Abstract
Preeclampsia is a pregnancy disorder that seriously affects the outcome of mothers and infants and lacks effective prediction and diagnosis methods. ELABELA is the second endogenous ligand of the apelin receptor (APJ) and is associated with the pathogenesis of preeclampsia. In a previous study, the authors found that the downregulation of ELABELA expression is closely related to late‐onset preeclampsia, which may be a marker for the clinical diagnosis of late‐onset preeclampsia. In this study, the authors again collected 120 maternal blood samples, including 60 pregnant women with a medical diagnosis of late‐onset preeclampsia. ELISA results showed that the serum ELABELA concentration in late‐onset preeclampsia pregnant women (12.57 ± 7.77 ng/mL) was significantly lower than that in normal pregnant women (36.99 ± 23.58 ng/mL), which was consistent with previously reported results. Therefore, the authors used an ELABELA monoclonal antibody to label four colloidal gold nanoparticles with different diameters (15, 30, 55, and 150 nm) and developed a transverse‐flow immunochromatographic band for the rapid and accurate detection of serum ELABELA levels. The strip test shows that colloidal gold with a diameter of 30 nm can be used as a good ELABELA detection marker and had more than 90% positive detection effect. Therefore, the authors hope that the colloidal gold strip with ELABELA as the diagnostic index developed by us will be popularized and applied in clinical diagnosis.
Keywords: colloidal gold, ELABELA, immune hierarchy, preeclampsia
1. INTRODUCTION
Preeclampsia belongs to the category of hypertensive disorders of pregnancy and is a multisystemic disorder that occurs specifically after 20 weeks of gestation. The incidence of preeclampsia is approximately 5%–8%. 1 It can cause 500 000 fetal/neonatal deaths and 70 000 maternal deaths each year worldwide. 2 The hospital usually evaluates the risk level of preeclampsia through routine pregnancy tests, such as blood pressure and proteinuria of pregnant women after 20 weeks of pregnancy, as well as nonspecific clinical features. According to statistics, approximately 30% of pregnant women receive preeclampsia evaluation at some point during pregnancy. 3 Generally, if a pregnant woman has a headache, vertigo, vomiting, epigastric discomfort, visual disturbances, and other symptoms, she is considered to have suspected preeclampsia. However, the clinical features of preeclampsia are sometimes nonspecific, so it is urgent to find more accurate biomarkers to diagnose preeclampsia at the molecular level. 4
Currently, the soluble fms‐like tyrosine kinase 1 (sFlt‐1)/placenta growth factor (PlGF) ratio is being investigated as a diagnostic and predictive marker. 5 However, this ratio is mainly applicable to early‐onset preeclampsia (<34 weeks) rather than late‐onset preeclampsia (≥34 weeks). In fact, the incidence rate and mortality of late‐onset preeclampsia are much higher than those of early‐onset preeclampsia. 6 ELABELA is an endogenous secreted peptide that acts on the apelin receptor (APLNR). 7 It was first detected in preimplantation human blastocysts and controls the self‐renewal of embryonic stem cells. 1 In adults, its expression is restricted to a few tissues, including two endocrine organs, the kidney and the placenta. 1 ELABELA‐deficient mice exhibit preeclampsia‐like symptoms during gestation, including hypertension, proteinuria, and renal injury, all of which can be rescued by exogenous recombinant ELABELA. 8 Furthermore, our previous study found that serum ELABELA levels were significantly lower in late‐onset preeclampsia. Thus, ELABELA may be a novel circulating biomarker in late‐onset preeclampsia. 9
Since late‐onset preeclampsia is usually urgent, the traditional protein quality detection method is time‐consuming and laborious. It is particularly important to establish a convenient and effective ELABELA monitoring method. In recent years, immunochromatographic test strips have become popular tools for molecular diagnosis. 10 Among them, colloidal gold immunochromatographic strip assays can obtain results within 5−10 min, which is an efficient, low‐cost and fast sample detection method. 11 The purpose of this study is to combine the immunocolloidal gold technique by immobilizing the antigen of ELABELA onto a colloidal gold pad and use the principle of antigen‐antibody reaction to quickly detect changes in the concentration of ELABELA in pregnant women to predict the possibility of preeclampsia.
2. EXPERIMENTAL DETAILS
2.1. Materials and reagent
The hybridoma cells used in this experiment were obtained from Kingsley Biotechnology Co., Ltd. (Nanjing, China). Colloidal gold special fiberglass sample pad (XQ‐YB), colloidal gold special fiberglass bond pad (GRADE8964), PVC bottom plate (DB‐6), plastic clamps (A‐9), and nitrocellulose membrane (YNHS‐140B) were all purchased from Shanghai Jieyi Biological Co., LTD (Shanghai, China). Water absorption pads were purchased from Shanghai Jinbiao Biological Co., Ltd. (Shanghai, China). Chloroauric acid (HAuCl4·3H2O) and trisodium citrate were obtained from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China) and used without further purification. Rabbit anti‐mouse IgG was purchased from Longji Biotechnology Co., Ltd. (Xi'an, China).
2.2. Human subjects
Blood samples from 120 pregnant women were collected from Nanjing Maternal and Child Health Hospital and Pan'an County Maternal and Child Health Hospital. The study was performed after agreement from the local ethics committee and with the patient's informed consent. Among them, 60 samples were medically diagnosed as suspected of late‐onset preeclampsia. Because their blood pressure increased during pregnancy (≥140/90 mmHg), most of them were accompanied by increased urinary protein. At the same time, we observed a significant and to some extent clinically significant increase in BMI in the late‐onset preeclampsia group. At present, a large number of epidemiological studies have shown that prepregnancy obesity and excessive weight gain during pregnancy are significantly positively correlated with preeclampsia, especially mild and late‐onset preeclampsia. 12 The information on all pregnant women with eclampsia is shown in Table 1.
TABLE 1.
Serum samples: clinical characteristics of late‐onset preeclampsia versus normal pregnant woman.
| Patient characteristics | Late‐onset PE | Normal | p value |
|---|---|---|---|
| N | 60 | 60 | |
| Age | 32.03 ± 4.64 | 30.23 ± 3.82 | .0277 |
| Gestation at blood collection, wk | 36.20 ± 4.61 | 36.22 ± 7.03 | <.0001 |
| BMI, kg/m2 | 28.44 ± 4.37 | 25.20 ± 3.13 | .0116 |
| Highest SBP during admission, mmHg | 148.11 ± 14.46 | 110.62 ± 9.42 | <.0001 |
| Highest DBP during admission, mmHg | 97.58 ± 10.16 | 70.55 ± 6.91 | <.0001 |
Note: Data are shown as the mean ± SD (unless stated differently).
Abbreviations: BMI, body mass index; DBP, diastolic blood pressure; PE, preeclampsia; SBP, systolic blood pressure.
All blood samples were numbered and centrifuged at 3000 rpm at 4°C for 10 min. The upper serum was dispensed into 300‐mL centrifuge tubes and stored at −80°C for further use. Placental tissues were obtained from elective cesarean section. For each placental tissue, four small pieces of tissue from separate lobules were collected and rinsed three times in sterile PBS. Then, the decidua was removed. Samples from one placenta were divided into two groups. One group's samples were snap‐frozen in liquid nitrogen within 10 min of delivery and then stored at −80°C for protein extraction. The other group of samples was fixed with 4% paraformaldehyde for histology analysis.
2.3. The content of ELABELA in serum was determined by ELISA
The concentration of ELABELA in serum samples was measured with the [pGlu1]‐ELABELA‐32 (Human) ELABELA Kit of PHOENIX PHARMACEUTICALS according to the instructions. The concentration of each sample was determined by measuring the absorbance at 450 nm in a microplate reader. Each sample was run in triplicate.
2.4. Western blot analysis of ELABELA in the placenta
The placenta was cut into pieces, added to RIPA buffer containing protease inhibitor, and then homogenized by a tissue homogenizer. All operations were performed on ice. After centrifugation at 12 000 rpm for 15 min, the protein concentration in the supernatant was detected with a BCA protein assay kit (Biyuntian Biological Technology Co., Ltd.). Proteins were separated by SDS‒PAGE and transferred to PVDF membranes. After the membrane was incubated with primary antibodies and horseradish peroxidase‐conjugated secondary antibody, the signal was developed by enhanced chemiluminescence (ECL) and visualized by a Tanon 5200 imaging system.
2.5. Immunohistochemistry
The placentas were fixed in 4% paraformaldehyde for 24 h, embedded in paraffin and cut into 5‐μm slices. Antigen retrieval was performed by treatment with citric acid buffer (pH 6.0). Nonspecific antibody binding was blocked by incubation with 10% normal goat serum for 1 h. Rabbit anti‐ELABELA antibody (1:200 dilution) (Bioworld Technology, Nanjing, China) was then added and incubated overnight at 4°C. Sections were then washed with PBS and incubated with horseradish peroxidase‐conjugated goat anti‐rabbit IgG for 30 min at 37°C. After washing, the sections were incubated with 3,3′‐diaminobenzidine and counterstained with hematoxylin. Images were photographed under an Olympus BX51 microscope.
2.6. Synthesis of colloidal gold
Colloidal gold was synthesized using a one‐step reduction method in which trisodium citrate reduces HAuCl4. 13 The basic principle is to add a certain amount of reducing agent to a gold solution of a certain concentration to make gold ions into gold atoms. 14 In this study, four colloidal gold particles with different particle sizes were prepared by the trisodium citrate reduction method, which was developed by Frens in 1973. 15 Briefly, l00 mL of 0.01% (W/V) chloroauric acid (HAuCl4) solution was preheated to 200°C in a heat gathering water bath with a magnetic stirrer at 600 rpm/min and then raised to 240°C until the HAuCl4 solution boiled. At the boiling point, different amounts of 1% (W/V) trisodium citrate solution were rapidly added in continuous mode while stirring. The reaction solution boiled at 240°C for another 10 min until the color of the solution changed from light yellow to deep wine red. 16 The solution was cooled to room temperature and stored at 4°C for future use. In this experiment, the colloidal gold particle size was changed by changing the amount of trisodium citrate solution added, and the average size and distribution of colloidal gold particles were measured by a ζ potentiometer. 17
2.7. Preparation of ELABELA monoclonal antibodies
ELABELA polypeptide (ERPVNLTMRRKLRKHNCLQRRCMPLHSRVPFP) was synthesized, and then five Balb/C mice were immunized with peptide‐KLH (EDC conjugation method) and peptide‐KLH (GA conjugation method) in a 1:1 ratio, and five hybridoma cells were obtained. After resuscitation, culture‐subculture and reculture, a large amount of cell supernatant containing monoclonal antibodies could be obtained. Then, the monoclonal antibodies were separated and purified by column chromatography, concentrated to an appropriate concentration and placed in a refrigerator at −20°C for further use.
2.8. Optimal conditions for the conjugation of mAb to colloidal gold
For the conjugation of colloidal gold, a minimum amount of monoclonal antibody concentration was needed for stabilizing colloidal gold particles. 18 The colloid gold suspension was adjusted to pH 8.0 with 0.2‐mol/L potassium carbonate solution and pipetted into a series of tubes. Five centrifuge tubes were removed, and 200 μL of pH‐adjusted gold liquid was added to each tube. Then, different amounts of anti‐ELABELA mAb (0, 2, 4 ng, 6 and 8 ng) were added to the five centrifuge tubes and incubated at room temperature for 30 min. 18 After the addition of 20 μL of 10% NaCl solution, a color change was observed after 2 h, and the color of the samples gradually changed from brilliant red to dark blue with decreasing concentrations of anti‐ELABELA mAb. The optimum concentration of anti‐ELABELA mAb needed to prevent colloidal gold from aggregating was the lowest, which maintained the wine‐red color of the solution. Then, 6‐μg anti‐ELABELA mAb was added to 200 μL of colloidal gold solution with pH values varying from 7.0 to 9.5. Five minutes later, 20 μL of 10% NaCl solution was added. Two hours later, the minimum pH that maintained the red color without change was considered the optimum pH.
2.9. Antibody pairing
The sensitivity of colloidal gold strips depends largely on the binding efficiency of the antigen and antibody. Because the test strips in this experiment were prepared by the double‐antibody sandwich method, we need to screen out a pair of antibodies that can pair with each other and bind the antigen with the highest efficiency among the five monoclonal antibodies obtained. First, 0.5∼1 mg of monoclonal antibodies were removed and then biotin labeled. Finally, ELISA was used for antibody pairing. A total of 20 pairs of five monoclonal antibodies will be tested to identify the group that binds the ELABELA antigen most efficiently.
2.10. Preparation and assembly of colloidal gold test strip
The test strip consisted of four components assembled together on a plastic backing: a sample pad, a conjugate pad, an absorbent pad, and a nitrocellulose membrane containing test and control lines. 19
The sample pad made of glass fiber was saturated with 0.5% bovine serum albumin (BSA), 0.1% Tween‐20, 5% sucrose, and 93.4% PBS for at least 30 min. It was then air‐dried at 37°C for 2 h in an air‐blast drying oven and stored at room temperature sealed in a dried aluminum foil bag for future use. 13
The conjugate pad was prepared by fixing the colloidal gold coupling of anti‐ELABELA mAb on the polyester fiber, which was treated in the same way as the sample pad. Then, anti‐ELABELA mAb wire‐colloidal gold coupling was sprayed onto the polyester fiber with a volume of 20 μL/cm through a Biodot‐xyz3060 colloidal gold strip 3D gold spray film stripper (United States/BIODOT). The final conjugate pad was dried at 37°C for 2 h and stored at room temperature in a dried aluminum foil airtight bag until use.
The nitrocellulose membrane on which capture reagents such as another anti‐ELABELA mAb and rabbit anti‐mouse IgG were immobilized was an important element of the test strip. Another anti‐ELABELA mAb (2.5 mg/mL) and rabbit anti‐mouse IgG (1 mg/mL) were separately applied to a nitrocellulose membrane with a volume of 1 μL/cm and used as the test line and the control line, respectively. 20 The distance between two lines was 5 mm. The membrane was dried at 37°C for 2 h and stored at room temperature in a dried aluminum foil airtight bag until use.
Absorbent paper was used as the absorbent pad, and a PVC plate served as the bottom (backing) of the test strip. 21 The nitrocellulose membrane, absorption pad, conjugate pad, and sample pad were attached sequentially to the PVC plate with a 1–3‐mm overlap. 22 The nitrocellulose membrane was pasted onto the center of the PVC plate and overlapped 1 mm with the conjugate pad and the absorption pad on each side. Then, the sample pad was pasted on the plate by overlapping the conjugate pad with 3 mm. 22 The absorbent and sample pads were pasted on each side of the strip. The master card was cut into 4‐mm width strips, which were sealed in a plastic bag and stored at 4°C.
2.11. Test procedure and principle
In this study, the double antibody sandwich method was used to test the ELABELA polypeptide. A 150‐μL pretreated sample was added to the sample pad, and 0.01‐mol/L PBS solution (pH 7.4) was used as the blank control. During sample flow, the ELABELA antigen in the serum is first captured by antigen 1 on the gold nanometer and continues to move forward with the liquid solution. 23 When the solution flows through the detection line on the nitrocellulose membrane, the antigen‐ELABELA antibody colloidal gold complex is captured by ELABELA mAb 2 on the detection line and fixed on the membrane. The excess colloidal gold of ELABELA antibody in the solution will further migrate to the control line and be captured by rabbit anti‐mouse IgG. Previous studies have shown that the concentration of ELABELA polypeptides decreases in patients with preeclampsia. Therefore, if both lines turn red, the sample is recorded as negative. If the test line was less red than the control line or had no color, the sample was considered positive. In addition, if the quality control line is not red, it indicates that rabbit anti‐mouse IgG is not bound to the colloidal gold complex of ELABELA antibody, and the strip is invalid. The whole test process takes 15 min, and the results of all strips subjected to the double antibody sandwich method displayed at 15 min.
2.12. Statistical analysis
Data were analyzed using GraphPad Prism 9.0 (GraphPad Software, Inc., CA, USA). Serum data were log‐transformed before analysis. The significant differences were evaluated using two‐tailed Student's t test. Data are expressed as the means ± SEs, and a p value<.05 was considered significant.
3. RESULTS AND DISCUSSION
3.1. ELABELA was downregulated in late‐onset eclampsia
The concentration of ELABELA in serum samples of 120 pregnant women (including 60 samples of late‐onset preeclampsia and 60 samples of normal women) was measured with a human ELISA kit. The standard curve was drawn according to the measurement results of a series of ELABELA standard diluents (0, 0.01, 0.1, 1, 10, 100 ng/mL). The standard four‐parameter logistic linear regression analysis equation curve is Y = (1.29563 − 0.00587)/[1+(x/0.68585) ^ 0.94301] +0.00587, R2 = 0.99858, indicating that there is a good linear relationship between absorbance and ELABELA concentration. We calculated the concentration of ELABELA in serum samples through a regression equation and absorbance, as shown in Figure 1A. The average concentration of ELABELA in late‐onset preeclampsia was 12.57 ng/mL, with a median of 10.90 ng/mL. In normal pregnant women, the average concentration of ELABELA was 36.99 ng/mL, with a median of 31.62 ng/mL (Figure 1A). This is consistent with our published results, and the concentration of ELABELA in late‐onset preeclampsia pregnant women is significantly reduced. Then, western blotting was used to detect the expression of ELABELA in the placentas of late‐onset preeclampsia and normal pregnant women. β‐Actin was used as an internal reference. As shown in Figure 1B, the protein expression of ELABELA in the late‐onset preeclampsia group was significantly lower than that in the normal control group. To further analyze the localization of ELABELA in the placenta, the expression of ELABELA was detected by immunohistochemistry. As shown in Figure 1C, brown represents the location and amount of target protein expression, while blue represents the nuclei stained with hematoxylin. The results of immunohistochemistry showed that ELABELA was highly expressed in syncytiotrophoblast cells and moderately expressed in trophoblast cells (Figure 1C). The expression of ELABELA in the placenta of late‐onset preeclampsia was significantly lower than that of the control group, which was basically consistent with previous results. 9 In conclusion, the content of ELABELA in the placenta of late‐onset preeclampsia is decreased and can be used as a potential biomarker for the biological diagnosis of late‐onset preeclampsia.
FIGURE 1.
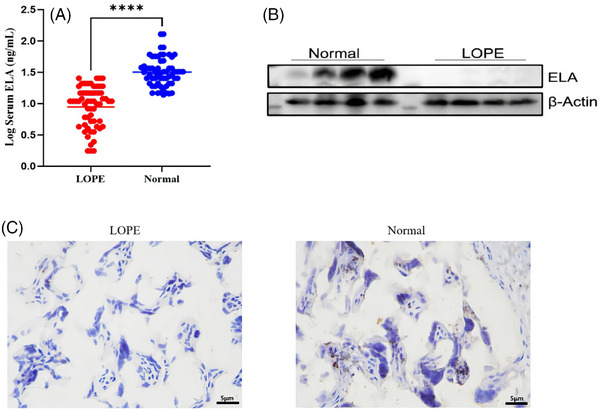
ELABELA levels in serum and placenta. (A) Serum ELABELA concentration in patients with late‐onset preeclampsia (LOPE, n = 60) and the corresponding normal women (n = 60) was detected by ELISA. (B) ELABELA protein expression in placental tissue obtained from late‐onset preeclampsia and normal pregnant women was detected by Western blot. β‐Actin was used as a loading control. (C) ELABELA expression in placental tissue of late‐onset preeclampsia (n = 5) and the control group (n = 5) was analyzed by immunohistochemistry using specific antibodies to ELABELA. The images are magnified 400×. ****p < .0001.
3.2. Pairing of monoclonal antibodies
After using purified recombinant ELABELA peptide‐immunized mice and screening, we obtained five hybridoma cell lines secreting the mAb against human ELABELA, named 12E, 58B, 52C, 65B, and 31D (Figure 2A,B). Electrophoresis showed that the heavy chain and light chain of the IgG antibody were approximately 50 and 25 kDa, respectively, which was consistent with the prediction (Figure 2C). Then, indirect ELISA was used to detect the level of the antibody immune response to the immunogen. The initial concentration was 1 mg/mL, and the dilution ratio was calculated according to the actual concentration. As shown in Table 2, the titers of the five antibodies were all greater than 1:52 000. S/B (signal/blank) and gt = 2.1 were the highest dilutions, and the blank OD450 was the average of two technical replicates (Table 2). Finally, the concentration of purified monoclonal antibody was measured by the BCA method, and the results are shown in Table 3. Except for 58B, the concentration of the other four antibodies reached above 1.0 mg/mL.
FIGURE 2.
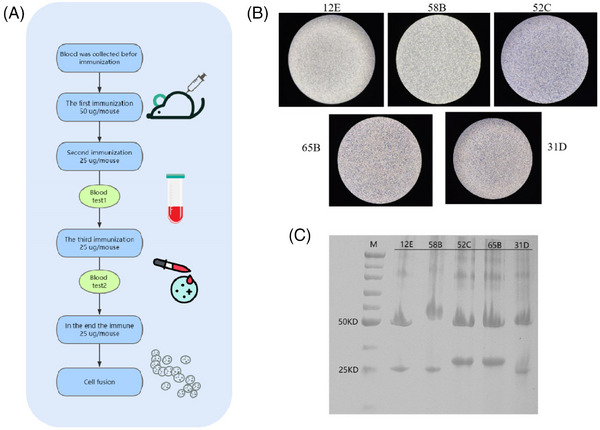
Pairing of monoclonal antibodies. (A) Procedure for immunizing mice. (B) Microscopic image of five hybridoma cells, all of which are semi—adherent, semi—suspended cells. (C) SDS‒PAGE analysis of the purified antibody revealed that the heavy and light chains of the IgG antibody were visible at approximately 50 and 25 kDa, respectively.
TABLE 2.
Titer testing of antibodies.
| Concentration (ng/mL) | 1000.00 | 500.00 | 250.00 | 125.00 | 62.50 | 31.25 | 15.62 | 7.81 | 3.90 | 1.95 | Blank |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 12E | 2.922 | 2.909 | 2.901 | 2.824 | 2.694 | 2.472 | 2.195 | 1.771 | 1.234 | 0.815 | 0.062 |
| 52C | 2.537 | 2.525 | 2.511 | 2.411 | 2.165 | 1.938 | 1.619 | 1.219 | 0.785 | 0.449 | 0.064 |
| 58B | 2.667 | 2.615 | 2.549 | 2.465 | 2.271 | 2.117 | 1.767 | 1.400 | 0.949 | 0.600 | 0.060 |
| 65B | 2.706 | 2.627 | 2.625 | 2.615 | 2.494 | 2.300 | 2.069 | 1.675 | 1.111 | 0.718 | 0.067 |
| 31D | 2.853 | 2.837 | 2.824 | 2.748 | 2.626 | 2.440 | 2.098 | 1.645 | 1.167 | 0.736 | 0.055 |
TABLE 3.
Concentrations of monoclonal antibodies.
| Name | Isotype | Concentration (mg/mL) | Purity |
|---|---|---|---|
| 12E | Mouse IgG1.k | 2.419 | 98% |
| 52C | Mouse IgG1.k | 1.609 | 98% |
| 58B | Mouse IgG1.k | 0.899 | 98% |
| 65B | Mouse IgG1.k | 1.103 | 95% |
| 31D | Mouse IgG1.k | 1.034 | 99% |
In this experiment, the colloidal gold strip was prepared by the double‐antibody sandwich method, so it is necessary to pair these five monoclonal antibodies. Then, 0.2 mg of the previously isolated and purified antibodies were removed for biotin labeling, and antibody pairing was performed by ELISA. There were 20 matching methods, and the matching results are shown in Table 4. The pairing results showed that 31D could successfully pair with the other four antibodies, while the other four antibodies could not pair with each other.
TABLE 4.
Antibody matching results.
| Antibody | 12E | 52C | 58B | 65B | 31D |
|---|---|---|---|---|---|
| 12E | / | × | × | × | √ |
| 52C | × | / | × | × | √ |
| 58B | × | × | / | × | √ |
| 65B | × | × | × | / | √ |
| 31D | √ | √ | √ | √ | / |
Note: “/” represents invalid pairing; “×” Indicates pairing failure; “√” means successful pairing.
3.3. Synthesis of colloidal gold and preparation of gold antibody
For colloidal gold bars, the smaller the gold nanoparticles are, the higher the specificity of the gold standard antibody, but the detection line color is lighter. In contrast, the larger the gold nanoparticles are, the lower the specificity of the gold standard antibody, and the darker the color of the test strip. 24 In addition, the binding efficiency of gold nanoparticles to the target protein is also related to the molecular weight and biological properties of the target protein. 14 , 21 Therefore, to select the colloidal gold particle size suitable for ELABELA monoclonal antibody, we prepared four colloidal gold particles with different particle sizes (Figure 3A). The UV‒visible spectra of the antibody‐free colloidal gold solution and mAb‐colloidal gold conjugate showed that due to the surface plasmon resonance of colloidal gold particles, an absorbance peak with high symmetry and narrow width was observed at 521 nm in curve a (Figure 3B). The results indicated that the prepared colloidal gold was monodispersed with a narrow size distribution. When ELABELA mAb was added, the surface resonance band (curve b) shifted slightly from 521 to 524 nm, which was caused by the interaction between the antibody and colloidal gold particles. This revealed a satisfactory binding nature of the antibody conjugates.
FIGURE 3.
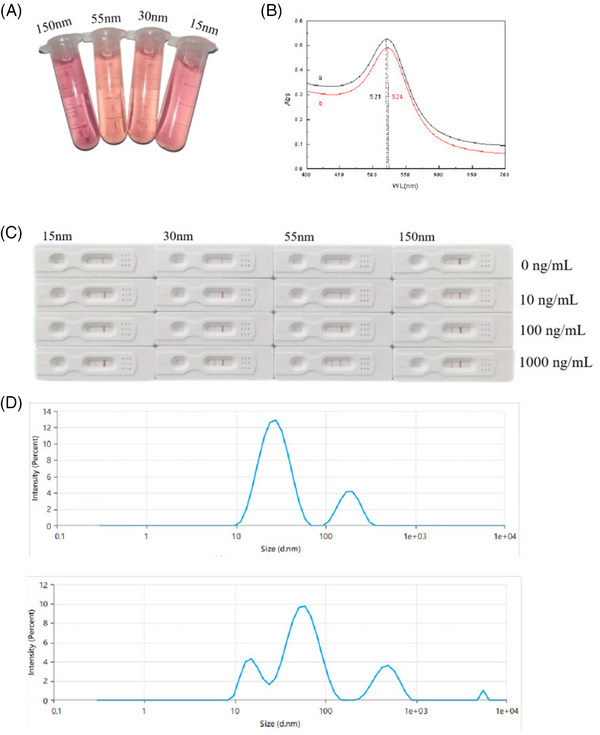
Synthesis of colloidal gold and preparation of gold antibody. (A) Colloidal gold solutions of different particle sizes show different colors according to different particle sizes. (B) UV‒visible spectra of the colloidal gold solution without antibody and the ELABELA mAb‐colloidal gold conjugate. The absorption peak is shifted from 521 to 523 nm. (C) From left to right, colloidal gold strips with sizes of 15, 30, 55, and 150 nm were used to test the sensitivity and specificity of the strips using the ELABELA standard with different concentration gradients. (D) Zeta potentiometer measures the size of colloidal gold particles and their dispersion in solution. The peak position represents the particle size of colloidal gold, and the number of peaks represents the homogeneity of colloidal gold particles.
Through the preliminary test strip experiment, it was found that when the size of gold nanoparticles was 15 nm, the color of the test strip was too light to be distinguishable by the naked eye. When the size of gold nanoparticles was 150 nm, the test paper showed a false‐positive. When the gold nanoparticles were 30 and 55 nm, the color of the test strip showed a significant gradient difference, which is convenient for the naked eye to distinguish the ELABELA concentration in serum (Figure 3C). Therefore, the colloidal gold used in this experiment was selected from 30‐ and 55‐nm colloidal gold particles. The distribution of 15‐nm gold nanoparticles was relatively uniform and narrow. However, the size distribution of the 55‐nm gold nanoparticles was not uniform, showing a bimodal distribution with a wide distribution band (Figure 3D). Therefore, 30‐nm colloidal gold particles will be used for subsequent experiments.
3.4. Sensitivity and specificity of test strips
According to our ELISA results, the concentration of ELABELA in preeclampsia is usually lower than 20 ng/mL (Figure 1A). Therefore, the test strip used in this experiment adjusted the amount of antibody on the detection line so that the detection limit of the test strip was 20 ng/mL. That is, when the concentration of ELABELA polypeptide was lower than 20 ng/mL, the test strip showed a single line; when the concentration of ELABELA polypeptide was higher than 20 ng/mL, the test strip showed a double line (Figure 4A). To determine the sensitivity of ELABELA‐mAb colloidal gold test strips, the ELABELA polypeptide standard was diluted to different concentrations (0, 10, 20, 30, 40 ng/mL). The results showed that when the concentration of ELABELA polypeptide was 10 ng/mL, a light red band appeared on the test line. When the concentration of ELABELA polypeptide was increased to 20 ng/mL, the band was clearly visible at the detection line (Figure 4B), indicating that the detection limit of this stick was 20 ng/mL.
FIGURE 4.
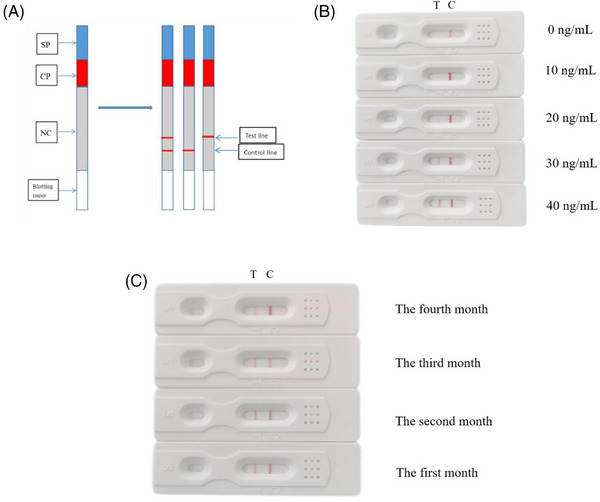
Sensitivity and specificity of test strips. (A) The strip is mainly composed of a sample pad (SP), binding pad (CP), nitrate cellulose film (NC), and absorbent paper. For the test strip detection principle, a double line represents that the ELABELA concentration in the sample is more than 20 ng/mL; a single line indicates that the ELABELA concentration in the sample is below 20 ng/mL. (B) The sensitivity of colloidal gold strips with a particle size of 30 nm was tested using different concentrations of ELABELA strips. When the concentration of ELABELA strips was 20 ng/mL, the strips showed obvious double lines. (C) The stability of the strip. (D) The picture shows the results of 120 serum samples tested by colloidal gold strip. The left picture shows the test results of 60 pregnant women with late‐onset preeclampsia, and the right picture shows the test results of 60 normal pregnant women.
The colloidal gold test strips were sealed and stored at room temperature for 4 months to evaluate their stability. We prepared and stored three batches of test strips and performed monthly specificity and sensitivity tests on the stored test strips over a period of 4 months, and the results showed that the test strips stored for a period of time were the same as those from new production (Figure 4C). Therefore, colloidal gold immunochromatographic strips can be stored at room temperature for at least 4 months without loss of sensitivity or specificity to ELABELA polypeptides.
Finally, to verify the accuracy of the test strip in clinical diagnosis, we measured the serum of 120 pregnant women collected, and the results are shown in Table 5. Among 60 preeclampsia maternal serum samples, 55 showed a single line and five showed a double line. Among the 60 serum samples of normal pregnant women, four showed a single line and 56 showed a double line. Therefore, the sensitivity and specificity of this test strip are between 90 and 93% (Table 5).
TABLE 5.
Accuracy of ELABELA colloidal gold test strips.
| Test strip characteristics | Late‐onset PE | Normal |
|---|---|---|
| N | 60 | 60 |
| Single line | 55 | 4 |
| Double line | 5 | 56 |
| Sensitivity and specificity | 91.67% | 91.33% |
Note: The detection limit of the test strip was 20 ng/mL. When the concentration of ELABELA in the sample was higher than 20 ng/mL, the test strip showed a double line. When the concentration of the sample in ELABELA was less than 20 ng/mL, the strip showed a single line.
4. DISCUSSION
Preeclampsia is a common disorder threatening the lives of pregnant women worldwide, and the mortality rate of patients with preeclampsia is approximately 1%. Although the prevalence of preeclampsia is between 2 and 7% in developed countries, it is as high as 10% in developing countries. 25 The pathogenesis of eclampsia may involve a variety of factors, such as maternal, placental, and fetal factors, including abnormal trophoblast invasion, abnormal immune regulation, endothelial cell damage, genetic factors, and nutritional factors. 26 At present, there is no single factor that can explain the etiology and mechanism of preeclampsia.
Preeclampsia can be divided into early‐onset and late‐onset preeclampsia according to the difference in occurrence time. Early‐onset preeclampsia occurs before 34 weeks of pregnancy and is associated with a higher neonatal incidence rate. 9 However, most preeclampsia occurs in the third trimester of pregnancy, which leads to the death of pregnant women. 9 The outcome of preeclampsia is very dangerous, but there are currently few diagnostic methods for preeclampsia. The PIGF/sFlt‐1 ratio is the most reliable biomarker for the diagnosis of preeclampsia thus far, but this is mainly used for early‐onset preeclampsia. Relatively speaking, the market still lacks reliable markers for late‐onset preeclampsia diagnosis.
In our previous study, we compared 22 late‐onset preeclampsia and 22 gestational age‐matched controls and found that the concentration of ELABELA in the serum of late‐onset preeclampsia pregnant women was significantly reduced. Real‐time PCR and immunohistochemistry results further confirmed that the expression of ELABELA in the placenta of late‐onset preeclampsia pregnant women was also significantly reduced. 9 This suggests that ELABELA could be used as a potential diagnostic biomarker for late‐onset preeclampsia. Ho and colleagues reported that the deletion of ELABELA can cause hypertension and proteinuria; on the other hand, the overall expression trends of placenta‐associated genes such as Hif‐1α, sFlt‐1, VEGFA, and PIGF were consistent with those of preeclampsia. In addition, exogenous supplementation of ELABELA to maternal mice significantly alleviated the clinical symptoms associated with preeclampsia. 8 In general, our research in clinical patients supports the research results obtained from ELABELA knockout mice. Of course, there are some contrary reports, such as Pritchard and colleagues, who found that ELABELA was increased in preeclampsia. 27 We speculate that the reasons for the opposite results are the time of sample collection and ethnic differences.
To further determine whether ELABELA can be used as a diagnostic marker of late‐onset preeclampsia, we collected blood samples from 60 late‐onset preeclampsia patients and 60 control pregnant women and analyzed the concentration of ELABELA in serum using an ELISA kit. The test results are consistent with our previous report, and the serum ELABELA level in patients with late‐onset preeclampsia was significantly reduced. 9 Therefore, we used ELABELA as a biomarker for the diagnosis of preeclampsia and prepared a colloidal gold strip that can detect ELABELA in serum to rapidly detect late‐onset preeclampsia in vitro. Based on the low concentration of ELABELA in pregnant women with preeclampsia, we differentiated normal pregnant women and late‐onset preeclampsia women samples by controlling the concentration of ELABELA antibody on the test line of the test strip. If the concentration of ELABELA in serum was higher than 20 ng/mL, the test strip showed two bands; in contrast, if it was lower than 20 ng/mL, the test strip showed one band. Combined with clinical diagnosis and ELISA test results, the positive rate of late‐onset preeclampsia diagnosed by our colloidal gold strip reached 90%.
Taken together, our study shows that ELABELA can be used as a biological diagnostic marker for detecting late‐onset eclampsia, and on this basis, we developed a colloidal gold immunochromatographic strip for detecting ELABELA. The test strip can safely, rapidly, and effectively predict suspected eclampsia in pregnant women, with an accuracy of more than 90%. It is expected to become a very valuable tool for preeclampsia diagnosis. However, as the onset of eclampsia is caused by multiple factors, it is unlikely that a single biomarker can accurately predict preeclampsia. 28 It may be necessary to find new biomarkers in early pregnancy and combine them with our colloidal gold strip to enhance the prediction ability of preeclampsia. In addition, it is also necessary to further study the molecular mechanism of gestational stage‐specific ELABELA concentration alteration on the development of preeclampsia.
AUTHOR CONTRIBUTIONS
In this study, Yang Na was responsible for the experimental research; Liu Kangsheng is responsible for clinical research; Zhang Wenli is responsible for the revision of important knowledge contents; Li Ying is responsible for manuscript preparation; Chen Meiling is responsible for the collection of experimental data; and Yang Lingdong is responsible for the review of manuscripts; Shen Suqin and Lu Chuanchuan are responsible for data analysis; Xu Kai, Peng Wei, and Deng Cheng are responsible for document retrieval; Lai Shanshan is the corresponding author. We confirm that this manuscript has not been published elsewhere and is not under consideration in whole or in part by another journal. All authors have approved the manuscript and agree with submission to The Journal of Clinical Hypertension.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
PATIENT CONSENT STATEMENT
The experimental protocol was approved by the Ethics Committee of Affiliated Jinling Hospital Medical School of Nanjing University.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
No.
CLINICAL TRIAL REGISTRATION
No.
ACKNOWLEDGMENTS
We thank Xia Li, Fang Liu, and Danyan Zheng for their help in sample collection and processing. Thanks to Yujing Han for her help in the development of colloidal gold strips and Huihao Tang, Ao Dai, and Hongying Hao for their help in the biological experiments. This work was supported by the National Natural Science Foundation of China (32270438, 31970388, 32170498), the National Key Research and Development Program of China (2021YFF0702000, 2018YFD0900602), the 1.3.5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYJC21050), the Science and Technology Department of Sichuan Province (2022YFH0116), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Yang N, Liu K, Zhang W, et al. Predicting late‐onset preeclampsia by detecting ELABELA content using an immunochromatographic colloidal gold test strip. J Clin Hypertens. 2023;25:932–942. 10.1111/jch.14724
Na Yang, Kangsheng Liu, Wenli Zhang, and Ying Li contributed equally to this study.
Contributor Information
Meilin Chen, Email: 867766139@qq.com.
Lindong Yang, Email: yanglingdong-123@163.com.
Shanshan Lai, Email: lss7259@163.com.
DATA AVAILABILITY STATEMENT
The authors will supply the relevant data in response to reasonable requests.
REFERENCES
- 1. Ho L, Tan SYX, Wee S, et al. ELABELA is an endogenous growth factor that sustains hESC self‐renewal via the PI3K/AKT pathway. Cell Stem Cell. 2015;17(4):435‐447. [DOI] [PubMed] [Google Scholar]
- 2. Rana S, Lemoine E, Granger JP, Karumanchi SA. Preeclampsia: pathophysiology, challenges, and perspectives. Circ Res. 2019;124(7):1094‐1112. [DOI] [PubMed] [Google Scholar]
- 3. Verlohren S, Brennecke SP, Galindo A, et al. Clinical interpretation and implementation of the sFlt‐1/PlGF ratio in the prediction, diagnosis and management of preeclampsia. Pregnancy Hypertens. 2022;27:42‐50. [DOI] [PubMed] [Google Scholar]
- 4. Lo JO, Mission JF, Caughey AB. Hypertensive disease of pregnancy and maternal mortality. Curr Opin Obstet Gyn. 2013;25(2):124‐132. [DOI] [PubMed] [Google Scholar]
- 5. Li Y, Sun L, Zheng X, Liu J, Zheng R, Lv Y. The clinical value of platelet parameters combined with sFlt‐1/PlGF in predicting preeclampsia. Ann Palliat Med. 2021;10(7):7619‐7626. [DOI] [PubMed] [Google Scholar]
- 6. Wallis AB, Saftlas AF, Hsia J, Atrash HK. Secular trends in the rates of preeclampsia, eclampsia, and gestational hypertension, United States, 1987–2004. Am J Hypertens. 2008;21(5):521‐526. [DOI] [PubMed] [Google Scholar]
- 7. Shimada N, Nakayama T, Umemura H, Kawana K, Yamamoto T, Uchigasaki S. A case‐control study of the APELA gene and hypertensive disorders of pregnancy. Medicina (Mex). 2022;58(5):591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ho L, van Dijk M, Chye STJ, et al. ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science. 2017;357(6352):707‐713. [DOI] [PubMed] [Google Scholar]
- 9. Zhou L, Sun H, Cheng R, Fan X, Lai S, Deng C. ELABELA, as a potential diagnostic biomarker of preeclampsia, regulates abnormally shallow placentation via APJ. Am J Physiol‐Endoc M. 2019;316(5):E773‐E781. [DOI] [PubMed] [Google Scholar]
- 10. Hou W, Wang S, Wang X. Development of colloidal gold immunochromatographic strips for detection of Riemerella anatipestifer. PLoS One. 2015;10(3):e0122952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Zeng L, Wu X, Liu L, Xu L, Kuang H, Xu C. Production of a monoclonal antibody for the detection of vitamin B1 and its use in an indirect enzyme‐linked immunosorbent assay and immunochromatographic strip. J Mater Chem B. 2020;8(9):1935‐1943. [DOI] [PubMed] [Google Scholar]
- 12. Sohlberg S, Stephansson O, Cnattingius S, Wikstrom A‐K. Maternal body mass index, height, and risks of preeclampsia. Am J Hypertens. 2012;25(1):120‐125. [DOI] [PubMed] [Google Scholar]
- 13. Wang XL, Wang L, Hasi CL, et al. A rapid colloidal gold immunochromatographic assay for the diagnosis of coronavirus disease 2019. Chin Med J (Engl). 2020;133(16):1986‐1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Uppal MA, Kafizas A, Ewing MB, Parkin IP. The effect of initiation method on the size, monodispersity and shape of gold nanoparticles formed by the Turkevich method. New J Chem. 2010;34(12):2906. [Google Scholar]
- 15. Nuntawong P, Ochi A, Chaingam J, Tanaka H, Sakamoto S, Morimoto S. The colloidal gold nanoparticle‐based lateral flow immunoassay for fast and simple detection of plant‐derived doping agent, higenamine. Drug Test Anal. 2021;13(4):762‐769. [DOI] [PubMed] [Google Scholar]
- 16. Wu M, Wu Y, Liu C, et al. Development and comparison of immunochromatographic strips with four nanomaterial labels: colloidal gold, new colloidal gold, multi‐branched gold nanoflowers and Luminol‐reduced Au nanoparticles for visual detection of Vibrio parahaemolyticus in seafood. Aquaculture. 2021;539:736563. [Google Scholar]
- 17. Celia Magno M, Venti F, Bergamin L, Gaglianone G, Pierfranceschi G, Romano E. A comparison between laser granulometer and sedigraph in grain size analysis of marine sediments. Measurement. 2018;128:231‐236. [Google Scholar]
- 18. Wu Y, Wu M, Liu C, et al. Colloidal gold immunochromatographic test strips for broad‐spectrum detection of Salmonella. Food Control. 2021;126:108052. [Google Scholar]
- 19. Terpos E, Ntanasis‐Stathopoulos I, Skvarč M. Clinical application of a new SARS‐CoV‐2 antigen detection kit (Colloidal Gold) in the detection of COVID‐19. Diagnostics. 2021;11(6):995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mao X, Wu Y, Chen H, Wang Y, Yu B, Shi G. A mix‐and‐detect method based on colloidal gold immunochromatographic assay for on‐site detection of zearalenone in edible oils. Anal Methods. 2020;12(46):5628‐5634. [DOI] [PubMed] [Google Scholar]
- 21. Cai P, Wang R, Ling S, Wang S. A high sensitive platinum‐modified colloidal gold immunoassay for tenuazonic acid detection based on monoclonal IgG. Food Chem. 2021;360:130021. [DOI] [PubMed] [Google Scholar]
- 22. Lin L, Xu X, Song S, et al. A colloidal gold immunochromatographic strip for quantitative detection of azoxystrobin in vegetables. New J Chem. 2021;45(20):9002‐9009. [Google Scholar]
- 23. Zhang X, Liu X, Wu X, et al. A colloidal gold test strip assay for the detection of African swine fever virus based on two monoclonal antibodies against P30. Arch Virol. 2021;166(3):871‐879. [DOI] [PubMed] [Google Scholar]
- 24. Nguyen DT, Kim DJ, So MG, Kim KS. Experimental measurements of gold nanoparticle nucleation and growth by citrate reduction of HAuCl4. Adv Powder Technol. 2010;21(2):111‐118. [Google Scholar]
- 25. Deniz R, Baykus Y, Ustebay S, Ugur K, Yavuzkir Ş, Aydin S. Evaluation of elabela, apelin and nitric oxide findings in maternal blood of normal pregnant women, pregnant women with pre‐eclampsia, severe pre‐eclampsia and umbilical arteries and venules of newborns. J Obstet Gynaecol. 2019;39(7):907‐912. [DOI] [PubMed] [Google Scholar]
- 26. Behram M, Oğlak SC, Dağ İ. Circulating levels of Elabela in pregnant women complicated with intrauterine growth restriction. J Gynecol Obstet Hum. 2021;50(8):102127. [DOI] [PubMed] [Google Scholar]
- 27. Pritchard N, Kaitu'u‐Lino TJ, Gong S, et al. ELABELA/APELA levels are not decreased in the maternal circulation or placenta among women with preeclampsia. Am J Pathol. 2018;188(8):1749‐1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kuc S, Wortelboer EJ, van Rijn BB, Franx A, Visser GHA, Schielen PCJI. Evaluation of 7 serum biomarkers and uterine artery doppler ultrasound for first‐trimester prediction of preeclampsia: a systematic review. Obstet Gynecol Surv. 2011;66(4):225‐239. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors will supply the relevant data in response to reasonable requests.


