements. Clin Exp Hypertens. 2005;27(6):477‐489. doi: 10.1081/CEH-200067668 [DOI] [PubMed] [Google Scholar]
- 30. Sato M, Iwanaga T, Mamada H, et al. Involvement of uric acid transporters in alteration of serum uric acid level by angiotensin II receptor blockers. Pharm Res. 2008;25(3):639‐646. doi: 10.1007/s11095-007-9401-6 [DOI] [PubMed] [Google Scholar]
- 31. Monterroso VH, Rodriguez Chavez V, Carbajal ET, et al. Use of ambulatory blood pressure monitoring to compare antihypertensive efficacy and safety of two angiotensin II receptor antagonists, losartan and valsartan Losartan Trial Investigators. Adv Ther. 2000;17(2):117‐131. doi: 10.1007/BF02854844 [DOI] [PubMed] [Google Scholar]
- 32. González‐Ortiz M, Mora‐Martínez JM, Martínez‐Abundis E, Balcázar‐Muñoz BR. Effect of valsartan on renal handling of uric acid in healthy subjects. J Nephrol. 2000;13(2):126‐128. [PubMed] [Google Scholar]
- 33. Iwanaga T, Sato M, Maeda T, Ogihara T, Tamai I. Concentration‐dependent mode of interaction of angiotensin II receptor blockers with uric acid transporter. J Pharmacol Exp Ther. 2007;320(1):211‐217. doi: 10.1124/jpet.106.112755 [DOI] [PubMed] [Google Scholar]
- 34. Denic A, Glassock RJ, Rule AD. Structural and functional changes with the aging kidney. Adv Chronic Kidney Dis. 2016;23(1):19‐28. doi: 10.1053/j.ackd.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tajiri K, Shimizu Y. Liver physiology and liver diseases in the elderly. World J Gastroenterol. 2013;19(46):8459‐8467. doi: 10.3748/wjg.v19.i46.8459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Appel LJ, Moore TJ, Obarzanek E, et al. A clinical trial of the effects of dietary patterns on blood pressure DASH collaborative research group. N Engl J Med. 1997;336(16):1117‐1124. doi: 10.1056/NEJM199704173361601 [DOI] [PubMed] [Google Scholar]
- 37. Juraschek SP, White K, Tang O, Yeh HC, Cooper LA, Miller ER. Effects of a dietary approach to stop hypertension (dash) diet intervention on serum uric acid in African Americans with hypertension. Arthritis Care Res (Hoboken). 2018;70(10):1509‐1516. doi: 10.1002/acr.23515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tang O, Miller ER, Gelber AC, Choi HK, Appel LJ, Juraschek SP. DASH diet and change in serum uric acid over time. Clin Rheumatol. 2017;36(6):1413‐1417. doi: 10.1007/s10067-017-3613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


