Summary
Balance between metabolic and reproductive processes is important for survival, particularly in mammals that gestate their young. How the nervous system coordinates this balance is an active area of study. Herein, we demonstrate that somatostatin (SST) neurons of the tuberal hypothalamus alter feeding in a manner sensitive to metabolic and reproductive states in mice. Whereas chemogenetic activation of SST neurons increased food intake across sexes, ablation decreased food intake only in female mice during proestrus. This ablation effect was only apparent in animals with low body mass. Fat transplantation and bioinformatics analysis of SST neuronal transcriptomes revealed white adipose as a key modulator of these effects. These studies indicate that SST hypothalamic neurons integrate metabolic and reproductive cues by responding to varying levels of circulating estrogens to modulate feeding differentially based on energy stores. Thus, gonadal steroid modulation of neuronal circuits can be context dependent and gated by metabolic status.
Subject areas: Physiology, Neuroscience, Cellular neuroscience
Graphical abstract
Highlights
-
•
Activation of somatostatin neurons increased feeding across sex
-
•
Somatostatin neuron ablation decreased feeding in low body mass, proestrus females
-
•
Tuberal nucleus somatostatin neurons are sensitive to estrogens and adipokines
-
•
Somatostatin neurons integrate reproductive and metabolic cues to alter feeding
Physiology; Neuroscience; Cellular neuroscience
Introduction
The homeostatic processes of metabolism and reproduction are mutually dependent on one another. Reproductive milestones, including pregnancy and quiescence, result in major metabolic shifts,1,2,3 and metabolic status can gate reproductive function in menstrual and estrual mammals. Pubertal onset has been shown to require a critical threshold of body fat,4,5 and increasing adiposity is associated with a decreasing age of pubertal onset in individuals with ovaries in particular.6 During reproductive years, undernutrition can acutely disrupt menstrual cyclicity7 via hypogonadotropic hypogonadism8 and result in fewer successful pregnancies.9 Rodent models have been used to investigate the effects of sex variables on metabolic tissues. Estradiol has been shown to regulate key metabolic processes such as adiposity,1 thermogenic capacity and locomotion,1,10,11,12,13 and feeding,14 while sex chromosome complement has been demonstrated to affect fat deposition and energy output.15 However, few studies have investigated the mechanisms by which both metabolic and reproductive status reciprocally interact to modulate behavior.
A candidate region for the seat of this interaction is the tuberal hypothalamus, the central region of the hypothalamus typically thought to be comprised of the mediobasal hypothalamus (arcuate nucleus, ventromedial nucleus, and median eminence), dorsomedial nucleus, lateral hypothalamic area, and the lateral tuberal nucleus (TN) (N.B.: to prevent confusion, references to the specific lateral tuberal nucleus are abbreviated TN, whereas “tuberal hypothalamus” and “tuberal hypothalamic” refer to the greater anatomical spatial designation -- the “middle” of the hypothalamus on the rostral-caudal axis -- encompassing the listed nuclei.). Positioned near the third ventricle and partially outside of the blood–brain barrier,16 the mediobasal hypothalamus is situated as a nexus region with the ability to sample circulating homeostatic hormones and relay relevant information to other regions of the brain. Importantly, both this region and the greater tuberal hypothalamus are sensitive to and vital for both reproductive and metabolic homeostasis. For instance, in addition to the role of kisspeptin neurons in the arcuate nucleus as key modulators of estrogen-mediated negative feedback in the hypothalamic-pituitary-ovarian axis,17,18,19 they also require epigenetic de-repression for menarche.20,21 The arcuate nucleus is also home to the canonical homeostatic feeding neurons—appetitive agouti-related peptide (AgRP)/neuropeptide Y (NPY) neurons and satiety-related proopiomelanocortin neurons—which can not only detect and respond to sensory and metabolic cues including leptin, ghrelin, and insulin22,23,24 but also regulate non-feeding metabolic processes such as bone mass25 and insulin resistance via modulation of brown adipose.26 The ventromedial nucleus of the hypothalamus, and in particular the ventrolateral subregion, is important for various functions across sexes, including mating behavior (reviewed by Kammel and Correa27). Distinct, estrogen-sensitive cellular populations in the ventrolateral ventromedial nucleus contribute to various aspects of metabolism, including locomotion10,28,29 and thermoregulation.30 Given the substantive neuronal heterogeneity within a small brain region, it is unsurprising that these populations form complex functional interactions. For example, simulation of starvation via chronic chemogenetic activation of appetitive arcuate AgRP neurons has been demonstrated to acutely disrupt estrous cyclicity,31 providing a neuronal contributor to the link between metabolic state and reproductive function.
Somatostatin (SST) is a key neuropeptide long known to be involved in the regulation of feeding.32,33,34,35,36,37,38,39,40,41 Its expression marks many distinct populations within the tuberal hypothalamus. Two such prominent populations are seen in the arcuate nucleus (ARC) and TN. While the ARC is an uncontested member of the mediobasal hypothalamus, the TN straddles the mediobasal hypothalamus and lateral hypothalamic area. It is an understudied region marked by expression of SST, which has been mostly characterized in rats. Somatostatin neurons in both the ARC (ARCSST) and TN (TNSST) regulate food intake,42,43,44,45 and SST expression within the greater hypothalamus is regulated by circulating gonadal steroids.46 Studies across various animals have found that ARCSST neurons both colocalize with estrogen receptor47 and seem to be responsive to estrogens.48 While the TN has not been directly analyzed for gonadal steroid receptor presence, studies have found the TN to be closely apposed to two highly estrogen-sensitive regions, ventrolateral region of the ventromedial hypothalamus49 and the lateral hypothalamic area,42 suggesting that the TN may also be estrogen sensing.49,50,51,52,53 Furthermore, SST signaling in the ARC influences or can be influenced by the hypothalamic-pituitary-gonadal axis across a variety of species.47,48,54,55 Thus, SST neurons in these regions are excellent prospective candidates for integrating metabolic and reproductive cues to affect feeding based on anatomical location, sensitivity to circulating reproductive hormones, detection of metabolic hormones such as ghrelin,43 and promotion of feeding behavior. Indeed, whole-body knockouts of SST exhibit weight gain that is exacerbated by sex category and high-fat diet.56
Here, we use mice to interrogate the role that SST neurons of tuberal hypothalamus play in integrating metabolic and reproductive cues to affect feeding. SST neurons exhibited differential control of feeding in female and male mice (defined by anogenital distance at weaning and postmortem inspection of the gonads), with neuronal ablation decreasing food intake only in females. This effect was primarily due to a decrease in food intake during proestrus, when circulating ovarian hormones are at high concentrations, and was only observed in animals with a low body mass. To determine whether adiposity could mediate the effect of body mass on food intake, fat transplantation experiments were performed. Increased white adipose tissue was sufficient to alter SST neuronal modulation of food intake. Together, these data reveal a context-dependent role for SST neurons of the tuberal hypothalamic region in the regulation of food intake, by which SST neurons tune feeding behavior in response to metabolic and reproductive states.
Results
Chemogenetic activation of TNSST neurons increases food intake in female and male mice
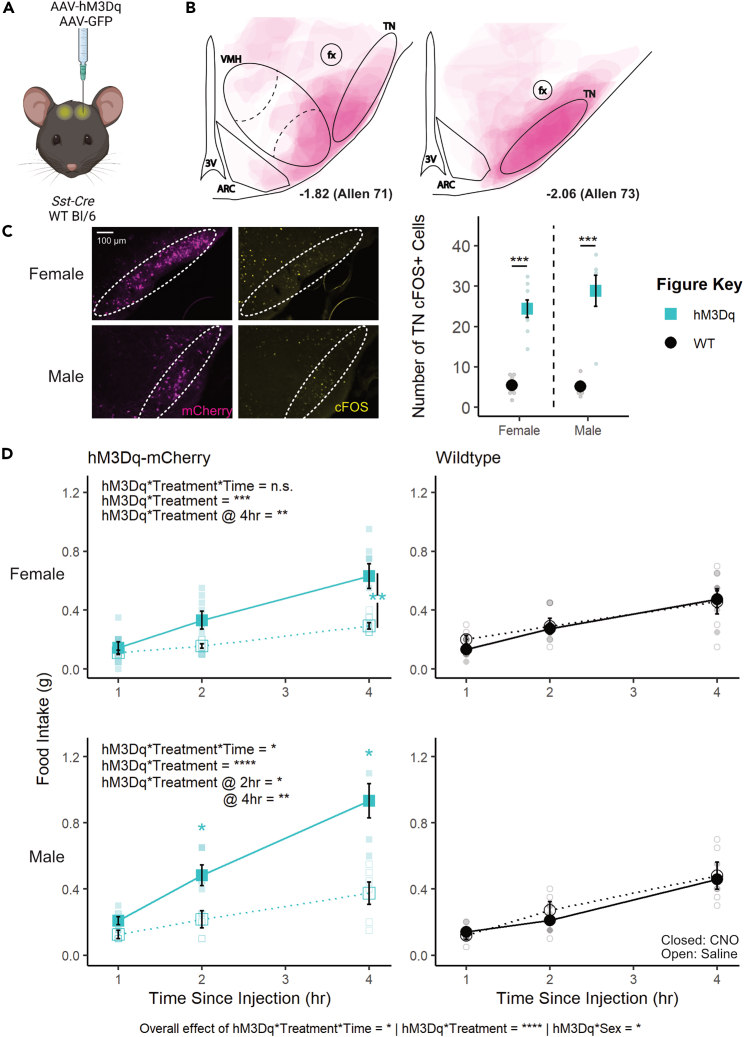
To test the role of TNSST neurons across sexes, an adeno-associated virus (AAV) expressing a Cre-dependent Gq-coupled hM3Dq57 was stereotaxically injected to the TN of Sst-Cre mice (Figures 1A–1C). Overall, activation of TNSST neurons using the small-molecule ligand clozapine-N-oxide (CNO) increased daytime food intake over a 4-h testing period in both females and males, with no sex differences apparent (hM3Dq∗treatment∗time∗sex, F(2,105) = 0.5982, p = 0.5517; Figure 1D). Control animals without expression of hM3Dq-mCherry confirmed no effect of CNO alone, while within-subjects comparisons of animals expressing hM3Dq in TNSST neurons indicated an increase in feeding upon CNO-induced cellular activation. There was a significant interaction between hM3Dq presence and treatment (saline v. CNO), both over time (treatment∗hM3Dq∗time, F(2,105) = 3.2964, p = 0.0409) and irrespective of time (treatment∗hM3Dq, F(1,105) = 35.2054, p < 0.0001). The effect of neuronal activation (genotype-by-treatment interaction) was most prominent across sexes at 4 h post-CNO injection (females: F(1,12) = 10.0208, p = 0.0081; males: F(1,9) = 12.1521, p = 0.0069), though males also exhibited a significant activation-by-treatment interaction at 2 h post-CNO administration (F(1,9) = 8.6957, p = 0.0163). Post hoc within-subjects analyses of animals bilaterally transduced with hM3Dq-mCherry indicated a significant increase in food intake during activation by CNO as compared to treatment with saline control (females overall: t(31) = 2.8486, p = 0.007732; males at 2 h: t(5) = 2.9701, p = 0.0311; males at 4 h: t(5) = 3.3263, p = 0.0286). Thus, activating TNSST neurons elicits feeding across sexes.
Figure 1.
Transient activation of TNSST neurons increased food intake across sexes
(A) Schematic of experimental paradigm. AAVs encoding hM3Dq-mCherry or GFP within flip-excision (FLEX) cassettes were injected bilaterally into the lateral tuberal nucleus (TN) of Sst-Cre or wild-type mice. Created with BioRender.com.
(B) Composite schematic of hM3Dq-mCherry expression.
(C) Fluorescent images of mCherry (magenta) and cFOS (yellow) in the TN of Sst-Cre mice and quantification of cFOS+ cells in these TN 90 min after CNO injection. Mice infected with h3MDq show an increase of cFOS-positive cells 90 min after CNO injection regardless of animal sex (overall effect of hM3Dq-mCherry presence, F(1,21) = 73.634, p < 0.0001; no interaction effect, F(1,21) = 0.939, p = 0.3435). Dashed ovals indicate boundaries of the TN. Scale bar = 100 μm.
(D) Activation of TNSST neurons in both female and male mice leads to higher food intake within the 4-h daytime testing period (left column, interaction between hM3Dq-mCherry presence and treatment: F(2,105) = 3.2964, p = 0.0409). Within sex, only males exhibited an effect of activation and treatment over time (F(2,45) = 3.2793, p = 0.0468), though both females and males exhibited an effect of hM3Dq presence and treatment independent of time (F(1,60) = 12.7928, p = 0.0007 and F(1,45) = 25.1794, p < 0.0001, respectively). Males also exhibited a significant hM3Dq-by-treatment interaction at 2 h post-CNO (F(1,9) = 8.69, p = 0.0163). CNO did not affect food intake in wild-type control mice (right column). Mean ± SEM; ANOVA and post hoc t tests where applicable ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001. F Control n = 6; M Control n = 5, F hM3Dq n = 8; M hM3Dq n = 6.
Caspase ablation of SST neurons decreases food intake only in females
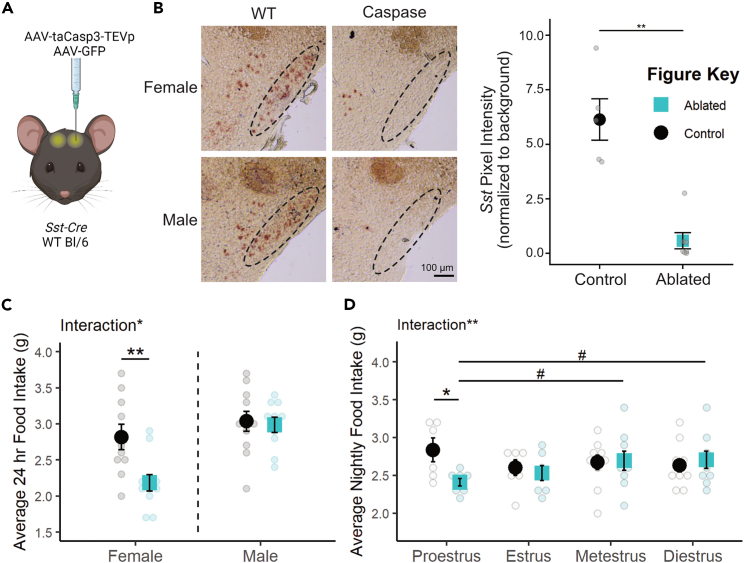
To determine if permanent inactivation of SST neurons in tuberal hypothalamic regions alters feeding across sexes, an AAV expressing a Cre-dependent modified caspase virus (taCasp3-TEVp58) was stereotaxically delivered to the TN of Sst-Cre mice (Figure 2A). Bilateral elimination of Sst expression was validated by in situ hybridization and pixel intensity quantification (t(5.24) = 5.44, p = 0.00247; Figure 2B). While ablation was largely targeted to the TN, there was also a significant decrease in Sst stain in the ARC (ablation-by-region interaction: F(3,36) = 7.31388, p = 0.001; post hoc TN t(5.24) = 5.44, padj = 0.00988; post hoc arcuate t(10) = 3.52, padj = 0.0167; Figure S1A). Mice were subjected to two 96-h food assays along with a battery of other metabolic tests. Final food intake, accounting for spillage, is depicted as an average over 24 h (Figure 2C). ANOVA revealed an overall effect of sex where males consume more food than females, as expected (F(1,38) = 14.1896, p = 0.0006).
Figure 2.
Caspase ablation of SST neurons decreased food intake only in females
(A) Schematic of experimental paradigm. AAVs encoding taCasp3-TEVp or GFP within flip-excision (FLEX) cassettes were injected bilaterally into the TN of Sst-Cre or wild-type mice. Created with BioRender.com.
(B) Representative bright-field images and quantification of Sst transcript expression in the TN of caspase-ablated and control animals. Dotted line indicates the boundary of the TN. Scale bar = 100 μm. Viral spread to ARC is quantified in Figure S1A.
(C) Permanent SST neuronal ablation decreases average daily food intake in females but not males. F Control n = 10; M Control n = 11; F Ablated n = 11; M Ablated n = 10.
(D) This decrease in food intake is detected only in the night of proestrus. Proestrus: Control n = 6, Ablated n = 8; Estrus: Control n = 7, Ablated n = 7; Metestrus: Control n = 10, Ablated n = 9; Diestrus: Control n = 10, Ablated n = 9. Mean ± SEM; ANOVA and post hoc t tests where applicable; between subjects: ∗p < 0.05, ∗∗p < 0.01; within subjects: #p < 0.10.
Interestingly, the effect of SST neuron ablation differed by sex (sex-by-ablation interaction: F(1,38) = 4.6852, p = 0.0368), an effect which post hoc t tests revealed to be carried by a decrease in food intake specifically in females (t(15.671) = -3.0561, p = 0.007686, Figure 2C). This sex difference was not previously reported, but collapsing these data across sex results in an overall decrease in food intake with SST neuron ablation (t(39.86) = -2.2713, p = 0.0286), consistent with previous studies.43
In order to ensure this effect is due to ingestion and not repetitive motion such as gnawing,42 crumb mass was compared across groups. There was an overall effect of sex (F(1,38) = 7.16394, p = 0.0109) such that female mice produced more crumbs on an average day than male mice (Figure S1B). However, there was no effect of genotype (F(1,38) = 3.14328, p = 0.0843) or interaction between sex and genotype (F(1,38) = 2.70519, p = 0.1083) on crumb mass.
The female-specific effect of SST neuron ablation is modulated by estrous cycle stage. There was a significant interaction between SST neuron ablation and estrous stage (F(1,45) = 8.1581, p = 0.0065) which was predominantly due to a decrease in food intake specifically during the night of proestrus (t(5.902) = -2.6044, p = 0.04104, Figure 2D). Within-subjects analysis of mice in the neuronal ablation group suggested that nighttime food intake during proestrus was also slightly lower than consumption during metestrus (t(7) = -1.976, p = 0.0887) and diestrus (t(7) = -2.3276, p = 0.0528), although these effects across the cycle did not reach statistical significance.
Despite effects on food intake, no other metabolic measures were altered by TNSST neuron ablation (see Table S1 for statistical results). SST neuron ablation did not affect telemetry measures of activity/movement (Figure S1C) or core body temperature (Figure S1D), or response to fasting glucose tolerance test (Figure S1E). SST neuron ablation did not affect body mass (Figure S1F), suggesting that the selective decrease in food intake during proestrus was not sufficient to alter body mass.
Body mass, specifically adiposity, influences the effect of SST neuronal ablation on food intake
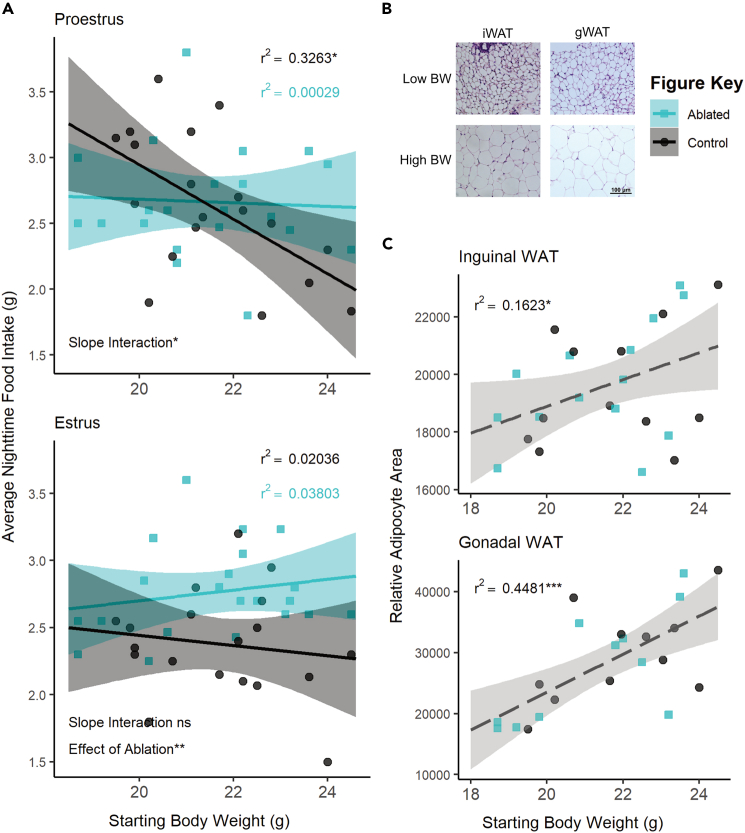
There was an overall interaction between body mass and estrous phase (F(1,150) = 3.9433, p = 0.04889) and between body mass and ablation (F(1,150) = 16.9924, p < 0.0001; see Table S1 for full results). Analyzing the relationship between body mass and food intake within each estrous stage revealed significant negative correlations between body mass and food intake in wild-type animals during nights of proestrus (r2 = 0.3263; F(1,17) = 8.235, p = 0.01062; Figure 3A) and metestrus (r2 = 0.2801; F(1,16) = 6.227, p = 0.0239; Figure S2A), stages with higher relative circulating estradiol levels.59 There were no correlations between food intake and body mass in estrus and diestrus, stages with lower relative circulating estradiol levels (Figure 3A; Figure S2A; Table S1). SST neuron ablation uncoupled this relationship with body mass in high-estradiol stages (proestrus: r2 = 0.00029, F(1,19) = 0.05492, p = 0.08172; metestrus: r2 = 0.09921, F(1,19) = 2.093, p = 0.1643), suggesting that this neuronal population is required for the body mass-dependent modulation of food intake during these stages.
Figure 3.
The effect of SST neuron ablation in proestrus depends on body mass or adiposity
(A) Regression analysis within proestrus (top panel) and estrus (bottom panel) across all ovary-intact animals reveals an interaction between body mass and nightly food intake in females. Significant negative correlations in wild-type animals are seen in the high-estradiol phase of proestrus but not in caspase-ablated females. Control n = 19, Ablated n = 21 across stages.
(B) Representative bright-field images of inguinal and gonadal white adipose tissue (iWAT & gWAT, respectively) in high and low body weight (BW) females. Scale bar = 100 μm.
(C) Terminal iWAT (left) and gWAT (right) adipocyte size both positively correlate with starting body weight regardless of TNSST neuron ablation (Interaction of slopes: iWAT F(1,22) = 0.2337 p = 0.6336, gWAT F(1,18) = 0.4552 p = 0.5085; difference in y-intercept: iWAT F(1,22) = 0.1417 p = 0.7103, gWAT F(1,18) = 0.000 p = 9971). Linear regression lines represent compiled data across ablation status. iWAT: Control n = 12, Ablated n = 14; gWAT: Control n = 11, Ablated n = 11. Linear regression ±95% CI; ANCOVA; ∗p < 0.05, ∗∗p < 0.01.
Ovariectomy to test the influence of ovarian secretions failed to replicate the effects of SST neuron ablation. We did not detect an interaction between ablation and gonad status (F(1,69) = 0.0109, p = 0.9173, Figure S2B), but overall food intake was lower in ovariectomized mice (F(1,69) = 4.4746, p = 0.038, Figure S2B). Body weight was not associated with food intake in either ablated or control animals (24 h: F(1,34) = 0.0076, p = 0.9311; Nightly: F(1,34) = 1.1595, p = 0.289147; Figure S2C), though examining nightly food intake only revealed an effect of ablation status on food intake overall (F(1,34) = 8.4044, p = 0.006512; Figure S2C). Given the influence of body weight on the effect of SST ablation (Figure 3A), it is possible that the lack of an effect in sham mice was at least partially due to higher body weight and altered metabolic profiles in this experiment.
Since fat mass is known to influence feeding, adiposity was examined postmortem. Postmortem adipocyte size of subcutaneous inguinal and visceral perigonadal white adipose tissue (iWAT and gWAT, respectively) positively correlated with the body mass determined at the onset of feeding assays (iWAT: F(1,22) = 4.3346, p = 0.04919; gWAT: F(1,18) = 14.9872, p = 0.001119; Figures 3B and 3C) regardless of SST neuron ablation (ANCOVA revealed no significant interaction of slopes or difference in y-intercept, see Table S1). Body mass accounted for a larger percentage of variation in visceral gWAT adipocyte size (r2 = 0.4481; F(1,20) = 16.24, p = 0.0006557) than it did for subcutaneous iWAT (r2 = 0.1623; F(1,24) = 4.649, p = 0.0413), suggesting a possible increased contribution of visceral adiposity to the effect of TNSST neuron ablation.
TNSST neurons are sensitive to estrogens and adiposity signals
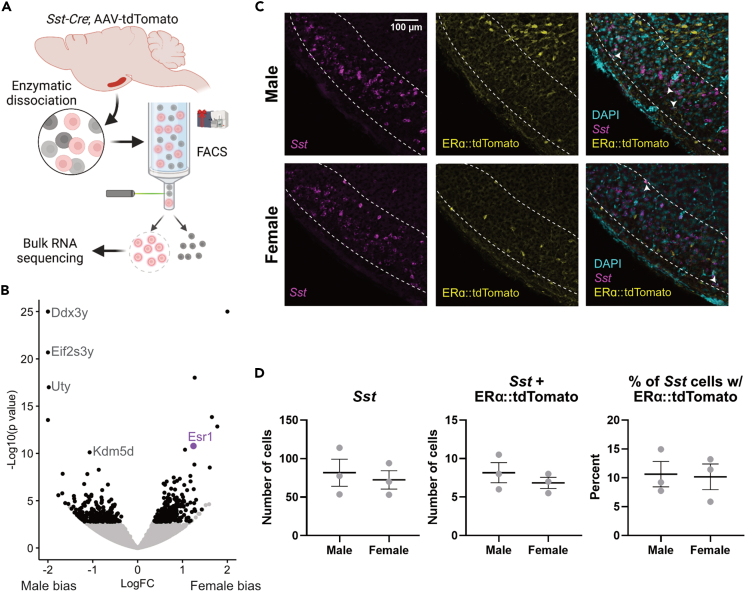
To test if TNSST neurons, specifically, can respond to estrogens and/or signals from white adipose tissue, we profiled the transcriptome of fluorescently labeled TNSST neurons using flow cytometry followed by bulk RNA sequencing (Flow-Seq; Figure 4A). Transcriptomic analysis of isolated TNSST neurons uncovered numerous differentially expressed genes between females and males, with the gene for estrogen receptor alpha, Esr1, being more highly expressed in females (Wχ2 = 6.736, adj p = 2.356 × 10−8; Figure 4B). However, we were unable to detect ERα immunoreactivity in the TN using standard antibodies (Millipore Sigma 06-935). Instead, we confirmed expression from the Esr1 locus through the injection of a Cre-dependent tdTomato reporter into the TN of female and male Esr1-Cre mice. Subsequent colocalization of tdTomato with Sst via in situ hybridization revealed co-expression of Esr1 in approximately 10% Sst-expressing cells across sexes (Figures 4C and 4D), confirming that at least a subset of TNSST neurons is sensitive to estrogens.
Figure 4.
A subset of TNSST neurons is sensitive to estrogens
(A) Schematic of Flow-Seq analysis (also applies to Figure 5 genetic input). AAV-flex-tdTomato was stereotaxically injected into the TN of Sst-Cre mice. Lateral hypothalamic region was grossly dissected and enzymatically dissociated before segregating Sst+ red neurons via flow cytometry. Resultant cells were sent for bulk RNA sequencing. Created using BioRender.com.
(B) Numerous genes are more differentially expressed between females and males (black dots), including canonical Y-associated genes (Ddx2y, Eif2s3y, Uty, and Kdm5d; gray text) and Esr1 (purple dot).
(C) Representative fluorescent images from Esr1-Cre mice showing colocalization of Sst (magenta), Esr1::tdTomato (yellow), and DAPI nuclear stain (cyan). White arrows indicate Sst-Esr1 double-labeled cells. Scale bar = 100 μm.
(D) Quantification of colocalization confirms Esr1/Sst co-expression but reveals no sex difference. Mean ± SEM.
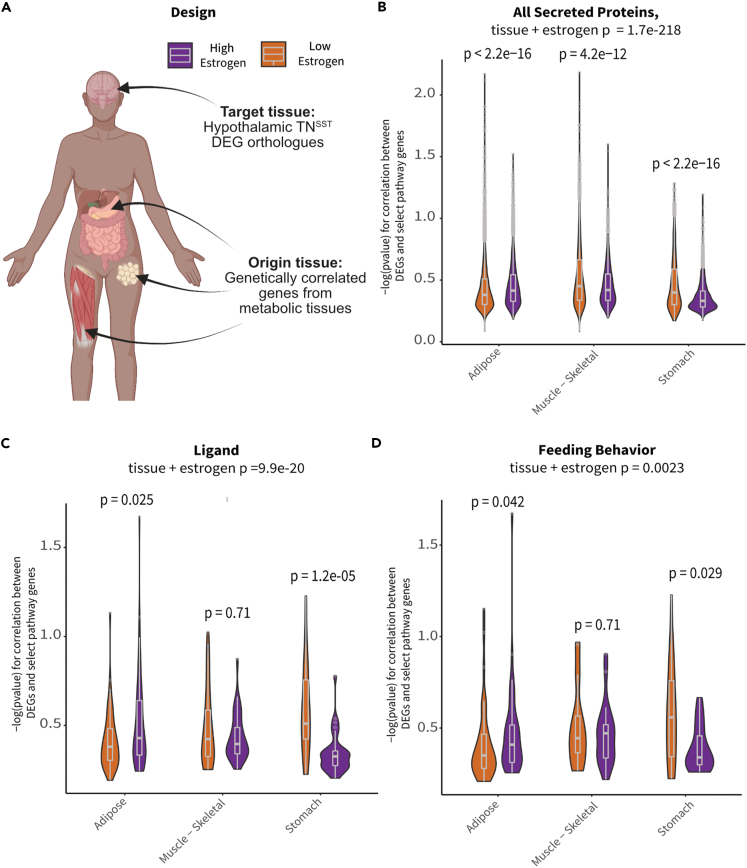
To determine if TNSST neurons communicate with adipose tissue or vice versa, we used a co-correlation analysis method based on genetic variation. High-expressing genes from TNSST neurons (based on counts > glial fibrillary acidic protein expression) were used as the “target” pathways for human orthologs within the GTEx database60 and subjected to cross-tissue genetic co-correlational analyses61,62 (Figure 5A). As previous data indicated that TNSST neuron responsivity to metabolic cues might be localized to periods of higher circulating estradiol (Figure 2D) and TNSST may be able to directly sense circulating estradiol levels (Figure 4), individuals in the GTEx database were binned into groups with either “high” or “low” circulating estradiol levels via weighted aggregation of pan-tissue Z scores corresponding to estrogen-responsive gene expression (Figures S3A–S3D). Binning individuals into groups with indicators of “low” or “high” estrogen signaling revealed weakly associated groups (as per biweight midcorrelation63; bicor coefficient = −0.32, p = 0.0023), suggesting that strong cross-tissue interactions differed depending on estrogen signaling status. Next, genetic co-correlation analyses from adipose (subcutaneous & omental), skeletal muscle, stomach, and small intestine to hypothalamic highly expressed genes were conducted. A lack of significant co-correlations with small intestine and several other tissues resulted in these tissues being omitted from the rest of analyses. A contributing factor for this lack of significance may be the limited number of individuals with matching tissue expression (Figure S3E). Given that subsets of strong cross-tissue correlations remained between the other tissues and highly expressed hypothalamic gene orthologs, relevant pathways which might contribute to signaling were examined accordingly (Figures 5B–5D). Significant interactions for co-correlations between estrogen signaling group and tissue were observed across tissues for all secreted proteins (Kruskal-Wallis test for interaction between estrogen category + tissue p = 1.7 × 10−218; Figure 5B), known ligands (Kruskal-Wallis interaction p = 9.9 × 10−20; Figure 5C), peptide hormones (Kruskal-Wallis interaction p = 1.8 × 10−13, data not shown), and feeding behavior pathways (Kruskal-Wallis interaction p = 0.0023; Figure 5D). In addition, several pathways showed specificity in strength of co-correlations from one tissue to another. For example, individuals in the higher inferred estrogen signaling group exhibited higher co-correlations between TNSST and adipose within secreted proteins (p < 2.2 × 10−16), ligand (p = 0.025), and feeding behavior pathways (p = 0.042) as compared to individuals in the low-estrogen signaling group. Interestingly, this relationship was reversed for all secreted proteins in skeletal muscle, with individuals in the low-estrogen signaling group exhibiting higher co-correlations (p = 4.2 × 10−12). These results indicate increased communication between adipose and TNSST neurons during periods of high estrogen signaling, and a switch to skeletal muscle communication when estrogen signaling is low. Across all gene sets, individuals with inferred low estrogen signaling exhibited higher co-correlations with stomach as compared to individuals with high estrogen signaling (all secreted proteins: p < 2.2 × 10−16; ligands: p = 1.2 × 10−5; peptide hormones: p = 1.8 × 10−4; and feeding behavior: p = 0.029). Together, these human genetic co-correlation data indicate that TNSST neurons modulate feeding pathways through preferential communication with adipose when estrogen signaling is high and stomach hormones when estrogen signaling is decreased. These observations are in line with known responsivity of TNSST neurons to stomach peptide hormone and known regulator of feeding ghrelin,43 but indicate that this communication pathway may be more salient when estradiol levels or estrogen signaling is low. Further, these analyses of human data may suggest a similar integration of metabolic cues alongside reproductive hormones in humans as well as mice.
Figure 5.
TNSST neurons display increased hormonal pathway co-correlations with white adipose tissue in individuals with inferred high estrogen signaling
(A) Schematic overview of co-correlation analysis. High-expressing TNSST genes from mouse Flow-Seq experiments were co-correlated across various peripheral metabolic tissues across high- and low-estradiol groups identified in the GTEx database. Created with BioRender.com.
(B) Inferred estradiol levels affected co-correlation pathways relevant to all secreted proteins. High-estradiol individuals showed increased co-correlations in adipose tissue whereas those with lower estradiol showed increases in skeletal muscle and stomach communication.
(C) Similar trends in adipose and stomach co-correlations across estradiol groupings were seen for ligand pathways.
(D) Co-correlations across tissues showed differential impact on genes associated with feeding pathways. Individuals with higher estradiol showed increased co-correlations within these pathways with adipose tissue and decreased co-correlations with stomach as compared to those with lower estradiol levels. (B)–(D) depict violin density plots with superimposed box-and-whisker plots denoting medium, first and third quartiles, and minimum and maximum values. Non-parametric ANOVA and post hoc t test p values reported on graphs.
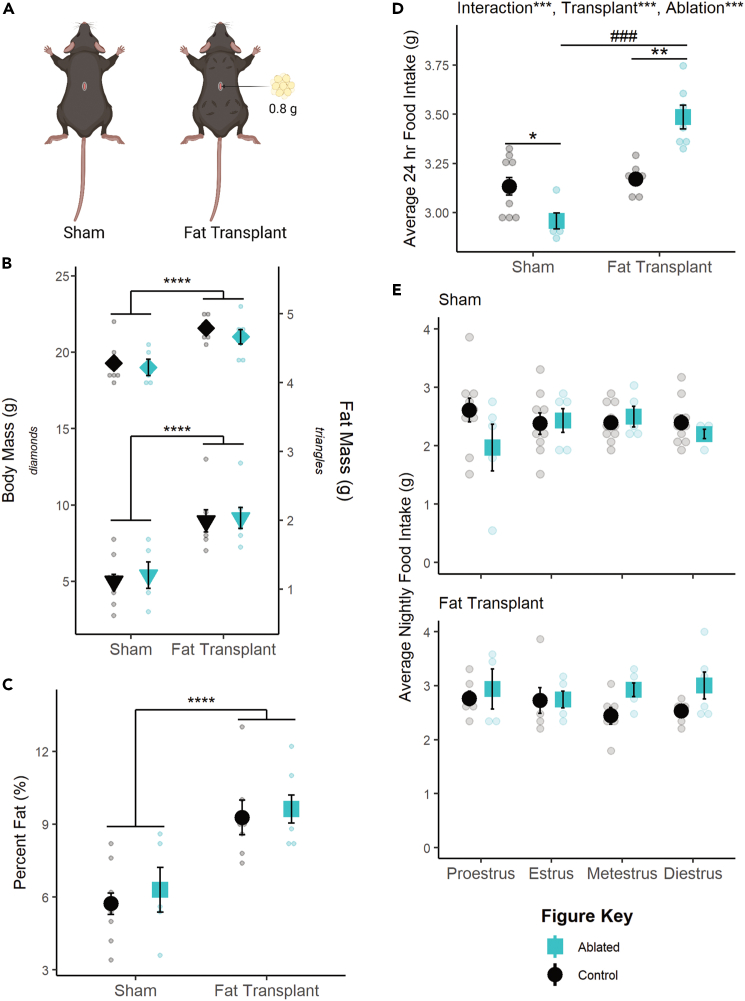
To test the causal, directional relationship between fat and tuberal hypothalamic SST neurons in the modulation of food intake, caspase ablation studies were repeated in combination with fat transplantation. Approximately 1.5 weeks following transplantation of ∼0.8 g subcutaneous fat (Figure 6A), recipient mice exhibited significantly increased body mass (F(1,25) = 28.3184, p < 0.0001; Figure 6B), raw fat mass (F(1,25) = 34.2342, p < 0.0001; Figure 6B), and percent fat mass (F(1,25) = 30.0008, p < 0.0001; Figure 6C) and no interaction with SST neuronal ablation in any case. Thus, fat transplantation increased adiposity similarly across neuronal ablation groups.
Figure 6.
Fat transplantation modulates the effect of SST neuron ablation
(A) Representative schematic of fat transplants. Four deposits of 0.2 g each were placed in the dorsal subcutaneous region. Created with BioRender.com.
(B) Fat transplant increases raw body mass (left axis, p < 0.0001) and fat mass (right axis, p < 0.001) regardless of SST neuronal ablation.
(C) This translates to an overall increase in adiposity (p < 0.0001).
(D) Fat transplantation reverses the effect of TNSST ablation, significantly increasing daily food intake compared to non-ablated controls.
(E) This effect of fat transplant (top panel) seems to be due to a lack of effect during proestrus, though data were underpowered to detect the effect of estrous stage in sham controls (bottom panel). Mean ± SEM; ANOVA and post hoc t tests where applicable; within adiposity group: ∗p < 0.05, ∗∗p < 0.01; ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001; between adiposity group: ###p < 0.001. Sham: Control n = 10, Ablated n = 5; Transplant: Control n = 7, Ablated n = 7. Sham: Proestrus Control n = 10 & Ablated n = 5, Estrus Control n = 9 & Ablated n = 5, Metestrus Control n = 10 & Ablated n = 5, Diestrus Control n = 10 & Ablated n = 4; Fat Transplant: Proestrus Control n = 6 & Ablated n = 5, Estrus Control n = 6 & Ablated n = 6, Metestrus Control n = 6 & Ablated n = 6, Diestrus Control n = 6 & Ablated n = 6.
Fat transplant also increased food intake in general (F(1,25) = 52.524, p < 0.0001), and the effect of SST neuronal ablation was affected by fat transplant (F(1,25) = 26.660, p < 0.0001; Figure 6D). Post hoc t tests revealed that SST neuronal ablation significantly decreased food intake in sham transplant animals (t(11.646) = -2.917, p = 0.01327) but significantly increased food intake in animals receiving fat transplant (t(4.8.2536) = 4.8427, p = 0.001175), similar to the previous relationship with body mass in proestrus (Figure 3A). However, we were unable to detect a significant interaction with fat transplantation and ablation status over the estrous cycle (Figure 6E), possibly due to high variability in nighttime food intake in sham-transplanted mice. Together, these findings indicate that fat transplantation masks the effect of SST neuron ablation and reveal a role for fat mass in modulating the function of tuberal hypothalamic SST neurons within the feeding circuit.
Discussion
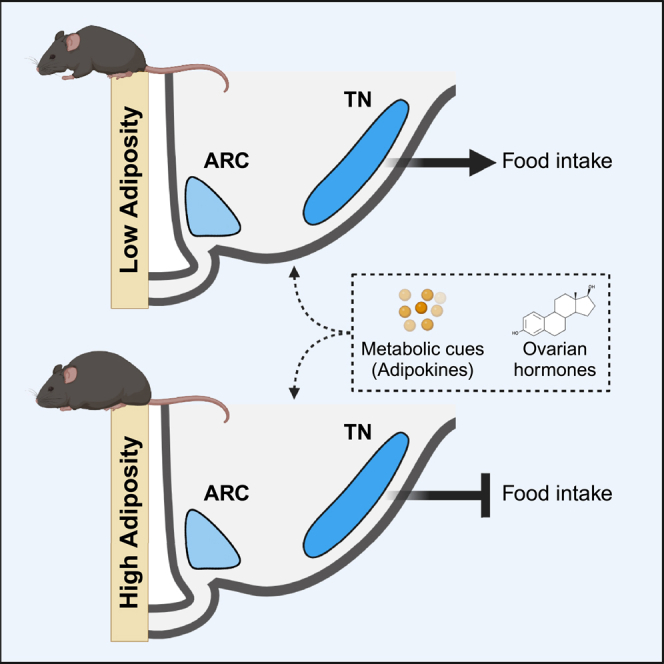
These data suggest that tuberal hypothalamic SST neurons are a locus in the brain that mediates metabolic and reproductive crosstalk. While activation of TNSST neurons increases food intake across sexes, permanent inactivation of ARCSST and TNSST neurons by ablation during adulthood results in decreased food intake only in females during the proestrus phase. This effect depends on body mass, as it is apparent only in lighter animals. In wild-type mice, body mass inversely correlates with food intake on the night of proestrus, but SST neuron ablation uncouples this relationship. Further analysis reveals that white adipose tissue abundance is a significant contributing factor. Not only does postmortem adipocyte size correlate with body mass in neuron ablation experiments, but also fat transplantation studies confirm that SST neuron ablation only decreases food intake in lean animals compared to their fat-transplanted counterparts. An interaction between cycling adipokines and gonadal hormones may be mediated by the direct effects of these circulating molecules on SST neurons, as these cells show some estrogen sensitivity via co-expression analyses. Furthermore, co-correlations between the hypothalamus and adipose tissue in humans and fat transplantation experiments in mice point to the importance of secreted proteins and ligands, suggesting SST neurons may detect and respond to adipokines. Future studies are needed to confirm and dissect the mechanisms of these cellular effects.
What adipokine factor is possibly being detected by the SST neurons (and specifically by the TNSST population) remains to be determined. Leptin positively correlates with overall adiposity,64 and it has been known to play a crucial role in reproductive responsiveness to metabolic condition, namely as the permissive signal required for pubertal onset.65,66,67 Adiponectin, an adipokine that negatively correlates with visceral fat mass in mammals,64 has long been shown to downregulate reproduction through direct impacts on the hypothalamus.68 Resistin is correlated with higher overall adiposity,69 exhibits numerous interactions with the hypothalamic-pituitary-gonadal axis,70,71,72 and is downregulated during the fertile periods of the mouse estrous cycle.73
Regardless of adipokine contributor, this crosstalk paradigm provides a plausible explanation for the varied effects of estradiol on food intake in mice. While endogenous fluctuations and experimental manipulations of estradiol consistently reveal that estrogens decrease food intake in rats and guinea pigs,74,75,76,77 the mouse literature is less definitive.76,78,79,80,81,82,83 Indeed, in our hands, ovariectomy decreased food intake across ablation groups, as has been seen previously in mice.81,83 Instead, the more consistent phenotype in mice is a decrease in energy expenditure following estradiol depletion.10,12,13,83 In light of this study, it is possible that the effects of estradiol on feeding across mouse studies, as observed by either endogenous estrous cycle fluctuations or ovariectomy manipulation, could be confounded by body mass and adiposity. Thus, factors like age at time of experiment, differences in fat distributions between species or strains, diet, or ovariectomy, and time from ovariectomy to estradiol replacement might present confounds based on changes to fat and/or lean mass.
How circulating estrogen levels contribute to this circuit also requires further investigation. Our human GTEx analyses show that co-correlations between genes expressed in TNSST and that in peripheral tissue shift from predominantly adipose-based in high estrogen conditions to skeletal muscle- and stomach-based in low estrogen signaling conditions (Figure 5). This suggests that higher estrogen levels may increase communication between TNSST neurons and white adipocyte depots, particularly as it relates to regulation of feeding behavior (Figure 5D). While this could be due to the actions of circulating estrogens on white adipose tissue itself (reviewed by Palmer and Clegg and Hevener et al.1,84), it is also possible that estrogens directly act on TNSST (and/or ARCSST) neurons to increase their sensitivity and/or responsivity to adipokines. Indeed, a subset of TNSST neurons exhibit estrogen sensitivity (Figure 4), and ARCSST neurons have been found to be estrogen sensitive47 and responsive48 in non-rodent models. Future studies would be needed to test for a possible direct effect on either of these populations in mice. Previous studies of the TN include both female and male mice but do not report Esr1 expression or estrogen sensitivity in Sst+ neurons.42,43 However, the idea that estrogen receptor is expressed in the TN and that the function of TN neurons is sensitive to estrous cycle stage is consistent with spatial mapping of Esr1+ neurons to a continuous region that spans the ventrolateral region of the ventromedial hypothalamus and the TN.85 It is also plausible that the concomitant decrease in effective communication with the stomach in high-estrogen signaling states is due to an interaction of estradiol with the hunger hormone ghrelin. TNSST neurons are directly responsive to ghrelin,43 while estradiol can blunt the orexigenic effects of ghrelin via reduced ghrelin treatment efficacy77 and reduced release of the hormone.86 Thus, estradiol may indirectly influence TNSST activity and subsequent food intake via regulation of ghrelin release and/or signaling.
Estradiol might also be detected elsewhere in the brain and impact TNSST and ARCSST neuronal modulation of feeding through integration at the circuit level. TNSST neurons project to many estrogen-sensitive nodes or nodes receiving direct input from estrogen-responsive regions, including the bed nucleus of the stria terminalis, parabrachial nucleus, and central amygdala.43 Importantly, bifurcated TN projections to the paraventricular nucleus and bed nucleus of the stria terminalis have been shown to mediate the feeding effects of TNSST neurons.43 As a known target for arcuate AgRP neurons—cells which have been shown to integrate reproductive and metabolic cues31—the paraventricular nucleus may contribute to the integration of metabolic and reproductive cues as a downstream integrative node. However, given that ablation of TNSST neurons eliminated the changes in the relationship between body weight and food intake over the estrous cycle, it is plausible that nodes of estradiol detection are TNSST neurons and/or neurons upstream of the TN. This circuit-wide integration of estradiol is a known mechanism of action for the gonadal hormone, with estrogens acting on many circuit nodes to coordinate behavioral output in a variety of cases, including reward/addiction87 and thermoregulation.88 It is therefore probable that the effects of estradiol on feeding function similarly, as the anorexigenic effects of estradiol have been localized to numerous feeding nodes such as the hypothalamic arcuate nucleus89,90,91,92,93 and the nucleus of the solitary tract of the brainstem.94,95 Retrograde tracing studies would help determine if these or estrogen-sensing regions (including the ventral subiculum, an upstream region targeting TNSST neurons44 known to express estrogen receptors96,97) are candidate nodes mediating the effects of estradiol seen in this study.
Alternatively, ARCSST neurons may detect fluctuating hormone levels to modulate the observed phenotype. In ewes, a subset of ARCSST neurons colocalize with ESR1 and increase their activity in response to estradiol treatment.47 Should this pattern of activation be confirmed in mice, it would suggest a mechanism whereby activation of ARCSST neurons during high periods of estradiol induces feeding under low adiposity conditions.
In all, this study adds to the growing literature interrogating the contributions of TNSST neurons to feeding behavior. Central SST (originally named growth hormone inhibiting hormone in the central nervous system)98 had long been known to affect food intake through somatostatin receptor 2.33,37,38,39,40,41,45,99,100,101,102 This effect was seemingly localized to the lateral tuberal nucleus, where TNSST neurons were found to integrate into the melanocortin feeding system, though the effect of these neurons on feeding was attributed to γ-aminobutyric acid (GABA) release as opposed to direct SST effects.43 However, given that dynamics of GABA, NPY, and AgRP release from arcuate neurons in the temporal regulation of food intake,103 it is possible that similar dynamics are apparent between GABA and SST in the TN. In line with previous studies,42,43 chemogenetic activation of TNSST increased food intake across animals. Lack of excess crumbs in subsequent caspase ablation experiments confirmed that this is not a side effect of gnawing; a possibility also excluded in a previous study looking at wood stick gnawing.43 However, a subsequent study did see an increase in gnawing behavior with chemogenetic activation, probably due to a larger target area encompassing large portions of the lateral hypothalamus.42 Furthermore, whereas caspase ablation did decrease food intake, previous studies pooled across the sexes.43 Similar pooling in our hands also resulted in an overall decrease in food intake with SST neuron ablation, indicating that females possibly carry the effect when there is low power to detect sex differences. Alternatively, the inclusion of ARCSST neurons in our ablation may have also resulted in the observed sex difference and integrative capacities of tuberal hypothalamic SST neurons at large.
In our hands, this ablation-mediated decrease in food intake did not result in any alteration to body weight. This is in line with a previous study demonstrating that while the decrease in food intake may be due to ablated animals gaining weight more slowly, there are no raw body weight differences that result.43 Previous studies have also been conflicted on the role of TNSST neurons on movement and locomotion. While one study demonstrated a decrease in total locomotion and increase in vertical movement with TNSST neuron ablation,43 another study found no change in walking and showed an increase in vertical movement with TNSST neuron activation.42 Our telemetry results suggest a lack of change in movement in general with SST neuron ablation. The differences among these studies may be due to the methods of movement quantification and/or viral spread of ablation. Similarly, while our study did not measure drinking behavior, it is unclear whether and how TNSST neuron ablation is involved in thirst processes, with one study demonstrating the necessity of TNSST neurons to maintain baseline drinking levels43 but another failing to show TNSST neuron activation as sufficient to induce increases in drinking.42
TNSST neurons were also found to contribute to food context learning in males (N.B.: in all external papers discussed, no definitions for sex category were ever provided. In mice, we assume that sexes were defined using anogenital distance.),44 indicating that the TN may straddle homeostatic and hedonic feeding mechanisms.14 And although many of these previous studies include female and male mice, they do not report Esr1 expression or estrogen sensitivity in TNSST neurons. Thus, the current paper adds to this growing literature by not only delineating an apparent sex difference but also context dependence in tuberal hypothalamic SST neuronal modulation of food intake.
We further speculate that tuberal hypothalamic SST neurons serve as a nexus of integration and a mediator of reproductive and metabolic interactions within the feeding circuit. In cycling rodents, fertile periods during the estrous cycle are accompanied by alterations to metabolic output, including a decrease in food intake,74,75,76,104 increase in locomotion,104,105,106,107 and increased core body temperature.105,107 These changes are hypothesized to suppress energy intake and promote active mate-seeking behavior and sexual receptivity. This study identifies tuberal hypothalamic SST neurons as possible mediators of such interactions, actively promoting energy intake during fertile periods when metabolic reserves may be insufficient to support reproduction.
Limitations of the study
Viral spread implicates both TNSST and ARCSST neurons in the conditional modulation of food intake. However, targeting toward TNSST neurons was more complete and robust across studies. Future studies dissecting the specific contributions of these two regions would greatly add to circuit delineation and perhaps illuminate the specific locations where metabolic and/or reproductive cues modulate circuitry function.
Additionally, while the integration of reproductive and metabolic states makes adaptive sense to occur in gestating mammals where metabolic requirements are vital to reproductive success, it is formally possible that this same mechanism could be engaged in non-gestating mammals (in this case, males) in certain contexts. For example, the effect might be masked in males due to their increased weight and adiposity at the time of testing at 8 weeks of age in this study. Future research could utilize mice with low adiposity to determine if the integrative capacity of the feeding circuit extends across sex.
Finally, while our approach to stratifying the GTEx database was creative and aligns with known data about deceased individuals therein, the estimations of circulating estrogens cannot be confirmed without either additional information regarding hormonal treatment, reproductive status, and/or salivary or blood serum hormone levels. Databases should consider adding additional information to their collections where financially feasible to support more nuanced analyses of variables related to sex and reproductive state.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| rabbit anti-cFOS | Synaptic Systems | Cat#226003; RRID: 2231974 |
| goat anti-rabbit Alexa Fluor 488 | Thermo Fisher | Cat#A11034; RRID: AB_2576217 |
| rabbit anti-DsRed | Takara Bio Clontech | Cat#632496; RRID: AB_10013483 |
| Bacterial and virus strains | ||
| AAV2-flex-taCasp3-TEVp | UNC Vector Core (Depositor: Nirao Shah & Jim Wells) Yang et al.58 |
|
| AAV8-hSyn-hM3D(Gq)-mCherry | Addgene (Depositor: Brian Roth) Krashes et al.57 |
Addgene viral prep Cat#50474-AAV8 |
| AAV2-FLEX-tdTomato | Addgene (Depositor: Edward Boyden) | Addgene plasmid Cat#28306 |
| AAV8-Syn-FLEX-Mac-GFP | Addgene (Depositor: Edward Boyden) | Addgene plasmid Cat#58852 |
| Chemicals, peptides, and recombinant proteins | ||
| Clozapine-n-oxide (CNO) | Millipore Sigma | Cat#0832 |
| Giemsa | Fisher | Cat#G146-10 |
| DIG RNA labeling kit | Roche | SKU#11277073910 |
| Fluorescein RNA Labeling Mix | Roche | SKU#11685619910 |
| RNA Clean & Concentrator | Zymo Research | Cat#R1017 |
| ZymoPURE II Plasmid Midiprep kit | Zymo Research | Cat#D4201 |
| Zymo DNA Clean & Concentrator | Zymo Research | Cat#D4033 |
| Blocking Reagent | Roche | Cat#11096176001 |
| TSA Plus Cyanine 5 System | Akoya Biosciences | Cat#NEL745001KT |
| Rneasy Micro kit | Qiagen | Cat#74004 |
| Deposited data | ||
| RNA seq data from neurons FACS isolated from mouse tuberal hypothalamus neurons | NCI Gene Expression Omnibus (GEO) | GSE224254 |
| GTEx | Broad Institute | Version 8 |
| Molecular Signatures Database | Broad Institute | Human Gene Set: HALLMARK_ESTROGEN_RESPONSE_EARLY |
| Datasets for estrogen signaling binning of human data | Github | https://github.com/Leandromvelez/sex-specific-endocrine-signals. |
| Experimental models: Organisms/strains | ||
| model organism: Sst-Cre: genotype STOCK Ssttm2.1(cre)Zjh/J | The Jackson Laboratory | Strain#013044 |
| model organism: Esr1-Cre: genotype B6N.129S6(Cg)-Esr1tm1.1(cre)And/J | The Jackson Laboratory | Strain#017911 |
| Recombinant DNA | ||
| cDNA for Sst (bases 7-550 for transcript variant 1, bases 317-860 for transcript variant 2) | Allen Brain Atlas | Probe: RP_Baylor_103041 |
| TA Cloning™ Kit, with pCR™2.1 Vector and One Shot™ TOP10F' Chemically Competent E. coli | Invitrogen | Cat#K203001 |
| Software and algorithms | ||
| VitalView Software | Starr Life Sciences | version 5.1 |
| lme() function (nlme package, version 3.1-157) | r-project.org | R version 4.2.1 |
| t_test() function (rstatix package, version 0.7.0) | r-project.org | R version 4.2.1 |
| Code for volcano plot | Github |
http://github.com/jevanveen/ratplots R function: deseq_volcano_plot_gs() |
| Analysis pipleline for estrogen signaling binning of human data and cross-tissue correlations with mouse orthologues | Github | https://github.com/Leandromvelez/sex-specific-endocrine-signals |
| Kallisto | Pachter Lab Bray et al.114 |
version 0.46.2 |
| Deseq2 | Galaxy | Version 2.11.40.6+galaxy1 |
| CellProfiler | Broad Institute | 4.2.1 |
Resource availability
Lead contact
Further information and Requests for resources and reagents should be direct to and will be fulfilled by the lead contact, Stephanie Correa (stephaniecorrea@ucla.edu).
Materials availability
The study did not generate new unique reagents.
Experimental model and study participant details
Mice
Female (defined as having small anogenital distance at weaning and presence of ovaries at time of death) and male (defined as having large anogenital distance at weaning and presence of testes postmortem) mice expressing the Sst-Cre driver transgene (JAX stock no. 013044, Ssttm2.1(cre)Zjh/J) were maintained on a C57BL/6J genetic background. Heterozygotes (Sst-Cre/+) and/or wildtype littermates (+/+) were used for all studies. Genotypes were determined as per JAX protocol 28317. Female and male mice expressing the Esr1-Cre driver transgene (JAX stock no. 017911, B6N.129S6(Cg)-Esr1tm1.1(cre)And/J) were maintained on a C57BL/6J genetic background. Heterozygotes (Esr1-Cre/+) were used for colocalization studies. Genotypes were determined as per primers from JAX protocol 27213. Experiments were performed on cycling females and gonadally-intact males unless otherwise stated. Mice were maintained on a 12:12 light cycle, with ad libitum access to food and water (unless otherwise specified), under controlled humidity conditions at 22-23C, and in single-housed cages with non-caloric paper bedding to ensure accurate food intake assessment. All studies were carried out in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. UCLA is AALAS accredited, and the UCLA Institutional Animal Care and Use Committee (IACUC) approved all animal procedures.
Method details
Estrous cycle staging
Vaginal lavages were performed on females daily, between ZT 0 and ZT 4, using 30 μL of standard phosphate buffered saline (PBS). Samples were deposited onto slides and allowed to dry prior to staining. Males were subjected to similar handling during this time to ensure roughly equivalent handling stress. Giemsa staining was carried out to visualize cellular composition of the vaginal cavity. Stock Giemsa stain was prepared at least one week in advance of use. An 18.5% solution of Giemsa powder (Fisher G146-10) in glycerin was heated to 60°C and cooled before diluting 9:14 with 100% methanol. Stock was diluted 1:30 in PBS before use, shaking vigorously before stain. Slides were incubated for one hour at room temperature. Prior to staining, slides were briefly fixed in 100% methanol. Staging was assessed via light microscopy to determine the relative abundance of leukocytes, nucleated epithelial cells, and cornified epithelial cells108 and stages were assigned with morning samples indicating the prior night’s estrous stage, to align the cytology stage to the behavioral changes that occur in the dark (night) of estrus.109 This staging method was confirmed by examining patterns of core body temperature during the dark phase across the estrous cycle, which also changes during estrus.105
Surgical procedures
Mice received analgesics (0.074 mg/kg buprenorphine two times daily, 7.11 mg/kg carprofen one time daily) on the day of and one day post-surgery. Mice were anaesthetized with 3% isoflurane and maintained within a range of 1.25-2.5%. AAVs were bilaterally injected into the TN of adult mice (coordinates relative to Bregma: A-P -1.65 mm, lateral ±0.75, D-V -5.45; scaled when Bregma-Lambda distance was not equivalent to 4.2 mm) at a rate of 5 nL/s using a glass-pulled needle. See below table for titers and injection volumes. Controls consisted of both wildtype animals injected with the experimental virus (virus controls) and Cre positive animals injected with cell-filling GFP (genotype controls). Ovariectomy surgeries included complete removal of gonads from adult mice. Gonadectomies occurred immediately prior to stereotaxic viral injections within the same surgical period. In telemetry experiments, G2 eMitters (Starr Life Sciences) were implanted intraperitoneally on the same day as viral injection. Experiments were conducted following at least two weeks recovery from surgical proceedings.
List of viral vectors used
| Experiment | Virus | Depositor & procurement | Titer (vg/mL) | Volume (nL) | Citation |
|---|---|---|---|---|---|
| Caspase ablation | AAV2-flex-taCasp3-TEVp | Nirao Shah & Jim Wells, UNC Vector Core | 1–8 x 1012 | 200–250 | Yang et al.58 |
| Transient activation | AAV8-hSyn-hM3D(Gq)-mCherry | Brian Roth, Addgene viral prep # 50474-AAV8 | ≥4 × 1012 | 150–200 | Krashes et al.57 |
| Fluorescent localization | AAV2-FLEX-tdTomato | Edward Boyden, Addgene plasmid #28306 | ≥5 × 1012 | 200 for Flow-Seq; 400 for Esr1-Cre |
|
| Fluorescent controls | AAV8-Syn-FLEX-Mac-GFP | Edward Boyden, Addgene plasmid #58852 | 1:5 dilution of stock | Matching volume to experimental animals |
Caspase ablation experiments
Gross movement and core body temperature were passively measured every other week for eight weeks using VitalView software (Starr Life Sciences). Body weight was measured every week. Food assay was performed when mice were not on telemetry pads. At ZT 0.5 on the start day of the experiment, 2/3 of the non-caloric paper bedding was removed. A pre-measured amount of food was delivered, and mouse body weight measured. Food in hopper was weighed at ZT 0.5 and ZT 11.5 every day until experiment conclusion. After 96 hours, food and all bedding, including interspersed crumbs, were collected. Measurements for total food were obtained by subtracting the final weight of food pellets and crumbs from the original weight of food provided. Measurements for crumbs were obtained by subtracting the final weight of food pellets and crumbs from the final weight of food in the cage hopper. Both measurements were divided by 4 to obtain the average food eaten or crumbs produced per day (24 h food intake). For estrous cycle experiments, 13-hour nightly food intake (ZT 11.5 – ZT 0.5) was utilized instead as we were more confident in nightly staging based on the behavioral changes that occur during the night of estrus109 and our ability to confirm estrus staging with core body temperature in the dark phase of estrus.105 For some experiments, 4-5 hour fasted glucose tolerance tests were performed prior to sacrifice. In ovariectomy experiments, two food assays were performed back-to-back, non-fasted resting glucose levels were collected, body composition was measured via NMR, and indirect calorimetry was performed in Oxymax metabolic chambers (Columbus Instruments) at room temperature. Upon experiment completion, all brains were collected using RNase-free conditions. Inguinal white adipose tissue (iWAT) and gonadal white adipose tissue (gWAT) were collected for histology analyses.
Transient activation food intake assay
Clozapine-n-oxide (CNO; MilliporeSigma #0832) was used to activate TNSST neurons in Sst-Cre animals expressing hM3Dq-mCherry. Stock solution of 20 mg/mL in DMSO was stored at -20°C and diluted to a working solution of 0.03 mg/mL in sterile saline also stored at -20°C. Saline control (0.15% DMSO) or CNO (10 μL/g body weight, dose of 0.3 mg/kg) working solution were administered IP in a counterbalanced design. Experiments were completed in duplicate replicate trials. Mice were transferred to experimental room at least 15 minutes prior to experimentation. Experiments were begun between ZT 2-3 and terminated between ZT 6-7. Following injection, food intake was measured at 0.5, 1, 2, and 4 hr. Vaginal lavage was performed on female mice after experiment conclusion to prevent stress interference with food intake. All mice were injected with CNO 90 minutes prior to sacrifice to enable neuronal activation validation via cFOS immunohistochemistry.
Fat transplantations
Donor fat was taken from various visceral (i.e., periuterine perigonadal, retroperitoneal, and omental) depots of wildtype female C57BL/6J mice and implanted into female mice recently stereotaxically injected under standard surgical conditions. Four depots of 0.15-0.25g were placed subcutaneously on the dorsal surface through a single incision mid-back, for a final transplantation total of 0.6-0.9g of white adipose. Fat for each depot was divided into at least three individual pieces to promote vascularization. The visceral-to-subcutaneous paradigm was used due to the deleterious metabolic effects of this graft.110 Food intake was assayed 2-3 weeks following transplantation to allow for sufficient angiogenesis111 and graft stabilization without endogenous fat depot compensation.112 Upon sacrifice, grafts were examined to confirm tissue was not necrotic.
Histology
In situ hybridization (ISH) and immunostaining (IHC)
Sst sense and antisense probes were transcribed using a DIG or FITC RNA labeling kit (Roche) and purified with RNA Clean & Concentrator (Zymo Research). PCR products were amplified using Allen Brain Institute-derived reference primer sequences and cloned into pCR 2.1 TOPO (Invitrogen). Plasmid DNA was then isolated from bacterial cultures (ZymoPURE II Plasmid Midiprep kit), linearized, and purified (Zymo DNA Clean & Concentrator). Validation of caspase ablation was carried out on 35μm-thick coronal slices via chromogen ISH using BCIP (5-bromo-4-chloro-3-indoyl phosphate) and INT (iodonitrotetrazolium). Validation of hM3Dq targeting and activation was accomplished by visualization of native mCherry expression and IHC stain for cFOS. Briefly, slides were blocked and incubated with rabbit anti-cFOS (1:200, Synaptic Systems # 226003, RRID: 2231974) primary antibody overnight at 4°C. The next day, sections were incubated for 1 hour at room temperature with goat anti-rabbit Alexa Fluor 488 secondary (1:500, Thermo Fisher Scientific # A11034, RRID: AB_2576217) and counterstained with DAPI. For colocalization experiments, Esr1-Cre mice were bilaterally injected with 400 μl AAV2-flex-tdTomato into the TN coordinates. Native tdTomato fluorescence destroyed by combined ISH protocol was recovered by rabbit anti-DsRed (1:1000, Takara Bio Clontech # 632496, RRID: AB_10013483) antibody and switched to the green channel using an Alexa Fluor 488 secondary. Dual Sst ISH & tdTomato IHC protocol was accomplished via TSA amplification. Briefly, 35 μm sections were fixed, permeabilized with Triton X-100, and acetylated before overnight ISH probe incubation at 65°C. The next day, tissue was then washed, blocked with Blocking Reagent (MilliporeSigma 11096176001Roche) and heat inactivated sheep serum, and incubated with anti-DsRed overnight at 4°C. The final day, tissue was washed before ISH signal was developed with the TSA Plus Cyanine 5 System (Akoya Biosciences # NEL745001KT). Slides were then stripped of horseradish peroxidase and blocked with normal goat serum before incubating with goat anti-rabbit Alexa Fluor 488 (1:400) for 2 hours at room temperature.
DREADD viral injection mapping
Sections were anatomically matched using the Allen Mouse Brain Atlas (mouse.brain-map.org), and the anatomical areas with hM3Dq-mCherry positive cells were manually identified and outlined in Illustrator. Areas containing dense hM3Dq-mCherry expression were outlined with 5% opacity, and areas containing sparse hM3Dq-mCherry expression were outlined with 3% opacity. Thus, areas with more hM3Dq-mCherry expression across animals appear more opaque than areas with less hM3Dq-mCherry.
Quantification and statistical analysis
Caspase ablation quantification
Sections were anatomically matched using the Allen Mouse Brain Atlas (mouse.brain-map.org), and then the TN, ARC, lateral hypothalamic area (LHA), and ventromedial nucleus (VMH) were manually outlined in ImageJ. The mean pixel intensity was then measured for each region and normalized to the local background. The mean normalized Sst signal in each region was then compared using a 2-way linear mixed-effects model with ablation status (control vs ablated) and region (TN, ARC, LHA, and VMH) as factors and animal as a nested random effect in R (version 4.2.1) using the lme() function (nlme package, version 3.1-157). Following a significant (p<0.05) ablation status x region interaction, we performed multiple t-tests to quantitatively compare Sst signal in each region individually. T-tests were performed and p-values calculated using the t_test() function (rstatix package, version 0.7.0), using the Holm method to correct for multiple comparisons.
Adipocyte size quantification
Inguinal and white adipose tissue was collected post-mortem and drop-fixed in 4% paraformaldehyde (PFA) for at least 18 hours. Tissue was then washed in PBS before being stored in PBS at 4°C until tissue analysis. For histological processing, tissue was placed in tissue processing cassettes and submerged in 70% ethanol before being embedded in paraffin, sectioned at 4 μM, and stained with hematoxylin & eosin (H&E) by the UCLA Translational Pathology Core Laboratory. Three regions of interest per tissue-type per mouse were imaged by light microscopy at 20x magnification. Adipocyte area was quantified using a custom pipeline in CellProfiler. Inclusion parameters were cell diameters of 100-300 pixel units and a global threshold strategy with minimum cross-entropy.
Colocalization analysis
Sst and Esr1 co-expression was determined using CellProfiler (version 4.2.1). First, a contour was drawn around a matched section of the TN using anatomical landmarks (i.e., shape of arcuate nucleus and third ventricle). For each hemisphere, DAPI-stained nuclei were detected and intensity thresholding was used to determine Sst+ cells. Incorrectly labeled cells were manually erased or added. Sst+ cells were then filtered based on Esr1::tdTomato signal intensity. Counts were made of total Sst+ cells, as well as Sst+/Esr1+ and SST+/ Esr1- cells. The counts were averaged across the two hemispheres, and percent was calculated as ([Sst+/Esr1+] / Total SST) x 100.
Bioinformatics analysis
Sst-Cre female and male mice were bilaterally stereotaxically injected with AAV expressing Cre-dependent tdTomato (see table in surgical procedures). Following at least two weeks for viral expression, animals were sacrificed and TN was microdissected under fluorescent illumination. Dissected TN was dissociated using a papain-based enzymatic process (Worthington Biochemical) and then TNSST neurons were enriched and collected via flow cytometry. Cells were sorted from debris and doublets were excluded by gating on forward-scatter and side-scatter profiles. Live nucleated cells were then selected by DAPI-negative (live) and DRAQ5-positive (nucleated) staining. Finally, tdTomato-positive cells were selected based on relatively high levels of red fluorescence (as in van Veen & Kammel et al., 2020). RNA was isolated from 500-2500 cells by Rneasy Micro kit (Qiagen). Cells were then submitted for bulk RNA sequencing. Single-end reads (∼10 million unique reads per mouse) were assembled to the mouse transcriptome (version mm10) using kallisto (version 0.46.2). Differentially expressed genes and normalized read counts were identified using Deseq2 Galaxy Version 2.11.40.6+galaxy1. Volcano plots were produced by the custom R function “deseq_volcano_plot_gs()” available through the following package: http://github.com/jevanveen/ratplots. Raw reads of the RNA sequencing data were also examined for hypothalamus-peripheral tissue co-correlations across stomach, small intestine, skeletal muscle, visceral fat, and subcutaneous fat as hypothalamic reads using the GTEx database as previously described.61,62 In addition, estrogen-responsive genes used to infer “low” vs “high estrogen signaling were gathered from: https://www.gsea-msigdb.org/gsea/msigdb/cards/HALLMARK_ESTROGEN_RESPONSE_EARLY.html.113 To clarify the analysis, estrogen signaling binning per individual and subsequent cross-tissue correlations with mouse DEG orthologues, all processed datasets, scripts used to analyze, and detailed walk-through is available at: https://github.com/Leandromvelez/sex-specific-endocrine-signals.
Statistical analyses
All statistics were carried out in R. Sex differences were determined by interaction terms between genotype and sex (caspase ablation experiments) or genotype, treatment, and sex (chemogenetic experiments). In DREADD experiments, data were transformed using the square root function to achieve the assumption of normality necessary for multifactorial ANOVA; raw data are present in Figure 1. In caspase ablation and fat transplantation experiments, animals meeting both the criteria of outlier by Cook’s distance, as well as “miss” (no hit or unilateral hit as defined by more than 5% of targeted cells still present) were excluded. For fat transplantation studies, only sham animals with <10% fat mass at the beginning of the feeding assay were included. All data were checked and transformed, if necessary, to meet normalcy criteria. In all analyses, ANOVA met threshold for interaction prior to running within-group post-hoc t-tests. In linear regressions, ANCOVAs were used. For all figures, ∗p<0.05, ∗∗p<0.01, ∗∗∗p<0.001, and ∗∗∗∗p<0.0001 between ablation groups or r2; #p<0.10 and ####p<0.0001 within ablation groups.
Acknowledgments
This research was supported by the UCLA Life Sciences Division, NIH R01 AG066821, NIH R01 DK136073, the NIH Specialized Centers of Research Excellence (SCORE) grant U54 DK120342, and the National Center for Advancing Translational Sciences (NCATS) under the UCLA Clinical and Translational Science Institute grant UL1TR001881. M.G.M. was supported by an NSF GRFP (DGE-2034835), Dissertation Year Fellowship from the UCLA Graduate Division, and the UCLA Kenneth I. Shine Fellowship. S.L.A. was supported by the CARE program of the UCLA Undergraduate Research Center (NIH NIGMS IMSD GM055052; PI: Hasson). M.M.S. was supported by NIH grant DK130640. The authors also wish to thank Drs. A. Arnold, K. Wassum, and E. Hsiao for helpful feedback. M.G.M. would also like to thank all members of the Correa lab who assisted with timed food assays.
Author contributions
Conceptualization: M.G.M., S.M.C.; methodology: M.G.M., A.L.C., K.R., J.E.V., M.M.S., S.M.C.; software: M.G.M., L.M.V., P.B.V., H.B.W., J.E.V.; validation: M.G.M., P.B.V., N.P.S.; formal analysis: M.G.M., L.M.V., P.B.V., J.E.V., M.M.S.; investigation: M.G.M., R.L.S., A.L.C., L.R.C., P.B.V., N.P.S., J.W.P., S.L.A., S.S.A., B.T.; data curation: L.M.V., J.E.V.; writing – original draft: M.G.M., J.E.V., M.M.S., S.M.C.; writing – review & editing: M.G.M., R.L.S., A.L.C., L.R.C., K.R., J.E.V., M.M.S., S.M.C.; visualization: M.G.M., L.R.C., P.B.V., M.M.S.; supervision, M.G.M., K.R., J.E.V., M.M.S., S.M.C.; funding acquisition: M.G.M., S.M.C.
Declaration of interests
The authors declare no competing interests.
Inclusion and diversity
We support inclusive, diverse, and equitable conduct of research. One or more of the authors of this paper self-identifies as an underrepresented ethnic minority in their field of research or within their geographical location. One or more of the authors of this paper self-identifies as a gender minority in their field of research. One or more of the authors of this paper self-identifies as a member of the LGBTQIA+ community. One or more of the authors of this paper self-identifies as living with a disability. One or more of the authors of this paper received support from a program designed to increase minority representation in their field of research.
Published: September 14, 2023
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2023.107918.
Supplemental information
ANOVA results and descriptive statistics, including sample sizes for each sex and treatment group, degrees of freedom, F statistics for each factor in the model, and exact p-values, for each experiment.
Data and code availability
-
•
RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. The accession umber is listed in the key resources table. This paper also analyzes existing, publicly available data. Accession numbers for the datasets are listed in the key resources table. All data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at Github and is publicly available as of the date of publication. Github links are available in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
References
- 1.Palmer B.F., Clegg D.J. The sexual dimorphism of obesity. Mol. Cell. Endocrinol. 2015;402:113–119. doi: 10.1016/J.MCE.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baz B., Riveline J.-P., Gautier J.-F. Endocrinology of Pregnancy: Gestational diabetes mellitus: definition, aetiological and clinical aspects. Eur. J. Endocrinol. 2016;174:R43–R51. doi: 10.1530/EJE-15-0378. [DOI] [PubMed] [Google Scholar]
- 3.Mauvais-Jarvis F. In: Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity. Mauvais-Jarvis F., editor. Springer Cham; 2017. Menopause, estrogens, and glucose homeostasis in women; pp. 217–227. [DOI] [Google Scholar]
- 4.Frisch R.E., Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science. 1970;169:397–399. doi: 10.1126/SCIENCE.169.3943.397. [DOI] [PubMed] [Google Scholar]
- 5.Frisch R.E. Weight at Menarche: Similarity for Well-Nourished and Undernourished Girls at Differing Ages, and Evidence for Historical Constancy. Pediatrics. 1972;50:445–450. [PubMed] [Google Scholar]
- 6.Burt Solorzano C.M., McCartney C.R. Obesity and the pubertal transition in girls and boys. Reproduction. 2010;140:399–410. doi: 10.1530/REP-10-0119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson J.F., Gosden R.G., Felicio L.S. Effect of Dietary Restriction on Estrous Cyclicity and Follicular Reserves in Aging C57BL/6J Mice. Biol. Reprod. 1985;32:515–522. doi: 10.1095/BIOLREPROD32.3.515. [DOI] [PubMed] [Google Scholar]
- 8.Muñoz M.T., Argente J. Anorexia nervosa in female adolescents: endocrine and bone mineral density disturbances. Eur. J. Endocrinol. 2002;147:275–286. doi: 10.1530/eje.0.1470275. [DOI] [PubMed] [Google Scholar]
- 9.Ball Z.B., Barnes R.H., Visscher M.B. The Effects of Dietary Caloric Restriction on Maturity and Senescence, with Particular Reference to Fertility and Longevity. Am. J. Physiol. 1947;150:511–519. doi: 10.1152/AJPLEGACY.1947.150.3.511. [DOI] [PubMed] [Google Scholar]
- 10.Correa S.M., Newstrom D.W., Warne J.P., Flandin P., Cheung C.C., Lin-Moore A.T., Pierce A.A., Xu A.W., Rubenstein J.L., Ingraham H.A. An Estrogen-Responsive Module in the Ventromedial Hypothalamus Selectively Drives Sex-Specific Activity in Females. Cell Rep. 2015;10:62–74. doi: 10.1016/j.celrep.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martínez de Morentin P.B., González-García I., Martins L., Lage R., Fernández-Mallo D., Martínez-Sánchez N., Ruíz-Pino F., Liu J., Morgan D.A., Pinilla L., et al. Estradiol regulates brown adipose tissue thermogenesis via hypothalamic AMPK. Cell Metab. 2014;20:41–53. doi: 10.1016/j.cmet.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Musatov S., Chen W., Pfaff D.W., Mobbs C.V., Yang X.-J., Clegg D.J., Kaplitt M.G., Ogawa S. Silencing of estrogen receptor alpha in the ventromedial nucleus of hypothalamus leads to metabolic syndrome. Proc. Natl. Acad. Sci. USA. 2007;104:2501–2506. doi: 10.1073/pnas.0610787104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Y., Nedungadi T.P., Zhu L., Sobhani N., Irani B.G., Davis K.E., Zhang X., Zou F., Gent L.M., Hahner L.D., et al. Distinct hypothalamic neurons mediate estrogenic effects on energy homeostasis and reproduction. Cell Metab. 2011;14:453–465. doi: 10.1016/j.cmet.2011.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Massa M.G., Correa S.M. Sexes on the brain: Sex as multiple biological variables in the neuronal control of feeding. Biochim. Biophys. Acta, Mol. Basis Dis. 2020;1866 doi: 10.1016/j.bbadis.2020.165840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen X., McClusky R., Chen J., Beaven S.W., Tontonoz P., Arnold A.P., Reue K. The Number of X Chromosomes Causes Sex Differences in Adiposity in Mice. PLoS Genet. 2012;8 doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ciofi P. The arcuate nucleus as a circumventricular organ in the mouse. Neurosci. Lett. 2011;487:187–190. doi: 10.1016/J.NEULET.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 17.Smith J.T., Cunningham M.J., Rissman E.F., Clifton D.K., Steiner R.A. Regulation of Kiss1 Gene Expression in the Brain of the Female Mouse. Endocrinology. 2005;146:3686–3692. doi: 10.1210/EN.2005-0488. [DOI] [PubMed] [Google Scholar]
- 18.Mittelman-Smith M.A., Williams H., Krajewski-Hall S.J., Lai J., Ciofi P., McMullen N.T., Rance N.E. Arcuate Kisspeptin/Neurokinin B/Dynorphin (KNDy) Neurons Mediate the Estrogen Suppression of Gonadotropin Secretion and Body Weight. Endocrinology. 2012;153:2800–2812. doi: 10.1210/EN.2012-1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pillon D., Caraty A., Fabre-Nys C., Lomet D., Cateau M., Bruneau G. Regulation by Estradiol of Hypothalamic Somatostatin Gene Expression: Possible Involvement of Somatostatin in the Control of Luteinizing Hormone Secretion in the Ewe. Biol. Reprod. 2004;71:38–44. doi: 10.1095/biolreprod.103.023689. [DOI] [PubMed] [Google Scholar]
- 20.Lomniczi A., Ojeda S.R. The Emerging Role of Epigenetics in the Regulation of Female Puberty. Endocr. Dev. 2016;29:1–16. doi: 10.1159/000438840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright H., Aylwin C.F., Toro C.A., Ojeda S.R., Lomniczi A. Polycomb represses a gene network controlling puberty via modulation of histone demethylase Kdm6b expression. Sci. Rep. 2021;11:1–17. doi: 10.1038/s41598-021-81689-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oldfield B.J., Mirabella P.N., Stefanidis A. In: Neuroendocrinology of Appetite. Dickson S.L., Mercer J.G., editors. Wiley-VCH GmbH; 2016. Neuroanatomy of feeding pathways; pp. 1–23. [Google Scholar]
- 23.Chen Y., Lin Y.-C., Kuo T.-W., Knight Z.A. Sensory Detection of Food Rapidly Modulates Arcuate Feeding Circuits. Cell. 2015;160:829–841. doi: 10.1016/J.CELL.2015.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Burnett C.J., Li C., Webber E., Tsaousidou E., Xue S.Y., Brüning J.C., Krashes M.J. Hunger-Driven Motivational State Competition. Neuron. 2016;92:187–201. doi: 10.1016/J.NEURON.2016.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim J.G., Sun B.-H., Dietrich M.O., Koch M., Yao G.-Q., Diano S., Insogna K., Horvath T.L. AgRP Neurons Regulate Bone Mass. Cell Rep. 2015;13:8–14. doi: 10.1016/j.celrep.2015.08.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steculorum S.M., Ruud J., Karakasilioti I., Backes H., Engström Ruud L., Timper K., Hess M.E., Tsaousidou E., Mauer J., Vogt M.C., et al. AgRP Neurons Control Systemic Insulin Sensitivity via Myostatin Expression in Brown Adipose Tissue. Cell. 2016;165:125–138. doi: 10.1016/j.cell.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kammel L.G., Correa S.M. Selective sexual differentiation of neuron populations may contribute to sex-specific outputs of the ventromedial nucleus of the hypothalamus. J. Neuroendocrinol. 2020;32:e12801. doi: 10.1111/jne.12801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krause W.C., Rodriguez R., Gegenhuber B., Matharu N., Rodriguez A.N., Padilla-Roger A.M., Toma K., Herber C.B., Correa S.M., Duan X., et al. Oestrogen engages brain MC4R signalling to drive physical activity in female mice. Nature. 2021;599:131–135. doi: 10.1038/s41586-021-04010-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Narita K., Murata T., Matsuoka S. The ventromedial hypothalamus oxytocin induces locomotor behavior regulated by estrogen. Physiol. Behav. 2016;164:107–112. doi: 10.1016/j.physbeh.2016.05.047. [DOI] [PubMed] [Google Scholar]
- 30.van Veen J.E., Kammel L.G., Bunda P.C., Shum M., Reid M.S., Massa M.G., Arneson D., Park J.W., Zhang Z., Joseph A.M., et al. Hypothalamic oestrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat. Metab. 2020;2:351–363. doi: 10.1038/s42255-020-0189-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Padilla S.L., Qiu J., Nestor C.C., Zhang C., Smith A.W., Whiddon B.B., Rønnekleiv O.K., Kelly M.J., Palmiter R.D. AgRP to Kiss1 neuron signaling links nutritional state and fertility. Proc. Natl. Acad. Sci. USA. 2017;114:2413–2418. doi: 10.1073/pnas.1621065114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aponte G., Leung P., Gross D., Yamada T. Effects of somatostin on food intake in rats. Life Sci. 1984;35:741–746. doi: 10.1016/0024-3205(84)90342-4. [DOI] [PubMed] [Google Scholar]
- 33.Danguir J. Food intake in rats is increased by intracerebroventricular infusion of the somatostatin analogue SMS 201–995 and is decreased by somatostatin antiserum. Peptides. 1988;9:211–213. doi: 10.1016/0196-9781(88)90030-7. [DOI] [PubMed] [Google Scholar]
- 34.Karasawa H., Yakabi S., Wang L., Taché Y. Orexin-1 receptor mediates the increased food and water intake induced by intracerebroventricular injection of the stable somatostatin pan-agonist, ODT8-SST in rats. Neurosci. Lett. 2014;576:88–92. doi: 10.1016/J.NEULET.2014.05.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lin M.T., Chen J.J., Ho L.T. Hypothalamic Involvement in the Hyperglycemia and Satiety Actions of Somatostatin in Rats. Neuroendocrinology. 1987;45:62–67. doi: 10.1159/000124704. [DOI] [PubMed] [Google Scholar]
- 36.Shibasaki T., Kim Y.S., Yamauchi N., Masuda A., Imaki T., Hotta M., Demura H., Wakabayashi I., Ling N., Shizume K. Antagonistic effect of somatostatin on corticotropin-releasing factor-induced anorexia in the rat. Life Sci. 1988;42:329–334. doi: 10.1016/0024-3205(88)90642-X. [DOI] [PubMed] [Google Scholar]
- 37.Stengel A., Rivier J., Taché Y. Central actions of somatostatin-28 and oligosomatostatin agonists to prevent components of the endocrine, autonomic and visceral responses to stress through interaction with different somatostatin receptor subtypes. Curr. Pharm. Des. 2013;19:98–105. doi: 10.2174/13816128130114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stengel A., Goebel M., Wang L., Rivier J., Kobelt P., Mönnikes H., Taché Y. Activation of brain somatostatin 2 receptors stimulates feeding in mice: analysis of food intake microstructure. Physiol. Behav. 2010;101:614–622. doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stengel A., Goebel M., Wang L., Rivier J., Kobelt P., Monnikes H., Taché Y. Selective central activation of somatostatin receptor 2 increases food intake, grooming behavior and rectal temperature in rats. J. Physiol. Pharmacol. 2010;61:399–407. [PMC free article] [PubMed] [Google Scholar]
- 40.Stengel A., Karasawa H., Taché Y. The role of brain somatostatin receptor 2 in the regulation of feeding and drinking behavior. Horm. Behav. 2015;73:15–22. doi: 10.1016/J.YHBEH.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tachibana T., Cline M.A., Sugahara K., Ueda H., Hiramatsu K. Central administration of somatostatin stimulates feeding behavior in chicks. Gen. Comp. Endocrinol. 2009;161:354–359. doi: 10.1016/J.YGCEN.2009.01.022. [DOI] [PubMed] [Google Scholar]
- 42.Mickelsen L.E., Bolisetty M., Chimileski B.R., Fujita A., Beltrami E.J., Costanzo J.T., Naparstek J.R., Robson P., Jackson A.C. Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci. 2019;22:642–656. doi: 10.1038/s41593-019-0349-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luo S.X., Huang J., Li Q., Mohammad H., Lee C.-Y., Krishna K., Kok A.M.-Y., Tan Y.L., Lim J.Y., Li H., et al. Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science. 2018;361:76–81. doi: 10.1126/science.aar4983. [DOI] [PubMed] [Google Scholar]
- 44.Mohammad H., Senol E., Graf M., Lee C.-Y., Li Q., Liu Q., Yeo X.Y., Wang M., Laskaratos A., Xu F., et al. A neural circuit for excessive feeding driven by environmental context in mice. Nat. Neurosci. 2021;24:1132–1141. doi: 10.1038/s41593-021-00875-9. [DOI] [PubMed] [Google Scholar]
- 45.Campbell J.N., Macosko E.Z., Fenselau H., Pers T.H., Lyubetskaya A., Tenen D., Goldman M., Verstegen A.M.J., Resch J.M., McCarroll S.A., et al. A molecular census of arcuate hypothalamus and median eminence cell types. Nat. Neurosci. 2017;20:484–496. doi: 10.1038/nn.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Werner H., Koch Y., Baldino F., Gozes I. Steroid regulation of somatostatin mRNA in the rat hypothalamus. J. Biol. Chem. 1988;263:7666–7671. [PubMed] [Google Scholar]
- 47.Scanlan N., Dufourny L., Skinner D.C. Somatostatin-14 Neurons in the Ovine Hypothalamus: Colocalization with Estrogen Receptor and Somatostatin-28(1-12) Immunoreactivity, and Activation in Response to Estradiol. Biol. Reprod. 2003;69:1318–1324. doi: 10.1095/biolreprod.103.017848. [DOI] [PubMed] [Google Scholar]
- 48.Fergani C., Routly J.E., Jones D.N., Pickavance L.C., Smith R.F., Dobson H. Activation of Cells Containing Estrogen Receptor Alpha or Somatostatin in the Medial Preoptic Area, Arcuate Nucleus, and Ventromedial Nucleus of Intact Ewes During the Follicular Phase, and Alteration after Lipopolysaccharide1. Biol. Reprod. 2014;91:141–275. doi: 10.1095/biolreprod.114.122408. [DOI] [PubMed] [Google Scholar]
- 49.Canteras N.S., Simerly R.B., Swanson L.W. Organization of projections from the ventromedial nucleus of the hypothalamus: A Phaseolus vulgaris-Leucoagglutinin study in the rat. J. Comp. Neurol. 1994;348:41–79. doi: 10.1002/CNE.903480103. [DOI] [PubMed] [Google Scholar]
- 50.Pfaff D., Keiner M. Atlas of estradiol-concentrating cells in the central nervous system of the female rat. J. Comp. Neurol. 1973;151:121–158. doi: 10.1002/cne.901510204. [DOI] [PubMed] [Google Scholar]
- 51.Simerly R.B., Chang C., Muramatsu M., Swanson L.W. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: An in situ hybridization study. J. Comp. Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 52.Muschamp J.W., Hull E.M. Melanin concentrating hormone and estrogen receptor-alpha are coexstensive but not coexpressed in cells of male rat hypothalamus. Neurosci. Lett. 2007;427:123–126. doi: 10.1016/j.neulet.2007.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tritos N.A., Segal-Lieberman G., Vezeridis P.S., Maratos-Flier E. Estradiol-Induced Anorexia Is Independent of Leptin and Melanin-Concentrating Hormone. Obes. Res. 2004;12:716–724. doi: 10.1038/oby.2004.84. [DOI] [PubMed] [Google Scholar]
- 54.Sugimoto A., Tsuchida H., Ieda N., Ikegami K., Inoue N., Uenoyama Y., Tsukamura H. Somatostatin-Somatostatin Receptor 2 Signaling Mediates LH Pulse Suppression in Lactating Rats. Endocrinology. 2019;160:473–483. doi: 10.1210/en.2018-00882. [DOI] [PubMed] [Google Scholar]
- 55.Dufourny L., Delmas O., Teixeira-Gomes A.-P., Decourt C., Sliwowska J.H. Neuroanatomical connections between kisspeptin neurones and somatostatin neurones in the female and male rat hypothalamus: A possible involvement of SSTR1 in kisspeptin release. J. Neuroendocrinol. 2018;30 doi: 10.1111/jne.12593. [DOI] [PubMed] [Google Scholar]
- 56.Luque R.M., Cordoba-Chacon J., Pozo-Salas A.I., Porteiro B., De Lecea L., Nogueiras R., Gahete M.D., Castaño J.P. Obesity- and gender-dependent role of endogenous somatostatin and cortistatin in the regulation of endocrine and metabolic homeostasis in mice. Sci. Rep. 2016;6 doi: 10.1038/srep37992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krashes M.J., Koda S., Ye C., Rogan S.C., Adams A.C., Cusher D.S., Maratos-Flier E., Roth B.L., Lowell B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Invest. 2011;121:1424–1428. doi: 10.1172/JCI46229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang C.F., Chiang M.C., Gray D.C., Prabhakaran M., Alvarado M., Juntti S.A., Unger E.K., Wells J.A., Shah N.M. Sexually Dimorphic Neurons in the Ventromedial Hypothalamus Govern Mating in Both Sexes and Aggression in Males. Cell. 2013;153:896–909. doi: 10.1016/j.cell.2013.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handelsman D.J., Gibson E., Davis S., Golebiowski B., Walters K.A., Desai R. Ultrasensitive serum estradiol measurement by liquid chromatography-mass spectrometry in postmenopausal women and mice. J. Endocr. Soc. 2020;4 doi: 10.1210/jendso/bvaa086. bvaa086–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.GTEx Consortium. Thomas J., Salvatore M., Phillips R., Lo E., Shad S., Hasz R., Walters G., Garcia F., Young N., et al. The Genotype-Tissue Expression (GTEx) project. Nat. Genet. 2013;45:580–585. doi: 10.1038/ng.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Seldin M.M., Koplev S., Rajbhandari P., Vergnes L., Rosenberg G.M., Meng Y., Pan C., Phuong T.M.N., Gharakhanian R., Che N., et al. A Strategy for Discovery of Endocrine Interactions with Application to Whole-Body Metabolism. Cell Metabol. 2018;27:1138–1155. doi: 10.1016/J.CMET.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Velez L.M., Van C., Moore T., Zhou Z., Johnson C., Hevener A.L., Seldin M.M. Genetic variation of putative myokine signaling is dominated by biological sex and sex hormones. Elife. 2022;11 doi: 10.7554/eLife.76887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Langfelder P., Horvath S. WGCNA: An R package for weighted correlation network analysis. BMC Bioinf. 2008;9:559. doi: 10.1186/1471-2105-9-559/FIGURES/4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fontana R., Della Torre S. The Deep Correlation between Energy Metabolism and Reproduction: A View on the Effects of Nutrition for Women Fertility. Nutrients. 2016;8:87. doi: 10.3390/NU8020087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chehab F.F., Lim M.E., Lu R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996;12:318–320. doi: 10.1038/ng0396-318. [DOI] [PubMed] [Google Scholar]
- 66.Cheung C.C., Thornton J.E., Kuijper J.L., Weigle D.S., Clifton D.K., Steiner R.A. Leptin Is a Metabolic Gate for the Onset of Puberty in the Female Rat. Endocrinology. 1997;138:855–858. doi: 10.1210/ENDO.138.2.5054. [DOI] [PubMed] [Google Scholar]
- 67.Roa J., Tena-Sempere M. Connecting metabolism and reproduction: Roles of central energy sensors and key molecular mediators. Mol. Cell. Endocrinol. 2014;397:4–14. doi: 10.1016/J.MCE.2014.09.027. [DOI] [PubMed] [Google Scholar]
- 68.Rodriguez-Pacheco F., Martinez-Fuentes A.J., Tovar S., Pinilla L., Tena-Sempere M., Dieguez C., Castaño J.P., Malagon M.M. Regulation of Pituitary Cell Function by Adiponectin. Endocrinology. 2007;148:401–410. doi: 10.1210/EN.2006-1019. [DOI] [PubMed] [Google Scholar]
- 69.Yang Z.-H., Miyahara H., Takeo J., Katayama M. Diet high in fat and sucrose induces rapid onset of obesity-related metabolic syndrome partly through rapid response of genes involved in lipogenesis, insulin signalling and inflammation in mice. Diabetol. Metab. Syndr. 2012;4:32. doi: 10.1186/1758-5996-4-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mathew H., Castracane V.D., Mantzoros C. Adipose tissue and reproductive health. Metabolism. 2018;86:18–32. doi: 10.1016/J.METABOL.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 71.Tsatsanis C., Dermitzaki E., Avgoustinaki P., Malliaraki N., Mytaras V., Margioris A.N. The impact of adipose tissue-derived factors on the hypothalamic-pituitary-gonadal (HPG) axis. Hormones (Basel) 2015;14:549–562. doi: 10.14310/HORM.2002.1649. [DOI] [PubMed] [Google Scholar]
- 72.Nogueiras R., Gualillo O., Caminos J.E., Casanueva F.F., Diéguez C. Regulation of resistin by gonadal, thyroid hormone, and nutritional status. Obes. Res. 2003;11:408–414. doi: 10.1038/oby.2003.55. [DOI] [PubMed] [Google Scholar]
- 73.Gui Y., Silha J.V., Murphy L.J. Sexual dimorphism and regulation of resistin, adiponectin, and leptin expression in the mouse. Obes. Res. 2004;12:1481–1491. doi: 10.1038/oby.2004.185. [DOI] [PubMed] [Google Scholar]
- 74.Asarian L., Geary N. Cyclic Estradiol Treatment Normalizes Body Weight and Restores Physiological Patterns of Spontaneous Feeding and Sexual Receptivity in Ovariectomized Rats. Horm. Behav. 2002;42:461–471. doi: 10.1006/HBEH.2002.1835. [DOI] [PubMed] [Google Scholar]
- 75.Asarian L., Geary N. Sex differences in the physiology of eating. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013;305:R1215–R1267. doi: 10.1152/ajpregu.00446.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eckel L.A. The ovarian hormone estradiol plays a crucial role in the control of food intake in females. Physiol. Behav. 2011;104:517–524. doi: 10.1016/j.physbeh.2011.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Clegg D.J., Brown L.M., Zigman J.M., Kemp C.J., Strader A.D., Benoit S.C., Woods S.C., Mangiaracina M., Geary N. Estradiol-Dependent Decrease in the Orexigenic Potency of Ghrelin in Female Rats. Diabetes. 2007;56:1051–1058. doi: 10.2337/DB06-0015. [DOI] [PubMed] [Google Scholar]
- 78.Petersen S. The temporal pattern of feeding over the oestrous cycle of the mouse. Anim. Behav. 1976;24:939–955. doi: 10.1016/S0003-3472(76)80023-1. [DOI] [PubMed] [Google Scholar]
- 79.Geary N., Asarian L., Korach K.S., Pfaff D.W., Ogawa S. Deficits in E2-Dependent Control of Feeding, Weight Gain, and Cholecystokinin Satiation in ER-α Null Mice. Endocrinology. 2001;142:4751–4757. doi: 10.1210/endo.142.11.8504. [DOI] [PubMed] [Google Scholar]
- 80.Naaz A., Zakroczymski M., Heine P., Taylor J., Saunders P., Lubahn D., Cooke P.S. Effect of Ovariectomy on Adipose Tissue of Mice in the Absence of Estrogen Receptor Alpha (ERα): a Potential Role for Estrogen Receptor Beta (ERβ) Horm. Metab. Res. 2002;34:758–763. doi: 10.1055/s-2002-38259. [DOI] [PubMed] [Google Scholar]
- 81.Witte M.M., Resuehr D., Chandler A.R., Mehle A.K., Overton J.M. Female mice and rats exhibit species-specific metabolic and behavioral responses to ovariectomy. Gen. Comp. Endocrinol. 2010;166:520–528. doi: 10.1016/j.ygcen.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nishio E., Hayashi T., Nakatani M., Aida N., Suda R., Fujii T., Wakatsuki T., Honda S., Harada N., Shimono Y. Lack of association of ovariectomy-induced obesity with overeating and the reduction of physical activities. Biochem. Biophys. Rep. 2019;20 doi: 10.1016/j.bbrep.2019.100671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rogers N.H., Perfield J.W., Strissel K.J., Obin M.S., Greenberg A.S. Reduced Energy Expenditure and Increased Inflammation Are Early Events in the Development of Ovariectomy-Induced Obesity. Endocrinology. 2009;150:2161–2168. doi: 10.1210/en.2008-1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hevener A.L., Clegg D.J., Mauvais-Jarvis F. Impaired estrogen receptor action in the pathogenesis of the metabolic syndrome. Mol. Cell. Endocrinol. 2015;418:306–321. doi: 10.1016/J.MCE.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo L., Yao S., Kim D.-W., Cetin A., Harris J., Zeng H., Anderson D.J., Weissbourd B. Connectional architecture of a mouse hypothalamic circuit node controlling social behavior. Proc. Natl. Acad. Sci. USA. 2019;116:7503–7512. doi: 10.1073/pnas.1817503116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yokota-Nakagi N., Takahashi H., Kawakami M., Takamata A., Uchida Y., Morimoto K. Estradiol Replacement Improves High-Fat Diet-Induced Obesity by Suppressing the Action of Ghrelin in Ovariectomized Rats. Nutrients. 2020;12:907. doi: 10.3390/nu12040907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becker J.B., Chartoff E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology. 2019;44:166–183. doi: 10.1038/s41386-018-0125-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang Z., Divittorio J.R., Joseph A.M., Correa S.M. The Effects of Estrogens on Neural Circuits That Control Temperature. Endocrinology. 2021;162 doi: 10.1210/ENDOCR/BQAB087. bqab087–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Santollo J., Torregrossa A.-M., Eckel L.A. Estradiol acts in the medial preoptic area, arcuate nucleus, and dorsal raphe nucleus to reduce food intake in ovariectomized rats. Horm. Behav. 2011;60:86–93. doi: 10.1016/J.YHBEH.2011.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roepke T.A., Malyala A., Bosch M.A., Kelly M.J., Rønnekleiv O.K. Estrogen Regulation of Genes Important for K + Channel Signaling in the Arcuate Nucleus. Endocrinology. 2007;148:4937–4951. doi: 10.1210/en.2007-0605. [DOI] [PubMed] [Google Scholar]
- 91.Roepke T.A., Bosch M.A., Rick E.A., Lee B., Wagner E.J., Seidlova-Wuttke D., Wuttke W., Scanlan T.S., Rønnekleiv O.K., Kelly M.J. Contribution of a Membrane Estrogen Receptor to the Estrogenic Regulation of Body Temperature and Energy Homeostasis. Endocrinology. 2010;151:4926–4937. doi: 10.1210/en.2010-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Stincic T.L., Grachev P., Bosch M.A., Rønnekleiv O.K., Kelly M.J. Estradiol Drives the Anorexigenic Activity of Proopiomelanocortin Neurons in Female Mice. eNeuro. 2018;5 doi: 10.1523/ENEURO.0103-18.2018. ENEURO.0103, 18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Stincic T.L., Rønnekleiv O.K., Kelly M.J. Diverse actions of estradiol on anorexigenic and orexigenic hypothalamic arcuate neurons. Horm. Behav. 2018;104:146–155. doi: 10.1016/J.YHBEH.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Asarian L., Geary N. Modulation of appetite by gonadal steroid hormones. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006;361:1251–1263. doi: 10.1098/rstb.2006.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Maske C.B., Jackson C.M., Terrill S.J., Eckel L.A., Williams D.L. Estradiol modulates the anorexic response to central glucagon-like peptide 1. Horm. Behav. 2017;93:109–117. doi: 10.1016/j.yhbeh.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Milner T.A., Ayoola K., Drake C.T., Herrick S.P., Tabori N.E., McEwen B.S., Warrier S., Alves S.E. Ultrastructural localization of estrogen receptor β immunoreactivity in the rat hippocampal formation. J. Comp. Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 97.Caba M., Beyer C., González-Mariscal G., Morrell J.I. Immunocytochemical detection of estrogen receptor-alpha in the female rabbit forebrain: topography and regulation by estradiol. Neuroendocrinology. 2003;77:208–222. doi: 10.1159/000069508. [DOI] [PubMed] [Google Scholar]
- 98.Painson J.-C., Tannenbaum G.S. Sexual Dimorphism of Somatostatin and Growth Hormone-Releasing Factor Signaling in the Control of Pulsatile Growth Hormone Secretion in the Rat. Endocrinology. 1991;128:2858–2866. doi: 10.1210/endo-128-6-2858. [DOI] [PubMed] [Google Scholar]
- 99.Beranek L., Hajdu I., Gardi J., Taishi P., Obál F., Jr., Krueger J.M. Central administration of the somatostatin analog octreotide induces captopril-insensitive sleep responses. Am. J. Physiol. 1999;277:R1297–R1304. doi: 10.1152/ajpregu.1999.277.5.R1297. [DOI] [PubMed] [Google Scholar]
- 100.Lin M.T., Uang W.N., Ho L.T., Chuang J., Fan L.J. Somatostatin: a hypothalamic transmitter for thermoregulation in rats. Pflugers Arch. 1989;413:528–532. doi: 10.1007/BF00594185. [DOI] [PubMed] [Google Scholar]
- 101.Stengel A., Coskun T., Goebel M., Wang L., Craft L., Alsina-Fernandez J., Rivier J., Taché Y. Central Injection of the Stable Somatostatin Analog ODT8-SST Induces a Somatostatin 2 Receptor-Mediated Orexigenic Effect: Role of Neuropeptide Y and Opioid Signaling Pathways in Rats. Endocrinology. 2010;151:4224–4235. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Stengel A., Coskun T., Goebel-Stengel M., Craft L.S., Alsina-Fernandez J., Wang L., Rivier J., Taché Y. Chronic injection of pansomatostatin agonist ODT8-SST differentially modulates food intake and decreases body weight gain in lean and diet-induced obese rats. Regul. Pept. 2011;167:201–208. doi: 10.1016/J.REGPEP.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Krashes M.J., Shah B.P., Koda S., Lowell B.B. Rapid versus Delayed Stimulation of Feeding by the Endogenously Released AgRP Neuron Mediators GABA, NPY, and AgRP. Cell Metab. 2013;18:588–595. doi: 10.1016/J.CMET.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Brobeck J.R., Wheatland M., Strominger J.L. Variations in regulation of energy exchange associated with estrus, diestrus and pseudopregnancy in rats. Endocrinology. 1947;40:65–72. doi: 10.1210/endo-40-2-65. [DOI] [PubMed] [Google Scholar]
- 105.Sanchez-Alavez M., Alboni S., Conti B. Sex- and age-specific differences in core body temperature of C57Bl/6 mice. Age (Dordrecht, Netherlands) 2011;33:89–99. doi: 10.1007/s11357-010-9164-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Steiner M., Katz R.J., Carroll B.J., Katz R.J., Carroll B.J. Pergamon Press and Brain Research Publ; 1982. Detailed Analysis of Estrous-Related Changes in Wheel Running and Self-Stimulation. [DOI] [PubMed] [Google Scholar]
- 107.Kent S., Hurd M., Satinoff E. Interactions between body temperature and wheel running over the estrous cycle in rats. Physiol. Behav. 1991;49:1079–1084. doi: 10.1016/0031-9384(91)90334-K. [DOI] [PubMed] [Google Scholar]
- 108.Cora M.C., Kooistra L., Travlos G. Vaginal Cytology of the Laboratory Rat and Mouse:Review and Criteria for the Staging of the Estrous Cycle Using Stained Vaginal Smears. Toxicol. Pathol. 2015;43:776–793. doi: 10.1177/0192623315570339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Becker J.B., Arnold A.P., Berkley K.J., Blaustein J.D., Eckel L.A., Hampson E., Herman J.P., Marts S., Sadee W., Steiner M., et al. Strategies and Methods for Research on Sex Differences in Brain and Behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- 110.Tran T.T., Yamamoto Y., Gesta S., Kahn C.R. Beneficial Effects of Subcutaneous Fat Transplantation on Metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gavrilova O., Marcus-Samuels B., Graham D., Kim J.K., Shulman G.I., Castle A.L., Vinson C., Eckhaus M., Reitman M.L. Surgical Implantation of Adipose Reverses Diabetes Lipoatrophic Mice. J. Clin. Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Rooks C., Bennet T., Bartness T.J., Harris R.B.S. Compensation for an increase in body fat caused by donor transplants into mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004;286:1149–1155. doi: 10.1152/ajpregu.00634.2003.-Rodents. [DOI] [PubMed] [Google Scholar]
- 113.Liberzon A., Birger C., Thorvaldsdóttir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database Hallmark. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bray N., Pimentel H., Melsted P., Pachter L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016;34:525–527. doi: 10.1038/nbt.3519. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
ANOVA results and descriptive statistics, including sample sizes for each sex and treatment group, degrees of freedom, F statistics for each factor in the model, and exact p-values, for each experiment.
Data Availability Statement
-
•
RNA-seq data have been deposited at GEO and are publicly available as of the date of publication. The accession umber is listed in the key resources table. This paper also analyzes existing, publicly available data. Accession numbers for the datasets are listed in the key resources table. All data reported in this paper will be shared by the lead contact upon request.
-
•
All original code has been deposited at Github and is publicly available as of the date of publication. Github links are available in the key resources table.
-
•
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.