Abstract
Manuel Romero-Gómez and colleagues discuss how diet and modifiable factors can help prevent of non-alcoholic fatty liver disease and the importance of engaging all society through awareness, education, and policy change
Key messages.
Non-alcoholic fatty disease (NAFLD) is a public health issue strongly related to obesity and metabolic disorders
A poor diet promotes the development of NAFLD, and proper nutrition aids prevention
Ultra-processed foods, sugar sweetened beverages, and saturated fats are the main contributors; dietary patterns, such as the Mediterranean diet, could be the best prevention
Prevention of NAFLD by avoidance of obesity is a challenging task that requires the participation of all stakeholders, from the government to parents, teachers, social media, food industry, and healthcare professionals
Non-alcoholic fatty liver disease (NAFLD) is characterised by fat accumulation in hepatocytes in people who drink little or no alcohol and is strongly related to metabolic disorders like obesity, type 2 diabetes, dyslipidaemia, and arterial hypertension.1 The global prevalence of NAFLD has increased over time, now reaching more than 30% of the general adult population, with an estimated annual growth of 0.7%.2 NAFLD is a complex, heterogeneous, and dynamic disease, and progression to fibrosis (scarring of the liver) occurs in around 10-15% of patients with NAFLD.3 NAFLD is one of the main risk factors for developing liver cancer, which represents the third highest cause of death by cancer in the latest Globocan report.4
People with obesity are 3.5 times as likely to develop NAFLD,5 mainly because both diseases share common pathophysiological pathways. A diet rich in saturated fatty acids (such as ultra-processed foods, full fat dairy products, red meat, and using coconut and palm oils) and sugars supplies a large amount of fatty acids to the liver and contributes to the onset and progression of liver damage as well as adipose tissue hypertrophy, dysfunction, systemic inflammation, and dysbiosis.1 A longitudinal study from 1997 to 2016 of a US cohort of adults (with and without NAFLD) showed that people with obesity and NAFLD had a higher risk of cirrhosis and its complications, cardiovascular events, and neoplasms beyond the liver, mainly gynaecological and gastrointestinal tumours.6 On the other hand, weight loss of 10% of body weight through lifestyle modifications, such as a low fat hypocaloric diet combined with exercise, promotes resolution of steatosis and inflammation and regression of fibrosis in NAFLD.7
NAFLD continues to be neglected, mainly owing to lack of awareness and education about preventing the disease, among the public as well as physicians.8 A large European primary care record study in 2018 confirmed the under-diagnosis and under-reporting of NAFLD, implying low disease awareness among primary care physicians and limited resource allocation.9 A cross sectional study among 108 primary care clinicians in Australia similarly found an under-appreciation of NAFLD and underestimation of its prevalence.10 There are currently no globally accepted, evidence based practical dietary recommendations for the prevention of NAFLD. Further, NAFLD is not mentioned in international and national guidelines on obesity and type 2 diabetes and is missing from the World Health Organization webpage on obesity complications.11 This results in poor management of liver related complications in patients with obesity and diabetes. We argue that promoting the prevention of NAFLD, with an emphasis on nutrition and a healthy lifestyle, will start to change this landscape and improve health outcomes, particularly among people at risk of NAFLD, with obesity and type 2 diabetes, dyslipidaemia, or arterial hypertension.
Modification of dietary habits
The prevention of NAFLD relies on adherence to a healthy lifestyle incorporating diet and moderate physical activity. This applies to the everyone—the general population, as well as those at risk of NAFLD or with NAFLD already. NAFLD national and international multi-society clinical practice guidelines from hepatology, diabetes, or obesity societies from all over the world, recommend the use of lifestyle modifications to manage NAFLD.12
Dietary recommendations to prevent NAFLD should include calorie reduction and the exclusion of processed and ultra-processed foods, saturated fat, high fructose foods, and sugar sweetened beverages. Ultra-processed foods, such as crisps, mass produced bread, breakfast cereals, biscuits, and soft drinks, have lower nutritional quality, higher energy density and contain higher levels of saturated fats, sugars, and salt and additives than fresh, healthier foods (vegetables, fruits, legumes, nuts, vegetable oils especially olive oil, fish, low fat meats, and dairy products).13 Prospective cohort studies show a dose-response association between soft drink consumption and NAFLD, 14 15 and a short term clinical trial examining the effect of a low free sugar diet compared with usual diet on NAFLD among adolescent boys aged 11 to 16 years showed the benefits of sugar or fructose restriction on liver fat.16
The growing global intake of ultra-processed foods poses a great challenge to preventing NAFLD. Several studies have shown an association between the dietary share of ultra-processed foods and the risk of metabolic disorders and NAFLD,17 and the consumption of ultra-processed foods is associated with an overconsumption of calories and weight gain.18 In parallel, because ultra-processed foods are generally less expensive than fresh foods, their consumption is higher among lower socioeconomic groups, with growing intake among children.19 A cross sectional analysis of the 2017-18 National Health and Nutrition Examination Surveys in the US found that the risk of NAFLD was lower among people with a college education, which was also associated with either a healthier diet quality or increased physical activity.20 Thus, educational and socioeconomic inequalities are risk factors for NAFLD.21
Diet has a key role in the development of NAFLD and can also help prevent it.22 The Mediterranean diet (rich in vegetables, fruit, beans, lentils nuts, whole grains, and fish) can reduce the risk of NAFLD and improve cardiometabolic health, as well as manage the effects of NAFLD in patients.23 24 This is proposed to be partially due to the anti-inflammatory and antioxidant effects of nutrients and bioactive compounds, such as polyphenols, flavonoids, carotenoids, and vitamins present in its foods. The Mediterranean diet is rich in mono-unsaturated fatty acids, abundant in olive oil, and has a balance of polyunsaturated fatty acids intake that prioritises omega-3 (fatty fish, nuts, and flaxseed) over omega-6 polyunsaturated fatty acids (safflower, sunflower, soybean, and corn oils). These fatty acids regulate the antioxidant signalling pathway and modulate inflammatory processes. The Mediterranean diet also has a beneficial effect on the composition and diversity of microbiota, and animal studies have shown that the gut microbiota has an important role in NAFLD pathogenesis.25 26
There are several evidence based dietary approaches to lose weight and prevent or manage NAFLD. In addition to the Mediterranean diet, which is the most studied, hypocaloric low carbohydrate diets and low fat diets are also effective in reducing liver fat and related biomarkers.27 Clinical trials assessing these diets are often limited by short term follow-up and the use of NAFLD surrogate biomarkers rather than liver biopsy.26 27 A definite diagnosis is mandatory, and clinical trial tackling long term lifestyle interventions must take into account a plethora of confounding factors.
Diets with a similar patter to the Mediterranean diet can be find around the world.28 The Japanesse diet pattern is mostly fish based with vegetables, fruits, grains, and legumes, coffee, and tea.29 The traditional Chinese diet is a near vegetarian diet centred around vegetables, rice, noodles, and a moderate amount of fish, poultry, and tofu.30 Both diets have been associated with a reduced risk of NAFLD.29 30
Growing evidence suggests that the DASH (dietary approaches to stop hypertension) diet contributes to reduced NAFLD risk. A cross sectional analysis of a population based cohort study in China found that adherence to the DASH diet was associated with a lower presence of NAFLD in adults aged 40 to 75.31 The DASH diet includes similar nutrients and foods to the Mediterranean diet but it encourages reduced fat intake, whereas the Mediterranean diet promotes the consumption of healthy fats, particularly monounsaturated fats. But there are no clinical trials comparing these dietary patterns, and more data are warranted to reach evidence based recommendations on lifestyle interventions to prevent NAFLD. Moreover, obesity and NAFLD share many pathways of progression, but they do not completely overlap.
A societal approach to preventing NAFLD
NAFLD shares risk factors with other prevalent metabolic diseases, particularly those related to diet and lifestyle. Thus, like these diseases, tackling NAFLD will require a multifaceted approach targeting children, parents, teachers, politicians, stakeholders, doctors, patients, and all of society in general (fig 1). This also implies the need to establish societal, political, and public health policies that can aid in the prevention and management of NAFLD including tackling the socioeconomic factors related to the risk of NAFLD. Many social and policy measures have been studied and evaluated for the prevention of non-communicable diseases related to NAFLD, but not for NAFLD specifically. A societal prevention programme is now needed to move NAFLD beyond a neglected disease.
Fig 1.
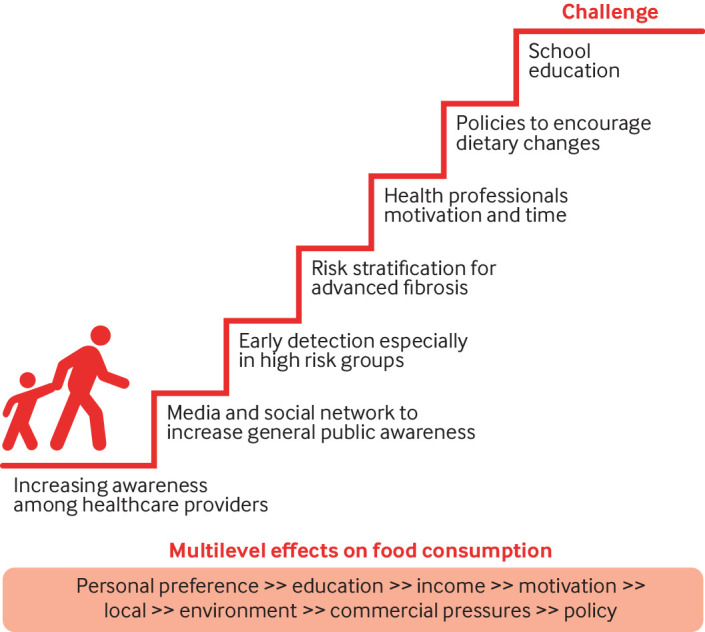
A societal approach to NAFLD prevention by nutrition
A first step would be to establish clear nutrition and dietary guidelines for the prevention of NAFLD. Although a lot of information on the most suitable diets exists, the information does not always reach the general public, and even if it does, it can be difficult for people to use the information in their daily lives. Health professionals should be one of the main drivers of nutritional recommendations to prevent and manage NAFLD, especially among patients at higher risk. But health professionals do not always have the time, knowledge, or motivation to support their patients to implement lifestyle changes.31 Therefore, health professionals should work on multidisciplinary teams, and the integration of NAFLD management recommendations in diabetes and obesity guidelines should be strongly encouraged.
The early detection and referral of people at risk of NAFLD is a critical component of care. The potential for under-diagnosis and missed opportunities increases when patients are referred to hepatologists after the development of cirrhosis, when the scope for intervention is markedly reduced and the relative risk of liver related mortality increases dramatically.32 33
From the patient’s perspective, healthcare systems should be reoriented away from a disease based model to patient centred multidisciplinary care for NAFLD, incorporating comprehensive dietary treatment by nutrition experts. The presence of a multidisciplinary team that can provide education on healthy eating skills, discuss potential barriers to lifestyle change (such as life stressors and “obesogenic environments”), and find shared solutions might help increase patient motivation to make successful lifestyle modifications. Several studies of multidisciplinary secondary care clinics for NAFLD show evidence of benefits to patient care such as improving cardio-hepato-metabolic risk, BMI, and transaminase levels.34 35 By contrast, a US observational cohort study found that, in most cases, overweight or obese adults with NAFLD receiving routine care did not see a clinical dietitian, and only half of patients received diet and exercise recommendations. Only 32% of overweight or obese patients achieved >5% weight reduction over a median follow-up period of 39 months. Weight regain back to baseline was common (21.2%).36
An educational intervention programme for patients with NAFLD provided by healthcare professionals showed statistically significant effects on quality of life, knowledge, and clinical outcomes at six months of follow-up, although the effect size was small.37 This reiterates that education programmes alone are not enough for patients to implement lifestyle modifications, and a multidisciplinary team is needed to deal with overall patient circumstances.
Public health campaigns can raise awareness in the general population about NAFLD, its risk factors, and preventive measures. These efforts can reinforce the benefits of living a healthy lifestyle, which includes a well balanced diet and regular physical activity, while emphasising the need to establish realistic goals and that even modest changes are clinically helpful. Education and interventions for healthy lifestyles can start at an early age in families and continue in school. The media, social media, and the internet can also be good sources of health information and help build public awareness about NAFLD. An evaluation of 47 Korean websites with educational information about NAFLD, however, found the information to be of poor quality.38 As a starting point, and to counter inappropriate claims, health professionals should provide patients at risk of NAFLD with links to credible sources of information.
Education interventions for the public can extend to the implementation of a nutritional front-of-package labelling system.39 40 This is a form of supplementary nutrition information that promotes healthy diets by helping consumers understand the nutritional values of food and make healthier food choices. Various labelling schemes have been developed by countries that are currently being analysed for their effectiveness at shifting consumers’ choices towards healthier diets.41
Consideration should be given to the development of measures to regulate the advertising and marketing of unhealthy foods and beverages, particularly to children. Limiting the promotion of high sugar, high fat items might help to reduce the prevalence of NAFLD. Advertising can sometimes be a cue to consumption. A meta-analysis of the effects of exposure to unhealthy food and non-alcoholic beverage advertising (television and internet) found that acute exposure to food advertising increased food intake in children but not in adults.42 These data support public health policy to reduce children’s exposure to unhealthy food advertising. Such efforts would include legislation to restrict advertising and aggressive marketing to children.43 In June 2023, for example, new regulations were introduced in the UK to restrict the placement of products that are high in fat, salt, and sugar in sections of retail stores that are attractive and available to children.44 Governments are also implementing statutory policies to restrict high fat, sugar, and salt food marketing to children, with most regulations seeking to protect children under 15 years.45 A 2019 narrative review of governmental policies on unhealthy food marketing found 16 countries had statutory food marketing regulations for children, with restrictions on TV advertising, mainly during children’s programmes, the most common regulation.46 Schools were also a common setting for restrictions on unhealthy food marketing. Advertising restrictions on other media and communication channels such as cinema, mobile phones, print (magazines and newspapers), product packaging, and the internet were less common. Although few studies had evaluated government policies on unhealthy food marketing to children, the review found that existing evaluations showed small or no policy related reductions in unhealthy food advertising, probably owing to the limited scope of the restrictions across media and marketing techniques.46 Because most policy evaluations are observational in nature and mainly examine short term results, such as changes in exposure to food marketing, the review also found limited evidence of long term effects on children’s food purchases, dietary intake, or weight status.46
Governments can enact laws to regulate the food and beverage industry, such as placing taxes on sugary beverages, implementing clear labelling of added sugars, favouring healthy food reformulation, and limiting marketing of harmful foods to minors. These policies have been implemented in several countries and seem to have a positive effect on the prevalence of non-communicable diseases.47 Systematic reviews and meta-analyses on the effects of food reformulation, across Europe and the United States40 48 showed that reformulated products led to improved nutrient intakes in 73% of studies. Several studies also found an overall improvement in cardiovascular risk factors, but more evidence is needed to understand the effects of food reformulation on other health outcomes, including NAFLD. In June 2023 a UK parliamentary debate on preventing obesity and NAFLD considered the importance of reformulation of unhealthy food for NAFLD prevention49; the British Liver Trust, the All-Party Parliamentary Group on Liver Health, and Liver Cancer UK called for government regulation on this issue.
Currently, there is no direct evidence linking food taxation to NAFLD risk, prevention, or treatment. But for other non-communicable diseases closely related to NAFLD, such as obesity and type 2 diabetes, taxation reduced the consumption of sugar sweetened drinks, although intake of untaxed unhealthy foods increased.50 Nevertheless, the evidence is unclear, with a 2020 Cochrane review indicating that despite a reduction in the consumption of taxed sugar added foods, there is uncertainty regarding the effectiveness of taxing unprocessed sugar or sugar added food on obesity and type 2 diabetes prevention.51 Specific taxes on sugar, across all foods and not only in sweetened beverages, produced a significant health improvement. These taxes should go together with other taxes on saturated fats and salt, as well as a subsidy for fruit and vegetables.52
A healthy lifestyle reduces the risk of developing NAFLD and could also promote NAFLD regression.53 Although the preventive potential of lifestyle modifications on NAFLD has been intensively investigated and its benefits are consistent, there have been no large scale, long term, population based studies testing the effects of structured lifestyle intervention programmes on the prevention of NAFLD. Such studies are difficult to conduct but are needed to establish public health benefits and support future strategies for liver disease prevention. Tackling NAFLD necessitates a multifaceted approach requiring collaboration across governments, healthcare institutions, communities, families, and individuals. Policy interventions when paired with individual efforts to adopt a healthy lifestyle, can help to prevent and control NAFLD.
Contributors and sources: MR-G, as guarantor of the article, is full professor of medicine and director of the programme for translational research on liver diseases at IBIS. He has published hundreds of papers in peer reviewed journals about NAFLD, mainly on lifestyle intervention, new diagnostic methods, and therapies. He patented diagnostic methods and participated in several international projects including LITMUS and NASH-PI. He invited SZ-S, a full professor of epidemiology and preventive medicine, an international expert nutritionist, and head of the School of Public Health Medical School at the University of Haifa. She has an extensive and fruitful career in NAFLD research and clinical management. FM is a full professor of nutrition at the University Pablo de Olavide in Seville. As an international expert, he has participated in the European Food Safety Authority and leads the Spanish Network for Diabetes Research (CiberDEM). EB is a renowned physician and clinical investigator in NAFLD, with over 200 published high quality articles from the University of Torino, Italy. BS is an emeritus professor of physiology in the School of Medicine and an extraordinary professor of regenerative medicine. In his tenure as minister of health and consumer affairs of Spain he implemented public health programmes to stop smoking, healthy nutrition in schoolchildren, and so on. He is a well recognised scientist and visionary leader who published more than 150 papers on advanced therapies for diabetes, stem cells, and nutrition. After brainstorming, we divided the manuscript into several parts written by each of us. MR-G was in charge of organising the final version of the manuscript that was approved by all of us.
Funding: PI22/01342 MERGE and ICI21/0016 projects granted by ISCIII, PID2020-116731RB-C21 from Ministerio de Ciencia e Innovación, Spanish Government, Spain, and GVA-COVID19/2021/047 from Generalitat Valenciana, Spain.
Patient involvement: The manuscript has been reviewed and authored by a patient: BS. In addition to their academic and clinical role, BS provides input from their experience as a patient with biopsy proved NAFLD.
Competing interests: We have read and understood BMJ policy on the declaration of interests and have the following interests to declare: MR-G declares consulting for Abbvie, Alpha-sigma, Allergan, Astra-Zeneca, Axcella, BMS, Boehringer-Ingelheim, Gilead, Intercept, Inventia, Kaleido, MSD, Novo-Nordisk, Pfizer, Prosciento, Rubió, Siemens, Shionogi, Sobi, Zydus. Research grants: Gilead, Intercept, Siemens, Theratechnologies. BS declares consulting for Abbvie, Celgene, Gilead, Roche, and Novo; filed patents on the treatment of covid-19, diabetes, and inflammatory diseases. SZ-S has given a one time presentation for and received support for attending a meeting from AbbVie, and a one time consultation fee from Siemens, outside of the submitted work.
This article is part of a collection proposed by Swiss Re, which also provided funding for the collection, including open access fees. The BMJ commissioned, peer reviewed, edited, and made the decision to publish. Nita Forouhi, Dariush Mozaffarian, and David Ludwig provided advice and guided the selection of topics. The lead editors for the collection were Navjoyt Ladher, Rachael Hinton, and Emma Veitch.
References
- 1. Romero-Gómez M. Non-alcoholic steatohepatitis. Med Clin (Barc) 2022;159:388-95. 10.1016/j.medcli.2022.06.017. [DOI] [PubMed] [Google Scholar]
- 2. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73-84. 10.1002/hep.28431 [DOI] [PubMed] [Google Scholar]
- 3.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643-54.e1-9 [DOI] [PMC free article] [PubMed]
- 4. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021;71:209-49. 10.3322/caac.21660 [DOI] [PubMed] [Google Scholar]
- 5. Li L, Liu DW, Yan HY, Wang ZY, Zhao SH, Wang B. Obesity is an independent risk factor for non-alcoholic fatty liver disease: evidence from a meta-analysis of 21 cohort studies. Obes Rev 2016;17:510-9. 10.1111/obr.12407 [DOI] [PubMed] [Google Scholar]
- 6. Allen AM, Hicks SB, Mara KC, Larson JJ, Therneau TM. The risk of incident extrahepatic cancers is higher in non-alcoholic fatty liver disease than obesity—A longitudinal cohort study. J Hepatol 2019;71:1229-36. 10.1016/j.jhep.2019.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vilar-Gomez E, Martinez-Perez Y, Calzadilla-Bertot L, et al. Weight Loss Through Lifestyle Modification Significantly Reduces Features of Nonalcoholic Steatohepatitis. Gastroenterology 2015;149:367-78.e5, quiz e14-5. 10.1053/j.gastro.2015.04.005 [DOI] [PubMed] [Google Scholar]
- 8. Lazarus JV, Anstee QM, Hagström H, et al. Defining comprehensive models of care for NAFLD. Nat Rev Gastroenterol Hepatol 2021;18:717-29. 10.1038/s41575-021-00477-7 [DOI] [PubMed] [Google Scholar]
- 9. Alexander M, Loomis AK, Fairburn-Beech J, et al. Real-world data reveal a diagnostic gap in non-alcoholic fatty liver disease. BMC Med 2018;16:130. 10.1186/s12916-018-1103-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Patel PJ, Banh X, Horsfall LU, et al. Underappreciation of non-alcoholic fatty liver disease by primary care clinicians: limited awareness of surrogate markers of fibrosis. Intern Med J 2018;48:144-51. 10.1111/imj.13667 [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. Obesity. https://www.who.int/health-topics/obesity#tab=tab_2
- 12. Schattenberg JM, Anstee QM, Caussy C, Bugianesi E, Popovic B. Differences between current clinical guidelines for screening, diagnosis and management of nonalcoholic fatty liver disease and real-world practice: a targeted literature review. Expert Rev Gastroenterol Hepatol 2021;15:1253-66. 10.1080/17474124.2021.1974295 [DOI] [PubMed] [Google Scholar]
- 13. Touvier M, da Costa Louzada ML, Mozaffarian D, et al. Ultra-processed foods and cardiometabolic health: public health policies to reduce consumption cannot wait. BMJ 2023;383:e075294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhao L, Zhang X, Coday M, et al. Sugar-sweetened and artificially sweetened beverages and risk of liver cancer and chronic liver disease mortality. JAMA 2023;330:537-46. 10.1001/jama.2023.12618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang S, Gu Y, Bian S, et al. Soft drink consumption and risk of nonalcoholic fatty liver disease: results from the Tianjin Chronic Low-Grade Systemic Inflammation and Health (TCLSIH) cohort study. Am J Clin Nutr 2021;113:1265-74. 10.1093/ajcn/nqaa380 [DOI] [PubMed] [Google Scholar]
- 16. Schwimmer JB, Ugalde-Nicalo P, Welsh JA, et al. Effect of a low free sugar diet vs usual diet on nonalcoholic fatty liver disease in adolescent boys: a randomized clinical trial. JAMA 2019;321:256-65. 10.1001/jama.2018.20579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhang S, Gan S, Zhang Q, et al. Ultra-processed food consumption and the risk of non-alcoholic fatty liver disease in the Tianjin Chronic Low-grade Systemic Inflammation and Health Cohort Study. Int J Epidemiol 2022;51:237-49. 10.1093/ije/dyab174 [DOI] [PubMed] [Google Scholar]
- 18. Hall KD, Ayuketah A, Brychta R, et al. Ultra-processed diets cause excess calorie intake and weight gain: an inpatient randomized controlled trial of ad libitum food intake. Cell Metab 2019;30:226. 10.1016/j.cmet.2019.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Golovaty I, Tien PC, Price JC, Sheira L, Seligman H, Weiser SD. Food insecurity may be an independent risk factor associated with nonalcoholic fatty liver disease among low-income adults in the United States. J Nutr 2020;150:91-8. 10.1093/jn/nxz212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vilar-Gomez E, Nephew LD, Vuppalanchi R, et al. High-quality diet, physical activity, and college education are associated with low risk of NAFLD among the US population. Hepatology 2022;75:1491-506. 10.1002/hep.32207 [DOI] [PubMed] [Google Scholar]
- 21. Karlsen TH, Sheron N, Zelber-Sagi S, et al. The EASL-Lancet Liver Commission: protecting the next generation of Europeans against liver disease complications and premature mortality. Lancet 2022;399:61-116. 10.1016/S0140-6736(21)01701-3 [DOI] [PubMed] [Google Scholar]
- 22. Romero-Gómez M, Zelber-Sagi S, Trenell M. Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 2017;67:829-46. 10.1016/j.jhep.2017.05.016 [DOI] [PubMed] [Google Scholar]
- 23. Hassani Zadeh S, Mansoori A, Hosseinzadeh M. Relationship between dietary patterns and non-alcoholic fatty liver disease: A systematic review and meta-analysis. J Gastroenterol Hepatol 2021;36:1470-8. 10.1111/jgh.15363 [DOI] [PubMed] [Google Scholar]
- 24. Kawaguchi T, Charlton M, Kawaguchi A, et al. Effects of Mediterranean diet in patients with nonalcoholic fatty liver disease: a systematic review, meta-analysis, and meta-regression analysis of randomized controlled trials. Semin Liver Dis 2021;41:225-34. 10.1055/s-0041-1723751 [DOI] [PubMed] [Google Scholar]
- 25. Abenavoli L, Boccuto L, Federico A, et al. Diet and non-alcoholic fatty liver disease: the Mediterranean way. Int J Environ Res Public Health 2019;16:3011. 10.3390/ijerph16173011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Anania C, Perla FM, Olivero F, Pacifico L, Chiesa C. Mediterranean diet and nonalcoholic fatty liver disease. World J Gastroenterol 2018;24:2083-94. 10.3748/wjg.v24.i19.2083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hansen CD, Gram-Kampmann EM, Hansen JK, et al. Effect of calorie-unrestricted low-carbohydrate, high-fat diet versus high-carbohydrate, low-fat diet on type 2 diabetes and nonalcoholic fatty liver disease : a randomized controlled trial. Ann Intern Med 2023;176:10-21. 10.7326/M22-1787 [DOI] [PubMed] [Google Scholar]
- 28. Dominguez LJ, Di Bella G, Veronese N, Barbagallo M. Impact of Mediterranean diet on chronic non-communicable diseases and longevity. Nutrients 2021;13:2028. 10.3390/nu13062028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Matsumoto Y, Fujii H, Harima M, et al. Severity of liver fibrosis is associated with the Japanese diet pattern and skeletal muscle mass in patients with nonalcoholic fatty liver disease. Nutrients 2023;15:1175. 10.3390/nu15051175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li X, Peng Z, Li M, et al. A healthful plant-based diet is associated with lower odds of nonalcoholic fatty liver disease. Nutrients 2022;14:4099. 10.3390/nu14194099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Xiao ML, Lin JS, Li YH, et al. Adherence to the Dietary Approaches to Stop Hypertension (DASH) diet is associated with lower presence of non-alcoholic fatty liver disease in middle-aged and elderly adults. Public Health Nutr 2020;23:674-82. 10.1017/S1368980019002568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Castera L, Laouenan C, Vallet-Pichard A, et al. QUID-NASH investigators . High prevalence of NASH and advanced fibrosis in type 2 diabetes: a prospective study of 330 outpatients undergoing liver biopsies for elevated ALT, using a low threshold. Diabetes Care 2023;46:1354-62. 10.2337/dc22-2048 [DOI] [PubMed] [Google Scholar]
- 33. Huang DQ, Noureddin N, Ajmera V, et al. Type 2 diabetes, hepatic decompensation, and hepatocellular carcinoma in patients with non-alcoholic fatty liver disease: an individual participant-level data meta-analysis. Lancet Gastroenterol Hepatol 2023;8:829-36. 10.1016/S2468-1253(23)00157-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moolla A, Motohashi K, Marjot T, et al. A multidisciplinary approach to the management of NAFLD is associated with improvement in markers of liver and cardio-metabolic health. Frontline Gastroenterol 2019;10:337-46. 10.1136/flgastro-2018-101155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. DeVore S, Kohli R, Lake K, et al. A multidisciplinary clinical program is effective in stabilizing BMI and reducing transaminase levels in pediatric patients with NAFLD. J Pediatr Gastroenterol Nutr 2013;57:119-23. 10.1097/MPG.0b013e318290d138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Malespin MH, Barritt AS, 4th, Watkins SE, et al. Weight loss and weight regain in usual clinical practice: results from the TARGET-NASH observational cohort. Clin Gastroenterol Hepatol 2022;20:2393-2395.e4. 10.1016/j.cgh.2021.01.023. [DOI] [PubMed] [Google Scholar]
- 37. Glass L, Asefa H, Volk M, Lok AS, Tincopa MA. disease knowledge, health-related quality of life, and lifestyle behavior change in patients with nonalcoholic fatty liver disease: impact of an educational intervention. Dig Dis Sci 2022;67:2123-33. 10.1007/s10620-021-07052-9 [DOI] [PubMed] [Google Scholar]
- 38. So IT, Lee YJ, Jung HI, Hwang JS, Jang BK. The quality of non-alcoholic fatty liver disease information resources for patients on the internet in Korea. Korean J Intern Med 2021;36:86-96. 10.3904/kjim.2018.359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.World Health Organization. Reformulation of food and beverage products for healthier diets: policy brief. 10 Jun 2022. https://www.who.int/publications/i/item/9789240039919
- 40. Gressier M, Swinburn B, Frost G, Segal AB, Sassi F. What is the impact of food reformulation on individuals’ behaviour, nutrient intakes and health status? A systematic review of empirical evidence. Obes Rev 2021;22:e13139. 10.1111/obr.13139 [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. State of play of WHO guidance on front-of-the-pack labelling. 27 Sep 2021. https://www.who.int/news/item/27-09-2021-state-of-play-of-who-guidance-on-front-of-the-pack-labelling.
- 42. Boyland EJ, Nolan S, Kelly B, et al. Advertising as a cue to consume: a systematic review and meta-analysis of the effects of acute exposure to unhealthy food and nonalcoholic beverage advertising on intake in children and adults. Am J Clin Nutr 2016;103:519-33. 10.3945/ajcn.115.120022 [DOI] [PubMed] [Google Scholar]
- 43.World Health Orgnization. Launch of the WHO guideline on Policies to protect children from the harmful impact of food marketing. 3 Jul 2023. https://www.who.int/news-room/events/detail/2023/07/03/default-calendar/launch-of-the-who-guideline-on-policies-to-protect-children-from-the-harmful-impact-of-food-marketing [PubMed]
- 44. Muir S, Dhuria P, Roe E, Lawrence W, Baird J, Vogel C. UK government’s new placement legislation is a ‘good first step’: a rapid qualitative analysis of consumer, business, enforcement and health stakeholder perspectives. BMC Med 2023;21:33. 10.1186/s12916-023-02726-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.World Health Organization. Policies to protect children from the harmful impact of food marketing: WHO guideline. 2023. https://www.who.int/news/item/03-07-2023-who-recommends-stronger-policies-to-protect-children-from-the-harmful-impact-of-food-marketing. [PubMed]
- 46. Taillie LS, Busey E, Stoltze FM, Dillman Carpentier FR. Governmental policies to reduce unhealthy food marketing to children. Nutr Rev 2019;77:787-816. 10.1093/nutrit/nuz021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gressier M, Sassi F, Frost G. Healthy foods and healthy diets. how government policies can steer food reformulation. Nutrients 2020;12:1992. 10.3390/nu12071992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gressier M, Sassi F, Frost G. Healthy foods and healthy diets. how government policies can steer food reformulation. Nutrients 2020;12:1992. 10.3390/nu12071992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.House of Commons Library. Debate on preventing obesity and fatty liver disease. 7 Jun 2023. https://commonslibrary.parliament.uk/research-briefings/cdp-2023-0121/
- 50. Batis C, Castellanos-Gutiérrez A, Sánchez-Pimienta TG, et al. Comparison of dietary intake before vs after taxes on sugar-sweetened beverages and nonessential energy-dense foods in Mexico, 2012 to 2018. JAMA Netw Open 2023;6:e2325191. 10.1001/jamanetworkopen.2023.25191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pfinder M, Heise TL, Hilton Boon M, et al. Taxation of unprocessed sugar or sugar-added foods for reducing their consumption and preventing obesity or other adverse health outcomes. Cochrane Database Syst Rev 2020;4:CD012333. 10.1002/14651858.CD012333.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Blakely T, Cleghorn C, Mizdrak A, et al. The effect of food taxes and subsidies on population health and health costs: a modelling study. Lancet Public Health 2020;5:e404-13. 10.1016/S2468-2667(20)30116-X [DOI] [PubMed] [Google Scholar]
- 53. Yu C, Gao J, Ge X, et al. Healthy lifestyle is associated with reduced mortality in patients with non-alcoholic fatty liver disease. Nutrients 2022;14:3785. 10.3390/nu14183785 [DOI] [PMC free article] [PubMed] [Google Scholar]


