Abstract
Tarini Shankar Ghosh and Ana Maria Valdes evaluate the evidence for clinical effects of microbiome altering interventions on cardiometabolic traits
Key messages.
The gut microbiome influences cardiometabolic risk, acting as a sensor of metabolic changes and as a modulator and translator of those changes via its metabolites
The gut microbiome can be modulated by different types of diet and dietary supplementation interventions, including those with probiotics, prebiotics, and synbiotics
Over 70% of clinical intervention studies found significant improvements in cardiometabolic traits, though only 63% reported changes to the gut microbiome
Prebiotic interventions are the most likely to result in changes to gut microbiome composition, followed by dietary interventions, and then probiotic interventions
There is no difference in either cardiometabolic outcomes or changes in gut microbiome related outcomes between single strain and multi-strain probiotic and synbiotic interventions
Cardiometabolic diseases are one of the main causes of morbidity and mortality in western countries and are increasing in low and middle income countries.1 Dietary intake is one of the main determinants of cardiometabolic health1 and of microbiome composition.2 The gut microbiome is known to play an important part in the development of cardiometabolic diseases, including hypertension, diabetes, and obesity.2 This is thought to be linked with the ability of the gut microbiome to modulate inflammation, insulin sensitivity, and blood lipid levels, and is hypothesised to be mediated by specific microbially produced metabolites such as short chain fatty acids (SCFAs), secondary bile acids, phenylacetylglutamine, and trimethylamine-N-oxide.2
As well as their direct influence, gut microbes can also modulate the response of the human host to various therapeutic interventions.3 Numerous claims have been made about how the gut microbiome affects the health outcomes of cardiometabolic disorders, including obesity, type 2 diabetes, hypertension, dyslipidaemia, and non-alcoholic fatty liver disease. It is unclear, however, which interventions targeting the gut microbiome may genuinely promote cardiometabolic health through changes to the composition or function of the gut microbiome. We evaluate the evidence from human studies of the effects of clinical interventions targeting the gut microbiome on cardiometabolic outcomes, highlighting the relevance of specific therapeutic regimens in reducing cardiometabolic disease risk through the gut microbiome, and some of the apparent paradoxes of recent studies.
Three facets of the gut microbiome
Defining a “healthy” gut microbiome remains challenging. However, the following definition has been proposed: a healthy gut microbiome is one which successfully maintains long term stability, resists invasive infections, provides its host with essential nutrients, such as vitamins and fermentation byproducts, and aids in maintaining host metabolic and immunological homoeostasis.4
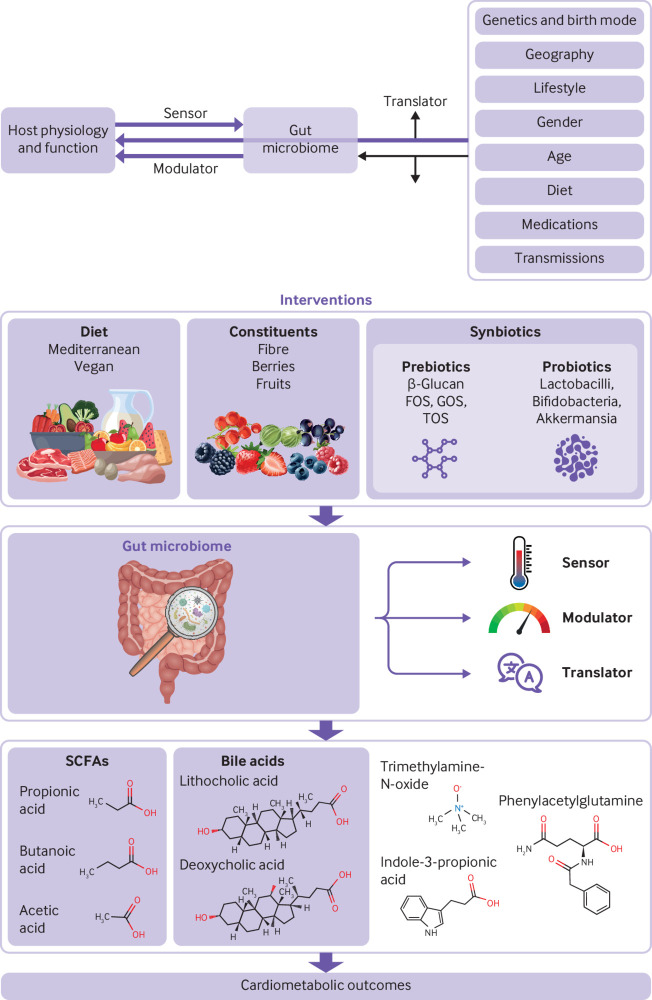
We can regard the gut microbiome as an externally modifiable organ of the human body (fig 1), with three distinct facets of its functional role on human health. Firstly, the gut microbiome acts as a sensor, capable of detecting physiological or environmental changes in the human gut and responding to such changes. An example is the high quantities of secondary bile acids produced by gut microbes in response to excessive dietary fat. Secondly, the gut microbiome can act as a modulator by directly influencing the physiological state of the host. Examples of this include the production of SCFAs, a class of microbial metabolites which reduce the production of proinflammatory cytokines.5 Furthermore, it affects the levels and types of bile acids in the gut and in blood. These bile acids modulate the activity of the bile acid receptor (or farnesoid X receptor), which is expressed in the liver and regulates lipid and glucose metabolism. Impairment and dysregulation of the gut microbiome increase gut microbe production of secondary bile acids, indoles, and other molecules such as phenylacetylglutamine and trimethylamine N-oxide.6 These molecules can be seen as diagnostic targets for sensing the host’s cardiometabolic disease risk. In this way, the gut microbiome acts as both a “sensor” and “modulator.”
Fig 1.
Mechanisms of action of the gut microbiome on human physiology and examples of dietary interventions and their effects on cardiometabolic outcomes via specific microbial metabolites. FOS=fructooligosaccharides; GOS=galactooligosaccharides; TOS=trans-galactooligosaccharides
Thirdly, the gut microbiome can act as a “translator,” meaning that the presence and abundance of certain gut microbes might cause the human host to respond in a particular way to specific external cues, such as diet. Such responses would not occur or would be different in the absence of these microbes. For example, specific gut microbes, including some Clostridium sp (C asparagiforme, C citroniae, C hathewayi, and C sporogenes), Desulfovibrio, and Enterobacteriaceae (Escherichia coli and Acinetobacter sp), can respond to a diet rich in choline, an essential nutrient for omnivores, and convert it to trimethylamine, which is then metabolised in the liver to trimethylamine N-oxide, a microbiome derived proatherogenic metabolite.7 Similarly, specific gut microbiome members, such as C sporogenes, can convert dietary protein derived phenylalanine to phenylacetic acid, which is then converted by liver enzymes to phenylacetylglutamine, a metabolite linked with hypertension and increased risk of stroke.8
Gut microbiome as a modifiable translator of therapeutic interventions
The direct effect of microbial metabolites on human health implies the potential for modulating the gut microbiome for therapeutic purposes. SCFAs, arguably the most widely studied gut microbial metabolites, have a role in reducing the risk of cardiometabolic diseases, including obesity, type 2 diabetes, and inflammation.6 9 10 Several studies have shown that people with a diet characterised by high fat, high protein, and lower fibre intake have disrupted gut permeability and a gut microbiome composition characterised by higher abundance of specific Bacteroides and Alistipes sp, Bilophila, Desulfovibrio, and the inflammation associated Ruminococcus gnavus, all of which are associated with an increased risk of cardiometabolic diseases.2 11 Importantly, the gut microbiome is therapeutically modifiable through intervention with probiotics, prebiotics, synbiotics, dietary change, or specific dietary components (box 1).
Box 1. Therapeutic approaches to modify the gut microbiome.
Prebiotic is the term used to define a substrate (molecule) that is selectively used by host microorganisms to confer a health benefit. These substrates typically include dietary fibre but may refer to other substances such as lactulose which can be metabolised by bacteria in the gut to promote a health benefit. While all dietary fibres are indigestible by human enzymes and pass through the digestive system intact, not all fibres have prebiotic properties. Only prebiotics promote the growth of beneficial gut bacteria. Non-prebiotic dietary fibres may yield other health benefits that are not mediated by gut microbes
Probiotics are live bacteria and yeasts that are beneficial to human health when given in a viable form and in sufficient quantities. They can be found in certain foods or as food supplements. Unlike prebiotics, which serve as food for gut bacteria, probiotics are live organisms that directly contribute to gut health
Post-biotics are preparations of inanimate microorganisms or their components that confer a health benefit on the host.12 In some scientific articles, the inactivated microbial cells of probiotics are called para-biotics or para-probiotics
Synbiotics are a combination of prebiotics and probiotics that work together to improve gut health. By providing both food for beneficial bacteria and live bacteria themselves, synbiotics may have a stronger effect on gut health than prebiotics or probiotics alone
Dietary intervention entails making changes to a person’s overall diet to achieve a particular health outcome. Dietary interventions may entail changes such as increasing the intake of fruit and vegetables, reducing the consumption of processed foods, or increasing fibre intake
Dietary supplementation may entail adding probiotics or prebiotics or some other type of food ingredient (such as vitamins) to a person’s diet
Dietary fibre and short chain fatty acids
Dietary fibre intake is important in reducing the risk of cardiometabolic conditions such as obesity, type 2 diabetes, and cardiovascular disease.13 This is thought to be because of its effects on the gut microbiome and the production of SCFAs. Dietary fibre also modulates secondary bile acid production, which has a negative effect on cardiometabolic health.14
Any polysaccharide (long chain carbohydrate) containing 10 or more monomeric units is resistant to human digestive enzymes. Many of the complex polysaccharides found in dietary fibre and resistant starch belong to this class; hence they are indigestible by humans but fermented by the gut microbiome. Several keystone species (such as Bifidobacterium sp and Ruminococcus bromii) act as primary degraders through fermentation of indigestible polysaccharides.15 This fermentation process entails microbial production of SCFAs, including acetate, propionate, and butyrate. SCFAs have several beneficial effects, including serving as an energy source for colonic cells, improving insulin sensitivity, reducing inflammation, and promoting satiety.16
These mechanisms are commonly thought to be responsible for the established links between dietary fibre intake and reduced risk of cardiometabolic disease.13 However, increased dietary fibre intake does not necessarily translate into increased production of SCFAs in humans, as a recent systematic review of dietary fibre interventions has shown.17 That systematic search identified 42 published randomised controlled trials that had tested the effect of dietary fibre supplementation (for any type of dietary fibre) on levels of faecal or serum SCFAs. Only 38% of the randomised controlled trials reported significant increases in one or more SCFAs, while 62% did not. The effect of dietary fibre interventions on increased production of SCFAs was highly dependent on both the dose and the chemical structure of dietary fibre consumed.17 Thus, the benefits of generic dietary fibre consumption on human health are almost certainly not exclusively the result of higher production of SCFAs by gut microbes. Other benefits include increased satiety, improved bowel movements, and reduced glucose spikes of meals. Moreover, an observable increased production of SCFAs by gut microbes in humans might occur only with certain types of fibre.
In addition, the effect of dietary fibre might also depend on the host’s gut microbiome composition.3 18 For example, a fibre based intervention was shown to have paradoxical effects on inflammation in some patients with ulcerative colitis whose gut microbiomes lacked the taxa that facilitate fermentation of fibre.19 In this study, a widely used fibre based prebiotic, β-fructan, led to accumulation of unfermented β-fructan in the gut of people without the fibre degrading bacteria, resulting in increased inflammation. However, few studies have reported host microbiome specific effects. This is likely because of the statistical challenges that the sample sizes of most randomised controlled trials represent for assessing interactions between baseline microbiome and intervention efficacy.
Interventions that target the gut microbiome
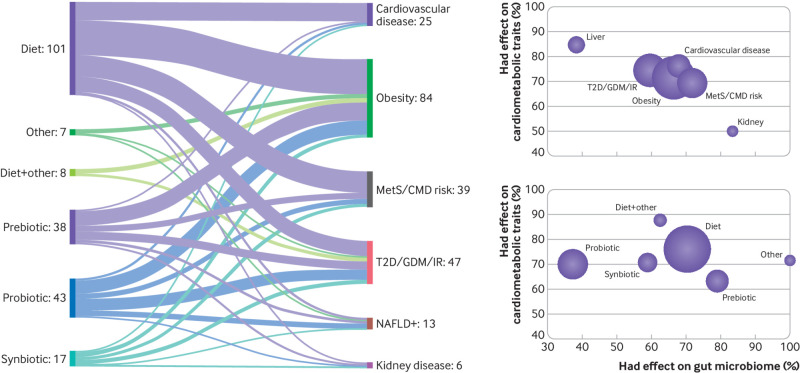
Given that the gut microbiome is a complex community consisting of phylogenetically distinct lineages,4 can targeting these complex bacterial consortiums through various treatments result in meaningful cardiometabolic health improvements? We looked for examples of interventions aimed at improving cardiometabolic outcomes by targeting the gut microbiome (web appendix). We found 214 studies that assessed changes in cardiometabolic traits by targeting the gut microbiome in a total of 16 375 participants (fig 2, box 2). Most of the studies were in Europe (60) and North America (40) and none were from South Asia or Africa. Most interventions entailed dietary changes (109/214) and were focused on obesity and metabolic syndrome (123/214). Of the remainder, 25 studies examined cardiovascular disease; 19 organ specific (kidney and liver) conditions; and seven exercise, faecal matter transplantation, or surgery. Thus, it seems that interventions that focus on the gut microbiome are seen as an extension of nutrition and “healthy eating” changes, not as medical interventions for diagnosed clinical conditions.
Fig 2.
Summary of studies examining effect of modifications to gut microbiome on cardiometabolic indicators. Sankey plot (left) shows the number of dietary intervention studies aimed at targeting cardiometabolic conditions. Height of each rectangle on the left hand side is proportional to the number of studies that used the corresponding intervention. Height of each rectangle on the right hand side is proportional to the number of studies aimed at the corresponding condition. Thickness of the lines connecting the rectangles indicates the number of people who moved from one condition to a specific dietary intervention. Cardiovascular disease comprises all cardiovascular traits, including atherosclerosis, hypertension, stroke, dyslipidaemia, heart failure, and any generic “cardiovascular” conditions. Obesity includes studies that focused only on obesity. Bubble plots show the percentage of studies reporting a significant effect on gut microbiome composition or function (top right) and the percentage showing a significant effect on cardiometabolic health by type of clinical outcome and type of intervention (bottom right). Size of the bubble represents the number of studies in each category. T2D/GDM/IR=type 2 diabetes, gestational diabetes mellitus, or insulin resistance; NAFLD+ studies focused on non-alcoholic fatty liver disease (NAFLD) or liver health in general MetS=metabolic syndrome, CMD=cardiometabolic disease.
Box 2. Survey of gut microbiome targeted interventions for tackling cardiometabolic risk*.
Efficacy on host clinical outcomes
72.5% of the studies reported significant improvements. The group specific efficacies, in ascending order, were prebiotics (63%), probiotics and synbiotics (70% each), diet alone (76%), other interventions (eg, faecal matter transplantation, physical activity) (75%), and dietary interventions in combination with another intervention(s) (87%). Despite variations, the differences in the proportion of studies reporting statistical effects between interventions with the highest efficacy (dietary interventions alone or with another intervention) versus those with lowest efficacy (prebiotic) interventions was marginal (P<0.094). Additionally, the prebiotic studies had significantly smaller cohort sizes and shorter durations than the diet or probiotic studies, indicating that they were not only statistically underpowered but that the shorter treatment duration may have prevented detection of therapeutic effects.
Efficacy changing gut microbiome composition
63.5% of all studies reported significant changes in gut microbial abundances or in microbial metabolite concentrations. Probiotics had the lowest efficacy (37%), followed, in ascending order, by synbiotics (59%), diet with another intervention (62%), diet alone (70%), prebiotics (82%), and other interventions (including faecal matter transplantation) (100%). Although this is partly the result of the inclusion criteria, which allowed the inclusion of probiotic studies even if no gut microbial composition assessment was part of the study, when only studies that measured gut microbial abundances or metabolites were considered, the number of probiotic and synbiotic studies reporting significant alterations in the gut microbiome were still the lowest (57% and 67%, respectively). There was a significant decrease in intervention efficacies of probiotics compared with prebiotics (P<0.034).
Single strain versus multi strain probiotics
With respect both to host cardiometabolic improvements and to changes to the gut microbiome, there was no significant differences in efficacy between probiotic/synbiotic interventions that used a single strain and those that used multiple strains (single strain efficacy 76% versus multiple strain efficacy 63%, Fishers’ exact test P>0.05 for host cardiometabolic improvement); (single strain efficacy 47% v multiple strain efficacy 53%, P>0.05 for gut microbiome change).
Gut microbial changes mediating effects on cardiometabolic health
Of 132 studies that reported a significant effect on the host phenotype and investigated if the effect was mediated by gut microbes, 81 (62%) reported or implied this to be the case. However, there was a lack of consistency in the way this was measured and reported, with approaches ranging from correlations to regressions to machine-learning based models.
*See web appendices for search strategy and further details of included studies
The proportion of studies reporting a significant improvement in clinical outcomes did not differ among the various types of cardiometabolic areas investigated (obesity, cardiovascular disease, metabolic syndrome, liver disease and so on) (fig 2). However, studies of probiotic interventions (both single and multiple strain formulations) were significantly less likely to report significant changes to the gut microbiome than studies using either dietary or prebiotic interventions (fig 2). A key limitation of probiotic based interventions already highlighted20 21 is the tendency for bacteria in the probiotic formulation to fail to form bacterial colonies in the gut of the host. This is confirmed by the interventional studies we analyse here.
It is therefore likely that the beneficial effects of probiotic supplementation on various cardiometabolic disease traits may be, at least in part, mediated by their “postbiotic” effects. Postbiotics are substances or components of the microbial cells (such as parts of their membranes) that provide a direct benefit to the host. In support of this possibility, it has been shown that pasteurised probiotic preparations (with microbial cell components but not with live microbes) improve cardiometabolic disease outcomes.12
Paradoxically, the dietary interventions category shows the largest proportion of studies that resulted in both changes in gut microbiome composition and in cardiometabolic disease improvements. Microbial strains, such as probiotics, or specific fibres which fulfil the definition of “prebiotics” seem to be less effective. This points to gaps in our understanding as to what truly drives beneficial changes in gut microbiome composition that result in cardiometabolic health benefits.
Conclusions
There is now a large body of evidence evaluating the effects of prebiotic, synbiotic, probiotic supplementation, or other dietary interventions aimed at improving cardiometabolic outcomes by targeting the gut microbiome. Although most studies (72.4%) report significant improvements in one or more cardiometabolic traits, the number of such interventions that tackle cardiovascular or organ specific diseases (such as atherosclerosis, hypertension, and non-alcoholic fatty liver disease), not just obesity, is fairly modest. A noticeable geographical disparity also exists, with few trials particularly in South Asia and Africa. Our analysis indicates that probiotic interventions are less effective in improving the microbiome than broader interventions, such as dietary interventions or prebiotics, that target a broader range of nutrients and multiple lineages of the gut microbiome simultaneously. This may be partly because the host gut’s microbiome is resistant to colonisation by new therapeutic strains. In such cases, probiotics derived from the host gut microbiome, such as Akkermansia muciniphila, could represent promising alternatives. Despite the paradoxes, the overall high rate of effective interventions indicates that targeting the gut microbiome through various interventions can modulate the host response to multiple disorders such as metabolic syndrome, obesity, and type 2 diabetes.22
The current lack of consistency in outcomes and study designs still represents a challenge in the identification of specific therapeutic targets, or target transducing modules, in the gut microbiome. It also highlights the need for a consensus on mechanistic markers, such as the various microbial metabolites robustly implicated in cardiometabolic risk. Therefore, well designed large scale studies with adequate biomarkers of microbiome functional changes aimed at answering specific cardiometabolic questions, along with uniform and integrated data driven protocols for investigating aspects such as the mediation or translatory effects of the gut microbiome, are required to develop much needed guidelines for practice in this area.
Web extra.
Extra material supplied by authors
Web appendix 1: Details of studies of interventions targeting gut microbiome
Web appendix: 2: Search strategy and characteristics of studies
This article was amended on 13 October 2023 to correct the labelling in the legend for figure 2.
Contributors and sources TSG and AMV had the idea for the article, TSG and AMV performed the literature searches, TSG and AMV wrote the article, AMV is the guarantor.
Competing interests: We have read and understood BMJ policy on declaration of interests and declare that AMV is a consultant and member of the scientific advisory board for Zoe, CP Kelco, Chuckling Goat, Sanofi.
Provenance and peer review: Commissioned; externally peer reviewed.
This article is part of a collection proposed by Swiss Re, which also provided funding for the collection, including open access fees. The BMJ commissioned, peer reviewed, edited, and made the decision to publish. Nita Forouhi, Dariush Mozaffarian, and David Ludwig provided advice and guided the selection of topics. The lead editors for the collection were Navjoyt Ladher, Rachael Hinton, and Emma Veitch.
References
- 1. Miranda JJ, Barrientos-Gutiérrez T, Corvalan C, et al. Understanding the rise of cardiometabolic diseases in low- and middle-income countries. Nat Med 2019;25:1667-79. 10.1038/s41591-019-0644-7 [DOI] [PubMed] [Google Scholar]
- 2. Valdes AM, Walter J, Segal E, Spector TD. Role of the gut microbiota in nutrition and health. BMJ 2018;361:k2179. 10.1136/bmj.k2179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ghosh TS, Rampelli S, Jeffery IB, et al. Mediterranean diet intervention alters the gut microbiome in older people reducing frailty and improving health status: the NU-AGE 1-year dietary intervention across five European countries. Gut 2020;69:1218-28. 10.1136/gutjnl-2019-319654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wilmanski T, Rappaport N, Diener C, Gibbons SM, Price ND. From taxonomy to metabolic output: what factors define gut microbiome health? Gut Microbes 2021;13:1-20. 10.1080/19490976.2021.1907270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chambers ES, Byrne CS, Morrison DJ, et al. Dietary supplementation with inulin-propionate ester or inulin improves insulin sensitivity in adults with overweight and obesity with distinct effects on the gut microbiota, plasma metabolome and systemic inflammatory responses: a randomised cross-over trial. Gut 2019;68:1430-8. 10.1136/gutjnl-2019-318424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zeisel SH, Warrier M. Trimethylamine N-oxide, the microbiome, and heart and kidney disease. Annu Rev Nutr 2017;37:157-81. 10.1146/annurev-nutr-071816-064732 [DOI] [PubMed] [Google Scholar]
- 7. Falony G, Vieira-Silva S, Raes J. Microbiology meets big data: the case of gut microbiota-derived trimethylamine. Annu Rev Microbiol 2015;69:305-21. 10.1146/annurev-micro-091014-104422 [DOI] [PubMed] [Google Scholar]
- 8. Zhu Y, Dwidar M, Nemet I, et al. Two distinct gut microbial pathways contribute to meta-organismal production of phenylacetylglutamine with links to cardiovascular disease. Cell Host Microbe 2023;31:18-32.e9. 10.1016/j.chom.2022.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mayorga-Ramos A, Barba-Ostria C, Simancas-Racines D, Guamán LP. Protective role of butyrate in obesity and diabetes: new insights. Front Nutr 2022;9:1067647. 10.3389/fnut.2022.1067647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yao J, Chen Y, Xu M. The critical role of short-chain fatty acids in health and disease: a subtle focus on cardiovascular disease-NLRP3 inflammasome-angiogenesis axis. Clin Immunol 2022;238:109013. 10.1016/j.clim.2022.109013 [DOI] [PubMed] [Google Scholar]
- 11. Kolodziejczyk AA, Zheng D, Elinav E. Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol 2019;17:742-53. 10.1038/s41579-019-0256-8 [DOI] [PubMed] [Google Scholar]
- 12. Salminen S, Collado MC, Endo A, et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat Rev Gastroenterol Hepatol 2021;18:649-67. 10.1038/s41575-021-00440-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet 2019;393:434-45. 10.1016/S0140-6736(18)31809-9 [DOI] [PubMed] [Google Scholar]
- 14. Louca P, Meijnikman AS, Nogal A, et al. The secondary bile acid isoursodeoxycholate correlates with post-prandial lipemia, inflammation, and appetite and changes post-bariatric surgery. Cell Rep Med; 2023: 100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Drey E, Kok CR, Hutkins R. Role of Bifidobacterium pseudocatenulatum in degradation and consumption of xylan-derived carbohydrates. Appl Environ Microbiol 2022;88:e0129922. 10.1128/aem.01299-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xiong R-G, Zhou D-D, Wu S-X, et al. Health benefits and side effects of short-chain fatty acids. Foods 2022;11:2863. 10.3390/foods11182863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vinelli V, Biscotti P, Martini D, et al. Effects of dietary fibers on short-chain fatty acids and gut microbiota composition in healthy adults: a systematic review. Nutrients 2022;14:2559. 10.3390/nu14132559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang DD, Nguyen LH, Li Y, et al. The gut microbiome modulates the protective association between a Mediterranean diet and cardiometabolic disease risk. Nat Med 2021;27:333-43. 10.1038/s41591-020-01223-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Armstrong HK, Bording-Jorgensen M, Santer DM, et al. Unfermented β-fructan fibers fuel inflammation in select inflammatory bowel disease patients. Gastroenterology 2023;164:228-40. 10.1053/j.gastro.2022.09.034 [DOI] [PubMed] [Google Scholar]
- 20. Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and microbiome features. Cell 2018;174:1388-1405.e21. 10.1016/j.cell.2018.08.041 [DOI] [PubMed] [Google Scholar]
- 21. Ghosh TS, Arnoux J, O’Toole PW. Metagenomic analysis reveals distinct patterns of gut lactobacillus prevalence, abundance, and geographical variation in health and disease. Gut Microbes 2020;12:1-19. 10.1080/19490976.2020.1822729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Depommier C, Everard A, Druart C, et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 2019;25:1096-103. 10.1038/s41591-019-0495-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Anhê FF, Jensen BAH, Perazza LR, Tchernof A, Schertzer JD, Marette A. Bacterial postbiotics as promising tools to mitigate cardiometabolic diseases. J Lipid Atheroscler 2021;10:123-9. 10.12997/jla.2021.10.2.123 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Web appendix 1: Details of studies of interventions targeting gut microbiome
Web appendix: 2: Search strategy and characteristics of studies