Abstract
HMR 3004 is a new hydrazono ketolide characterized by a 3-keto function instead of the cladinose moiety. The effect of this antimicrobial agent on inducible and constitutive macrolide-lincosamide-streptogramin B (MLSB) resistance was tested in a lacZ reporter system under control of several ermAM-like attenuator variants. For one constitutively resistant Streptococcus agalactiae strain, three inducibly resistant Streptococcus pneumoniae strains, and one inducibly resistant Enterococcus faecalis strain, the attenuators fused with lacZ were cloned into the shuttle plasmid pJIM2246 and the plasmid was introduced into Staphylococcus aureus RN4220. For the wild-type attenuators, HMR 3004 was a very weak inducer, unlike its cladinose counterpart RU 6652 and erythromycin. As expected, for the fusion originating from the constitutively resistant S. agalactiae strain, the level of uninduced β-galactosidase synthesis was high. For one S. pneumoniae attenuator, mutations in the 3′ end of the attenuator that weakened the stem-loop structure that sequesters the ribosome-binding site and start codon for ermAM methylase could explain the high level of uninduced β-galactosidase produced. For streptococci, the activity of HMR 3004 correlated with the basal level of β-galactosidase synthesized. The weak inducer activity of HMR 3004 explained its activity against inducibly MLSB-resistant S. pneumoniae but did not correlate with the moderate activity of the antibiotic against inducibly resistant E. faecalis.
The emergence of resistance to erythromycin in enterococci, staphylococci, pneumococci, and other streptococci is reported worldwide (28). Modification of the ribosomal target of the macrolides remains a frequent mechanism of resistance, although active efflux of erythromycin, recently reported in Streptococcus pyogenes and Streptococcus pneumoniae, appears increasingly prevalent (7, 26, 27). N-6 dimethylation of a specific adenine residue in 23S rRNA confers cross-resistance to macrolides, lincosamides, and streptogramins, the so-called MLSB phenotype (16). MLSB resistance is encoded by the prototype ermAM gene from plasmid pAM77 in Streptococcus sanguis (17). Hybridization experiments and nucleotide sequencing have shown that determinants closely related to the ermAM gene are widespread in streptococci, pneumococci, and enterococci, in which they are borne by transposons similar to Tn1545 (22, 30) or Tn917 (29) or by plasmids (15). Expression of MLSB resistance may be inducible or constitutive, depending upon a putative regulatory region preceding the ermAM gene. This region is composed, from 5′ to 3′, of sequences coding for a putative 36-amino-acid leader peptide followed by a set of inverted repeats forming 14 possible axes of symmetry (17). Since this structure resembles one, preceding the ermC gene, that is responsible for translational attenuation of MLSB resistance in Staphylococcus aureus, a similar mechanism has been proposed for the expression of the ermAM gene (17, 33). In this model, the ribosome-binding site and the start codon for the methylase are sequestered within a stem-loop structure and are therefore inaccessible to the ribosomes, thus preventing methylase synthesis. It has been proposed that erythromycin provokes the stalling of ribosomes while the leader peptide is translated. Stalling, in turn, disrupts the secondary structure which sequester the initiation sequences for the methylase. Phenotypic expression of inducible MLSB resistance differs between streptococci and staphylococci. In staphylococci, inducible resistance is dissociated between erythromycin or other commercially available 14- and 15-membered macrolides, which are inducers, and 16-membered macrolides and lincosamides (e.g., clindamycin), which are not (19, 32). By contrast, there is cross-resistance between MLSB antibiotics, which are efficient inducers in streptococci (11, 17).
Several macrolides derived from erythromycin, such as clarithromycin, dirithromycin, and azithromycin, have recently been introduced in therapy. Although these antimicrobial agents display significantly improved pharmacokinetic properties, they do not overcome MLSB resistance (1). Recently, a new class of macrolides with 14-membered lactone rings, the ketolides, which are derivatives of erythromycin A characterized by a 3-keto function instead of the cladinose moiety, has been shown to be active against most erythromycin-resistant gram-positive organisms (2, 3, 10). One of the most active ketolides is HMR 3004, which is a hydrazono ketolide (2). In recent studies, this antimicrobial agent demonstrated activity against most streptococci and pneumococci cross-resistant to erythromycin, spiramycin (a 16-membered macrolide), and clindamycin (2, 3, 10). This activity was attributed to the lack of induction of resistance to MLSB by this compound (3). In the investigation described in this report, we have compared the ability of HMR 3004 to induce β-galactosidase activity to those of its cladinose counterpart, RU 66252, and of erythromycin in ermAM-lacZ gene fusions from strains for which the MICs of HMR 3004 varied.
MATERIALS AND METHODS
Bacterial strains.
The activity of HMR 3004 was tested against 40 clinical isolates of streptococci and enterococci including 11 Enterococcus faecalis, 2 Enterococcus faecium, 14 S. pneumoniae, 6 oral streptococcal and 7 Streptococcus agalactiae isolates resistant to erythromycin and lincomycin (31). The strains were isolated from patients hospitalized in various hospitals in Europe and the United States at different times, and no link could be established between the patients, whose infections were considered epidemiologically unrelated. The erythromycin-susceptible strains E. faecalis JH2-2 (18), S. agalactiae HM50, and S. pneumoniae HM51 (31) and strain E. faecalis JH2-2/pAMβ1, which was constitutively resistant to erythromycin (20), were used as controls.
Antibiotic susceptibility testing.
The MICs of the antibiotics were determined by the agar dilution method with Mueller-Hinton medium (Sanofi Diagnostics Pasteur, Marnes-la-Coquette, France) supplemented with 5% horse blood and an inoculum of 104 cells per spot (8). The plates were incubated for 24 h at 37°C in 5% CO2. The following antibiotics were provided by their manufacturers: erythromycin, chloramphenicol, HMR 3004, and RU 66252 (Hoechst-Marion-Roussel, Romainville, France), spiramycin (Rhône-Poulenc, Rorer, Antony, France), and lincomycin (Upjohn Laboratories, Val de Reuil, France). RU 66252 is structurally similar to HMR 3004 except for a substitution of the 3-keto function by a cladinose sugar.
Gene fusions.
The regulatory regions and the first 24 nucleotides of the ermAM-like genes of four inducible strains (S. pneumoniae HM30, HM34, and HM28 and E. faecalis HM3) and of a constitutive strain (S. agalactiae HM1081) were amplified by PCR with oligonucleotides SR3 (5′-CTTAGAAGCAAACTTAAGAGTGTGT-3′) and SR5 (5′-GGTTGAGTACCTTTTCATTCGTTAA-3′), which are primers intended to amplify the regulatory regions of most ermAM-related genes on the basis of sequence comparison. The amplification products were purified and sequenced by the dideoxy chain termination technique (24). The PCR products were treated with T4 DNA polymerase to create blunt extremities and were cloned into the SmaI site of the fusion vector pMC1871 (which encodes tetracycline resistance) (Pharmacia Biotech, Saint Quentin-en-Yvelines, France) which contains a promoterless lacZ gene. The constructs were introduced by transformation into Escherichia coli MC1061 (9), and the transformants were plated onto media containing 10 μg of tetracycline per ml. The colonies were screened for β-galactosidase activity in brain heart infusion agar (bioMérieux, La Balme les Grottes, France) containing an inducing concentration of erythromycin (25 μg/ml) and β-d-galactopyranoside. For each strain, the recombinant plasmid was purified from one of the clones producing β-galactosidase in the presence of erythromycin. The recombinant plasmids were digested with PstI, and the fused ermAM-lacZ fragments were subcloned in the shuttle vector pJIM2246, which confers resistance to chloramphenicol (23), and were introduced by transformation into E. coli MC1061. The hybrid plasmids were purified and introduced by electroporation into S. aureus RN4220. Total and plasmid DNAs were isolated from E. coli by using commercial kits (Qiagen kit; Qiagen, Courtabeuf, France) and from S. aureus as described previously (13).
β-Galactosidase induction assays.
Staphylococci were grown to an optical density of 0.8 at 600 nm in brain heart infusion broth containing chloramphenicol (25 μg/ml). Cultures not induced or induced with nearly one-fifth of the MIC of a macrolide (0.03 μg of erythromycin or RU 66252 per ml and 0.005 μg of HMR 3004 per ml) were washed and lysed by sonication in TDTT (Tris-HCl 50 mM [pH 7.8], dithiothreitol 20 mM), and the cell debris was removed by centrifugation at 20,000 × g for 10 min. The protein concentration in the supernatants (S20) was determined by the technique of Bradford (5). β-Galactosidase activity was assessed as described previously (21). The chloramphenicol acetylase activities of the S20 extracts of induced and noninduced cells were measured as a control (25).
RESULTS
Activity of HMR 3004 against streptococci and enterococci.
The 40 streptococci and enterococci studied were resistant to erythromycin (MICs, 16 to 8,000 μg/ml) (Table 1). Expression of MLSB resistance in these strains was previously characterized by microbiological techniques and was found to be inducible in 24 strains of streptococci and 11 strains of enterococci and constitutive in five strains (2 E. faecalis, 1 S. agalactiae HM1081, and 2 S. pneumoniae strains) (31). The MICs of HMR 3004 were dispersed and ranged from <0.016 to 4 μg/ml for streptococci and from 2 to greater than 32 μg/ml for enterococci (Table 1). However, the activity of HMR 3004 differed depending on the inducible or constitutive expression of MLSB resistance. The MICs of HMR 3004 for 21 pneumococci and streptococci inducibly resistant to erythromycin were less than or equal to 0.25 μg/ml, as was true for the susceptible control strains (Table 1) and for previously reported erythromycin-susceptible strains (2, 3, 10); this was not true for three strains, however, including S. pneumoniae HM30 (MICs, 0.5 to 2 μg/ml). By contrast, the activity of HMR 3004 against enterococci inducibly resistant to MLSB antibiotics was markedly diminished in comparison to that against the erythromycin-susceptible control strain (Table 1). After induction with 0.1 μg of erythromycin per ml, the MICs of HMR 3004 for streptococci with inducible MLSB resistance increased from 0.03 to 2 μg/ml to 0.12 to 32 μg/ml and MICs of inducible enterococci increased from 2 to 16 μg/ml to 16 to 32 μg/ml (Table 1).
TABLE 1.
MICs of erythromycin and HMR 3004 for enterococci and streptococci
| Species (no. of strains) | MIC (μg/ml)
|
||
|---|---|---|---|
| Erythromycin | HMR 3004 | HMR 3004, induceda | |
| Control strains | |||
| E. faecalis JH2-2 | 1 | 0.03 | 0.03 |
| E. faecalis JH2-2/pAMβ1 | >8,000 | 32 | 32 |
| S. agalactiae HM50 | 0.25 | 0.03 | NDb |
| S. pneumoniae HM51 | 0.12 | 0.03 | 0.12 |
| Strains inducibly MLSB resistant | |||
| Enterococci (11) | 8,000 | 2–16 | 16–32 |
| Oral streptococci (6) | 256–>8,000 | 0.06–2 | 4–32 |
| S. agalactiae (6) | 16–4,000 | 0.03–2 | 0.12–8 |
| S. pneumoniae (12) | 128–8,000 | <0.01–1 | 1–4 |
| Strains constitutively MLSB resistant | |||
| Enterococci (2) | 8,000 | 32 | ≥32 |
| S. agalactiae HM1081 (1) | 2,000 | 8 | 8 |
| S. pneumoniae (2) | 8,000 | 4 | 4 |
Induced, the MICs of RU 640004 were determined after induction with 0.1 or 1 μg of erythromycin per ml.
ND, not done.
HMR 3004 was much less active against the six constitutively resistant strains (MICs, 4 to ≥32 μg/ml), including the control strain E. faecalis JH2-2/pAMβ1, although it was still considerably more active than erythromycin. As expected, the MICs of the ketolide did not further increase after culture in the presence of erythromycin (Table 1).
Gene fusion studies.
The abilities of erythromycin, HMR 3004, and RU 66252 to induce the synthesis of β-galactosidase were studied against four strains with inducible MLSB resistance (S. pneumoniae HM28, HM30, and HM34 and E. faecalis HM3) and one strain with constitutive MLSB resistance (S. agalactiae HM1081) in fusion experiments. The MICs of erythromycin, HMR 3004, and RU 66252 are presented in Table 2. HMR 3004 was poorly active against the inducibly resistant strains S. pneumoniae HM30 and E. faecalis HM3.
TABLE 2.
Activities of HMR 3004 and RU 66252 against MLSB-resistant strains used in fusion experiments
| Speciesa | MIC (μg/ml)
|
||
|---|---|---|---|
| Erythromycin | HMR 3004 | RU 66252 | |
| S. pneumoniae HM28 (MLSBi) | 128 | 0.12 | 8 |
| S. pneumoniae HM34 (MLSBi) | 1,000 | 0.12 | 16 |
| S. pneumoniae HM30 (MLSBi) | 2,000 | 2 | 8 |
| E. faecalis HM3 (MLSBi) | 1,000 | 4 | 8 |
| S. agalactiae HM1081 (MLSBc) | 2,000 | 8 | 8 |
MLSBi, inducible resistance to MLSB antibiotics; MLSBc, constitutive resistance to MLSB.
The sequence upstream from the ermAM-related genes was amplified by using SR3 and SR5 oligodeoxynucleotides as primers and total DNA from the five strains as a template. The inducibly resistant strains yielded a ca. 380-bp fragment, whereas the constitutively resistant strain S. agalactiae HM1081 yielded a 170-bp PCR product. The sequences of the PCR products and of the corresponding portion of ermAM from pAM77 are presented in Fig. 1. Alignment of the sequence of S. agalactiae HM1081 with that of the 5′ end of the ermAM gene from plasmid pAM77 revealed within the regulatory region a large deletion which could explain the constitutive expression of MLSB resistance in strain HM1081. In the other strains, duplication of a TAAA motif within the sequence for the leader peptide generated a stop codon which could lead to the synthesis of a shorter peptide. However, this truncation of the leader peptide did not result in constitutive expression. The amplification products fused with the lacZ gene on the shuttle vector pJIM2246 were introduced into S. aureus RN4220, and the β-galactosidase activity in S20 extracts from cells noninduced or induced with erythromycin or ketolides was measured (Table 3). In the absence of induction, all fused genes directed the synthesis of various basal levels of β-galactosidase. Consistent with the constitutive expression of MLSB resistance in S. agalactiae HM1081, the basal level of β-galactosidase synthesis in S. aureus RN4220 containing the corresponding ermAM-lacZ fusion was high and showed only a small increase after culture in the presence of erythromycin (Table 3). By contrast, the low level of production of β-galactosidase directed by fusions from inducibly resistant strains HM3, HM28, and HM34 increased after culture in the presence of erythromycin or RU 62252 by 5.4 to 9.7 times. For S. pneumoniae HM30, a higher basal level of β-galactosidase was measured; however, production of the enzyme increased slightly (by 1.7 times) after growth in the presence of an inducer. For strains inducibly resistant to MLSB antibiotics, HMR 3004 was a very weak inducer which increased β-galactosidase synthesis by 1.3 to 1.9 times (1.2 times for HM30) only. In all experiments, the activity of chloramphenicol acetyltransferase (expressed as micromoles per minute per milligram of protein) encoded by plasmid pJIM2246 was monitored in S20 extracts from induced or noninduced cells and remained stable.
FIG. 1.
Sequence alignment of attenuators of ermAM-related genes. pAM77, S. sanguis (17); Ef HM3, E. faecalis HM3; Pn HM28, Pn HM30, and Pn HM34, S. pneumoniae HM28, HM30, and HM34, respectively; Sa HM1081, S. agalactiae HM1081. Only differences from the sequence of ermAM are shown. Deletions are indicated by dashes. Putative promoters (−35, −10), ribosome-binding sites (SD1 and SD2), and sequences for the control peptide and methylase are underlined. Stop codons are underlined and are in italics.
TABLE 3.
β-Galactosidase activity in S20 extracts from induced and noninduced S. aureus RN4220 cells containing ermAM-lacZ fusions
| Origin of fusion gene | β-Galactosidase activity (units/g of protein/10−3)a
|
|||
|---|---|---|---|---|
| Without induction | After induction with the following drug (inducing concn [μg/ml]):
|
|||
| Erythro- mycin (0.03) | RU 66252 (0.03) | HMR 3004 (0.005) | ||
| S. pneumoniae HM28 | 258 ± 27 | 1,686 ± 28 | 2,499 ± 151 | 486 ± 72 |
| S. pneumoniae HM34 | 447 ± 61.5 | 3,012 ± 135 | 3,203 ± 27 | 597 ± 90 |
| S. pneumoniae HM30 | 1593 ± 80 | 2,685 ± 394 | 2,659 ± 61 | 2,048 ± 60 |
| E. faecalis HM3 | 393 ± 12 | 2,118 ± 197 | 2,362 ± 73 | 592 ± 80 |
| S. agalactiae HM1081 | 3,230 ± 110 | 3,776 ± 126 | 3,325 ± 125 | 2,830 ± 70 |
Results are means of a minimum of three independent experiments.
DISCUSSION
The mechanism of inducible expression of resistance to MLSB antibiotics in streptococci and enterococci has been assigned to the structures of sequences upstream from the ermAM genes on the basis of their similarity to the regulatory region of the ermC gene from staphylococcal plasmid pE194 (17, 33). Deletion of most of the regulatory region led to constitutive expression of resistance in S. agalactiae HM1081 and of β-galactosidase production in the corresponding gene fusion.
The ketolide HMR 3004 was active against most erythromycin-resistant streptococci (Table 1) (2, 3, 10). This property could be related either to the affinity of the drug for methylated ribosomes or to a lack of inducing capacity. It has been shown that certain 11,12-carbonate analogs of erythromycin and 11,12-carbamate analogs of clarithromycin retained, at least in part, affinity for methylated ribosomes (12, 14). However, the differences in the MICs of HMR 3004 for strains with inducible and constitutive MLSB resistance suggested that the in vitro efficacy of the antibiotic against most erythromycin-resistant streptococci was related to the fact that the drug has a weak or no inducing ability (3). Consistent with that proposal, the MICs of HMR 3004 significantly increased after induction of the cells with erythromycin. Fusion experiments confirmed that HMR 3004 was a very weak inducer of β-galactosidase synthesis. The observation that its cladinose counterpart, RU 66252, was a potent inducer indicated that replacement of a cladinose sugar by a keto function plays a key role in the change of the inducing capability of the ketolide. This moiety has been shown to be responsible, at least in part, for the induction of MLSB resistance in staphylococci (4). The relationship between the structure of the ketolides and the loss of the capacity to induce the ribosomal methylase remains to be thoroughly studied.
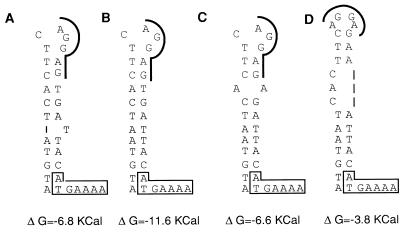
HMR 3004 was moderately active against S. pneumoniae HM30 and E. faecalis HM3, although these strains displayed inducible resistance to erythromycin (Table 2). Analysis of the 3′ ends of the putative attenuators from five inducible strains showed that a stem-loop structure which might sequester the initiation sequences for the methylase in the absence of inducer could form (Fig. 2). However, point mutations in the attenuator of strain HM30 resulted in a stem-loop structure which was potentially less stable than those of the other inducible strains. This could have resulted in an elevated basal level of β-galactosidase production and, presumably, an elevated basal level of the methylase in strain HM30 and thus to relative resistance to HMR 3004. Alternatively, several substitutions in the promoter region were observed (Fig. 1), and these could account for the increased basal level of expression of ermAM. It has been shown that an increase in the basal levels of the ermC methylase due to mutations in the regulatory region or to an increase in the copy number of the plasmid allowed the host to grow on media containing a noninducing macrolide (34). By contrast, analysis of the regulatory region of ermAM from E. faecalis HM3 did not provide any clue as to the reduced activity of HMR 3004 against this strain with inducible resistance to MLSB antibiotics.
FIG. 2.
Potential secondary structures of the 3′ ends of attenuators from the strains with inducible MLSB resistance. (A) S. sanguis (pAM77); (B) E. faecalis HM3; (C) S. pneumoniae HM28 and HM34; (D) S. pneumoniae HM30. The free energy (ΔG) contribution is expressed in kilocalories. The ribosome-binding sites for methylase are underlined; the first nucleotides for methylase are boxed.
Of note, the MICs of HMR 3004 against constitutively resistant streptococci were increased (Table 1) but remained, by far, lower than those of the other macrolides. The higher intrinsic activity of this compound against susceptible streptococci may explain the lower MICs for resistant organisms (2).
ACKNOWLEDGMENTS
This work was supported by a grant from Hoechst-Marion-Roussel.
We thank Pierre Renault for the gift of plasmid pJIM2246 and Patrice Courvalin for reading the manuscript.
REFERENCES
- 1.Adrian F, Gomez-Luz S, Perez-Trallero E, Liñares J, Alcaide F, Gomez-Luz R. Program and abstracts of the 36th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1996. Study of MLS-resistance phenotypes in Streptococcus spp and the genetic basis of this resistance, abstr. C86; p. 50. [Google Scholar]
- 2.Agouridas C, Benedetti Y, Denis A, Le Martret O, Chantot J F. Program and abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1995. Ketolides: a new distinct semi-synthetic class of macrolides, abstr. F157; p. 140. [Google Scholar]
- 3.Agouridas C, Bonnefoy A, Chantot J F. Antibacterial activity of RU 64004 (HMR 3004), a novel ketolide derivative active against respiratory pathogens. Antimicrob Agents Chemother. 1997;41:2149–2158. doi: 10.1128/aac.41.10.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen N E. Macrolide resistance in Staphylococcus aureus: induction of macrolide resistant protein synthesis. Antimicrob Agents Chemother. 1977;11:661–668. doi: 10.1128/aac.11.4.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradford M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Breidt F, Dubnau D. Identification of cis-acting sequences required for translational autoregulation of the ermC methylase. J Bacteriol. 1990;172:3661–3668. doi: 10.1128/jb.172.7.3661-3668.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clancy J, Petitpas J, Fadia D H, Wei Y, Cronan M, Kamath A, Bergeron J, Retsema J A. Molecular cloning and functional analysis of a novel macrolide-resistance determinant, mefA, from Streptococcus pyogenes. Mol Microbiol. 1996;22:867–879. doi: 10.1046/j.1365-2958.1996.01521.x. [DOI] [PubMed] [Google Scholar]
- 8.Comité de l’Antibiogramme de la Societé Française de Microbiologie. 1996. 1996 report of the Comité de l’Antibiogramme de la Societé Française de Microbiologie. Technical recommendations for in vitro susceptibility testing. Clin. Microbiol. Infect. 2(Suppl. 1):11–25.
- 9.Dower W J, Miller J F, Ragsdale C W. High efficiency transformation of Escherichia coli by high voltage electroporation. Nucleic Acids Res. 1988;16:6127–6145. doi: 10.1093/nar/16.13.6127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ednie L, Spangler M, Jacobs S K, R. M, Appelbaum P C. Susceptibilities of 228 penicillin- and erythromycin-susceptible and -resistant pneumococci to RU 64004, a new ketolide, compared with susceptibilities to 16 other agents. Antimicrob Agents Chemother. 1997;41:1037–1041. doi: 10.1128/aac.41.5.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fantin B, Leclercq R, Garry L, Carbon C. Influence of inducible cross resistance to macrolides, lincosamides, and streptogramin B-type antibiotics in Enterococcus faecium on activity of quinupristin/dalfopristin in vitro and in rabbits with experimental endocarditis. Antimicrob Agents Chemother. 1997;41:931–935. doi: 10.1128/aac.41.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes P B, Baker W R, Freiberg L A, Hardy D J, McDonald E J. New macrolides active against Streptococcus pyogenes with inducible or constitutive type macrolide-lincosamide-streptogramin B resistance. Antimicrob Agents Chemother. 1989;33:78–81. doi: 10.1128/aac.33.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Göering R V, Ruff E A. Comparative analysis of conjugative plasmids mediating gentamicin resistance in Staphylococcus aureus. Antimicrob Agents Chemother. 1983;24:450–452. doi: 10.1128/aac.24.3.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldman R C, Kadam S K. Binding of novel macrolide structures to macrolides-lincosamides-streptogramin B resistant ribosomes inhibits protein synthesis in bacterial growth. Antimicrob Agents Chemother. 1989;33:1058–1066. doi: 10.1128/aac.33.7.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horaud, T., C. Le Bouguénec, and K. Pepper. 1985. Molecular genetics of resistance to macrolides, lincosamides, and streptogramins type B (MLS) in streptococci. J. Antimicrob. Chemother. 16(Suppl. A):111–135. [DOI] [PubMed]
- 16.Horinouchi S, Weisblum B. Posttranscriptional modification of mRNA conformation: mechanism that regulates erythromycin-induced resistance. Proc Natl Acad Sci USA. 1980;77:7079–7083. doi: 10.1073/pnas.77.12.7079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horinouchi S, Byeon W, Weisblum B. A complex attenuator regulates inducible resistance to macrolide, lincosamide and streptogramin type B antibiotics in Streptococcus sanguis. J Bacteriol. 1983;154:1252–1262. doi: 10.1128/jb.154.3.1252-1262.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jacob A E, Hobbs S J. Conjugal transfer of plasmid-borne multiple antibiotic resistance in Streptococcus faecalis var. zymogenes. J Bacteriol. 1974;117:360–372. doi: 10.1128/jb.117.2.360-372.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq R, Courvalin P. Bacterial resistance to macrolide, lincosamide, and streptogramin antibiotics by target modification. Antimicrob Agents Chemother. 1991;35:1267–1272. doi: 10.1128/aac.35.7.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin B, Alloing G, Méjean V, Claverys J P. Constitutive expression of erythromycin resistance mediated by ermAM determinant from pAMβ1 results from deletion of 5′ leader peptide sequences. Plasmid. 1987;18:250–253. doi: 10.1016/0147-619x(87)90068-0. [DOI] [PubMed] [Google Scholar]
- 21.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. p. 17. [Google Scholar]
- 22.Poyart-Salmeron C, Trieu-Cuot P, Carlier C, Courvalin P. Nucleotide sequence specific for Tn1545-like conjugative transposons in pneumococci and staphylococci resistant to tetracycline. Antimicrob Agents Chemother. 1991;35:1567–1660. doi: 10.1128/aac.35.8.1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Renault P, Corthier G, Goupil N, Delorme C, Ehrlich S D. Plasmid vectors for gram-positive bacteria switching from high and to low copy number. Gene. 1996;183:175–182. doi: 10.1016/s0378-1119(96)00554-9. [DOI] [PubMed] [Google Scholar]
- 24.Sanger F, Micklen S, Coulson A R. DNA sequencing with chain terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shaw W Y. Chloramphenicol acetyltransferase: enzymology and molecular biology. Crit Rev Biochem. 1983;14:1–46. doi: 10.3109/10409238309102789. [DOI] [PubMed] [Google Scholar]
- 26.Sutcliffe J, Tait-Kamradt A, Wondrack L. Streptococcus pneumoniae and Streptococcus pyogenes resistant to macrolides but sensitive to clindamycin: a common resistance pattern mediated by an efflux system. Antimicrob Agents Chemother. 1996;40:1817–1824. doi: 10.1128/aac.40.8.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tait-Kamradt A, Clancy J, Cronan M, Dib-Hajj F, Wondrack L, Yuan W, Sutcliffe J. mefE is necessary for the erythromycin-resistant M phenotype in Streptococcus pneumoniae. Antimicrob Agents Chemother. 1997;41:2251–2255. doi: 10.1128/aac.41.10.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomasz A M. Multiply antibiotic resistant pathogenic bacteria: a report on The Rockefeller University Workshop. N Engl J Med. 1994;30:1247–1251. doi: 10.1056/NEJM199404283301725. [DOI] [PubMed] [Google Scholar]
- 29.Tomich P K, An F Y, Clewell D B. Properties of erythromycin-inducible transposon Tn917 in Streptococcus faecalis. J Bacteriol. 1980;141:1366–1374. doi: 10.1128/jb.141.3.1366-1374.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trieu-Cuot P, Poyart-Salmeron C, Carlier C, Courvalin P. Nucleotide sequence of the erythromycin resistance gene of the conjugative transposon Tn1545. Nucleic Acids Res. 1990;18:3660. doi: 10.1093/nar/18.12.3660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vicarini H, Rosato A, Leclercq R. Analysis of regulatory region of ermAM genes in streptococci and enterococci highly resistant to macrolides and lincosamides. In: Horaud T, Bouvet A, Leclercq R, de Montclos H, Sicard M, editors. Streptococci and the host. New York, N.Y: Plenum Press; 1997. pp. 495–498. [DOI] [PubMed] [Google Scholar]
- 32.Weisblum B. Erythromycin resistance by ribosome modification. Antimicrob Agents Chemother. 1995;39:577–585. doi: 10.1128/AAC.39.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weisblum B. Insights into erythromycin action from studies of its activity as inducer of resistance. Antimicrob Agents Chemother. 1995;39:797–805. doi: 10.1128/aac.39.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weisblum B, Graham M Y, Gryczan T, Dubnau D. Plasmid copy number control: isolation and characterization of high-copy number mutants of plasmid pE194. J Bacteriol. 1979;137:635–643. doi: 10.1128/jb.137.1.635-643.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]