Abstract
Background and Objectives
An increased risk of cardiovascular and metabolic diseases (CVMDs) among patients with cancer suggests a potential link between CVMD and cancer. The impact of CVMD on the survival time of patients with esophageal and gastric cancer remains unknown. We aimed to determine the incidence of CVMD and its impact on the longterm outcomes in esophageal and gastric cancer patients.
Methods
A total of 2074 cancer patients were enrolled from January 1, 2007 to December 31, 2017 in two hospitals, including 1205 cases of esophageal cancer and 869 cases of gastric cancer, who were followed up for a median of 79.8 and 79.3 months, respectively. Survival time was analyzed using the Kaplan–Meier method before and after propensity score matching.
Results
The incidence of CVMD in patients with esophageal and gastric cancer was 34.1% (411/1205) and 34.3% (298/869), respectively. The effects of hypertension, diabetes, and stroke on the long-term survival of esophageal and gastric cancer patients were not significant (all P > 0.05). The survival time was significantly longer in esophageal cancer patients without ischemic heart disease than in patients with ischemic heart disease, both before matching (36.5 vs. 29.1 months, P = 0.027) and after matching (37.4 vs. 27.9 months, P = 0.011). The survival time in gastric cancer patients without ischemic heart disease was significantly longer than in patients with ischemic heart disease, both before (28.4 vs.17.5 months, P = 0.032) and after matching (29.5 vs.17.5 months, P = 0.02).
Conclusion
The survival time of esophageal and gastric cancer patients with ischemic heart disease was significantly reduced compared to that of esophageal and gastric cancer patients without ischemic heart disease.
Key words: long-term outcome, gastric cancer, esophageal cancer, cardiovascular and metabolic diseases, propensity score matching
Introduction
Cardiovascular and metabolic diseases (CVMDs) and cancer are the top two causes of death worldwide.[1, 2, 3, 4] There is growing concern regarding the impact of CVMDs on the long-term prognosis of cancer patients, as the incidence of CVMDs has been reported to be increasing in cancer patients.[5, 6, 7] In 2020, esophageal and gastric cancer-related deaths accounted for more than one-tenth of cancer-related deaths, becoming the sixth and third leading causes of cancer-related deaths worldwide, respectively.[8,9] Previous studies have revealed that the risk of CVMDs is higher in patients with cancer (including breast cancer, Hodgkin lymphoma, leukemia, non-Hodgkin lymphoma, and lung cancer) than in noncancerous controls.[10, 11, 12] A recent report suggested that 18.0% of cancer patients had at least one type of CVMD, and the most common CVMD among all cancer patients was hypertension, followed by diabetes, stroke, and ischemic heart disease.[13] Although cumulative studies have revealed an increased risk of CVMDs in cancer patients, the impact of CVMDs on the survival time of cancer patients remains unclear. More evidence is needed to clarify the association between CVMDs and survival time in cancer patients, as comorbidity with cancer and CVMDs are usually excluded from clinical trials and most studies have focused on cancer-specific prognoses. Both tumors and CVMDs may influence the overall survival (OS) time of patients with cancer. Thus, it is desirable to collect and analyze the detailed clinical factors in esophageal and gastric cancer patients with CVMDs and to determine the impact of CVMDs on the long-term outcomes of these patients. To this end, clinical information on esophageal and gastric cancers from two hospitals in Xinxiang, a high-incidence area of esophageal and gastric cancer, was collected and analyzed using a pseudo-randomized method with propensity score matching.
Methods
Patients and study design
This cohort included patients diagnosed with esophageal and gastric cancer at the First Affiliated Hospital of Xinxiang Medical College and the Third Affiliated Hospital of Xinxiang Medical College, China. Data were collected retrospectively from January 1, 2007 to December 31, 2017. The inclusion criteria were as follows: (1) newly diagnosed primary esophageal and gastric cancers, (2) available detailed diagnostic and treatment records in these hospitals, (3) age > 20 years, and (4) no history of other malignancies. The exclusion criteria were as follows: (1) lack of follow-up data, (2) unknown therapeutics, and (3) incomplete clinical information. The protocol was approved by the institutional review boards of the participating hospitals. All procedures were performed in accordance with the Declaration of Helsinki. Informed written consent was obtained from all patients before inclusion in the study.
Data collection
The esophageal and gastric cancer cohorts were followed up. Patient information included age, sex, smoking history, drinking history, family history of tumors, relapse, lymph node metastasis at diagnosis, distant metastasis at diagnosis, tumor stage, tumor differentiation, treatment regimen, and survival time. The tumor stage was determined according to the 8th American Joint Committee on Cancer classification system.[14] Detailed information on CVMDs of patients was also collected, including hypertension, diabetes, ischemic heart disease, and stroke. The International Classification of Diseases standard ICD-10 was applied to these four diseases.
Follow-up
The follow-up data were based on medical records from these hospitals and phone call interviews. Follow-up was conducted once a year and ended in December 2021. OS was calculated from the time of diagnosis to the date of death or end of the follow-up.
Statistical analysis
We divided all cancer patients into two groups: those with and without CVMD. Statistical analysis was performed using the chi-squared test or Fisher’s exact test for categorical variables. To better determine the impact of CVMDs on cancer patient survival and reduce the interference of other factors, we performed a propensity score matching analysis between the two groups. Propensity score matching was performed using a 1:1 nearest-neighbor matching algorithm with a 0.02 caliper to minimize selection bias. A logistic regression propensity score model for the presence of CVMD was created, and the following variables were included in the propensity score model: age, sex, smoking history, drinking history, family tumor history, relapse, lymph node metastasis at diagnosis, distant metastasis at diagnosis, tumor stage, tumor differentiation, and treatment regimen. Absolute standardized differences (ASDs) of covariates before and after matching were calculated to evaluate propensity score matching effectiveness. Covariables were considered well balanced if the ASD was < 10%.[15, 16, 17] The Kaplan–Meier method was used to estimate the OS between the two groups, and the statistical significance of differences was assessed using the log-rank test. P < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 15.1 (Stata Corp., College Station, TX, USA) and Statistical Package for the Social Sciences (SPSS) 26.0 (IBM Corp., Armonk, NY, USA).
Results
Baseline and clinicopathological characteristics
Based on the inclusion criteria, 1352 patients with esophageal cancer and 935 patients with gastric cancer were enrolled in the study. Among these, 147 esophageal cancer patients and 66 gastric cancer patients were excluded due to a lack of follow-up data, unknown treatment regimens, or incomplete clinicopathological data, resulting in 1205 esophageal cancer patients and 869 gastric cancer patients eligible for this study (Figure 1). All patients with esophageal and gastric cancer were divided into groups: those with and without CVMD. Patients in the groups with CVMD were matched individually to those in the groups without CVMD using propensity scores. Consequently, 404 pairs of matched esophageal cancer patients and 264 pairs of matched gastric cancer patients were created.
Figure 1.
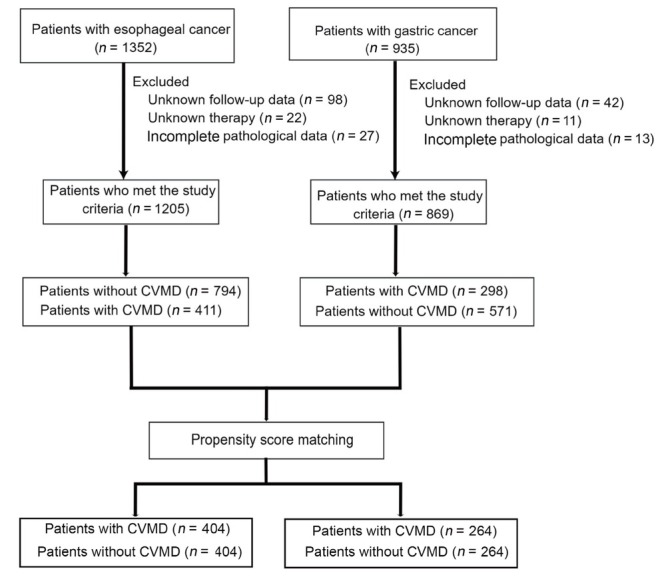
Diagram of the study design. CVMDs: cardiovascular and metabolic diseases.
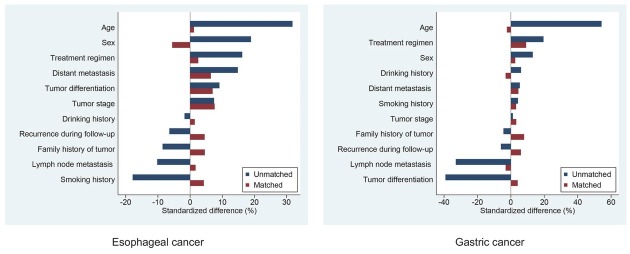
The baseline characteristics of the overall and propensity score-matched esophageal cancer patient cohorts are shown in Table 1. Of these patients, 733 (60.8%) were men, 343 (28.5%) were aged < 60 years, 680 (56.4%) were aged 60–75 years, and 182 (15.1%) were aged > 75 years. Sex, age, smoking history, distant metastasis, and treatment regimens were significantly different between the two groups before matching. No significant differences in baseline characteristics were found among the 404 matched pairs of patients with esophageal cancer. In 404 pairs of propensity score-matched patients, the standardized differences for all covariates were < 10%, suggesting a substantial balance between the two groups (Figure 2).
Table 1.
Baseline characteristics of the overall and propensity score-matched esophageal cancer patient cohorts
| Characteristics |
Before matching (n = 1205)
|
After matching (n = 808)
|
||||
|---|---|---|---|---|---|---|
| Patients without CVMD (n = 794) | Patients with CVMD (n = 411) | P value | Patients without CVMD (n = 404) | Patients with CVMD (n = 404) | P value | |
| Sex | 0.002 | 0.437 | ||||
| Male | 508 (64.0) | 225 (54.7) | 214 (53.0) | 225 (55.7) | ||
| Female | 286 (36.0) | 186 (45.3) | 190 (47.0) | 179 (44.3) | ||
| Age | < 0.001 | 0.471 | ||||
| ≤ 60 years | 263 (33.1) | 80 (19.5) | 90 (22.3) | 80 (19.8) | ||
| 61–74 years | 429 (54.0) | 251 (61.0) | 234 (57.9) | 251 (62.1) | ||
| ≥ 75 years | 102 (12.9) | 80 (19.5) | 80 (19.8) | 73 (18.1) | ||
| Smoking history | 0.004 | 0.526 | ||||
| No | 510 (64.2) | 298 (72.5) | 299 (74.0) | 291 (72.0) | ||
| Yes | 284 (35.8) | 113 (27.5) | 105 (26.0) | 113 (28.0) | ||
| Drinking history | 0.775 | 0.836 | ||||
| No | 683 (86.0) | 356 (86.6) | 351 (86.9) | 349 (86.4) | ||
| Yes | 111 (14.0) | 55 (13.4) | 53 (13.1) | 55 (13.6) | ||
| Family history of tumor | 0.162 | 0.504 | ||||
| No | 577 (72.7) | 314 (76.4) | 315 (78.0) | 307 (76.0) | ||
| Yes | 217 (27.3) | 97 (23.6) | 89 (22.0) | 97 (24.0) | ||
| Recurrence during follow-up | 0.296 | 0.485 | ||||
| No | 722 (90.9) | 381 (92.7) | 379 (93.8) | 374 (92.6) | ||
| Yes | 72 (9.1) | 30 (7.3) | 25 (6.2) | 30 (7.4) | ||
| Lymph node metastasis | 0.096 | 0.806 | ||||
| No | 565 (71.2) | 311 (75.7) | 307 (76.0) | 304 (75.2) | ||
| Yes | 229 (28.8) | 100 (24.3) | 97 (24.0) | 100 (24.8) | ||
| Distant metastasis | 0.013 | 0.37 | ||||
| No | 673 (84.8) | 325 (79.1) | 332 (82.2) | 322 (79.7) | ||
| Yes | 121 (15.2) | 86 (20.9) | 72 (17.8) | 82 (20.3) | ||
| Tumor stage | 0.058 | 0.769 | ||||
| I | 338 (42.6) | 176 (42.8) | 185 (45.8) | 173 (42.8) | ||
| II | 200 (25.2) | 90 (21.9) | 91 (22.5) | 90 (22.3) | ||
| III | 135 (17.0) | 59 (14.4) | 56 (13.9) | 59 (14.6) | ||
| IV | 121 (15.2) | 86 (20.9) | 72 (17.8) | 82 (20.3) | ||
| Tumor differentiation | 0.267 | 0.606 | ||||
| Well differentiated | 379 (47.7) | 176 (42.8) | 189 (46.8) | 176 (43.6) | ||
| Moderately differentiated | 281 (35.4) | 158 (38.5) | 149 (36.9) | 154 (38.1) | ||
| Poorly differentiated | 134 (16.9) | 77 (18.7) | 66 (16.3) | 74 (18.3) | ||
| Treatment regimen | 0.008 | 0.723 | ||||
| Without chemo-radiotherapy | 492 (62.0) | 222 (54.0) | 227 (56.2) | 222 (55.0) | ||
| With chemo-radiotherapy | 302 (38.0) | 189 (46.0) | 177 (43.8) | 182 (45.0) |
Data are presented as n (%). CVMDs: cardiovascular and metabolic diseases.
Figure 2.
Standardized differences in baseline characteristics between cancer patients with and without CVMD, before and after propensity score matching. CVMDs: cardiovascular and metabolic diseases.
Table 2 shows the baseline characteristics of the patients with gastric cancer before and after propensity score matching. Of the patients with gastric cancer, 651 (74.9%) were men, 371 (42.7%) were aged < 60 years, 399 (45.9%) were aged 60–75 years, and 99 (11.4%) were aged > 75 years. Age, lymph node metastasis, tumor differentiation, and treatment regimens were significantly different between the two groups before matching. None of the factors were significantly different among the 264 matched pairs of gastric cancer patients. After matching, the standardized differences for all measured covariates were < 10%, suggesting a good balance between the two groups (Figure 2).
Table 2.
Baseline characteristics of the overall and propensity score-matched gastric cancer patient cohorts
| Characteristics |
Before matching (n = 869)
|
After matching (n = 528)
|
||||
|---|---|---|---|---|---|---|
| Patients without CVMD (n = 571) | Patients with CVMD (n = 298) | P value | Patients without CVMD (n = 264) | Patients with CVMD (n = 264) | P value | |
| Sex | 0.064 | 0.768 | ||||
| Male | 439 (76.9) | 212 (71.1) | 195 (73.9) | 192 (72.7) | ||
| Female | 132 (23.1) | 86 (28.9) | 69 (26.1) | 72 (27.3) | ||
| Age | < 0.001 | 0.073 | ||||
| ≤ 60 years | 298 (52.2) | 73 (24.5) | 83 (31.4) | 73 (27.7) | ||
| 61–74 years | 222 (38.9) | 177 (59.4) | 137 (51.9) | 161 (61.0) | ||
| ≥ 75 years | 51 (8.9) | 48 (16.1) | 44 (16.7) | 30 (11.3) | ||
| Smoking history | 0.55 | 0.719 | ||||
| No | 370 (64.8) | 187 (62.8) | 168 (63.6) | 164 (62.1) | ||
| Yes | 201 (35.2) | 111 (37.2) | 96 (36.4) | 100 (37.9) | ||
| Drinking history | 0.391 | 0.727 | ||||
| No | 486 (85.1) | 247 (82.9) | 218 (82.6) | 221 (83.7) | ||
| Yes | 85 (14.9) | 51 (17.1) | 46 (17.4) | 43 (16.3) | ||
| Family history of tumor | 0.537 | 0.349 | ||||
| No | 426 (74.6) | 228 (76.5) | 209 (79.2) | 200 (75.8) | ||
| Yes | 145 (25.4) | 70 (23.5) | 55 (20.8) | 64 (24.2) | ||
| Recurrence during follow-up | 0.414 | 0.452 | ||||
| No | 528 (92.5) | 280 (94.0) | 251 (95.1) | 247 (93.6) | ||
| Yes | 43 (7.5) | 18 (6.0) | 13 (4.9) | 17 (6.4) | ||
| Lymph node metastasis | < 0.001 | 0.725 | ||||
| No | 258 (45.2) | 183 (61.4) | 150 (56.8) | 154 (58.3) | ||
| Yes | 313 (54.8) | 115 (38.6) | 114 (43.2) | 110 (41.7) | ||
| Distant metastasis | 0.44 | 0.559 | ||||
| No | 557 (97.5) | 288 (96.6) | 259 (98.1) | 257 (97.3) | ||
| Yes | 14 (2.5) | 10 (3.4) | 5 (1.9) | 7 (2.7) | ||
| Tumor stage | 0.279 | 0.233 | ||||
| I | 120 (21) | 72 (24.1) | 56 (21.2) | 65 (24.6) | ||
| II | 230 (40.2) | 101 (33.9) | 118 (44.7) | 95 (36.0) | ||
| III | 207 (36.3) | 115 (38.6) | 85 (32.2) | 97 (36.7) | ||
| IV | 14 (2.5) | 10 (3.4) | 5 (1.9) | 7 (2.7) | ||
| Tumor differentiation | < 0.001 | 0.838 | ||||
| Well differentiated | 66 (11.6) | 53 (17.8) | 45 (17.1) | 40 (15.1) | ||
| Moderately differentiated | 267 (46.8) | 178 (59.7) | 154 (58.3) | 157 (59.5) | ||
| Poorly differentiated | 238 (41.6) | 67 (22.5) | 65 (24.6) | 67 (25.4) | ||
| Treatment regimen | 0.005 | 0.302 | ||||
| Without chemo-radiotherapy | 466 (81.6) | 219 (73.5) | 208 (78.8) | 198 (75.0) | ||
| With chemo-radiotherapy | 105 (18.4) | 79 (26.5) | 56 (21.2) | 66 (25.0) |
Data are presented as n (%). CVMDs: cardiovascular and metabolic diseases.
OS in esophageal and gastric cancer patients with and without CVMD
For esophageal cancer patients, the 5-year OS rate was 39% before matching, during a median follow-up period of 79.8 (interquartile range [IQR] 69.9–91.4) months. In addition, the 5-year OS rates in the groups with and without CVMD were 38% and 40%, respectively. For gastric cancer patients, the 5-year OS rate was 43% before propensity score matching, during a median follow-up period of 79.3 (IQR 67.6–92.5) months. The 5-year OS rates in the groups with and without CVMD were 40% and 45%, respectively.
In esophageal cancer patients, the median OS of groups without and with CVMD was 37.2 and 33.8 months, respectively, before matching (P = 0.41) and 39.9 and 33.8 months, respectively, after matching (P = 0.46). OS was not significantly different between the groups without CVMD and with CVMD before and after matching (Supplementary Figure 1). In the groups of gastric cancer patients without and with CVMD, the median OS was 27.8 and 27.4 months, respectively, before matching (P = 0.62) and 28.7 and 27.9 months, respectively, after matching (P = 0.57). The survival times of the two groups were not significantly different (Supplementary Figure 1).
Characteristics of CVMDs in esophageal and gastric cancer patients
The constitution of CVMDs in patients with esophageal and gastric cancer is shown in Supplementary Table 1. Common CVMDs included ischemic heart disease, hypertension, diabetes, and stroke. The prevalence of CVMDs in patients with esophageal cancer was 34.1% (411/1205), among which the prevalence of hypertension, diabetes, ischemic heart disease, and stroke was 22.7% (273/1205), 8.1% (98/1205), 8% (96/1205), and 6.9% (83/1205), respectively. The incidence of one, two, and multiple CVMDs in patients with esophageal cancer was 24.3% (293/1205), 8.2% (99/1205), and 1.6% (19/1205), respectively. The incidence was 34.3% (298/869) in gastric cancer patients, among which the prevalence of hypertension, diabetes, ischemic heart disease, and stroke was 24.5% (213/869), 6.8% (59/869), 7.2% (63/869), and 7.5% (65/869), respectively. The incidence for gastric cancer patients with one, two, and multiple CVMDs was 24.7% (215/869), 7.5% (65/869), and 2.1% (18/869), respectively. The median OS was 37.2 versus 36 versus 30.3 versus 32.4 months (P = 0.052) before matching and 39.9 versus 35.7 versus 30 versus 33.3 months (P = 0.081) after matching in esophageal cancer patients without and with one, two, or multiple subtypes of CVMDs, respectively (Supplementary Figure 2). OS was not significantly different between the groups. Similar procedures were performed for patients with gastric cancer with and without CVMD. The median OS was 27.8 versus 26.8 versus 27.9 versus 28.3 months (P = 0.74) before matching and 28.7 versus 27.7 versus 34.2 versus 28.2 months (P = 0.81) after matching in gastric cancer patients without and with one, two, or multiple subtypes of CVMDs, respectively (Supplementary Figure 2). The survival time was not significantly different between the various groups.
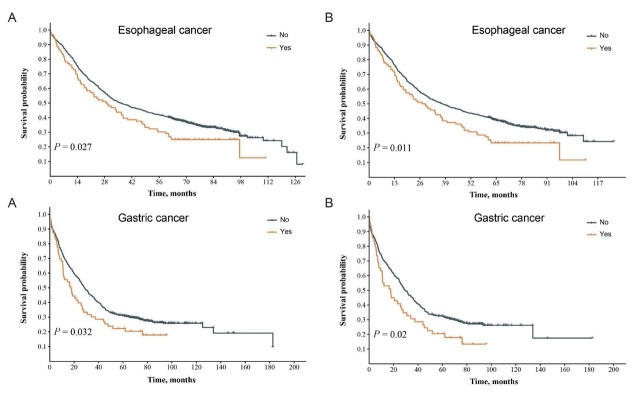
All patients with esophageal and gastric cancer were further grouped according to the subtype of CVMD, including hypertension, diabetes, ischemic heart disease, and stroke. Before propensity score matching, the median OS was 36.5 and 29.1 months in esophageal cancer patients without and with ischemic heart disease (P = 0.027), respectively. After matching, the median OS was 37.4 and 27.9 months in esophageal cancer patients without and with ischemic heart disease (P = 0.011), respectively. In gastric cancer, the median OS was 28.4 and 17.5 months in patients without and with ischemic heart disease (P = 0.032) before matching, respectively. After matching, the median OS was 29.5 and 17.5 months in gastric cancer patients without and with ischemic heart disease, respectively, showing a significant difference (P = 0.02). The above results demonstrated that ischemic heart disease significantly reduced the OS in both esophageal and gastric cancer patients (Figure 3).
Figure 3.
Survival time of esophageal and gastric cancer patients with or without ischemic heart disease. Kaplan–Meier survival plots of cancer patients with or without ischemic heart disease before (A) and after matching (B). P values were calculated using the log-rank test. “No” refers to the group of cancer patients without ischemic heart disease, and “Yes” refers to the group of cancer patients with ischemic heart disease.
The median OS was 32.4 months in esophageal cancer patients with hypertension and 37.2 months in esophageal cancer patients without hypertension (P = 0.53) before matching. After matching, the median OS was 33 months in esophageal cancer patients with hypertension and 37.7 months in patients without hypertension (P = 0.62). Similar results were obtained for gastric cancer patients using the same method. The median OS was 27.9 and 27.1 months in patients with and without hypertension, respectively, before matching (P = 0.73). After matching, the median OS was 28.7 and 27.4 months in patients with and without hypertension, respectively (P = 0.87). These results suggest that hypertension does not affect the OS of patients with esophageal and gastric cancer (Supplementary Figure 3).
In esophageal cancer patients with and without diabetes, the median OS was 33.9 and 35.4 months, respectively, before matching (P = 0.4), and after matching, the median OS was 36.5 and 35.5 months, respectively (P = 0.64). In gastric cancer patients with and without diabetes, the median OS was 28.4 and 27.6 months before matching, respectively (P = 0.88). The median OS was 28.2 and 28.4 months in patients with and without diabetes, respectively, after matching (P = 0.47). No impact of diabetes on OS was found in patients with esophageal and gastric cancer (Supplementary Figure 4).
In esophageal cancer patients with and without stroke, the median OS was 34.1 and 35.3 months, respectively, before matching (P = 0.36) and 36.8 and 35.5 months, respectively, after matching (P = 0.55). In gastric cancer patients with and without stroke, the median OS was 36.2 and 27.1 months, respectively, before matching (P = 0.16) and 36.3 and 27.6 months, respectively, after matching (P = 0.097). There was no significant difference in the OS of esophageal and gastric cancer patients with and without stroke before and after matching (Supplementary Figure 5).
Risk factors of esophageal and gastric cancer patients with ischemic heart disease, hypertension, diabetes, or stroke
A significant association was found between age, distant metastasis, tumor stage, and chemoradiotherapy and esophageal cancer patients with ischemic heart disease (all P < 0.05, Table 3). No significant association was found between sex, smoking, drinking, family history of tumor, recurrence, lymph node metastasis, or tumor differentiation and esophageal cancer patients with ischemic heart disease (all P > 0.05, Table 3).
Table 3.
Association between clinical features and ischemic heart disease in esophageal cancer patients
| Characteristics | Patients without ischemic heart disease (n = 1109) | Patients with ischemic heart disease (n = 96) | P value |
|---|---|---|---|
| Sex | 0.761 | ||
| Male | 676 (61.0) | 57 (59.4) | |
| Female | 433 (39.0) | 39 (40.6) | |
| Age | < 0.001 | ||
| ≤ 60 years | 334 (30.1) | 9 (9.4) | |
| 61–74 years | 626 (56.5) | 54 (56.2) | |
| ≥ 75 years | 149 (13.4) | 33 (34.4) | |
| Smoking history | 0.887 | ||
| No | 743 (67.0) | 65 (67.7) | |
| Yes | 366 (33.0) | 31 (32.3) | |
| Drinking history | 0.192 | ||
| No | 952 (85.8) | 87 (90.6) | |
| Yes | 157 (14.2) | 9 (9.4) | |
| Family history of tumor | 0.465 | ||
| No | 817 (73.7) | 74 (77.1) | |
| Yes | 292 (26.3) | 22 (22.9) | |
| Recurrence during follow-up | 0.667 | ||
| No | 1014 (91.4) | 89 (92.7) | |
| Yes | 95 (8.6) | 7 (7.3) | |
| Lymph node metastasis | 0.138 | ||
| No | 800 (72.1) | 76 (79.2) | |
| Yes | 309 (27.9) | 20 (20.8) | |
| Distant metastasis | 0.003 | ||
| No | 929 (83.8) | 69 (71.9) | |
| Yes | 180 (16.2) | 27 (28.1) | |
| Tumor stage | 0.004 | ||
| I | 473 (42.6) | 41 (42.7) | |
| II | 278 (25.1) | 12 (12.5) | |
| III | 178 (16.1) | 16 (16.7) | |
| IV | 180 (16.2) | 27 (28.1) | |
| Tumor differentiation | 0.214 | ||
| Well differentiated | 513 (46.2) | 42 (43.8) | |
| Moderately differentiated | 408 (36.8) | 31 (32.2) | |
| Poorly differentiated | 188 (17.0) | 23 (24.0) | |
| Treatment regimen | 0.001 | ||
| Without chemo-radiotherapy | 681 (61.4) | 33 (34.4) | |
| With chemo-radiotherapy | 428 (38.6) | 63 (65.6) |
Data are presented as n (%).
A significant association was found between sex, age, lymph node metastasis, tumor differentiation, and chemoradiotherapy and gastric cancer patients with ischemic heart disease (all P < 0.05, Table 4). No significant association was found between smoking, drinking, family history of tumor, recurrence, distant metastasis, or tumor stage and gastric cancer patients with ischemic heart disease (all P > 0.05, Table 4). These results suggest that both esophageal and gastric cancer patients with ischemic heart disease share the risk factors of age and chemoradiotherapy.
Table 4.
Association between clinical features and ischemic heart disease in gastric cancer patients
| Characteristics | Patients without ischemic heart disease (n = 806) | Patients with ischemic heart disease (n = 63) | P value |
|---|---|---|---|
| Sex | 0.006 | ||
| Male | 613 (76.1) | 38 (60.3) | |
| Female | 193 (23.9) | 25 (39.7) | |
| Age | < 0.001 | ||
| ≤ 60 years | 359 (44.5) | 12 (19.0) | |
| 61–74 years | 364 (45.2) | 35 (55.6) | |
| ≥ 75 years | 83 (10.3) | 16 (25.4) | |
| Smoking history | 0.659 | ||
| No | 515 (63.9) | 42 (66.7) | |
| Yes | 291 (36.1) | 21 (33.3) | |
| Drinking history | 0.960 | ||
| No | 680 (84.4) | 53 (84.1) | |
| Yes | 126 (15.6) | 10 (15.9) | |
| Family history of tumor | 0.630 | ||
| No | 605 (75.1) | 49 (77.8) | |
| Yes | 201 (24.9) | 14 (22.2) | |
| Recurrence during follow-up | 0.613 | ||
| No | 748 (92.8) | 60 (95.2) | |
| Yes | 58 (7.2) | 3 (4.8) | |
| Lymph node metastasis | 0.018 | ||
| No | 400 (49.6) | 41 (65.1) | |
| Yes | 406 (50.4) | 22 (34.9) | |
| Distant metastasis | 0.249 | ||
| No | 782 (96.8) | 63 (100) | |
| Yes | 24 (3.0) | 0 | |
| Tumor stage | 0.282 | ||
| I | 180 (22.3) | 12 (19.1) | |
| II | 309 (38.3) | 22 (34.9) | |
| III | 293 (36.4) | 29 (46.0) | |
| IV | 24 (3.0) | 0 | |
| Tumor differentiation | 0.002 | ||
| Well differentiated | 102 (12.7) | 17 (27.0) | |
| Moderately differentiated | 412 (51.1) | 33 (52.4) | |
| Poorly differentiated | 292 (36.2) | 13 (20.6) | |
| Treatment regimen | 0.001 | ||
| Without chemo-radiotherapy | 647 (80.3) | 38 (60.3) | |
| With chemo-radiotherapy | 159 (19.7) | 25 (39.7) |
Data are presented as n (%).
A significant association was found between sex, age, and smoking and esophageal cancer patients with hypertension (all P < 0.05, Supplementary Table 2). No significant association was found between other clinical factors and esophageal cancer patients with hypertension (all P > 0.05, Supplementary Table 2). The association was found to be significant between age, lymph node metastasis, and tumor differentiation and gastric cancer patients with hypertension (all P < 0.05, Supplementary Table 3). No significant association was found between other clinical factors and gastric cancer patients with hypertension (all P > 0.05, Supplementary Table 3). These results demonstrated that age was the only risk factor for both esophageal and gastric cancer patients with hypertension.
Smoking was the only factor significantly associated with esophageal cancer patients with diabetes (P < 0.05, Supplementary Table 4). No significant association was found between other clinical factors and esophageal cancer patients with diabetes (P > 0.05, Supplementary Table 4). Sex and age were significantly associated with gastric cancer patients with diabetes (P < 0.05, Supplementary Table 5). No significant association was found between other clinical factors and gastric cancer patients with diabetes (P > 0.05, Supplementary Table 5). No shared associated clinical factors were found between esophageal cancer and gastric cancer patients with diabetes.
A significant association was found between age, distant metastasis, and tumor stage and esophageal cancer patients with stroke (all P < 0.05, Supplementary Table 6). No significant association was found between other clinical factors and esophageal cancer patients with stroke (all P > 0.05; Supplementary Table 6). The association was found to be significant between age, tumor differentiation, and chemoradiotherapy and gastric cancer patients with stroke (all P < 0.05, Supplementary Table 7). No significant association was found between other clinical factors and gastric cancer patients with stroke (all P > 0.05; Supplementary Table 7). These results suggest that age is the only factor related to both esophageal and gastric cancer patients with stroke.
Discussion
Metabolic syndrome is a cluster of morbidities including central obesity, insulin resistance, and dyslipidemia. It has been shown to increase the risk of cancer-related death and CVMDs.[18, 19, 20] Accumulating evidence suggests a link between cancer and CVMDs, in addition to shared risk factors such as smoking, obesity, age, and inflammation.[21,22] Cancer and related therapeutics may increase the risk of thrombosis and adverse cardiovascular events in this population.[23, 24, 25] Thus, the risk factors for CVMDs are different in the general population and in patients with cancer. Other reports have suggested that CVMDs may increase the risk of cancer.[22,26,27] As CVMD has been excluded from cancer studies in most clinical trials, the association between CVMDs and different cancers remains unclear.
In this study, a total of 2074 patients with esophageal and gastric cancers were included, among which 34.1% (411/1205) of esophageal cancer patients and 34.3% (298/869) of gastric cancer patients had comorbidity with CVMDs, suggesting a heavy burden of CVMDs in the cohort of esophageal and gastric cancer patients. The results demonstrated that the OS was significantly shorter in both esophageal and gastric cancer patients with ischemic heart disease than in those without ischemic heart disease. These results indicate that it is necessary to prevent and treat ischemic heart disease during cancer treatment. The OS was not significantly different in esophageal and gastric cancer patients with or without hypertension, diabetes, and stroke. In this study, patients were followed up for a median of 79.8 and 79.3 months for esophageal and gastric cancers, respectively. Increasing the length of follow-up may lead to different conclusion for cancer patients with comorbidities of hypertension, stroke, or diabetes.
Further analysis of the association of clinical factors with esophageal and gastric cancer patients with ischemic heart disease, hypertension, diabetes, and stroke is required. An association was frequently found between each clinical factor and esophageal or gastric cancer patients with different diseases, including ischemic heart disease, hypertension, diabetes, and stroke; however, shared risk factors were seldom found in both esophageal and gastric cancer patients with other diseases. Our results demonstrated that old age and chemoradiotherapy are shared risk factors for both esophageal and gastric cancer patients with ischemic heart disease. Many studies have confirmed that chemoradiotherapy causes ischemic heart disease, and the incidence of ischemic heart disease increases with age. Age is the only shared risk factor for both esophageal and gastric cancer patients with hypertension or stroke. No shared risk factors were found for esophageal and gastric cancer patients with diabetes. Mortality risk was different in cancer patients with CVMD and in those with CVMD among the general population. Cancer itself and its treatment may increase the risk of mortality in patients with CVMD. The etiology and mechanisms of CVMD and cancer are complex. Similar risk factors, including smoking, obesity, and age, have been frequently reported in cancer patients and patients with atrial fibrillation, atherosclerosis, stroke, myocardial infarction, and hypertension. Recent evidence suggests that inflammation is the mutual pathophysiologic link between atherothrombosis and cancer; atherosclerosis and cancer may influence each other during progression.[21,28] An increased risk of both cancer and cardiovascular disease was found in rheumatoid arthritis, which is a high-level inflammatory disease, and anti-inflammatory therapies have shown promise in reducing cardiovascular events and cancer risk.[29,30] Canakinumab, an interleukin (IL)-1β inhibitor, significantly reduced cardiovascular-related death.[31,32] IL-1 mediates tumor angiogenesis, metastasis, and immune evasion.[33,34] The application of IL-6 and IL-1 antagonists increased the survival time of cancer patients in clinical trials[21] and reduced lung cancer mortality.[35] Inflammation may also lead to atherosclerosis and cancer via the production of reactive oxygen species.[36]
Cancer itself may increase the risk of thrombotic events, both arterial and venous.[23] The incidence of ischemic cardiovascular events varies among different types of cancers,[37] and treatment with various regimens may alter the thrombotic risk in different cancers.[21] Radiation may directly increase the risk of ischemic heart disease.[38, 39, 40] Cytotoxic drugs also cause cardiotoxicity and vascular toxicity, increasing atherosclerotic risk by damaging endothelial cells and plaque erosion.[41] Immune checkpoint inhibitors are novel therapeutic agents that have been reported to increase the risk of vascular events;[42] recent evidence suggests that immune checkpoint inhibitors increase the risk of cardiovascular events by threefold.[43]
In summary, it is reasonable to deduce that cancer itself, radiotherapy, and chemotherapy may cause inflammation, atherosclerosis, DNA damage, and cardiovascular thrombotic events, which generate or promote ischemic heart disease and death. Our results suggest that both esophageal and gastric cancer patients with ischemic heart disease have significantly reduced OS compared to those without ischemic heart disease. It is important to prevent and manage ischemic heart disease during the treatment of patients with esophageal and gastric cancer.
The limitations of this retrospective study were inevitable. For some patients, it was difficult to determine the specific cause of death without advanced laboratory testing. In addition, data on the potential confounding factors, including obesity, body mass index, and tumor location, were lacking. To further understand the interaction between cancer and CVMD, a large-scale, multicenter, prospective study is needed.
In conclusion, ischemic heart disease significantly reduced the survival time of esophageal and gastric cancer patients in Xinxiang, a high-incidence area of esophageal and gastric cancer. In this cohort, an effect of hypertension, diabetes, and stroke on the long-term survival of patients with esophageal and gastric cancers was not found.
Supplementary Material
Supplementary Materi
Footnotes
Supplementary Materials
Supplementary information is available only at the official site of the journal.
Author Contributions
Conception and study design: Zhou B, Wang ZX, and Guo MZ. Analysis of the data: Zhou B and Wang ZX. Interpretation of the data: Zhou B, Wang ZX, Guo MZ, Dou QF, Li WB, Li YY, Yan ZQ, and Sun PS. Drafting of the manuscript: Zhou B, Wang ZX, Guo MZ, Li XM, and Shen FF. Critical revision of the article for important intellectual content: Zhou B, Guo MZ, Dou QF, Li XM, Shen FF, Li YY, Li WB, and Zhang BJ. Final approval of the article: all authors. Statistical expertise: Zhou B, Wang ZX, and Guo MZ. Administrative, technical, or logistic support: Shen FF and Zhang BJ. Guo MZ and Zhou B take responsibility for all aspects of the manuscript, and authors take responsibility for their contributions.
Ethics Approval and Consent to Participate
The protocol was approved by the institutional review boards of the participating hospitals. All procedures were performed in accordance with the Declaration of Helsinki. Informed written consent was obtained from all patients before inclusion in the study.
Source of Funding
This study was supported by grants from the National Key Research and Development Program of China (2018YFA0208902, 2020YFC2002705), National Natural Science Foundation of China (81672318, U1604281), and Xinxiang Science and Technology Project (GG2020040).
Conflict of Interest
The authors declare that they have no conflict of interest.
Data Sharing Statement
No additional data available.
References
- 1.GBD. Mortality and Causes of Death Collaborators. Global, regional, and national life expectancy, all-cause mortality, and cause-specific mortality for 249 causes of death, 1980-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet (London, England) 2015;2016;388:1459. doi: 10.1016/S0140-6736(16)31012-1. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029. doi: 10.1002/cncr.33587. –. [DOI] [PubMed] [Google Scholar]
- 3.Gao J, Lu Y, Gokulnath P, Vulugundam G, Li G, Li J. Benefits of physical activity on cardiometabolic diseases in obese children and adolescents. J Transl Intern Med. 2022;10:236. doi: 10.2478/jtim-2022-0041. et al. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sun Y, Sun G, Chang Y. Current status and future direction of community-based management of hypertension in China. J Transl Intern Med. 2021;9:61. doi: 10.2478/jtim-2021-0022. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarfati D, Koczwara B, Jackson C. The impact of comorbidity on cancer and its treatment. CA Cancer J Clin. 2016;66:337. doi: 10.3322/caac.21342. –. [DOI] [PubMed] [Google Scholar]
- 6.Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271. doi: 10.3322/caac.21349. et al. –. [DOI] [PubMed] [Google Scholar]
- 7.Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ. Annual Report to the Nation on the status of cancer, 19752010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer. 2014;120:1290. doi: 10.1002/cncr.28509. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209. doi: 10.3322/caac.21660. et al. –. [DOI] [PubMed] [Google Scholar]
- 9.Sun K, Lv H, Chen B, Nie C, Zhao J, Wang S, Wang J, Xu W, Chen X. Dawning precision treatment for gastric cancer: The latest biomarkers. J Transl Intern Med. 2021;9:228. doi: 10.2478/jtim-2021-0023. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rugbjerg K, Mellemkjaer L, Boice JD, Køber L, Ewertz M, Olsen JH. Cardiovascular disease in survivors of adolescent and young adult cancer: a Danish cohort study, 1943-2009. J Natl Cancer Inst. 2014;106:dju110. doi: 10.1093/jnci/dju110. [DOI] [PubMed] [Google Scholar]
- 11.Armenian SH, Xu L, Ky B, Sun C, Farol LT, Pal SK. Cardiovascular Disease Among Survivors of Adult-Onset Cancer: A Community-Based Retrospective Cohort Study. J Clin Oncol. 2016;34:1122. doi: 10.1200/JCO.2015.64.0409. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strongman H, Gadd S, Matthews A, Mansfield KE, Stanway S, Lyon AR. Medium and long-term risks of specific cardiovascular diseases in survivors of 20 adult cancers: a population-based cohort study using multiple linked UK electronic health records databases. Lancet (London, England) 2019;394:1041. doi: 10.1016/S0140-6736(19)31674-5. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu D, Ma Z, Yang J, Zhao M, Ao H, Zheng X. Prevalence and prognosis significance of cardiovascular disease in cancer patients: a population-based study. Aging. 2019;11:7948. doi: 10.18632/aging.102301. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67:93. doi: 10.3322/caac.21388. et al. –. [DOI] [PubMed] [Google Scholar]
- 15.Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH. Long-Term Outcome of Endoscopic Resection vs. Surgery for Early Gastric Cancer: A Non-inferiority-Matched Cohort Study. Am J Gastroenterol. 2016;111:240. doi: 10.1038/ajg.2015.427. et al. –. [DOI] [PubMed] [Google Scholar]
- 16.Jeon HK, Kim GH, Lee BE, Park DY, Song GA, Kim DH. Long-term outcome of endoscopic submucosal dissection is comparable to that of surgery for early gastric cancer: a propensity-matched analysis. Gastric cancer. 2018;21:133. doi: 10.1007/s10120-017-0719-4. et al. –. [DOI] [PubMed] [Google Scholar]
- 17.Kim SY, Park JM, Cho HS, Cho YK, Choi MG. Assessment of Cimetropium Bromide Use for the Detection of Gastric Neoplasms During Esophagogastroduodenoscopy. JAMA Netw Open. 2022;5:e223827. doi: 10.1001/jamanetworkopen.2022.3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koren-Morag N, Goldbourt U, Tanne D. Relation between the metabolic syndrome and ischemic stroke or transient ischemic attack: a prospective cohort study in patients with atherosclerotic cardiovascular disease. Stroke. 2005;36:1366. doi: 10.1161/01.STR.0000169945.75911.33. –. [DOI] [PubMed] [Google Scholar]
- 19.Akinyemiju T, Moore JX, Judd S, Lakoski S, Goodman M, Safford MM, Pisu M. Metabolic dysregulation and cancer mortality in a national cohort of blacks and whites. BMC Cancer. 2017;17:856. doi: 10.1186/s12885-017-3807-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C, Sun XN, Zhao S, Scherer PE. Crosstalk between adipose tissue and the heart: An update. J Transl Intern Med. 2022;10:219. doi: 10.2478/jtim-2022-0039. - [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leiva O, AbdelHameid D, Connors JM, Cannon CP, Bhatt DL. Common Pathophysiology in Cancer, Atrial Fibrillation, Atherosclerosis, and Thrombosis: JACC: CardioOncology State-of-the-Art Review. JACC CardioOncol. 2021;3:619. doi: 10.1016/j.jaccao.2021.08.011. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau ES, Paniagua SM, Liu E, Jovani M, Li SX, Takvorian K. Cardiovascular Risk Factors are Associated with Future Cancer. JACC CardioOncol. 2021;3:48. doi: 10.1016/j.jaccao.2020.12.003. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leiva O, Newcomb R, Connors JM, Al-Samkari H. Cancer and thrombosis: new insights to an old problem. J Med Vasc. 2020;45(6s):6s8–6s16. doi: 10.1016/S2542-4513(20)30514-9. [DOI] [PubMed] [Google Scholar]
- 24.Hu YF, Liu CJ, Chang PM, Tsao HM, Lin YJ, Chang SL. Incident thromboembolism and heart failure associated with new-onset atrial fibrillation in cancer patients. Int J Cardiol. 2013;165:355. doi: 10.1016/j.ijcard.2012.08.036. et al. –. [DOI] [PubMed] [Google Scholar]
- 25.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV. Risk of Arterial Thromboembolism in Patients With Cancer. J Am Coll Cardiol. 2017;70:926. doi: 10.1016/j.jacc.2017.06.047. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013;62:881. doi: 10.1016/j.jacc.2013.04.088. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Banke A, Schou M, Videbaek L, Møller JE, Torp-Pedersen C, Gustafsson F. Incidence of cancer in patients with chronic heart failure: a longterm follow-up study. Eur J Heart Fail. 2016;18:260. doi: 10.1002/ejhf.472. et al. –. [DOI] [PubMed] [Google Scholar]
- 28.Manohar H, Potter AS, Koutroumpakis E, Deswal A, Palaskas NL. Can We Mitigate Coronary Heart Disease Risk in Patients with Cancer? Curr Atheroscler Rep. 2022;24:599. doi: 10.1007/s11883-022-01035-5. –. [DOI] [PubMed] [Google Scholar]
- 29.Mantel Ä, Holmqvist M, Andersson DC, Lund LH, Askling J. Association Between Rheumatoid Arthritis and Risk of Ischemic and Nonischemic Heart Failure. J Am Coll Cardiol. 2017;69:1275. doi: 10.1016/j.jacc.2016.12.033. –. [DOI] [PubMed] [Google Scholar]
- 30.Simon TA, Thompson A, Gandhi KK, Hochberg MC, Suissa S. Incidence of malignancy in adult patients with rheumatoid arthritis: a meta-analysis. Arthritis Res Ther. 2015;17:212. doi: 10.1186/s13075-015-0728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N Engl J Med. 2017;377:1119. doi: 10.1056/NEJMoa1707914. et al. –. [DOI] [PubMed] [Google Scholar]
- 32.Everett BM, MacFadyen JG, Thuren T, Libby P, Glynn RJ, Ridker PM. Inhibition of Interleukin-1β and Reduction in Atherothrombotic Cardiovascular Events in the CANTOS Trial. J Am Coll Cardiol. 2020;76:1660. doi: 10.1016/j.jacc.2020.08.011. –. [DOI] [PubMed] [Google Scholar]
- 33.Tengesdal IW, Menon DR. Targeting tumor-derived NLRP3 reduces melanoma progression by limiting MDSCs expansion. Proc Natl Acad Sci U S A. 2021;118:e20009151182021. doi: 10.1073/pnas.2000915118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gottschlich A, Endres S, Kobold S. Therapeutic Strategies for Targeting IL-1 in Cancer. Cancers (Basel) 2021;13:477. doi: 10.3390/cancers13030477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ridker PM, MacFadyen JG, Thuren T, Everett BM, Libby P, Glynn RJ. Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet (London, England) 2017;390:1833. doi: 10.1016/S0140-6736(17)32247-X. –. [DOI] [PubMed] [Google Scholar]
- 36.Moloney JN, Cotter TG. ROS signalling in the biology of cancer. Semin Cell Dev Biol. 2018;80:50. doi: 10.1016/j.semcdb.2017.05.023. –. [DOI] [PubMed] [Google Scholar]
- 37.Pastori D, Marang A, Bisson A, Menichelli D, Herbert J, Lip GYH. Thromboembolism, mortality, and bleeding in 2,435,541 atrial fibrillation patients with and without cancer: A nationwide cohort study. Cancer. 2021;127:2122. doi: 10.1002/cncr.33470. et al. –. [DOI] [PubMed] [Google Scholar]
- 38.Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, Brønnum D. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med. 2013;368:987. doi: 10.1056/NEJMoa1209825. et al. –. [DOI] [PubMed] [Google Scholar]
- 39.Yakupovich A, Davison MA, Kharouta MZ, Turian J, Seder CW, Batus M. Heart dose and coronary artery calcification in patients receiving thoracic irradiation for lung cancer. J Thorac Dis. 2020;12:223. doi: 10.21037/jtd.2020.01.52. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128:1927. doi: 10.1161/CIR.0b013e3182a88099. et al. –. [DOI] [PubMed] [Google Scholar]
- 41.Herrmann J, Yang EH, Iliescu CA, Cilingiroglu M, Charitakis K, Hakeem A. Vascular Toxicities of Cancer Therapies: The Old and the New-An Evolving Avenue. Circulation. 2016;133:1272. doi: 10.1161/CIRCULATIONAHA.115.018347. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bar J, Markel G, Gottfried T, Percik R, Leibowitz-Amit R, Berger R. Acute vascular events as a possibly related adverse event of immunotherapy: a single-institute retrospective study. Eur J Cancer. 2019;120:122. doi: 10.1016/j.ejca.2019.06.021. et al. –. [DOI] [PubMed] [Google Scholar]
- 43.Drobni ZD, Alvi RM, Taron J, Zafar A, Murphy SP, Rambarat PK. Association Between Immune Checkpoint Inhibitors With Cardiovascular Events and Atherosclerotic Plaque. Circulation. 2020;142:2299. doi: 10.1161/CIRCULATIONAHA.120.049981. et al. –. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Materi