Abstract
Triple-negative breast cancer (TNBC) is a heterogeneous and aggressive group of tumors that are defined by the absence of estrogen and progesterone receptors and lack of ERBB2 (formerly HER2 or HER2/neu) overexpression. TNBC accounts for 8%–13% of breast cancers. In addition, it accounts for a higher proportion of breast cancers in younger women compared with those in older women, and it disproportionately affects non-Hispanic Black women. TNBC has high metastatic potential, and the risk of recurrence is highest during the 5 years after it is diagnosed. TNBC exhibits benign morphologic imaging features more frequently than do other breast cancer subtypes. Mammography can be suboptimal for early detection of TNBC owing to factors that include the fast growth of this cancer, increased mammographic density in young women, and lack of the typical features of malignancy at imaging. US is superior to mammography for TNBC detection, but benign-appearing features can lead to misdiagnosis. Breast MRI is the most sensitive modality for TNBC detection. Most cases of TNBC are treated with neoadjuvant chemotherapy, followed by surgery and radiation. MRI is the modality of choice for evaluating the response to neoadjuvant chemotherapy. Survival rates for individuals with TNBC are lower than those for persons with hormone receptor–positive and human epidermal growth factor receptor 2–positive cancers. The 5-year survival rates for patients with localized, regional, and distant disease at diagnosis are 91.3%, 65.8%, and 12.0%, respectively. The early success of immunotherapy has raised hope regarding the development of personalized strategies to treat TNBC. Imaging and tumor biomarkers are likely to play a crucial role in the prediction of TNBC treatment response and TNBC patient survival in the future.
©RSNA, 2023
Quiz questions for this article are available in the supplemental material.
Introduction
Breast cancer is among the most commonly diagnosed cancers worldwide and is the second leading cause of cancer-related death among women in the United States (1). At one time, the histopathologic features were the basis for the traditional classification of breast cancer. However, advancements in the analysis of gene expression arrays during the past 2 decades have made it possible to molecularly characterize breast cancers as different subtypes. As gene-expression profiling is not widely available, a surrogate classification system based on clinical-pathologic features has been adopted. With this system, estrogen receptor (ER) and progesterone receptor levels are assessed by using immunohistochemistry, and human epidermal growth factor receptor 2 (HER2) levels are assessed by using immunohistochemistry or fluorescence in situ hybridization. These breast cancer subtypes are luminal A and luminal B cancers, which are positive for ER; HER2-enriched cancers that demonstrate amplification of ERRB2; and basal-like cancers, in which ER, progesterone receptor, and HER2 are absent.
Basal-like breast cancers are also characterized by overexpression of oncogenes that promote cell proliferation and carcinogenesis, such as the epidermal growth factor gene, EGFR. The most common basal-like breast cancer is triple-negative breast cancer (TNBC), which is negative for ER, progesterone receptor, and HER2. About 80% of TNBCs are basal-like cancers, and most basal-like cancers are TNBCs. Therefore, the terms basal-like cancer and triple-negative breast cancer are frequently used interchangeably, even though they are not synonymous (2). An aggressive subtype, TNBC accounts for 8%–13% of all breast cancers, affecting nearly 13 in 100 000 women yearly, and is the second most common breast cancer subtype among all age groups (3).
TNBC includes a wide spectrum of tumors with different molecular features, histologic characteristics, and clinical behavior. It accounts for a higher proportion of breast cancers in younger patients than in older patients, is more common in patients with germline BRCA and PALB2 mutations (4), and disproportionately affects non-Hispanic Black women, who also have a higher mortality rate associated with TNBC than do women of other races and ethnicities (5). Interval cancers, which are cancers diagnosed between regular mammographic screenings, are two times more likely than screening-detected cancers to be TNBC (6). Factors such as the fast growth of TNBC, increased mammographic density in young women, and lack of the typical features of malignancy at imaging of TNBC contribute to the delayed diagnosis of these cancers.
The mainstay of treatment for TNBC is neoadjuvant chemotherapy (NAC); rates of pathologic complete response (pCR) to NAC range from 40% to 50% (7). Patients who achieve pCR have a 5-year overall survival rate of 84%, whereas the 5-year overall survival rate for those who do not achieve pCR is 47% (8).
Although TNBC is chemosensitive, it is associated with a poor prognosis because it grows and spreads rapidly and is likely to recur. Howlader et al (9) reported that TNBC was associated with a worse 5-year survival rate (77.0%) than was hormone receptor–positive, HER2-negative malignancies (94.4%) between 2010 and 2014 (9). The 5-year survival rates associated with TNBC based on the extent of disease at initial diagnosis are 91.3% for localized disease, 65.8% for disease spread to regional lymph nodes, and 12.0% for distant disease (3). The rate of development of distant metastases among patients with TNBC who are treated with NAC, surgery, and radiation is approximately 27.4%, with a peak incidence in the 3rd year after diagnosis and a median survival of 13.3 months after the diagnosis of metastasis (10). TNBC has a propensity to metastasize to the brain and lung (9) and is less likely than other breast cancer subtypes to metastasize to bones (10).
The purpose of this article is to provide readers with an overview of TNBC, including the epidemiologic and genomic factors, histopathologic features, imaging findings, treatment, and prognoses associated with this malignancy.
Epidemiologic Factors
TNBC Trends Based on Patient Age
In a study in which data from the National Cancer Database were used, Plasilova et al (11) noted that 13.1% of 295 801 female patients with breast cancer and 5.9% of 3136 male patients with breast cancer who were diagnosed in 2010–2011 had TNBC. Across all races and ethnicities, hormone receptor–positive, HER2-negative cancer was the most common breast cancer subtype (11). TNBC accounted for a higher proportion of breast cancer cases among younger patients: 479 of 2059 (23.3%) cases among patients aged 30 years or younger, 3183 of 15 094 (21.1%) cases among patients aged 31–40 years, 7850 of 51 793 (15.2%) cases among patients aged 41–50 years, 10 423 of 72 543 (14.4%) cases among patients aged 51–60 years, 8912 of 77 870 (11.4%) cases among patients aged 61–70 years, and 7966 of 79 578 (10.0%) cases among patients older than 70 years.
Zhu et al (12) evaluated National Cancer Institute Surveillance, Epidemiology, and End Results (SEER) Program data on women who were diagnosed with TNBC from 2010 to 2011. The patients were divided into two groups based on their age: an older group, who were aged 70 years or older, and a younger group, who were younger than 70 years. The older group accounted for 20.4% (n = 2017) of all 9908 of the patients whose data were reviewed. Compared with the younger group, the older group had less aggressive tumors more often. For instance, this group had a lower probability of lymph node metastasis (30.5% vs 36.2% in the younger group; P < .001), an earlier TNM stage more frequently (stage I in 42.5% of cases vs in 35.2% of cases in the younger group; P < .001), and better differentiation (grade I or II in 28.4% of cases vs in 17.0% of cases in the younger group; P <.001). Despite these findings, there was an increased rate of early mortality in the older group. In terms of cancer-specific mortality, a larger number of older patients (5.9%) than younger patients (2.7%) died (P < .001) (12). After correcting for confounding factors, Zhu et al (12) noted that advanced age (>70 years) at diagnosis was independently predictive of poor overall survival and poor cancer-specific survival.
Racial Disparities
In an analysis of data on women with TNBC who were diagnosed during 2015–2019, Sung et al (13) found a downward trend in the incidence of TNBC across all races. The incidence of TNBC was highest among non-Hispanic Black women. TNBC incidences, expressed in numbers of cases per 100 000 individuals per year, were 25.2 cases among non-Hispanic Black women, 12.9 cases among White women, 11.2 cases among American Indian or Alaska Native women, 11.1 cases among Hispanic women, and 9.0 cases among Asian or Pacific Islander women (13).
Cho et al (14) compared TNBC cases among non-Hispanic Black women with those among White women. This group found that non-Hispanic Black women with TNBC were younger at diagnosis (aged 56.3 years vs 59.7 years among white women) and had higher rates of stage III disease (20.0% vs 15.2% in white women), tumor size larger than 5 cm (14.3% vs 9.6% in white women), and positive lymph nodes (39.0% vs 31.6% in white women). The mortality rate was greater for non-Hispanic Black women (16.0%) than for White women (13.2%); this result was partially explained by the fact that non-Hispanic Black women had a lower probability of undergoing surgery and chemotherapy (14).
The increased incidence of TNBC among non-Hispanic Black women may be attributed to several biologic and environmental factors. Mutations in BRCA1 and BRCA2 and other genetic mutations are more common in non-Hispanic Black women than in the white population and may contribute to their increased susceptibility to TNBC. Obesity and lack of breastfeeding also contribute to the high burden of TNBC among non-Hispanic Black women (4). In addition, sociodemographic and health care quality behaviors have been associated with the high incidence of TNBC among non-Hispanic Black women (13). The highest proportion of TNBC among non-Hispanic Black women is in the southern part of the United States (59.0%), a region characterized by lower socioeconomic status, worse health care behaviors, and worse health care services than other regions of the United States (13). However, the reasons for the disparities in outcomes among the non-Hispanic Black women with TNBC are not entirely clear, and further research is warranted. Compared with other women with TNBC, non-Hispanic Black women with this malignancy are diagnosed at a younger age and have larger tumors, more advanced tumor stages, and higher mortality rates.
Histologic Characteristics of TNBC
TNBCs include a spectrum of tumors with diverse histologic characteristics. Plasilova et al (11) evaluated a large database of patients with TNBC (n = 38 843) and found that 85% of TNBCs were invasive ductal carcinomas, whereas 1.4% were invasive lobular carcinomas (11). The remaining cases were those of rare histologic types. The invasive ductal carcinomas that are TNBCs are characterized by a high grade, pushing borders, prominent nuclear pleomorphisms, and geographic zones of necrosis (15). Rare subtypes of TNBC include metaplastic carcinomas and medullary carcinomas, both of which are high grade, and adenoid cystic carcinomas and secretory carcinomas, both of which are low grade.
Genetic Mutations in TNBC
TNBCs, as compared with other subtypes of breast cancer, are associated with germline BRCA mutations more often. Under normal conditions, BRCA1 and BRCA2 are tumor suppressor genes that promote gene integrity by repairing double-stranded DNA breaks. BRCA2 is the larger of the two genes, located on chromosome 13, and a mediator of homologous recombination. BRCA1 is located on chromosome 17 and plays a multifunctional role in DNA metabolism. Nearly 60% of breast cancers in premenopausal women who are BRCA1 mutation carriers are TNBCs (16), and 30% of TNBCs in women of Ashkenazi Jewish ancestry carry the BRCA1 mutation (17).
BRCA2 mutations are associated with TNBC but not as strongly; 16% of breast cancers in BRCA2 mutation carriers are TNBCs (18). It is interesting that the incidence of TNBC in BRCA1 mutation carriers decreases with age, whereas in BRCA2 mutation carriers, TNBC incidence increases with age (18).
Additional mutations have also been identified in patients with TNBC. Mutations in TP53 are seen in 62% of basal-like and 43% of non–basal-like TNBCs (19). Shimelis et al (20) identified germline pathogenic variants in BARD1, BRCA1, BRCA2, PALB2, and RAD51D that were associated with a high risk of TNBC and a greater than 20% absolute risk of breast cancer. A multigene hereditary panel may identify women at high risk for TNBC. Currently, the National Comprehensive Cancer Network guidelines recommend genetic risk assessment with possible genetic testing for all patients with TNBC who are aged 60 years or younger (21).
TNBC Subtypes Based on Genomic Profiling
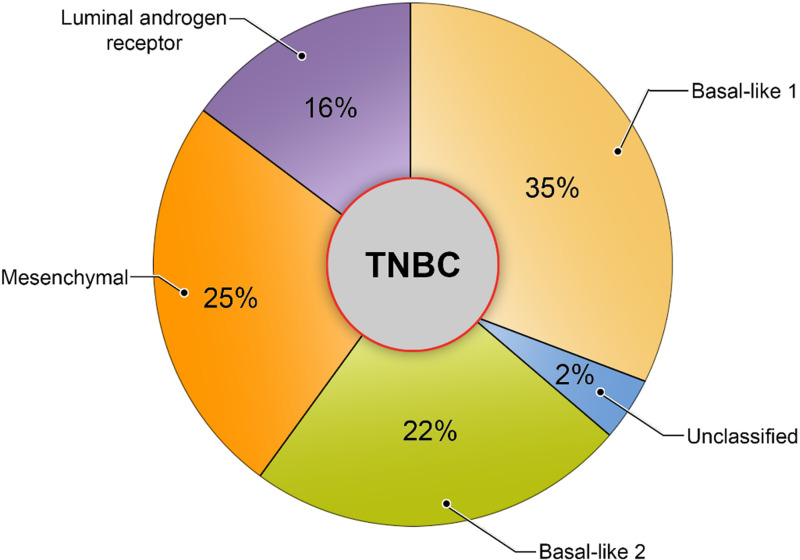
Efforts to classify TNBC by using gene expression profiling are underway, and there are several subtypes and classifying methods, all of which are aimed at identifying biologically relevant differences that would influence prognoses and treatment decisions. Among these efforts, one of the most studied is the Lehmann classification system, also known as the Vanderbilt classification (Lehmann). Lehmann et al (22) performed genomic profiling and categorized TNBC into four subtypes: basal-like 1, basal-like 2, mesenchymal, and luminal androgen receptor. They examined 306 patients with TNBC and found that 35% of them had basal-like 1 TNBC; 22%, basal-like 2 TNBC; 25%, mesenchymal TNBC; 16%, luminal androgen receptor TNBC; and 2%, an unclassified subtype (Fig 1).
Figure 1.
Lehmann subtype classification of TNBC (2016 version), with the proportions of TNBC tumors in each of the four subgroups cited (22).
Compared with the other subtypes, basal-like 1 TNBC is more likely to be of a higher grade, but it is characterized by a high rate of pCR to NAC and better survival compared with the other subtypes. In contrast, the basal-like 2 subtype is characterized by the lowest rate of pCR to NAC and a worse survival.
The nonbasal subtypes of TNBC, luminal androgen receptor and mesenchymal TNBCs, tend to initially manifest at a higher stage than the basal-like subtypes. Luminal androgen receptor TNBC is characterized by the expression of androgen receptor. This subtype is diagnosed most frequently in older women and is characterized by a higher rate of regional nodal spread and preferential metastasis to the bones. Women with luminal androgen receptor TNBC have a better chance of surviving despite having low rates of pCR to NAC. The mesenchymal subtype has sarcoma-like or squamous cell–like tissue and a relatively low rate of pCR and preferentially metastasizes to the lungs (23).
Immune Biomarkers in TNBC
Immunologic evasion through multiple mechanisms is a key factor in the development of malignancy. The immune system typically plays a significant role in preventing carcinogenesis. A growing understanding of the interactions between the tumor and the host, including the blockade of immunologic checkpoints and the activation of specific pathways and cells, has allowed the discovery of promising biomarkers that can predict what subset of patients will have a good prognosis and treatment response to chemotherapy (24). Among TNBC immune biomarkers identified to date are programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and tumor-infiltrating lymphocytes (TILs).
PD-1 and PD-L1
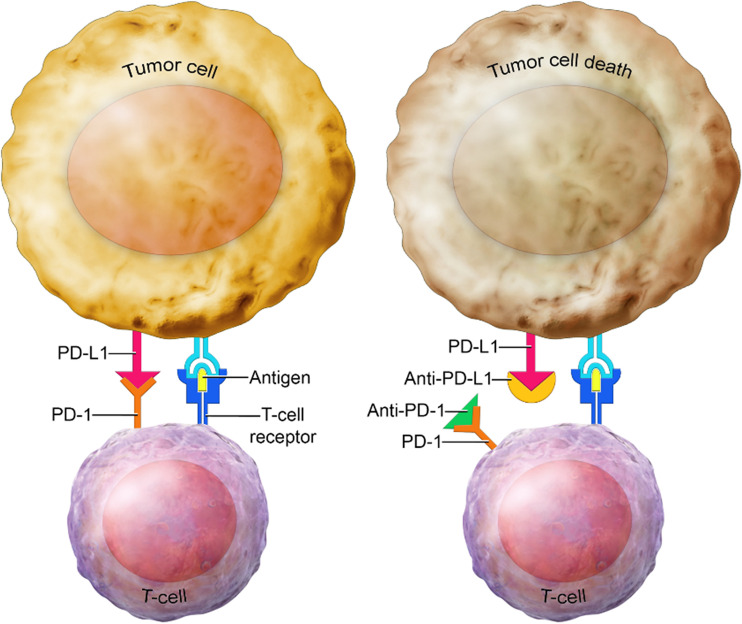
The immune system can differentiate between normal cells and foreign cells. Tumor cells can use upregulation of checkpoint proteins to evade the immune system. The checkpoint proteins act like switches that need to be turned on or off for the cell to start an immune response. PD-1 is a checkpoint protein expressed on T cells that can bind to PD-L1, a protein on tumor cells. When PD-1 binds to PD-L1, the T cell does not attack the tumor (Fig 2). Breast cancer, among other tumors, can use upregulation of PD-L1 expression to escape an antitumor immune response. PD-L1 is expressed in approximately 20% of TNBCs; it is used as a predictive biomarker for sensitivity to immunotherapy and is associated with a better prognosis. Different U.S. Food and Drug Administration–approved immunohistochemical tests are available to measure expression of immune biomarkers. Currently, the PD-L1 22C3 antibody test is used in breast cancer cases (24).
Figure 2.
Immune checkpoint inhibitors in TNBC. T cells normally attack the tumor, but the presence of PD-L1 proteins on tumor cells prevents T cells from causing death to tumor cells. PD-1 is a checkpoint protein on T cells that can bind to PD-L1, a protein on some cancer cells. When PD-1 (on the immune cell) binds to PD-L1 (on the tumor cell), the T cell does not attack the tumor. This is the basis of immunotherapy, whereby monoclonal antibodies block PD-1 or PD-L1 proteins. Pembrolizumab is a PD-1–targeted antibody, and combined with chemotherapy, it has been approved as the standard of care for patients with TNBC.
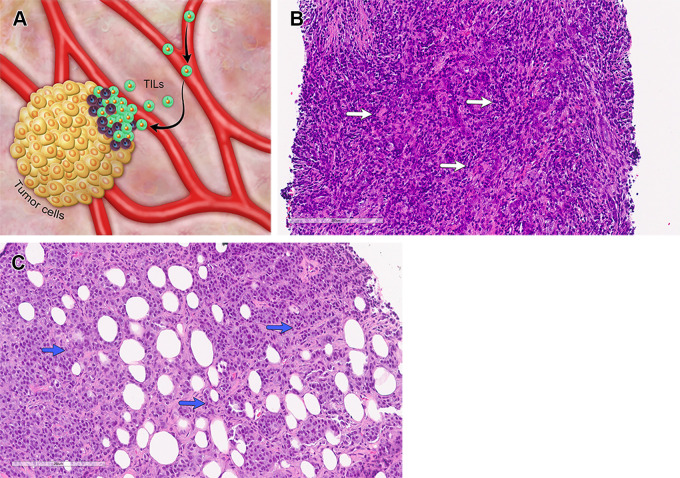
Tumor-infiltrating Lymphocytes
TILs are cytotoxic lymphocytes that infiltrate tumors and stroma (Fig 3A) as a host immune response. In TNBCs, the presence of TILs has been shown to be a predictive and prognostic biomarker. An increase in TILs, especially in the stroma, is predictive of a higher likelihood of pCR and better overall and disease-free survivals. TIL evaluation is performed on hematoxylin-eosin–stained tumor sections (Fig 3B, 3C), and a semiquantitative score is provided by using the 2014 International TILs Working Group guidelines (25). No consensus on the cutoff values for TIL levels has been established.
Figure 3.
TILs in TNBC. Normal breast tissue does not contain large aggregates of immune cells. (A) As cancer grows, lymphocytes recognize the cancer cells as abnormal and infiltrate the tumor. TILs are mononucleated lymphoid cells that infiltrate the tumor and its stroma and reflect the host immune response against the tumor cells. Quantification of TILs is performed on hematoxylin-eosin (H-E)–stained tissue sections from biopsy specimens obtained at the time of diagnosis and in the residual disease after NAC. Only those TILs located in the stromal portions between cancer cells are considered when counting. (B, C) Photomicrographs show two cases of TNBC, one with high (B) and one with low (C) levels of TILs. Arrows point to lymphocytes in the stroma associated with the invasive carcinoma. (H-E stain; original magnification, ×400.)
Investigators in several studies have tried to determine whether there is a correlation between the imaging features of TNBC and TIL levels. Ku et al (26) reported that round shape, circumscribed margins, homogeneous enhancement, and absence of multifocality on breast MR images were associated with high TIL levels, whereas irregular shape, noncircumscribed margins, multifocality, and heterogeneous enhancement were associated with low TIL levels. Candelaria et al (27) reported similar findings on sonographic images; in their study, oval or round shape, circumscribed margins, complex cystic and solid masses, and posterior acoustic enhancement were associated with high TIL levels (27). The findings in these studies indicate that there is a potential to use imaging features to complement the TIL information obtained from core needle biopsy tumor specimens.
Imaging Features of TNBC
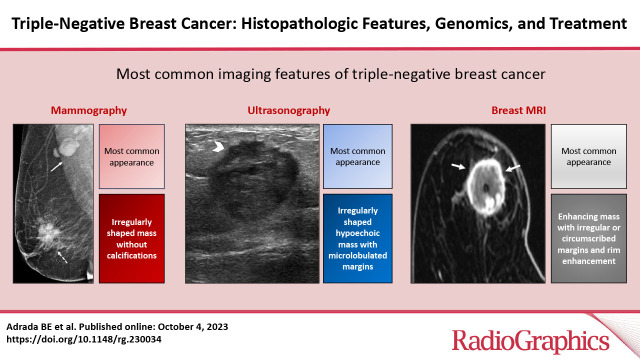
Mammographic Features
TNBC is detected more often at diagnostic mammography performed for evaluation of a clinical finding (eg, palpable mass, breast pain, nipple discharge) than at screening mammography. Sixty-eight percent of TNBCs, as compared with 48% of non-TNBCs, are clinically detected (28). The majority of TNBCs (48%–85%) manifest as irregularly shaped masses (Fig 4) without calcifications. Margins most commonly are ill defined or indistinct (45%–46% of cases) or spiculated (15%–21% of cases) (28–31).
Figure 4.
TNBC of the right breast in a 74-year-old woman. Right mediolateral oblique mammogram shows a 3-cm irregular noncalcified mass (dashed arrow) with spiculated margins and a 2.5-cm enlarged axillary lymph node (solid arrow).
A less common manifestation is a mass with associated calcifications (12%–29% of TNBC cases) (28–31). One study (31) showed a mass associated with calcifications to be a feature in 21% of TNBCs, 28% of luminal tumors, and 45% of HER2-positive breast cancers. Similarly, ductal carcinoma in situ, which commonly manifests as calcifications on mammograms, is less frequent in cases of TNBC than in cases of luminal and HER2-positive breast cancers. Yang et al (30) found that 18% of women with TNBC had associated in situ carcinoma. In contrast, 57% of women with HER2-positive breast cancers had associated in situ carcinoma (30).
While the majority of TNBCs manifest as irregularly shaped masses at mammography, approximately 8%–32% of them manifest as round or oval masses (Fig 5) (28–32). Asymmetries and architectural distortions are less common mammographic features of TNBC.
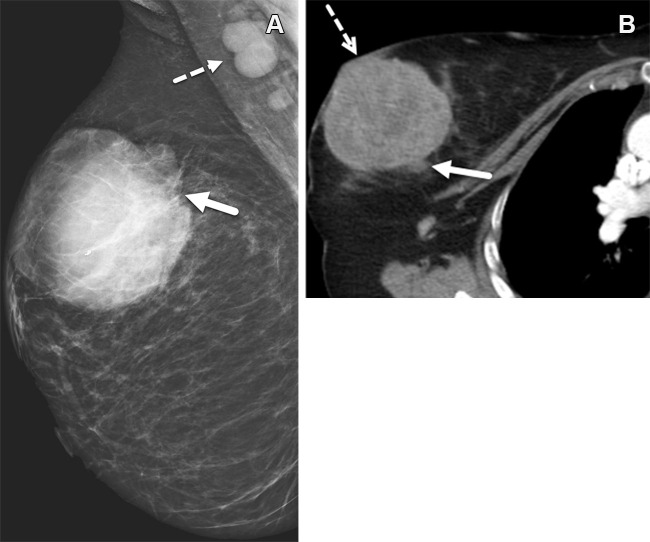
Figure 5.
Right breast TNBC in a 44-year-old woman who is a BRCA1 mutation carrier and has a Ki-67 index (proportion of Ki-67-expressing cells divided by the proportion of tumor cells) of 91%. (A) Right mediolateral oblique mammogram shows an 8-cm, high-density, noncalcified round mass (solid arrow) in the upper outer quadrant and associated axillary adenopathy (dashed arrow). (B) Axial chest CT image shows the mass (solid arrow) with microlobulated and indistinct margins invading the overlying skin (dashed arrow).
A relatively recent study by Aslan et al (33) showed variability in the imaging features of TNBC, depending on menopausal status. The TNBC tumors in premenopausal patients were more likely to appear as oval or round masses, while those in postmenopausal patients were more likely to manifest as irregularly shaped masses (33).
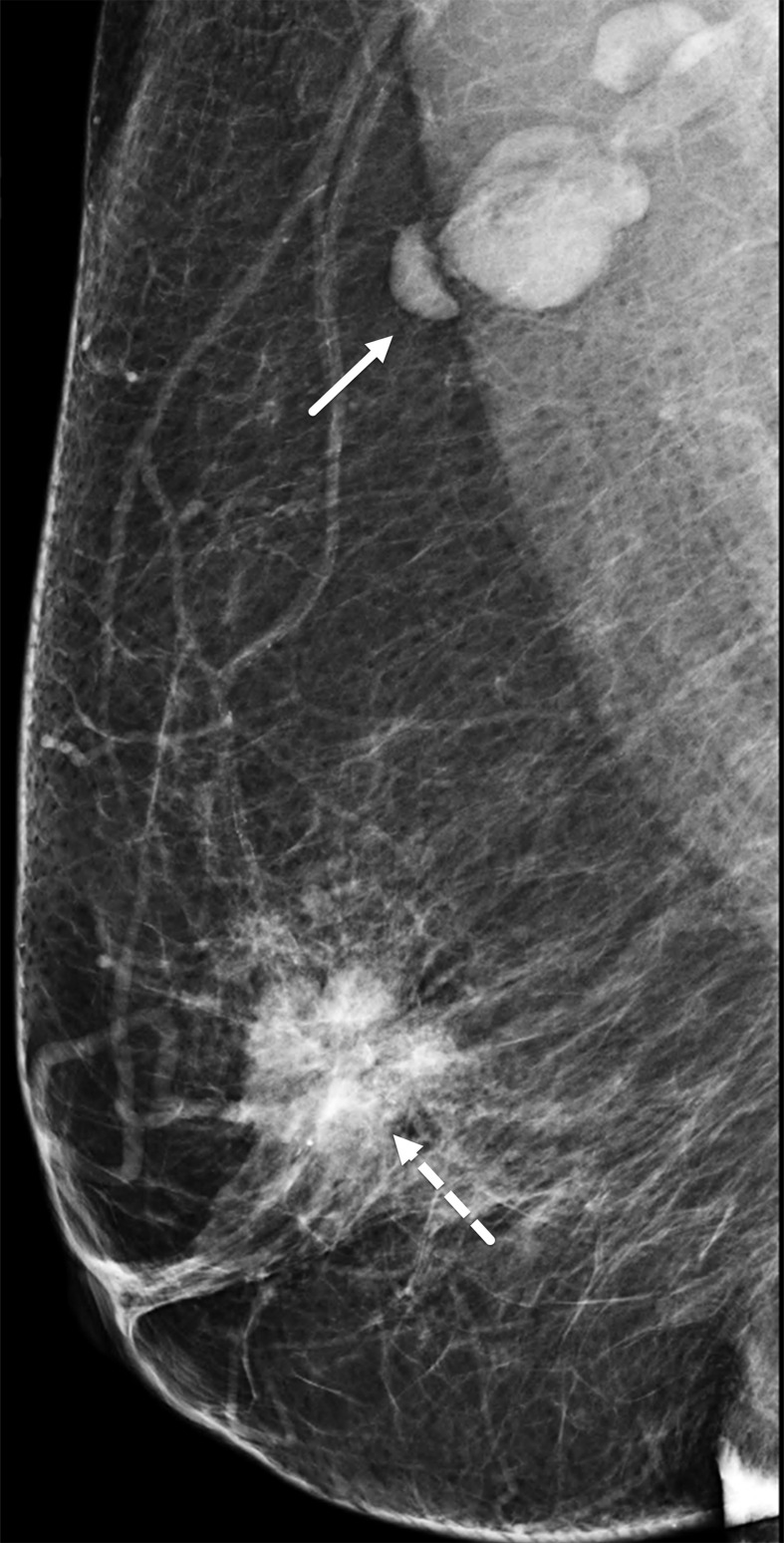
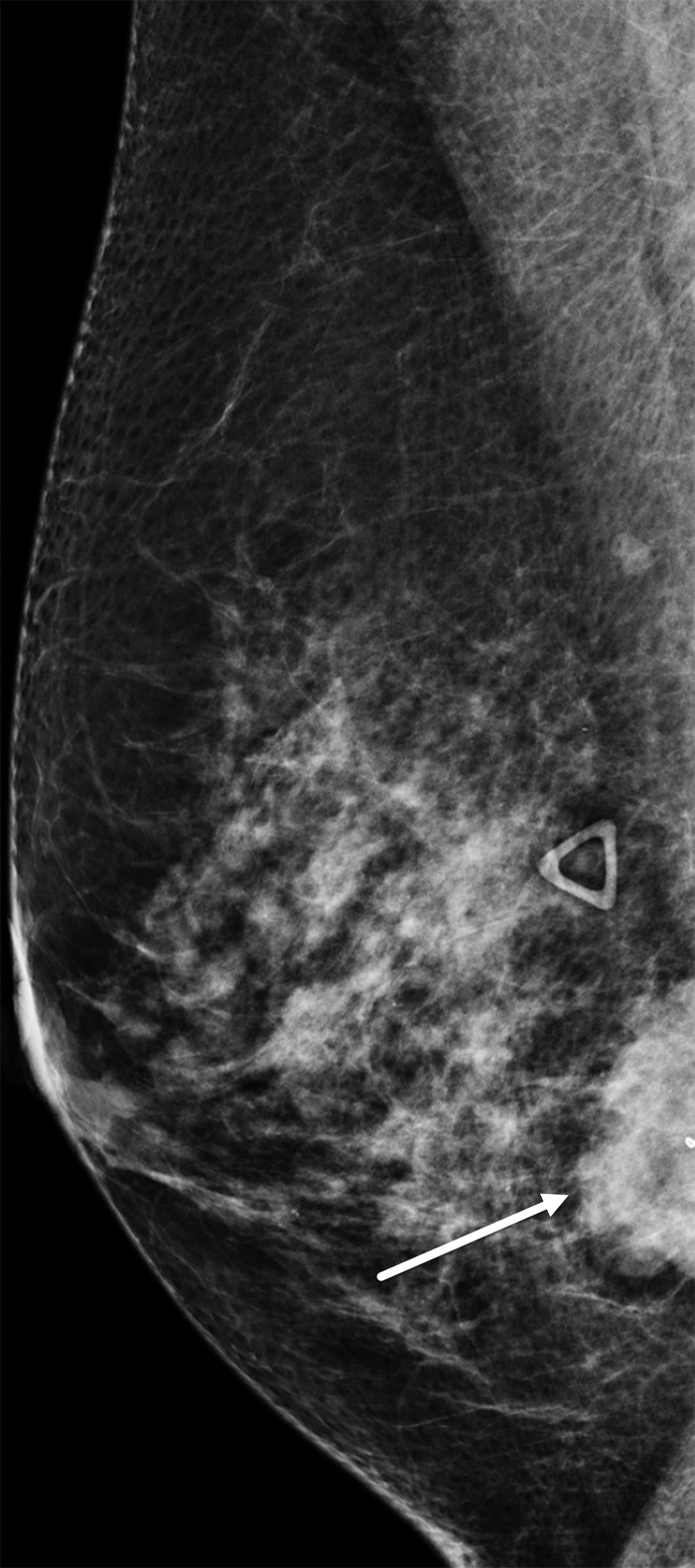
TNBCs have been reported to be mammographically occult in 9%–18% of cases (29,34), possibly owing to the absence of spiculated margins or architectural distortion or to the surrounding tissue density. TNBCs frequently can be seen in a posterior location (ie, close to the chest wall) (Fig 6), and in relatively recent studies, 47.0%–57.1% of TNBC cases were in the posterior third of the breast (33,35). The most common appearance of TNBC on mammograms is that of an irregularly shaped mass without calcifications.
Figure 6.
Right breast TNBC in a 54-year-old woman. Right breast mediolateral oblique mammogram shows a 3.5-cm, high-density, irregularly shaped mass (arrow) in the lower inner breast, correlating with the palpable abnormality (triangular marker). Note the posterior location of the tumor.
US Features
The typical sonographic manifestation of TNBC is an irregularly shaped (65%–83% of cases), hypoechoic (77%–89% of cases) mass (Fig 7A) with noncircumscribed margins (87% of cases) (28–31). The most common (38%–42% of cases) noncircumscribed margins are microlobulated (35,36).
Figure 7.
US features of TNBC in four patients. (A) Longitudinal image in a 50-year-old woman shows TNBC as a 3-cm round mass with microlobulated and angular margins (arrow). (B) Longitudinal image in a 40-year-old woman shows TNBC as a 3-cm oval mass that is parallel in orientation with posterior acoustic enhancement (arrows). Note that this mass has a microlobulated superficial margin. (C) Transverse image in a 30-year-old woman shows TNBC as a 5-cm oval complex cystic and solid mass (arrows). (D) Transverse image in a 64-year-old woman shows TNBC as a 2-cm irregularly shaped mass with angular margins (arrow).
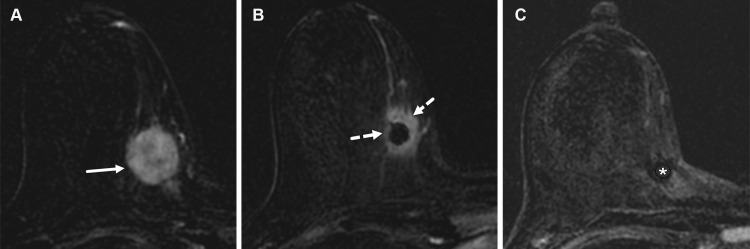
However, at US, TNBC may also appear with features typically seen in benign tumors, including circumscribed margins, parallel orientation, and posterior acoustic enhancement (Fig 7B). One study (29) found that 27% of TNBCs had circumscribed margins. In another study (35), two-thirds of TNBCs exhibited parallel orientation, and one-third of them exhibited posterior acoustic enhancement. Aslan et al (33) showed that TNBC tumors in premenopausal patients were more likely to be round or oval (Fig 7C), whereas those in postmenopausal patients were more likely to manifest as irregularly shaped masses (Fig 7D), indicating that menopausal status may play a role in the sonographic appearance of TNBC. Given that TNBC may resemble a benign tumor such as a fibroadenoma at US (appearing as an oval mass with parallel orientation and posterior acoustic enhancement), careful review of the margins of breast tumors at sonography is recommended to evaluate for any features that would necessitate biopsy (Fig 8).
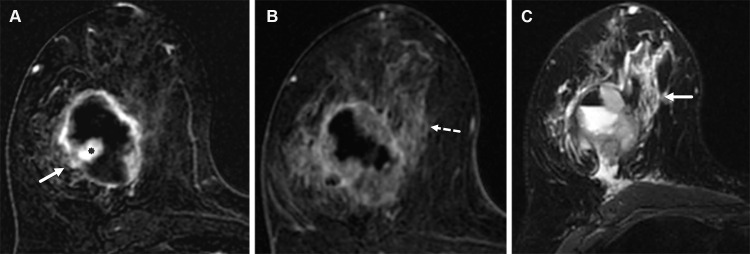
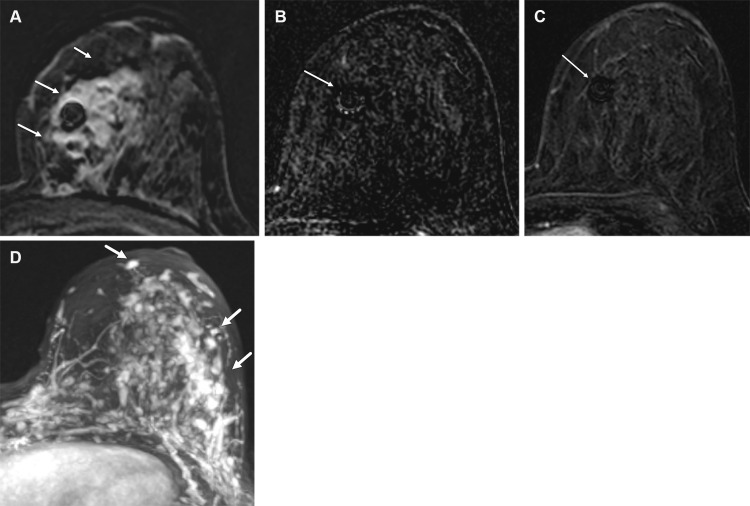
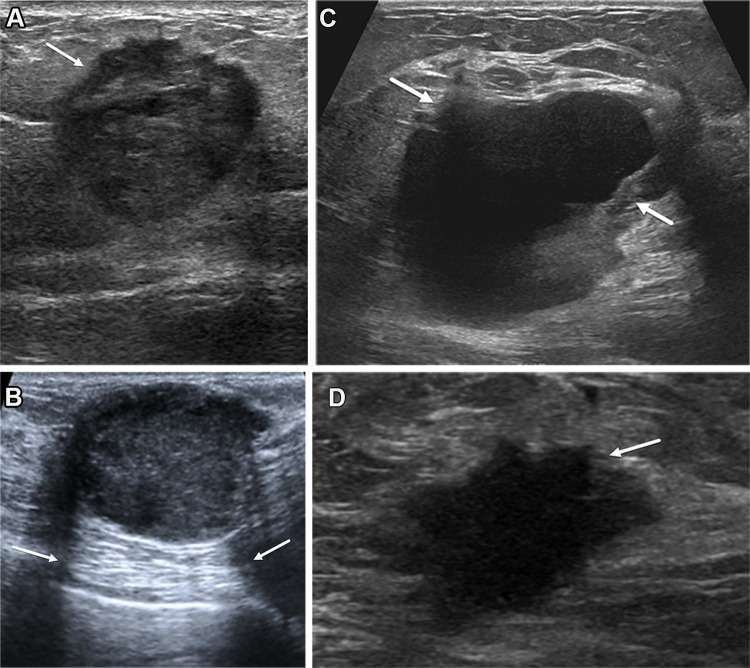
Figure 8.
Left breast palpable abnormality in a 39-year-old woman. (A) Left craniocaudal mammogram shows a 0.9-cm oval mass (dashed arrow) in the upper outer breast correlating with the palpable area of concern (triangular marker [*] ). This lesion was shown to represent an intramammary lymph node at subsequent US. A 1-cm oval mass (solid arrow) at the posterior depth was incidentally noted. (B) On a transverse US image, the 1-cm mass appears to have an oval shape and circumscribed margins (arrow). (C) Six-month follow-up US image shows a 1.4-cm irregularly shaped mass with microlobulated margins (arrow). US-guided biopsy revealed TNBC.
In the Dogan and Turnbull (34) study, the US findings were negative in 6.8% of cases of TNBC. In their study, three of the 44 (6.8%) cancers were sonographically occult. Two of these patients had calcifications detected at presentation, and the third patient had axillary metastasis from an occult breast cancer that was identifiable with MRI only (34). At US, TNBC most commonly manifests as an irregularly shaped hypoechoic mass with microlobulated margins. However, TNBC may have the sonographic appearance of a benign mass.
MRI Features
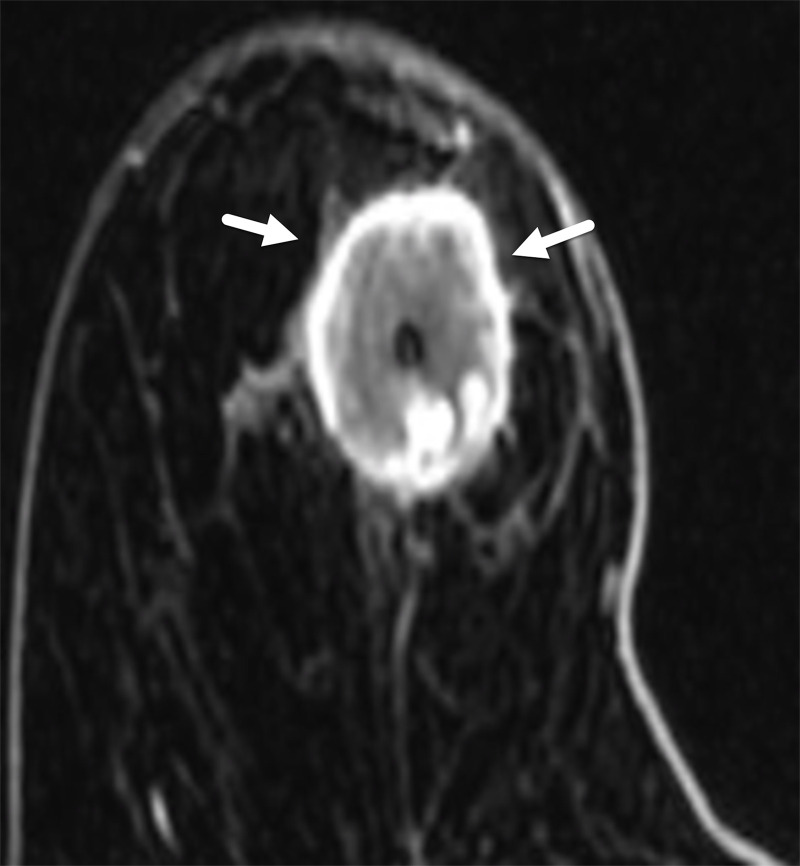
The most common MRI feature of TNBC is an enhancing mass, which is seen in 82%–95% of affected patients (37,38). While the margins can be noncircumscribed or circumscribed, circumscribed margins are more suggestive of TNBC. Rim enhancement (Fig 9) is the most common internal enhancement pattern in TNBC, seen in 41%–80% of patients (37,38). Rim enhancement reflects active tumoral angiogenesis, and its presence has been closely associated with lymphovascular invasion, a known risk factor for recurrence (39).
Figure 9.
MRI features of TNBC in a 66-year-old woman. Axial postcontrast T1-weighted subtraction MR image shows TNBC as a 3-cm round mass with thick and irregular rim enhancement (arrows).
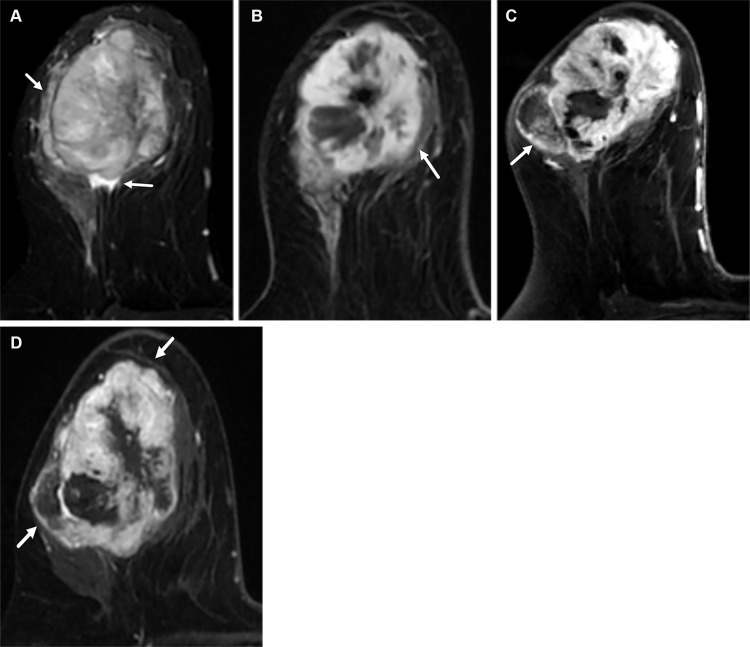
Additional imaging characteristics that are strongly suggestive of TNBC include high T2 intratumoral signal intensity (seen in 52% of cases) (40) and intratumoral necrosis (seen in 25%–48% of cases), which is characterized by the presence of areas of very high intratumoral signal intensity on T2-weighted MR images (Fig 10A). Costantini et al (40) found that peritumoral edema was more common with TNBC (52% of cases) than with HER2-positive (45% of cases) and luminal (13% of cases) cancers. Because peritumoral edema reflects the fast growth of TNBC, it has been tied to chemotherapy resistance (Fig 10B–10D) and early recurrence of TNBC (41).
Figure 10.
Metaplastic (spindle cell) TNBC of the right breast in a 57-year-old woman. (A) Axial T2-weighted MR image shows a 6-cm round mass with areas of very high T2 signal intensity (arrows) consistent with areas of intratumoral necrosis and surrounding peritumoral edema. The patient was started on NAC. (B–D) Serial postcontrast MR images show increased tumor size, indicating progression of disease and resistance to chemotherapy. Axial postcontrast T1-weighted pretreatment MR image (B) shows a 6-cm heterogeneously enhancing mass (arrow in B). Axial postcontrast T1-weighted midtreatment MR image (C) shows that the mass (arrow in C) has increased in size and now measures 7 cm. Axial postcontrast T1-weighted posttreatment MR image (D) shows that the mass (arrows in D) is continuously growing and now measures 8 cm. The surgical-pathologic specimen (not shown) revealed a residual mass measuring 8 cm in largest diameter.
Peritumoral edema can sometimes be misdiagnosed as areas of nonmass enhancement (Fig 11). Nonmass enhancement is not a typical TNBC imaging feature; it is seen in 16% of TNBCs versus in 29% of HER2-positive cancers and 17.3% of luminal cancers (40). Youk et al (42) reported that on diffusion-weighted MR images, the mean apparent diffusion coefficient (ADC) value for TNBC was higher (1.03) than that for ER-positive (0.89) and HER2-positive (0.84) cancers (P < .0001). TNBCs are more likely to be unifocal, but multifocal or multicentric disease is seen in 27% of cases (Fig 12) (43). At breast MRI, TNBC most commonly manifests as an enhancing mass with irregular or circumscribed margins. The most characteristic internal enhancement pattern is that of rim enhancement.
Figure 11.
TNBC of the right breast in a 63-year-old woman. (A) Axial postcontrast T1-weighted subtraction MR image shows a 5-cm complex cystic and solid enhancing mass (*) with thick irregular rim enhancement (arrow). (B) Axial late phase postcontrast T1-weighted subtraction MR image shows associated nonmass enhancement (arrow). (C) Axial T2-weighted MR image shows peritumoral edema (arrow). MRI-guided biopsy of the area of nonmass enhancement (not shown) showed reactive changes and lymphocytic perilobulitis, indicating that the nonmass enhancement represented peritumoral edema.
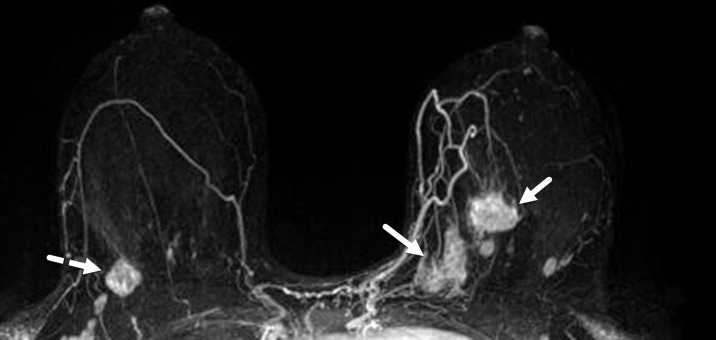
Figure 12.
TNBC in both breasts in a 32-year-old woman. Maximum intensity projection breast MR image shows a unifocal 2-cm mass (dashed arrow) in the right breast upper outer quadrant. Also demonstrated are multiple masses (solid arrows) of varying sizes in the left breast, consistent with multicentric disease.
PET/CT Features
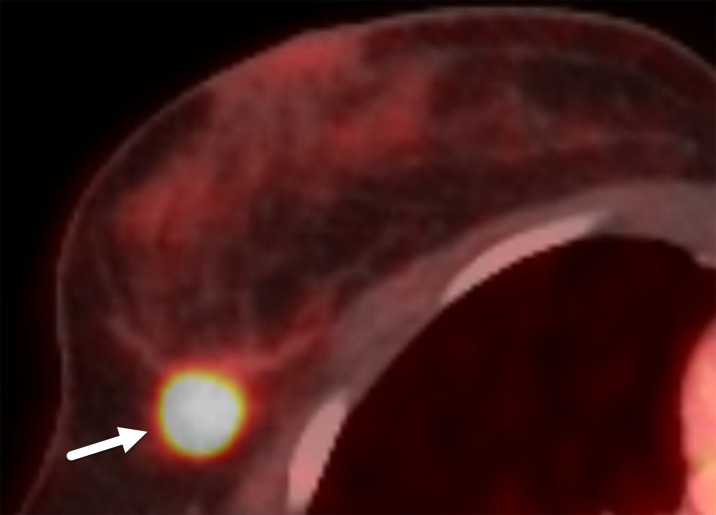
In patients with breast cancer, fluorine 18 fluorodeoxyglucose (FDG) PET/CT has been used for initial staging, identifying distant metastases, restaging local-regional recurrence, and monitoring response to treatment (44). Studies have demonstrated a correlation between the degree of FDG uptake and the subtype of breast cancer, with higher avidity seen in patients with poor prognostic features such as high tumor grade, hormone receptor negativity, triple negativity, and metaplastic tumors (45). In a study by Koo et al (46), TNBC and HER2-positive cancers showed higher maximal standardized uptake values than those of luminal A malignancies. This higher FDG avidity in TNBCs is believed to be related to their aggressive biologic behavior (Fig 13). In addition, significantly higher maximal standardized uptake values have been correlated with certain MRI breast features that are often seen in TNBC, such as high T2 signal intensity and a heterogeneous or rim enhancement pattern (47).
Figure 13.
Triple-negative invasive ductal carcinoma in a 33-year-old woman. Axial PET/CT image shows focal FDG uptake in the mass (arrow). PET/CT has high sensitivity for TNBC. Higher FDG uptake is related to enhanced glycolysis and a higher proliferation rate, which are characteristic of TNBC.
Imaging Features Predictive of TNBC Response to Treatment
Assessment of the TNBC response to NAC is dependent on the type of imaging modality being used. Conventional anatomic imaging modalities such as mammography and US rely on changes in tumor size, density, and morphology. Functional imaging modalities such as breast MRI can be used to evaluate not only changes in size and morphology but also vascularity and metabolic activity.
With MRI assessment of breast cancer response to NAC, the pattern of tumor shrinkage, in addition to the size of the tumor, should be evaluated and reported, as the shrinkage pattern is useful for surgical planning. Two types of shrinkage patterns are possible in patients who undergo NAC: concentric and nonconcentric. The concentric pattern is the most common in the setting of TNBC (Fig 14), occurring in 66.2% of cases, and is a favorable predictor for pCR (48) and achievement of negative surgical margins in patients who are selected for breast conservation surgery (49). Breast MRI outperforms conventional imaging and clinical examination in the prediction of residual disease after NAC (50). In a meta-analysis (51), breast MRI had an area under the receiver operating characteristic curve of 0.88 for predicting residual disease across all subtypes. However, Kim et al (52) found that the performance of breast MRI varied according to breast cancer subtype. In their study, the accuracy of MRI in predicting pCR was 85.6% for TNBC and 69.3% for hormone receptor–positive, HER2-positive disease.
Figure 14.
Triple-negative invasive ductal carcinoma of the right breast in a 40-year-old woman. (A) Axial postcontrast T1-weighted subtraction pretreatment MR image shows a 3-cm round mass (arrow) with homogeneous enhancement in the upper inner quadrant. (B) Axial postcontrast T1-weighted subtraction midtreatment MR image shows a 1-cm mass (arrows) with a pattern of concentric shrinkage. The central area with a signal void is related to susceptibility artifact from a biopsy clip. (C) Axial postcontrast T1-weighted subtraction posttreatment MR image shows a biopsy clip susceptibility artifact (*) but no evidence of enhancement, indicating complete resolution of the mass. The imaging findings are consistent with a complete imaging response. Final pathologic analysis revealed no residual carcinoma in the breast.
Breast MRI in the setting of TNBC has been shown to have high negative predictive values (60%–90%) (53,54), implying that if breast MRI shows a complete response after NAC, the likelihood of pCR at surgical-pathologic analysis is high. Breast MRI also facilitates the best agreement between the imaging-determined size of the residual tumor after NAC and the pathologically determined size of the residual tumor. The agreement between breast MRI–determined residual tumor size and pathologically determined residual tumor size is best for TNBC and worst for ER-positive, HER2-negative tumors (52,55).
To enable tailored treatment, efforts have been made to find imaging biomarkers that can be used to predict response to NAC. Findings at diffusion-weighted imaging are some of the imaging biomarkers that are being investigated for determining early treatment response. One study (56) showed that mean TNBC pretreatment apparent diffusion coefficient (ADC) values (± standard deviations) were lower in complete responders (1.060 × 10−3 mm2/sec ± 0.143) and higher in partial responders (1.227 × 10−3 mm2/sec ± 0.271). Partridge et al (57) found no difference in the absolute pretreatment ADC values between responders and nonresponders to neoadjuvant therapy. However, they found that the ADC value progressively increased among patients who achieved a pCR compared with patients who did not achieve a pCR and that there was a significant difference in the mean percentage change in ADC after treatment of the TNBC. The increase in ADC values after NAC reflects the decreased cellularity that allows water to diffuse freely (57). Breast MRI has a high negative predictive value for pCR in the setting of TNBC and is the imaging modality at which the imaging-determined residual tumor size is most concordant with the pathologically determined residual tumor size.
Clinical Management and Outcomes of TNBC
Lymph Node Involvement
Plasilova et al (11) reported that TNBC had a lower rate of lymph node involvement than other molecular subtypes of breast cancer after adjustments for tumor size and grade. This was confirmed by Liu et al (58) in a study involving 528 women with primary breast cancer. They found that TNBC and luminal A breast cancers were node negative more often (in 77.4% and 73.4% of cases, respectively) than luminal B and HER2-positive breast cancers (in 45.3% and 40.0% of cases, respectively). Nodal involvement with TNBC significantly affects overall survival. Hernandez-Aya et al (59) reviewed 1711 TNBCs and found that the 5-year overall survival rate decreased with increasing number of positive axillary lymph nodes, with 5-year overall survival rates of 80% for node-negative disease, 65% for N1 disease (one to three positive lymph nodes), 48% for N2 disease (four to nine positive lymph nodes), and as low as 44% for 10 or more positive lymph nodes.
Treatment
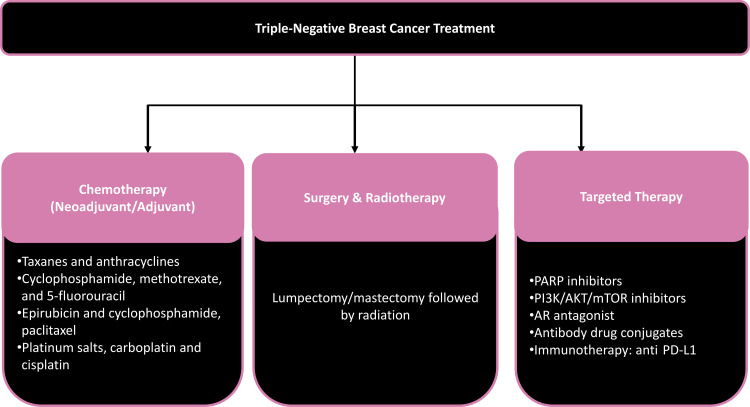
The local-regional treatment options for TNBC are similar to those for other types of breast cancer and include lumpectomy with radiation therapy and mastectomy with or without radiation therapy (Fig 15). Neoadjuvant systemic therapy is the standard of care for stages II–III TNBC but remains controversial for treatment of stage T1 TNBC (60). Owing to the absence of actionable targets in TNBC, such as estrogen, progesterone, and HER2 receptors, until very recently the only systemic treatment option for TNBC was traditional cytotoxic chemotherapy consisting of anthracycline- and cyclophosphamide-based treatment combined with taxane therapy, sometimes with the addition of platinum agents. pCR after NAC has been shown to correlate with excellent long-term response and survival, establishing pCR as an important prognostic marker for TNBC (61).
Figure 15.
Options for treatment of TNBCs. AR = androgen receptor, PARP = poly (ADP-ribose), PI3K/AKT/mTOR = phosphoinositide 3–kinase/protein kinase B/mammalian target of rapamycin.
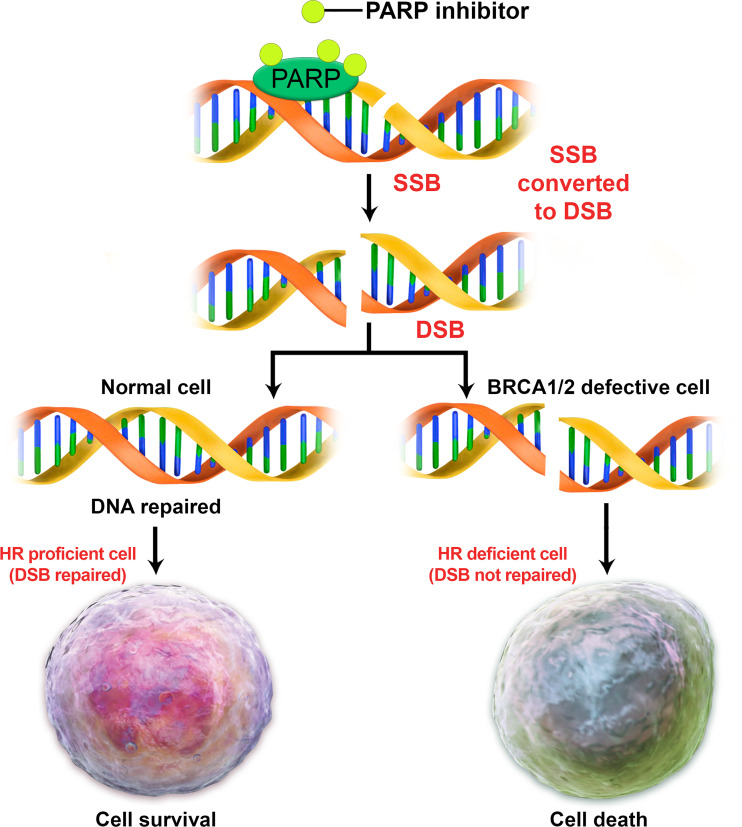
Improved understanding of TNBC biologic factors, tumoral intrinsic and microenvironment heterogeneity, and co-evolution of the tumor-immune system has led to the development of advanced targeted agents (61). One of the most promising targeted therapies for TNBC is inhibition of the poly (adenosine diphosphate–ribose) polymerase (PARP) enzyme system in BRCA1- and BCRA2-deficient cells. The rationale for this therapy is the inhibition of one of the mechanisms to repair DNA (Fig 16). PARP1 is involved in the repair of single-strand breaks (SSBs) in DNA. If the ability of a cell to repair SSBs is inhibited, as in the case with a PARP1 inhibitor, SSBs accumulate and become double-strand breaks (DSBs).
Figure 16.
Diagram illustrates how normal cells use several mechanisms to repair DNA. One of these mechanisms involves the use of BRCA1 and BRCA2 proteins to help repair DNA double-strand breaks (DSBs) by means of homologous recombination (HR). Another mechanism used to repair DNA is the poly (adenosine diphosphate–ribose) polymerase (PARP) system, which helps to repair DNA single-strand breaks (SSBs). Efficient SSB repair is essential for cell survival. Unrepaired SSBs can be converted to DSBs, which are toxic to cells. HR is the major pathway to repairing such DSBs during cell replication. HR-proficient cells can repair DSBs to ensure genome stability and cell survival, while HR-deficient cells (in BRCA mutation carriers) cannot repair DSBs and undergo apoptosis and eventually cell death.
DSBs are lethal to cells unless they are repaired by the homologous recombination repair pathway. The homologous recombination repair pathway is deficient in BRCA mutation carriers. Accordingly, TNBCs carrying BRCA mutations and/or other similar DNA repair pathway mutations are sensitive to PARP inhibitor therapy (62). Thus, the use of PARP inhibitors will hinder the repair of SSBs, and without a functioning homologous recombination repair pathway, two DNA repair systems will be damaged and result in cell death. This briefly explains how PARP inhibitors can kill tumor cells that are defective in BRCA genes without affecting the survival of cells with normal BRCA functioning. PARP1 inhibitors have been approved as targeted treatments for TNBC in patients with BRCA mutations (62). Their role in the treatment of sporadic TNBC is still under investigation.
Similarly, the discovery of the high activity of immune checkpoint inhibitors in TNBC led to multiple clinical trials being conducted to evaluate the role of anti–PD-1 and anti–PD-L1 inhibitors for the treatment of TNBC. The KEYNOTE-522 trial (63) showed higher rates of pCR with use of combined pembrolizumab, a PD-1–targeted antibody, and chemotherapy, as compared with the use of chemotherapy alone, leading to the U.S. Food and Drug Administration approval of pembrolizumab for treatment of TNBC in 2021. Multiple ongoing clinical trials are being conducted to evaluate other targeted agents for the treatment of TNBC (64).
Recurrence after Treatment of TNBC
There are three main types of breast cancer recurrence: local, regional, and distant. With local recurrence, the cancer recurs in the breast where it was initially diagnosed. With regional recurrence, the cancer recurs in nearby lymph nodes or the chest wall. With distant recurrence, also referred to as metastasis, the cancer recurs at another site in the body. Local and regional recurrences are often grouped together and termed local-regional recurrences. A part of what makes TNBC notoriously aggressive is the high rates of local and distant recurrences following initial regression with chemotherapy.
The rate of local-regional recurrence of TNBC is 7.4%–13.5% (65,66) and is higher than that of other breast cancer subtypes. Sixty percent of local-regional recurrences of TNBC involve the lymph nodes (67). The high rate of local-regional recurrence of TNBC is probably related to the fact that therapies targeting hormone receptor and HER2 are not effective against TNBC.
The rate of distant recurrence of TNBC is approximately 27.4% (67), and the risk of distant recurrence peaks the 3rd year after the diagnosis. The median survival time after the diagnosis of metastasis is 13.3 months (10). Lin et al (10) reported that 46% of cases of distant metastasis of TNBC are in the central nervous system and that 41% of these cases are in the lung.
The risk of recurrence of TNBC is unique in that it is the highest within the first 5 years after diagnosis. Investigators in one study (68) found that patients with TNBC had high recurrence rates 1–4 years after the initial diagnosis and that the risk of recurrence rapidly decreased to virtually no risk of recurrence at 8 years after the diagnosis. Conversely, the risk of recurrence for non–TNBCs is steady, continuing for more than 10 years after diagnosis. Recurrent TNBC usually progresses rapidly and exhibits a strong resistance to chemotherapy and radiation therapy (Fig 17).
Figure 17.
Triple-negative metaplastic carcinoma of the left breast in a 38-year-old woman. (A) Axial postcontrast T1-weighted MR image shows the malignancy as an 8-cm area of nonmass enhancement with regional distribution (arrows). (B) Axial postcontrast T1-weighted subtraction MR image in the early phase after NAC shows no residual enhancement. A biopsy clip–related susceptibility artifact (arrow) is noted. (C) Axial postcontrast T1-weighted subtraction MR image in the late phase after NAC shows no residual enhancement, with the biopsy clip–related susceptibility artifact (arrow) still seen. The patient underwent surgery, which revealed a 1-mm focus of residual carcinoma. (D) Maximum intensity projection breast MR image 1 year after the completion of segmental mastectomy and radiation therapy shows numerous areas of nonmass enhancement and multiple foci suggestive of recurrence involving four quadrants (arrows). US-guided biopsy confirmed the recurrence.
Imaging Biomarkers for TNBC Recurrence
Identification of patients who are at high risk for TNBC recurrence is important for guiding posttreatment surveillance. Therefore, efforts have been made to determine whether any TNBC MRI features are associated with the likelihood of recurrence. The functional tumor volume (FTV) measured by using breast MRI during the course of neoadjuvant treatment has been shown to be a strong predictor of recurrence. The FTV assessed after one cycle of chemotherapy can be helpful in predicting recurrence-free survival for patients with early-stage TNBC (69).
Currently there is extensive research on potential radiomic predictors of outcome. Such research has shown that tumors with higher heterogeneity are associated with poor survival. Koh et al (70) analyzed the three-dimensional radiomic features of TNBC. They extracted 3995 radiomic features, selected 32 features, and generated a radiomic score. The radiomic score was correlated with clinicopathologic features and accurately predicted disease-free survival. Investigators in one study (71) reported that radiomic models based on the combination of pre- and post-MRI features performed well in predicting recurrence within 3 years after NAC. However, the results of these studies need to be validated with multicenter studies and large cohorts of patients. Radiomic features are expected to be key prognostic factors in TNBC in the future.
Conclusion
TNBCs are an aggressive and heterogeneous group of cancers with a wide range of histologic, molecular, and imaging profiles and variable responses to treatment. TNBCs may have benign imaging features, which can lead to radiologists misdiagnosing them, and may rapidly progress to a higher stage if not properly diagnosed and treated.
Racial and age disparities have been observed among patients with TNBC, and it is critical that future research be designed to improve understanding of the factors that contribute to these disparities.
As a result of advances in genomic profiling, TNBC has been classified into molecular subtypes that respond differently to systemic therapy. Although traditional chemotherapy is currently the mainstay of systemic therapy for TNBC, it has limited effectiveness. Immunotherapy drugs and other advanced therapies, such as PARP inhibitors, have recently been added to the options that are available for treating patients with TNBC. Numerous clinical trials on TNBC therapy are underway. In addition, imaging and pathologic biomarkers to predict tumor recurrence and response to chemotherapy are under investigation. The use of these biomarkers may pave the way to personalized treatment for patients with TNBC.
Acknowledgments
Acknowledgments
The manuscript was edited by Stephanie Deming, ELS, Research Medical Library; and the illustrations were created by Kelly Kage, BS, MFA, Division of Diagnostic Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX.
Supported by a National Institutes of Health/National Cancer Institute MD Anderson Cancer Support Grant (P30CA016672).
Recipient of a Cum Laude award for an education exhibit at the 2022 RSNA Annual Meeting.
Disclosures of conflicts of interest.—: T.W.M. Consulting fees from Hologic, Siemens Healthineers, and Merit Medical. All other authors, the editor, and the reviewers have disclosed no relevant relationships.
Abbreviations:
- ER
- estrogen receptor
- FDG
- fluorine 18 fluorodeoxyglucose
- HER2
- human epidermal growth factor receptor 2
- NAC
- neoadjuvant chemotherapy
- pCR
- pathologic complete response
- PD-L1
- programmed cell death ligand 1
- PD-1
- programmed cell death protein 1
- TIL
- tumor-infiltrating lymphocyte
- TNBC
- triple-negative breast cancer
References
- 1. Centers for Disease Control and Prevention . Breast Cancer Statistics . Centers for Disease Control and Prevention website https://www.cdc.gov/cancer/breast/statistics/index.htm. Updated June 6, 2022. Accessed February 1, 2023 .
- 2. Trop I , LeBlanc SM , David J , et al . Molecular classification of infiltrating breast cancer: toward personalized therapy . RadioGraphics 2014. ; 34 ( 5 ): 1178 – 1195 . [DOI] [PubMed] [Google Scholar]
- 3. National Cancer Institute . Cancer Stat Facts: Female Breast Cancer Subtypes . National Institutes of Health, National Cancer Institute website https://seer.cancer.gov/statfacts/html/breast-subtypes.html. Accessed March 28, 2023 .
- 4. Howard FM , Olopade OI . Epidemiology of triple-negative breast cancer: a review . Cancer J 2021. ; 27 ( 1 ): 8 – 16 . [DOI] [PubMed] [Google Scholar]
- 5. Liedtke C , Mazouni C , Hess KR , et al . Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer . J Clin Oncol 2008. ; 26 ( 8 ): 1275 – 1281 . [Published correction appears in J Clin Oncol 2023;41(10):1809-1815.] [DOI] [PubMed] [Google Scholar]
- 6. McCarthy AM , Friebel-Klingner T , Ehsan S , et al . Relationship of established risk factors with breast cancer subtypes . Cancer Med 2021. ; 10 ( 18 ): 6456 – 6467 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tufano AM , Teplinsky E , Landry CA . Updates in neoadjuvant therapy for triple negative breast cancer . Clin Breast Cancer 2021. ; 21 ( 1 ): 1 – 9 . [DOI] [PubMed] [Google Scholar]
- 8. Spring LM , Fell G , Arfe A , et al . Pathologic Complete Response after Neoadjuvant Chemotherapy and Impact on Breast Cancer Recurrence and Survival: A Comprehensive Meta-analysis . Clin Cancer Res 2020. ; 26 ( 12 ): 2838 – 2848 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howlader N , Cronin KA , Kurian AW , Andridge R . Differences in breast cancer survival by molecular subtypes in the United States . Cancer Epidemiol Biomarkers Prev 2018. ; 27 ( 6 ): 619 – 626 . [DOI] [PubMed] [Google Scholar]
- 10. Lin NU , Claus E , Sohl J , Razzak AR , Arnaout A , Winer EP . Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases . Cancer 2008. ; 113 ( 10 ): 2638 – 2645 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Plasilova ML , Hayse B , Killelea BK , Horowitz NR , Chagpar AB , Lannin DR . Features of triple-negative breast cancer: analysis of 38,813 cases from the national cancer database . Medicine (Baltimore) 2016. ; 95 ( 35 ): e4614 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhu W , Perez EA , Hong R , Li Q , Xu B . Age-related disparity in immediate prognosis of patients with triple-negative breast cancer: a population-based study from SEER cancer registries . PLoS One 2015. ; 10 ( 5 ): e0128345 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sung H , Wiese D , Jatoi I , Jemal A . State variation in racial and ethnic disparities in incidence of triple-negative breast cancer among US women . JAMA Oncol 2023. ; 9 ( 5 ): 700 – 704 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cho B , Han Y , Lian M , et al . Evaluation of racial/ethnic differences in treatment and mortality among women with triple-negative breast cancer . JAMA Oncol 2021. ; 7 ( 7 ): 1016 – 1023 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyle P . Triple-negative breast cancer: epidemiological considerations and recommendations . Ann Oncol 2012. ; 23 ( suppl 6 ): vi7 – vi12 . [DOI] [PubMed] [Google Scholar]
- 16. Atchley DP , Albarracin CT , Lopez A , et al . Clinical and pathologic characteristics of patients with BRCA-positive and BRCA-negative breast cancer . J Clin Oncol 2008. ; 26 ( 26 ): 4282 – 4288 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Comen E , Davids M , Kirchhoff T , Hudis C , Offit K , Robson M . Relative contributions of BRCA1 and BRCA2 mutations to “triple-negative” breast cancer in Ashkenazi Women . Breast Cancer Res Treat 2011. ; 129 ( 1 ): 185 – 190 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mavaddat N , Barrowdale D , Andrulis IL , et al. ; Consortium of Investigators of Modifiers of BRCA1/2 . Pathology of breast and ovarian cancers among BRCA1 and BRCA2 mutation carriers: results from the Consortium of Investigators of Modifiers of BRCA1/2 (CIMBA) . Cancer Epidemiol Biomarkers Prev 2012. ; 21 ( 1 ): 134 – 147 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shah SP , Roth A , Goya R , et al . The clonal and mutational evolution spectrum of primary triple-negative breast cancers . Nature 2012. ; 486 ( 7403 ): 395 – 399 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shimelis H , LaDuca H , Hu C , et al . Triple-negative breast cancer risk genes identified by multigene hereditary cancer panel testing . J Natl Cancer Inst 2018. ; 110 ( 8 ): 855 – 862 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daly MB , Pilarski R , Berry M , et al . NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Genetic/Familial High-Risk Assessment: Breast and Ovarian . Version 3.2019 . National Comprehensive Cancer Network website . https://www2.tri-kobe.org/nccn/guideline/gynecological/english/genetic_familial.pdf. Accessed October 3, 2017 .
- 22. Lehmann BD , Jovanović B , Chen X , et al . Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection . PLoS One 2016. ; 11 ( 6 ): e0157368 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Masuda H , Baggerly KA , Wang Y , et al . Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes . Clin Cancer Res 2013. ; 19 ( 19 ): 5533 – 5540 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sukumar J , Gast K , Quiroga D , Lustberg M , Williams N . Triple-negative breast cancer: promising prognostic biomarkers currently in development . Expert Rev Anticancer Ther 2021. ; 21 ( 2 ): 135 – 148 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Salgado R , Denkert C , Demaria S , et al. ; International TILs Working Group 2014 . The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014 . Ann Oncol 2015. ; 26 ( 2 ): 259 – 271 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ku YJ , Kim HH , Cha JH , et al . Correlation between MRI and the level of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer . AJR Am J Roentgenol 2016. ; 207 ( 5 ): 1146 – 1151 . [DOI] [PubMed] [Google Scholar]
- 27. Candelaria RP , Spak DA , Rauch GM , et al . BI-RADS ultrasound lexicon descriptors and stromal tumor-infiltrating lymphocytes in triple-negative breast cancer . Acad Radiol 2022. ; 29 ( Suppl 1 ): S35 – S41 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Krizmanich-Conniff KM , Paramagul C , Patterson SK , et al . Triple receptor-negative breast cancer: imaging and clinical characteristics . AJR Am J Roentgenol 2012. ; 199 ( 2 ): 458 – 464 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang Y , Ikeda DM , Narasimhan B , et al . Estrogen receptor–negative invasive breast cancer: imaging features of tumors with and without human epidermal growth factor receptor type 2 overexpression . Radiology 2008. ; 246 ( 2 ): 367 – 375 . [DOI] [PubMed] [Google Scholar]
- 30. Yang WT , Dryden M , Broglio K , et al . Mammographic features of triple receptor-negative primary breast cancers in young premenopausal women . Breast Cancer Res Treat 2008. ; 111 ( 3 ): 405 – 410 . [DOI] [PubMed] [Google Scholar]
- 31. Ko ES , Lee BH , Kim HA , Noh WC , Kim MS , Lee SA . Triple-negative breast cancer: correlation between imaging and pathological findings . Eur Radiol 2010. ; 20 ( 5 ): 1111 – 1117 . [DOI] [PubMed] [Google Scholar]
- 32. Johnson KS , Conant EF , Soo MS . Molecular subtypes of breast cancer: a review for breast radiologists . J Breast Imaging 2021. ; 3 ( 1 ): 12 – 24 . [DOI] [PubMed] [Google Scholar]
- 33. Aslan AA , Gültekin S , Karakoç E , Tosun SN . Impact of menopausal status on imaging findings of patients with triple-negative breast cancer . J Breast Imaging 2022. ; 4 ( 4 ): 384 – 391 . [DOI] [PubMed] [Google Scholar]
- 34. Dogan BE , Turnbull LW . Imaging of triple-negative breast cancer . Ann Oncol 2012. ; 23 ( Suppl 6 ): vi23 – vi29 . [DOI] [PubMed] [Google Scholar]
- 35. Karbasian N , Sohrabi S , Omofoye TS , et al . Imaging Features of Triple Negative Breast Cancer and the Effect of BRCA Mutations . Curr Probl Diagn Radiol 2021. ; 50 ( 3 ): 303 – 307 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Boisserie-Lacroix M , Macgrogan G , Debled M , et al . Triple-negative breast cancers: associations between imaging and pathological findings for triple-negative tumors compared with hormone receptor-positive/human epidermal growth factor receptor-2-negative breast cancers . Oncologist 2013. ; 18 ( 7 ): 802 – 811 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sung JS , Jochelson MS , Brennan S , et al . MR imaging features of triple-negative breast cancers . Breast J 2013. ; 19 ( 6 ): 643 – 649 . [DOI] [PubMed] [Google Scholar]
- 38. Uematsu T , Kasami M , Yuen S . Triple-negative breast cancer: correlation between MR imaging and pathologic findings . Radiology 2009. ; 250 ( 3 ): 638 – 647 . [DOI] [PubMed] [Google Scholar]
- 39. Bae MS , Moon HG , Han W , et al . Early stage triple-negative breast cancer: imaging and clinical-pathologic factors associated with recurrence . Radiology 2016. ; 278 ( 2 ): 356 – 364 . [DOI] [PubMed] [Google Scholar]
- 40. Costantini M , Belli P , Distefano D , et al . Magnetic resonance imaging features in triple-negative breast cancer: comparison with luminal and HER2-overexpressing tumors . Clin Breast Cancer 2012. ; 12 ( 5 ): 331 – 339 . [DOI] [PubMed] [Google Scholar]
- 41. Lee YJ , Youn IK , Kim SH , Kang BJ , Park WC , Lee A . Triple-negative breast cancer: Pretreatment magnetic resonance imaging features and clinicopathological factors associated with recurrence . Magn Reson Imaging 2020. ; 66 : 36 – 41 . [DOI] [PubMed] [Google Scholar]
- 42. Youk JH , Son EJ , Chung J , Kim JA , Kim EK . Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes . Eur Radiol 2012. ; 22 ( 8 ): 1724 – 1734 . [DOI] [PubMed] [Google Scholar]
- 43. Grimm LJ , Johnson KS , Marcom PK , Baker JA , Soo MS . Can breast cancer molecular subtype help to select patients for preoperative MR imaging? Radiology 2015. ; 274 ( 2 ): 352 – 358 . [DOI] [PubMed] [Google Scholar]
- 44. Groheux D . FDG-PET/CT for Primary Staging and Detection of Recurrence of Breast Cancer . Semin Nucl Med 2022. ; 52 ( 5 ): 508 – 519 . [DOI] [PubMed] [Google Scholar]
- 45. Groheux D , Giacchetti S , Moretti JL , et al . Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer . Eur J Nucl Med Mol Imaging 2011. ; 38 ( 3 ): 426 – 435 . [DOI] [PubMed] [Google Scholar]
- 46. Koo HR , Park JS , Kang KW , et al . 18F-FDG uptake in breast cancer correlates with immunohistochemically defined subtypes . Eur Radiol 2014. ; 24 ( 3 ): 610 – 618 . [DOI] [PubMed] [Google Scholar]
- 47. Choi BB , Lee JS , Kim KH . Association between MRI features and standardized uptake value of 18F-FDG PET/CT in triple-negative breast cancer . Oncol Res Treat 2018. ; 41 ( 11 ): 706 – 711 . [DOI] [PubMed] [Google Scholar]
- 48. Eom HJ , Cha JH , Choi WJ , Chae EY , Shin HJ , Kim HH . Predictive clinicopathologic and dynamic contrast-enhanced MRI findings for tumor response to neoadjuvant chemotherapy in triple-negative breast cancer . AJR Am J Roentgenol 2017. ; 208 ( 6 ): W225 – W230 . [DOI] [PubMed] [Google Scholar]
- 49. Zheng CH , Xu K , Shan WP , et al . Meta-analysis of shrinkage mode after neoadjuvant chemotherapy for breast cancers: association with hormonal receptor . Front Oncol 2022. ; 11 : 617167 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Scheel JR , Kim E , Partridge SC , et al. ; ACRIN 6657 Trial Team and I-SPY Investigators Network . MRI, Clinical Examination, and Mammography for Preoperative Assessment of Residual Disease and Pathologic Complete Response After Neoadjuvant Chemotherapy for Breast Cancer: ACRIN 6657 Trial . AJR Am J Roentgenol 2018. ; 210 ( 6 ): 1376 – 1385 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Marinovich ML , Houssami N , Macaskill P , et al . Meta-analysis of magnetic resonance imaging in detecting residual breast cancer after neoadjuvant therapy . J Natl Cancer Inst 2013. ; 105 ( 5 ): 321 – 333 . [DOI] [PubMed] [Google Scholar]
- 52. Kim SY , Cho N , Park IA , et al . Dynamic contrast-enhanced breast MRI for evaluating residual tumor size after neoadjuvant chemotherapy . Radiology 2018. ; 289 ( 2 ): 327 – 334 . [DOI] [PubMed] [Google Scholar]
- 53. De Los Santos JF , Cantor A , Amos KD , et al . Magnetic resonance imaging as a predictor of pathologic response in patients treated with neoadjuvant systemic treatment for operable breast cancer. Translational Breast Cancer Research Consortium trial 017 . Cancer 2013. ; 119 ( 10 ): 1776 – 1783 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen CA , Hayward JH , Woodard GA , et al . Complete breast MRI response to neoadjuvant chemotherapy and prediction of pathologic complete response . J Breast Imaging 2019. ; 1 ( 3 ): 217 – 222 . [DOI] [PubMed] [Google Scholar]
- 55. Bae SJ , Ahn SG , Yoon CI , et al . Measuring tumor extent based on subtypes using magnetic resonance imaging: radiologic-pathologic discordance and high positive margin rates in breast cancer . J Breast Cancer 2019. ; 22 ( 3 ): 453 – 463 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Richard R , Thomassin I , Chapellier M , et al . Diffusion-weighted MRI in pretreatment prediction of response to neoadjuvant chemotherapy in patients with breast cancer . Eur Radiol 2013. ; 23 ( 9 ): 2420 – 2431 . [DOI] [PubMed] [Google Scholar]
- 57. Partridge SC , Zhang Z , Newitt DC , et al. ; ACRIN 6698 Trial Team and I-SPY 2 Trial Investigators . Diffusion-weighted MRI Findings Predict Pathologic Response in Neoadjuvant Treatment of Breast Cancer: The ACRIN 6698 Multicenter Trial . Radiology 2018. ; 289 ( 3 ): 618 – 627 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Liu N , Yang Z , Liu X , Niu Y . Lymph node status in different molecular subtype of breast cancer: triple negative tumours are more likely lymph node negative . Oncotarget 2017. ; 8 ( 33 ): 55534 – 55543 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hernandez-Aya LF , Chavez-Macgregor M , Lei X , et al . Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer . J Clin Oncol 2011. ; 29 ( 19 ): 2628 – 2634 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kumar P , Aggarwal R . An overview of triple-negative breast cancer . Arch Gynecol Obstet 2016. ; 293 ( 2 ): 247 – 269 . [DOI] [PubMed] [Google Scholar]
- 61. Bianchini G , De Angelis C , Licata L , Gianni L . Treatment landscape of triple-negative breast cancer - expanded options, evolving needs . Nat Rev Clin Oncol 2022. ; 19 ( 2 ): 91 – 113 . [DOI] [PubMed] [Google Scholar]
- 62. Gupta GK , Collier AL , Lee D , et al . Perspectives on triple-negative breast cancer: current treatment strategies, unmet needs, and potential targets for future therapies . Cancers (Basel) 2020. ; 12 ( 9 ): 2392 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Schmid P , Cortes J , Pusztai L , et al. ; KEYNOTE-522 Investigators . Pembrolizumab for Early Triple-Negative Breast Cancer . N Engl J Med 2020. ; 382 ( 9 ): 810 – 821 . [DOI] [PubMed] [Google Scholar]
- 64. Vagia E , Mahalingam D , Cristofanilli M . The landscape of targeted therapies in TNBC . Cancers (Basel) 2020. ; 12 ( 4 ): 916 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McGuire A , Lowery AJ , Kell MR , Kerin MJ , Sweeney KJ . Locoregional recurrence following breast cancer surgery in the trastuzumab era: a systematic review by subtype . Ann Surg Oncol 2017. ; 24 ( 11 ): 3124 – 3132 . [DOI] [PubMed] [Google Scholar]
- 66. Lowery AJ , Kell MR , Glynn RW , Kerin MJ , Sweeney KJ . Locoregional recurrence after breast cancer surgery: a systematic review by receptor phenotype . Breast Cancer Res Treat 2012. ; 133 ( 3 ): 831 – 841 . [DOI] [PubMed] [Google Scholar]
- 67. Wu X , Baig A , Kasymjanova G , et al . Pattern of local recurrence and distant metastasis in breast cancer by molecular subtype . Cureus 2016. ; 8 ( 12 ): e924 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reddy TP , Rosato RR , Li X , Moulder S , Piwnica-Worms H , Chang JC . A comprehensive overview of metaplastic breast cancer: clinical features and molecular aberrations . Breast Cancer Res 2020. ; 22 ( 1 ): 121 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Hylton NM , Gatsonis CA , Rosen MA , et al. ; ACRIN 6657 Trial Team and I-SPY 1 TRIAL Investigators . Neoadjuvant Chemotherapy for Breast Cancer: Functional Tumor Volume by MR Imaging Predicts Recurrence-free Survival—Results from the ACRIN 6657/CALGB 150007 I-SPY 1 TRIAL . Radiology 2016. ; 279 ( 1 ): 44 – 55 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Koh J , Lee E , Han K , et al . Three-dimensional radiomics of triple-negative breast cancer: prediction of systemic recurrence . Sci Rep 2020. ; 10 ( 1 ): 2976 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ma M , Gan L , Liu Y , et al . Radiomics features based on automatic segmented MRI images: prognostic biomarkers for triple-negative breast cancer treated with neoadjuvant chemotherapy . Eur J Radiol 2022. ; 146 : 110095 . [DOI] [PubMed] [Google Scholar]



![Left breast palpable abnormality in a 39-year-old woman. (A) Left craniocaudal mammogram shows a 0.9-cm oval mass (dashed arrow) in the upper outer breast correlating with the palpable area of concern (triangular marker [*] ). This lesion was shown to represent an intramammary lymph node at subsequent US. A 1-cm oval mass (solid arrow) at the posterior depth was incidentally noted. (B) On a transverse US image, the 1-cm mass appears to have an oval shape and circumscribed margins (arrow). (C) Six-month follow-up US image shows a 1.4-cm irregularly shaped mass with microlobulated margins (arrow). US-guided biopsy revealed TNBC.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/6dfb/10560981/8ea8765d15d6/rg.230034.fig8.jpg)